The Effect of Body Mass Index on Melanoma Biology, Immunotherapy Efficacy, and Clinical Outcomes: A Narrative Review
Abstract
1. Introduction
2. Results
2.1. Obesity and Melanoma Biology
2.1.1. Obesity-Induced Pathophysiological States and Immune Dysfunction
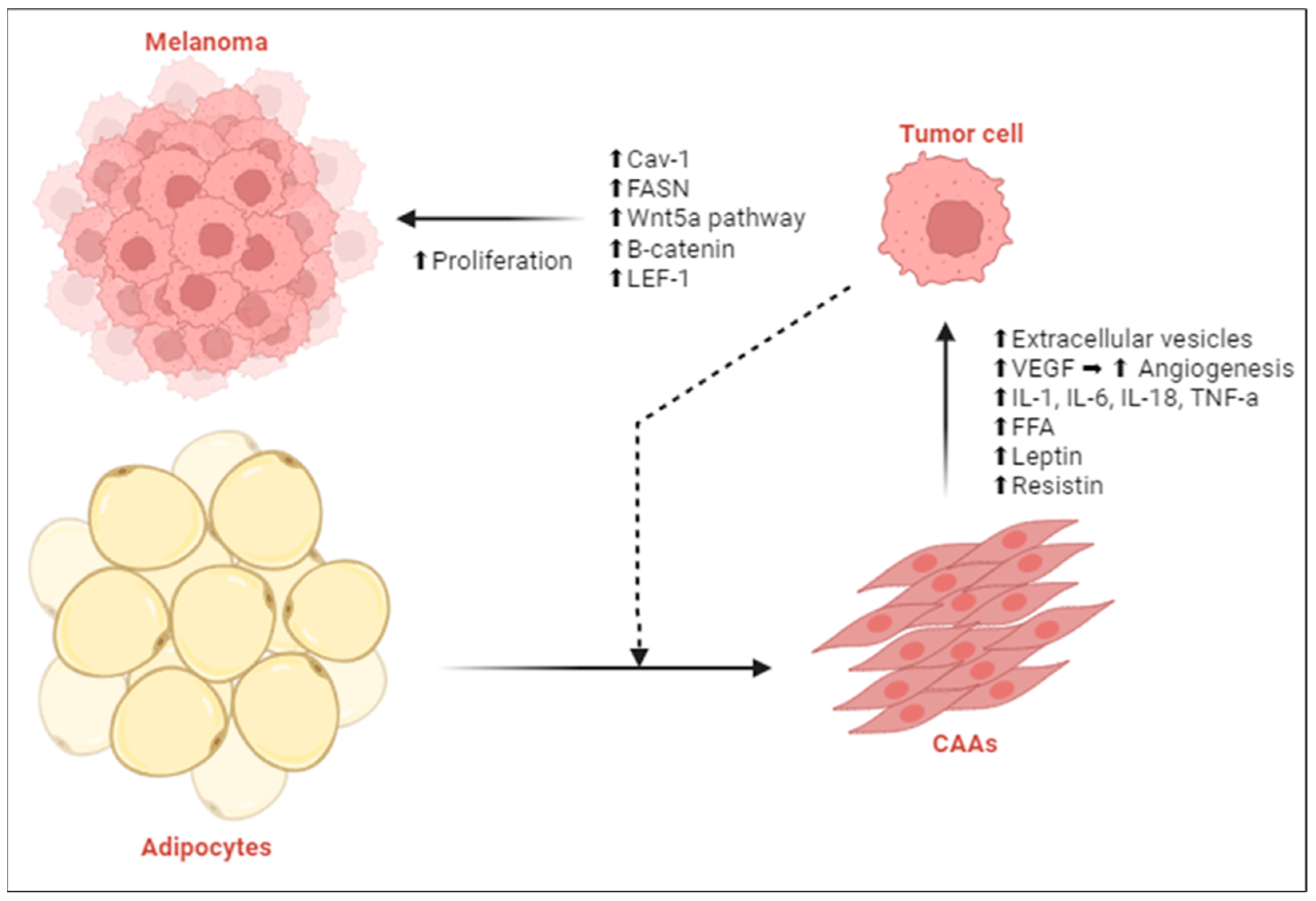
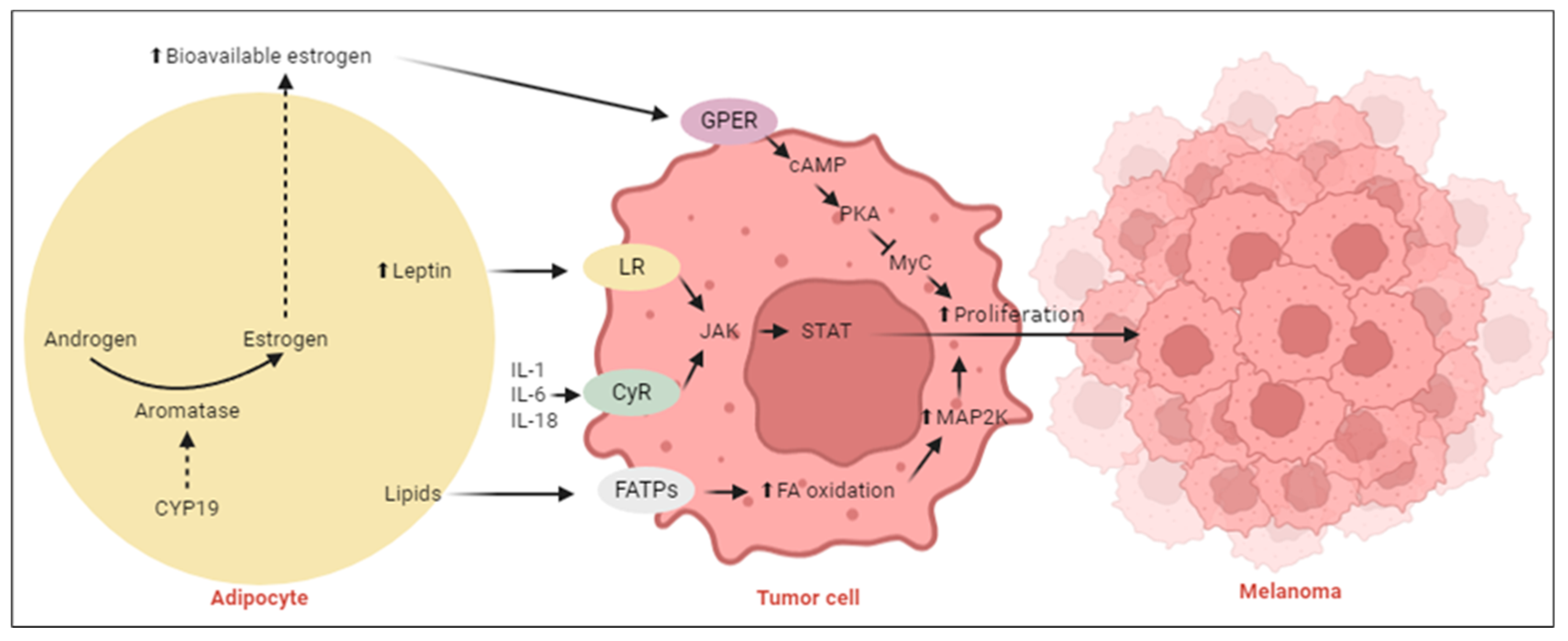
2.1.2. Adipocyte-Rich Tumor Microenvironment Alters Melanoma Metabolism
2.2. BMI and Melanoma Prognosis
3. Discussion
3.1. Melanoma Biology
3.2. Melanoma Prognosis
3.3. Confounding Variables: “Explaining the Inconsistencies”
3.3.1. Age-Related Variability and Gut Microbiome
3.3.2. Sex-Based Differences
3.3.3. Vitamin D, Sunlight, and Diet
3.3.4. Skeletal Muscle Mass and Sarcopenia
3.4. Clinical Implications: “When Fat Becomes Favorable”
3.5. Strengths and Limitations
3.6. Future Directions
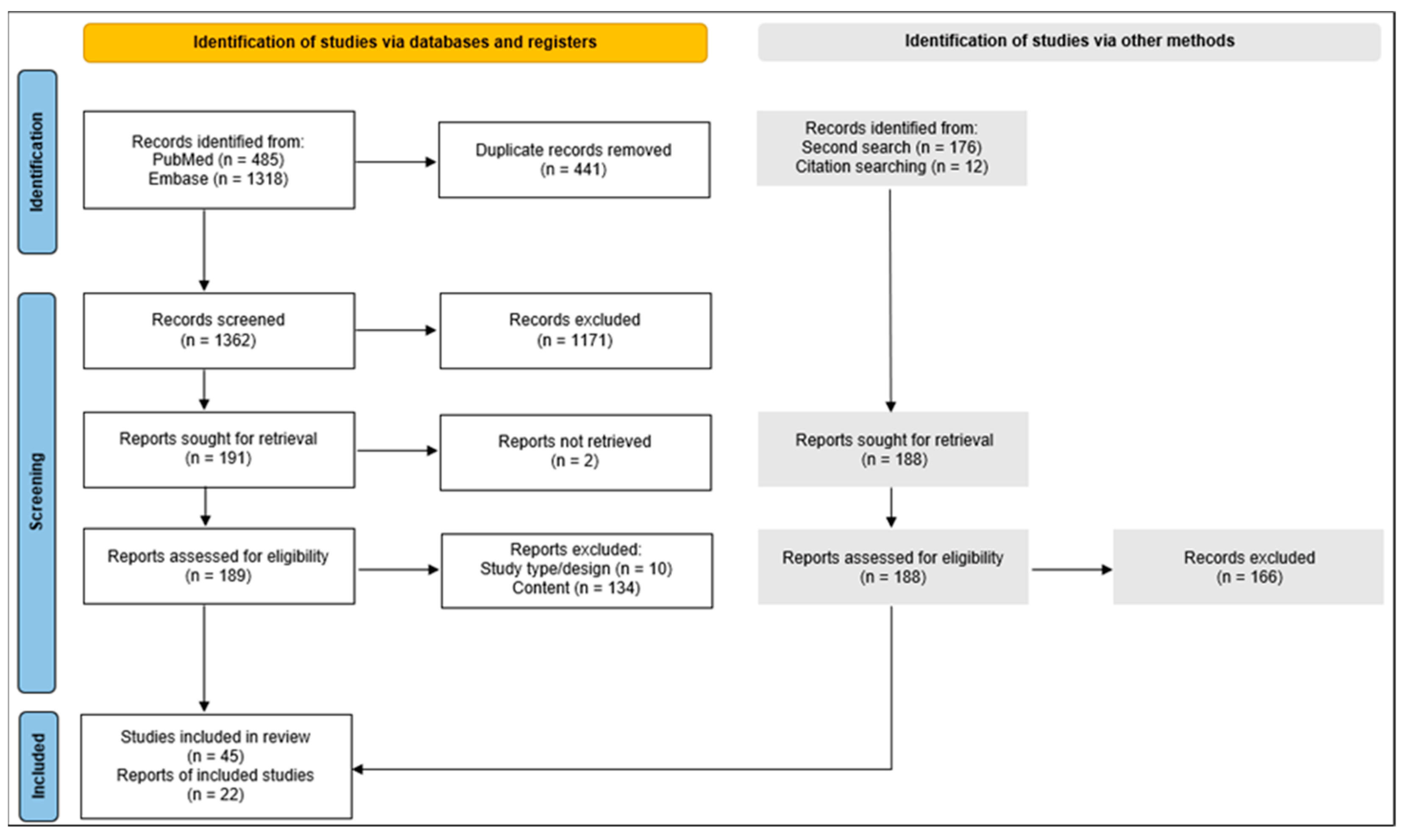
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMS3 | Acyl-CoA synthetase medium-chain 3 |
| AMPK | AMP-activated protein kinase |
| AR | Androgen receptor |
| BMI | Body mass index |
| CAA | Cancer-associated adipocytes |
| Cav-1 | Caveolin-1 |
| CM | Cutaneous melanoma |
| CRP | C-reactive protein |
| ER | Estrogen receptor |
| FAs | Fatty acids |
| FASN | Fatty acid synthase |
| FFA | Free fatty acid |
| ICI | Immune checkpoint inhibitor |
| IGF-1 | Insulin-like growth factor-1 |
| IL-1 | Interleukin-1 |
| IL-6 | Interleukin-6 |
| IL-18 | Interleukin-18 |
| irAEs | Immune-related adverse events |
| LEF-1 | Lymphoid enhancer-binding factor 1 |
| LDL | Low-density lipoproteins |
| mTOR | Mammalian target of rapamycin |
| OS | Overall survival |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROS | Reactive oxygen species |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor alpha |
| TNM | Tumor, Node, Metastasis |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| VEGF | Vascular endothelial growth factor |
| Wnt5a | Wnt family member 5A |
| β-cat | Beta-catenin |
Appendix A
| Database | Search String |
| PubMed | (“Melanoma”[Mesh] OR “melanoma*”[tiab] OR “melanocarcinoma* ”[tiab] OR “melano-carcinoma*”[tiab] OR “Melanoma, Cutaneous Malignant”[Supplementary Concept] OR “melanomalignoma*”[tiab] OR “naevocarcinoma*”[tiab] OR “nevocarcinoma*”[tiab] AND (“Body Mass Index”[Mesh] OR “body mass”[tiab] OR “quetelet*”[tiab] OR “BMI”[tiab] OR “thinness”[Mesh] OR “thinness”[tiab] OR “leanness”[tiab] OR “underweight”[tiab] OR “ideal body weight”[Mesh] OR “body weight”[tiab] OR “healthy weight”[tiab] OR “overweight”[Mesh] OR “overweight”[tiab] OR “obesity”[Mesh] OR “obes*”[tiab] OR “Adipose Tissue”[Mesh] OR “adipose tissue”[tiab] OR “fatty tissue”[tiab] OR “fat tissue”[tiab] OR “body fat”[tiab] OR “fat deposition”[tiab] OR “Body Fat Distribution”[Mesh] OR “body fat distribution”[tiab] OR “adiposity”[tiab] OR “beige fat”[tiab] OR “white fat”[tiab] OR “abdominal fat”[tiab] OR “subcutaneous fat”[tiab] OR “brown fat”[tiab] OR “Skinfold Thickness”[Mesh] OR “skinfold thickness*”[tiab] OR “skin fold thickness*”[tiab] OR “skin fold measurement*”[tiab ] OR “skinfold measurement*”[tiab])) NOT “Uveal melanoma”[Supplementary Concept] OR “uveal-melanoma*”[tiab] OR “eye-melanoma*”[tiab] OR “Mucous Membrane”[Mesh] OR “mucous-membrane*”[tiab] OR “mucosa”[tiab] OR “central nervous system melanoma*”[tiab]. |
| Embase | ((‘Melanoma’/exp OR ‘melanoma*’:ti,ab,kw OR ‘melanocarcinoma*’:ti,ab,kw OR ‘melano-carcinoma*’:ti,ab,kw OR ‘melanomalignoma*’:ti,ab,kw OR ‘cutaneous melanoma’/exp OR ‘naevocarcinoma*’:ti,ab,kw OR ‘nevocarcinoma*’:ti,ab,kw AND (‘Body mass’/exp OR ‘body mass’:ti,ab,kw OR ‘BMI’:ti,ab,kw OR ‘quetelet* index’:ti,ab,kw OR ‘BMI chart’/exp OR ‘BMI’:ti,ab,kw OR ‘underweight’/exp OR ‘underweight’:ti,ab,kw OR ‘thinness’:ti,ab,kw OR ‘leanness’:ti,ab,kw OR ‘ideal body weight’/exp OR ‘body weight’:ti,ab,kw OR ‘healthy weight’:ti,ab,kw OR ‘obesity’/exp OR ‘obes*’:ti,ab,kw OR ‘overweight’:ti,ab,kw OR ‘Adipose tissue’/de OR ‘adipose tissue’:ti,ab,kw OR ‘fatty tissue’:ti,ab,kw OR ‘fat tissue’:ti,ab,kw OR ‘body fat’/exp OR ‘body fat’:ti,ab,kw OR ‘fat deposition’:ti,ab,kw OR ‘body fat distribution’/exp OR ‘body fat distribution’:ti,ab,kw OR ‘adiposity’:ti,ab,kw OR ‘beige adipose tissue’/exp OR ‘beige fat’:ti,ab,kw OR ‘white adipose tissue’/exp OR ‘white fat’:ti,ab,kw OR ‘abdominal fat’/exp OR ‘abdominal fat’:ti,ab,kw OR ‘subcutaneous fat’/exp OR ‘subcutaneous fat’:ti,ab,kw OR ‘brown adipose tissue’/exp OR ‘brown fat’:ti,ab,kw OR ‘skinfold thickness’/exp OR ‘skinfold thickness*’:ti,ab,kw OR ‘skin fold thickness*’:ti,ab,kw OR ‘skin fold measurement*’:ti,ab,kw OR ‘skinfold measurement*’:ti,ab,kw)) NOT ‘Eye melanoma’/exp OR ‘eye melanoma*’:ti,ab,kw OR ‘uveal melanoma*’:ti,ab,kw OR ‘mucosa’/exp OR ‘mucosa’:ti,ab,kw OR ‘mucous membrane*’:ti,ab,kw OR ‘central nervous system melanoma’/exp OR ‘central nervous system melanoma*’:ti,ab,kw) NOT (‘conference abstract’:it OR ‘editorial’:it OR ‘letter’:it). |
Appendix B
| Inclusion criteria |
|
| Exclusion criteria |
|
References
- Long, G.V.; Swetter, S.M.; Menzies, A.M.; Gershenwald, J.E.; Scolyer, R.A. Cutaneous melanoma. Lancet 2023, 402, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, G.; Morrison, S.; Leong, S.P.; Kashani-Sabet, M.; Vetto, J.; White, R.; Schneebaum, S.; Pockaj, B.; Mozzillo, N.; et al. Factors predicting survival in thick melanoma: Do all thick melanomas have the same prognosis? Surgery 2020, 168, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed]
- Almeida Oliveira, F.; Klose, J.; Schulze, H.J.; Ribeiro Teixeira, M.; Dermietzel, A.; Wellenbrock, S.; Herter-Sprie, G.-S.; Hirsch, T.; Kueckelhaus, M. The Influence of Obesity on Melanoma and Sentinel Lymph Node Diagnosis: A Retrospective Monocentric Study in 1001 Patients. Cancers 2023, 15, 1806. [Google Scholar] [CrossRef] [PubMed]
- Skowron, F.; Bérard, F.; Balme, B.; Maucort-Boulch, D. Role of obesity on the thickness of primary cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Zepeda-Najar, C.; Lino-Silva, L.S.; Chávez-Hernandez, J.D.; Salcedo-Hernández, R.A.; Jimenez-Sánchez, J.P.; Fernández-Sánchez, C.E.; Valdez-Aguilar, C.D. The influence of body mass index on the survival of patients with melanoma. A cross-sectional study of 707 patients. Contemp. Oncol. 2021, 25, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Harrell Shreckengost, C.S.; Tariq, M.; Farley, C.R.; Zhang, C.; Delman, K.A.; Kudchadkar, R.R.; Lowe, M.C. The Impact of Obesity on Surgically Treated Locoregional Melanoma. Ann. Surg. Oncol. 2021, 28, 6140–6151. [Google Scholar] [CrossRef] [PubMed]
- Cassano, N.; Caccavale, S.; Vena, G.A.; Argenziano, G. Body Mass Index and Melanoma Prognosis. Dermatol. Pract. Concept. 2021, 11, e2021106. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cortellini, A.; Indini, A.; Tomasello, G.; Ghidini, M.; Nigro, O.; Salati, M.; Dottorini, L.; Iaculli, A.; Varricchio, A.; et al. Association of Obesity with Survival Outcomes in Patients with Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021, 4, e213520. [Google Scholar] [CrossRef]
- An, Y.; Wu, Z.; Wang, N.; Yang, Z.; Li, Y.; Xu, B.; Sun, M. Association between body mass index and survival outcomes for cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. J. Transl. Med. 2020, 18, 235. [Google Scholar] [CrossRef]
- Nie, R.C.; Chen, G.M.; Wang, Y.; Yuan, S.Q.; Zhou, J.; Duan, J.L.; Liu, W.W.; Chen, S.; Cai, M.Y.; Li, Y.F. Association between Body Mass Index and Survival Outcomes in Patients Treated with Immune Checkpoint Inhibitors: Meta-analyses of Individual Patient Data. J. Immunother. 2021, 44, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, J.A.F.; Pasquarelli-do-Nascimento, G.; Duarte, M.S.V.; Bonamino, M.H.; Magalhães, K.G. The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J. Biomed. Sci. 2022, 29, 12. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.; FAl Marjani, M.; Al Natour, D.; Abuelshayeb, L.; Rayan, A.; Mahmod, A. Obesity and Cancer: An Underestimated Toxic Relationship. Farmacia 2023, 71, 3. [Google Scholar] [CrossRef]
- Smith, L.K.; Arabi, S.; Lelliott, E.J.; McArthur, G.A.; Sheppard, K.E. Obesity and the Impact on Cutaneous Melanoma: Friend or Foe? Cancers 2020, 12, 1583. [Google Scholar] [CrossRef] [PubMed]
- Güvenç, C.; Antoranz, A.; Szumera-Ciećkiewicz, A.; Teterycz, P.P.; Rutkowski, P.R.; Rawson, R.V.; Scolyer, R.A.; Thompson, J.F.; Newton-Bishop, J.; Stas, M.; et al. Road to Metastasis: The TWEAK Pathway as a Discriminant between Metastasizing and Non-Metastasizing Thick Melanomas. Int. J. Mol. Sci. 2021, 22, 1583. [Google Scholar] [CrossRef] [PubMed]
- Barakat, R.; Oakley, O.; Kim, H.; Jin, J.; Ko, C.J. Extra-gonadal sites of estrogen biosynthesis and function. BMB Rep. 2016, 49, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Burgess, S. Causal role of high body mass index in multiple chronic diseases: A systematic review and meta-analysis of Mendelian randomization studies. BMC Med. 2021, 19, 320. [Google Scholar] [CrossRef] [PubMed]
- Olszańska, J.; Pietraszek-Gremplewicz, K.; Nowak, D. Melanoma Progression under Obesity: Focus on Adipokines. Cancers 2021, 13, 2281. [Google Scholar] [CrossRef]
- Malvi, P.; Chaube, B.; Singh, S.V.; Mohammad, N.; Vijayakumar, M.V.; Singh, S.; Chouhan, S.; Bhat, M.K. Elevated circulatory levels of leptin and resistin impair therapeutic efficacy of dacarbazine in melanoma under obese state. Cancer Metab. 2018, 6, 2. [Google Scholar] [CrossRef]
- Roccuzzo, G.; Moirano, G.; Fava, P.; Maule, M.; Ribero, S.; Quaglino, P. Obesity and immune-checkpoint inhibitors in advanced melanoma: A meta-analysis of survival outcomes from clinical studies. Semin. Cancer Biol. 2023, 91, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Bowers, L.W. The role of immune dysfunction in obesity-associated cancer risk, progression, and metastasis. Cell Mol. Life Sci. 2021, 78, 3423–3442. [Google Scholar] [CrossRef]
- Molinelli, E.; Ceccarelli, G.; Fantone, S.; Di Mercurio, E.; Gambini, D.; Maurizi, A.; Perugini, J.; Tossetta, G.; Brisigotti, V.; De Simoni, E.; et al. Melanoma and subcutaneous adipose tissue: Role of peritumoral adipokines in disease characterization and prognosis. Pigment. Cell Melanoma. Res. 2023, 36, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Incorvaia, L.; Rinaldi, G.; Badalamenti, G.; Cucinella, A.; Brando, C.; Madonia, G.; Fiorino, A.; Pipitone, A.; Perez, A.; Li Pomi, F.; et al. Prognostic role of soluble PD-1 and BTN2A1 in overweight melanoma patients treated with nivolumab or pembrolizumab: Finding the missing links in the symbiotic immune-metabolic interplay. Ther. Adv. Med. Oncol. 2023, 15, 17588359231151845. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Indini, A.; De Luca, M.; Merelli, B.; Mariuk-Jarema, A.; Teterycz, P.; Rogala, P.; Lugowska, I.; Cybulska-Stopa, B.; Labianca, A.; et al. Body mass index (BMI) and outcome of metastatic melanoma patients receiving targeted therapy and immunotherapy: A multicenter international retrospective study. J. Immunother. Cancer 2020, 8, e001117. [Google Scholar] [CrossRef]
- Khojandi, N.; Kuehm, L.M.; Piening, A.; Donlin, M.J.; Hsueh, E.C.; Schwartz, T.L.; Farrell, K.; Richart, J.M.; Geerling, E.; Pinto, A.K.; et al. Oxidized Lipoproteins Promote Resistance to Cancer Immunotherapy Independent of Patient Obesity. Cancer Immunol. Res. 2021, 9, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Grabacka, M.; Plonka, P.M.; Reiss, K. Melanoma-Time to fast or time to feast? An interplay between PPARs, metabolism and immunity. Exp. Dermatol. 2020, 29, 436–445. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, P.; Zhang, L. Expression of acyl-CoA synthetase medium-chain 3 is associated with obesity in melanoma patients and correlates with androgen receptor. Dermatol. Sinica 2023, 41, 87–93. [Google Scholar] [CrossRef]
- Zhu, X.; Zeng, Z.; Chen, M.; Chen, X.; Hu, D.; Jiang, W.; Du, M.; Chen, T.; Chen, T.; Liao, W.; et al. TREM2 as a Potential Immune-Related Biomarker of Prognosis in Patients with Skin Cutaneous Melanoma Microenvironment. Dis. Markers 2023, 2023, 8101837. [Google Scholar] [CrossRef]
- Zoico, E.; Darra, E.; Rizzatti, V.; Tebon, M.; Franceschetti, G.; Mazzali, G.; Rossi, A.P.; Fantin, F.; Zamboni, M. Role of adipose tissue in melanoma cancer microenvironment and progression. Int. J. Obes. 2018, 42, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Robado de Lope, L.; Alcíbar, O.L.; Amor López, A.; Hergueta-Redondo, M.; Peinado, H. Tumour-adipose tissue crosstalk: Fuelling tumour metastasis by extracellular vesicles. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 29158314. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.A.; Encarnação, C.; Franco, V.A.; Xavier Botelho, L.G.; Rodrigues, G.P.; Ramos-Andrade, I.; Barja-Fidalgo, C.; Renovato-Martins, M. Adipose Tissue-Derived Extracellular Vesicles and the Tumor Microenvironment: Revisiting the Hallmarks of Cancer. Cancers 2021, 13, 3328. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.W.; Menk, A.V.; Rivadeneira, D.B.; Augustin, R.C.; Xu, M.; Li, J.; Wu, X.; Mishra, A.K.; Gide, T.N.; Quek, C.; et al. Obesity Is Associated with Altered Tumor Metabolism in Metastatic Melanoma. Clin. Cancer Res. 2023, 29, 154–164. [Google Scholar] [CrossRef]
- Clement, E.; Lazar, I.; Muller, C.; Nieto, L. Obesity and melanoma: Could fat be fueling malignancy? Pigment. Cell Melanoma. Res. 2017, 30, 294–306. [Google Scholar] [CrossRef]
- You, Y.; Jiang, C.; Peng, K.; He, W.; Wang, L.; Jin, Y.; Xia, L. The predictive value of body mass index on prognosis and adverse events of cancers treated with immunotherapy: A systematic review and meta-analysis. Cancer Immunol. Immunother. 2021, 70, 2323–2335. [Google Scholar] [CrossRef]
- Xu, H.; Cao, D.; He, A.; Ge, W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: A pooled analysis of 4090 cancer patients. Int. Immunopharmacol. 2019, 74, 105745. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Santini, D.; Buti, S.; Tiseo, M.; Cannita, K.; Perrone, F.; Giusti, R.; De Tursi, M.; Zoratto, F.; et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/Programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: A multicentre analysis of immune-related adverse events. Eur. J. Cancer 2020, 128, 17–26. [Google Scholar] [PubMed]
- Bastacky, M.L.; Wang, H.; Fortman, D.; Rahman, Z.; Mascara, G.P.; Brenner, T.; Najjar, Y.G.; Luke, J.J.; Kirkwood, J.M.; Zarour, H.M.; et al. Immune-Related Adverse Events in PD-1 Treated Melanoma and Impact upon Anti-Tumor Efficacy: A Real World Analysis. Front. Oncol. 2021, 11, 749064. [Google Scholar] [CrossRef]
- Nitipir, C.; Orlov-Slavu, C.; Alecu, L.; Slavu, I.; Pantea-Stoian, A.; Celmare, I.D.; Olaru, M.; Calu, V.; Suceveanu, A.-I.; Mazilu, L.; et al. Possible Influence of Weight Gain and Creatinine Levels in Predicting Response to Nivolumab: A Multicenter Analysis. Metabolites 2020, 10, 510. [Google Scholar] [CrossRef]
- Kondo, T.; Nomura, M.; Otsuka, A.; Nonomura, Y.; Kaku, Y.; Matsumoto, S.; Muto, M. Predicting marker for early progression in unresectable melanoma treated with nivolumab. Int. J. Clin. Oncol. 2019, 24, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Deckers, E.A.; Kruijff, S.; Bastiaannet, E.; van Ginkel, R.J.; Hoekstra-Weebers, J.; Hoekstra, H.J. Obesity is not associated with disease-free interval, melanoma-specific survival, or overall survival in patients with clinical stage IB-II melanoma after SLNB. J. Surg. Oncol. 2021, 124, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, Y.; Dalle, S.; Mortier, L.; Dereure, O.; Dalac, S.; Dutriaux, C.; Leccia, M.-T.; Legoupil, D.; Saiag, P.; Brunet-Possenti, F.; et al. Relevance of body mass index as a predictor of systemic therapy outcomes in metastatic melanoma: Analysis of the MelBase French cohort data. Ann. Oncol. 2021, 32, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Hyung, S.; Lee, J.; Choi, S.H. Visceral adiposity and systemic inflammation in the obesity paradox in patients with unresectable or metastatic melanoma undergoing immune checkpoint inhibitor therapy: A retrospective cohort study. J. Immunother. Cancer 2022, 10, e005226. [Google Scholar] [CrossRef] [PubMed]
- Antoun, S.; Lanoy, E.; Ammari, S.; Farhane, S.; Martin, L.; Robert, C.; Planchard, D.; Routier, E.; Voisin, A.L.; Messayke, S.; et al. Protective effect of obesity on survival in cancers treated with immunotherapy vanishes when controlling for type of cancer, weight loss and reduced skeletal muscle. Eur. J. Cancer 2023, 178, 49–59. [Google Scholar] [CrossRef] [PubMed]
- McQuade, J.L.; Daniel, C.R.; Hess, K.R.; Mak, C.; Wang, D.Y.; Rai, R.R.; Park, J.J.; Haydu, L.E.; Spencer, C.; Wongchenko, M.; et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol. 2018, 19, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.S.; Waikar, S.S.; Johnson, A.E.W.; Buchbinder, E.I.; Haq, R.; Hodi, F.S.; Schoenfeld, J.D.; Ott, P.A. Complex inter-relationship of body mass index, gender and serum creatinine on survival: Exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J. Immunother. Cancer 2019, 7, 89. [Google Scholar] [CrossRef] [PubMed]
- Dakup, P.P.; Greer, A.J.; Gaddameedhi, S. Let’s talk about sex: A biological variable in immune response against melanoma. Pigment. Cell Melanoma. Res. 2022, 35, 268–279. [Google Scholar] [CrossRef]
- Sergentanis, T.N.; Antoniadis, A.G.; Gogas, H.J.; Antonopoulos, C.N.; Adami, H.O.; Ekbom, A.; Petridou, E.T. Obesity and risk of malignant melanoma: A meta-analysis of cohort and case-control studies. Eur. J. Cancer. 2013, 49, 642–657. [Google Scholar] [CrossRef]
- Smalley, K.S. Why do women with melanoma do better than men? eLife 2018, 7, e33511. [Google Scholar] [CrossRef] [PubMed]
- Hieken, T.J.; Glasgow, A.E.; Enninga, E.A.L.; Kottschade, L.A.; Dronca, R.S.; Markovic, S.N.; Block, M.S.; Habermann, E.B. Sex-Based Differences in Melanoma Survival in a Contemporary Patient Cohort. J. Womens. Health 2020, 29, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.B.; McQuade, J.L. Modifiable Host Factors in Melanoma: Emerging Evidence for Obesity, Diet, Exercise, and the Microbiome. Curr. Oncol. Rep. 2019, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Youn, S.; Eurich, D.T.; McCall, M.; Walker, J.; Smylie, M.; Sawyer, M.B. Skeletal muscle is prognostic in resected stage III malignant melanoma. Clin. Nutr. 2022, 41, 1066–1072. [Google Scholar] [CrossRef]
- Baiden-Amissah, R.E.M.; Tuyaerts, S. Contribution of Aging, Obesity, and Microbiota on Tumor Immunotherapy Efficacy and Toxicity. Int. J. Mol. Sci. 2019, 20, 3586. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, N.; Vallianou, N.; Kadillari, J.; Dalamaga, M. The interplay of obesity, gut microbiome and diet in the immune check point inhibitors therapy era. Semin. Cancer Biol. 2021, 73, 356–376. [Google Scholar] [CrossRef]
- Vandoni, G.; D’Amico, F.; Fabbrini, M.; Mariani, L.; Sieri, S.; Casirati, A.; Di Guardo, L.; Del Vecchio, M.; Anichini, A.; Mortarini, R.; et al. Gut Microbiota, Metabolome, and Body Composition Signatures of Response to Therapy in Patients with Advanced Melanoma. Int. J. Mol. Sci. 2023, 24, 11611. [Google Scholar] [CrossRef]
- De Smedt, J.; Van Kelst, S.; Janssen, L.; Marasigan, V.; Boecxstaens, V.; Stas, M.; Vanderschueren, D.; Guler, I.; Bogaerts, K.; Vandenberghe, K.; et al. Determinants of 25-hydroxyvitamin D Status in a Cutaneous Melanoma Population. Acta Derm. Venereol. 2022, 102, adv00692. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Aune, D.; Schlesinger, S. 25-Hydroxyvitamin D status, vitamin D intake, and skin cancer risk: A systematic review and dose-response meta-analysis of prospective studies. Sci. Rep. 2020, 10, 13151. [Google Scholar] [CrossRef]
- Stenehjem, J.S.; Støer, N.C.; Ghiasvand, R.; Grimsrud, T.K.; Babigumira, R.; Rees, J.R.; Nilsen, L.T.; Johnsen, B.; Thorsby, P.M.; Veierød, M.B.; et al. Prediagnostic serum 25-hydroxyvitamin D and melanoma risk. Sci. Rep. 2020, 10, 20129. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.C.B.; Malt, M.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Diet quality is associated with primary melanoma thickness. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1745–1750. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; D’Eusebio, C.; Ponzo, V.; Tonella, L.; Finocchiaro, C.; Fierro, M.T.; Quaglino, P.; Bo, S. Nutritional Interventions for Patients with Melanoma: From Prevention to Therapy-An Update. Nutrients 2021, 13, 4018. [Google Scholar] [CrossRef] [PubMed]
- Loosen, S.H.; van den Bosch, V.; Gorgulho, J.; Schulze-Hagen, M.; Kandler, J.; Jördens, M.S.; Tacke, F.; Loberg, C.; Antoch, G.; Brümmendorf, T.; et al. Progressive Sarcopenia Correlates with Poor Response and Outcome to Immune Checkpoint Inhibitor Therapy. J. Clin. Med. 2021, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Miyazawa, H.; Yanagi, T.; Maeda, T.; Kitamura, S.; Ujiie, H. Association between weight loss and death in patients with malignant melanoma: A retrospective study of 28 cases. J. Dermatol. 2024, 51, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]

| Reference | Obesity | Melanoma Biology |
|---|---|---|
| Olszanska [19] Malvi [20] Molinelli [23] | Adipokine dysregulation | ⬆ Inflammation ⬆ Immune dysfunction ⬆ Tumor growth ⬆ Melanoma proliferation |
| Kulkarni [22] | Macrophage polarization | ⬆ Melanoma proliferation |
| Talib [13] Smith [14] Assumpção [12] | Chronic inflammation | ⬆ Insulin resistance ⬆ Adipokine dysregulation ⬆ Tumor growth ⬆ Melanoma proliferation |
| Wang [24] Roccuzzo [21] Incorvaia [25] | T cell dysfunction | ⬆ Efficacy of immunotherapy |
| Khojandi [27] | Oxidized LDL | ⬆ T cell suppression ⬆ Melanoma’s immune escape |
| Grabacka [28] | PPARα upregulation | ⬆ Prognosis |
| Zheng [29] | ACSM3 upregulation | |
| Zhu [30] | TREM2 upregulation | |
| Zoico [31] | ⬆ CAAs | ⬆ Activation of Cav-1, Wnt5a, β-cat, LEF-1 ⬆ Tumor growth ⬆ Melanoma proliferation |
| Robado de Lope [32] Moraes [33] | ⬆ Extracellular vesicles | ⬆ Pro-inflammatory proteins and FAs ⬆ Tumor growth ⬆ Melanoma proliferation |
| Reference | BMI Cohorts | Melanoma Prognosis |
|---|---|---|
| Petrelli [9] |
| BMI ≥ 30 kg/m2 ➡ improved survival (OS) |
| You [36] |
| BMI ≥ 25 kg/m2 ➡ improved survival (OS, PFS), irAEs ⬆ |
| Cortellini [38] | ||
| Bastacky [39] | ||
| An [10] | BMI ≥ 25 kg/m2 ➡ improved survival (OS, PFS) | |
| Nie [11] | ||
| Zepeda-Najar [6] | ||
| Antoun [46] | ||
| McQuade [47] | ||
| Rutkowski [26] | No survival advantage | |
| Deckers [43] | ||
| Di Filippo [44] | ||
| Lee [45] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, J.; Garmyn, M.; Güvenç, C. The Effect of Body Mass Index on Melanoma Biology, Immunotherapy Efficacy, and Clinical Outcomes: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 6433. https://doi.org/10.3390/ijms25126433
Jansen J, Garmyn M, Güvenç C. The Effect of Body Mass Index on Melanoma Biology, Immunotherapy Efficacy, and Clinical Outcomes: A Narrative Review. International Journal of Molecular Sciences. 2024; 25(12):6433. https://doi.org/10.3390/ijms25126433
Chicago/Turabian StyleJansen, Jente, Marjan Garmyn, and Canan Güvenç. 2024. "The Effect of Body Mass Index on Melanoma Biology, Immunotherapy Efficacy, and Clinical Outcomes: A Narrative Review" International Journal of Molecular Sciences 25, no. 12: 6433. https://doi.org/10.3390/ijms25126433
APA StyleJansen, J., Garmyn, M., & Güvenç, C. (2024). The Effect of Body Mass Index on Melanoma Biology, Immunotherapy Efficacy, and Clinical Outcomes: A Narrative Review. International Journal of Molecular Sciences, 25(12), 6433. https://doi.org/10.3390/ijms25126433






