Anti-Obesity Properties of Blackberries Fermented with L. plantarum JBMI F5 via Suppression of Adipogenesis Signaling Mechanisms
Abstract
1. Introduction
2. Results
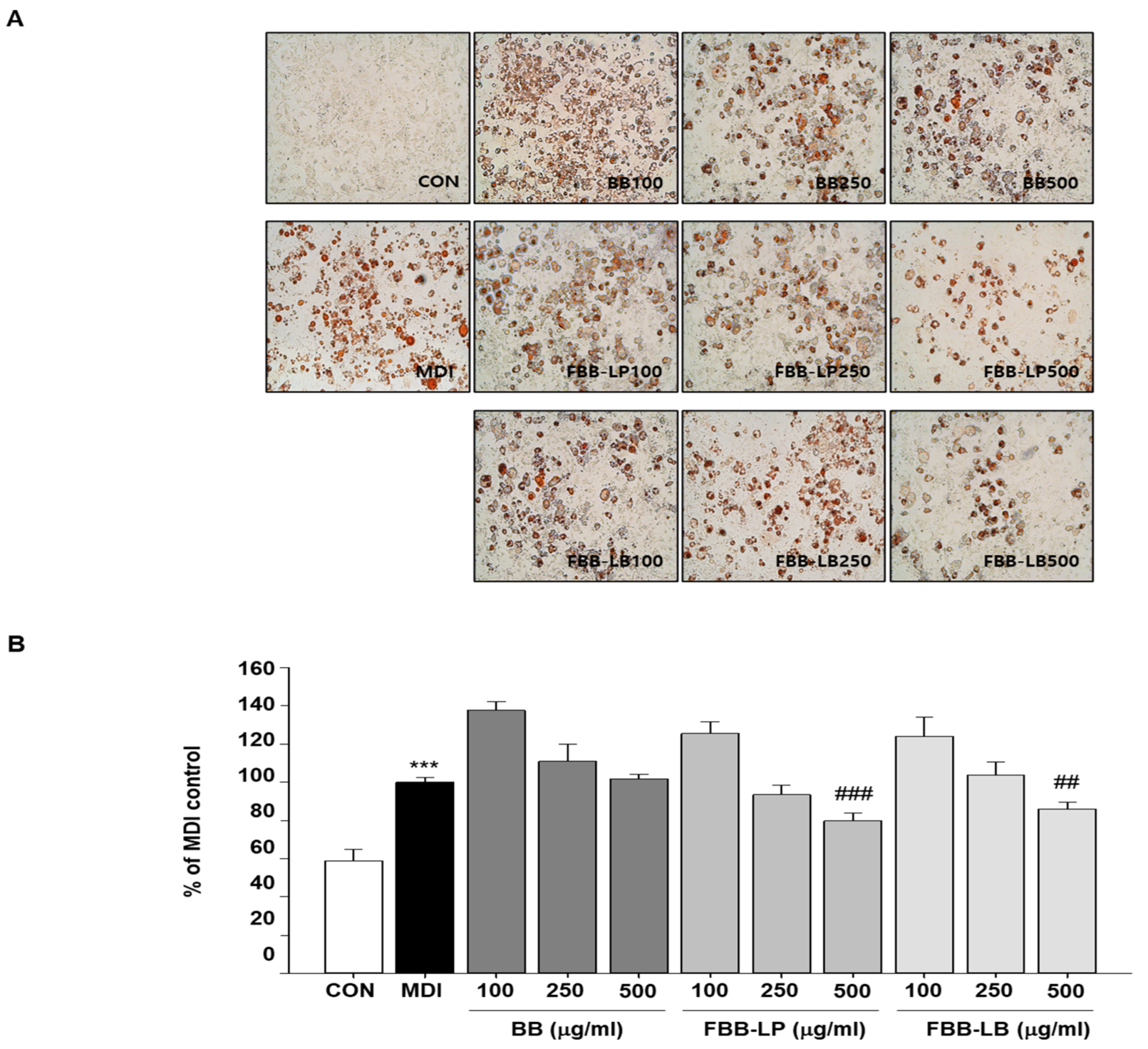
2.1. Effects of Fermented Blackberries Administration on Adipogenesis and Lipid Accumulation
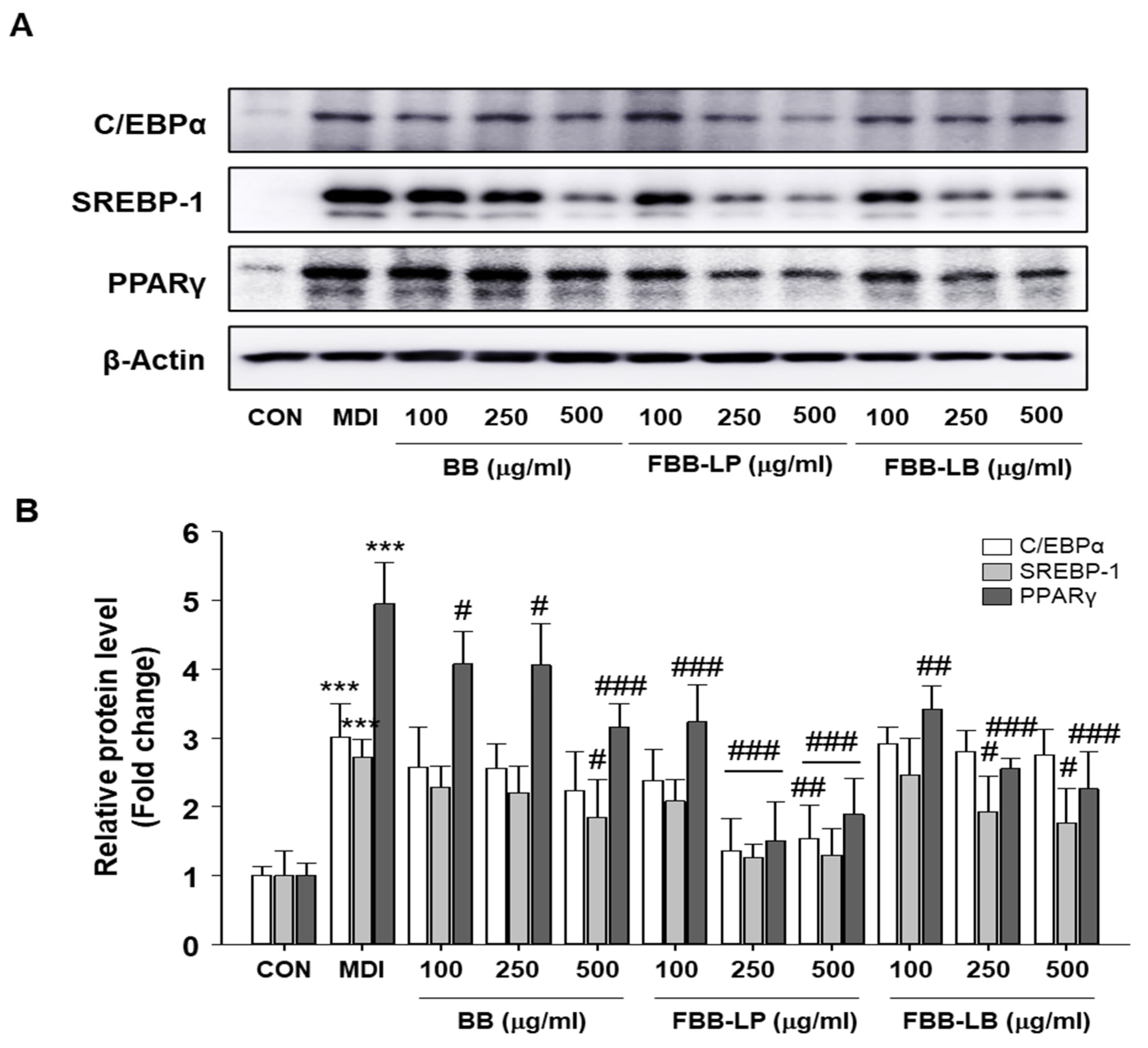
2.2. Effects of Fermented Blackberries on Expression of Adipocyte Differentiation Regulatory Proteins
2.3. Effects of Fermented Blackberries on Body Weight and eWAT Weight in HFD-Fed Mice
2.4. Effects of Fermented Blackberries on Adipogenesis and Lipid Accumulation in HFD-Fed Mice
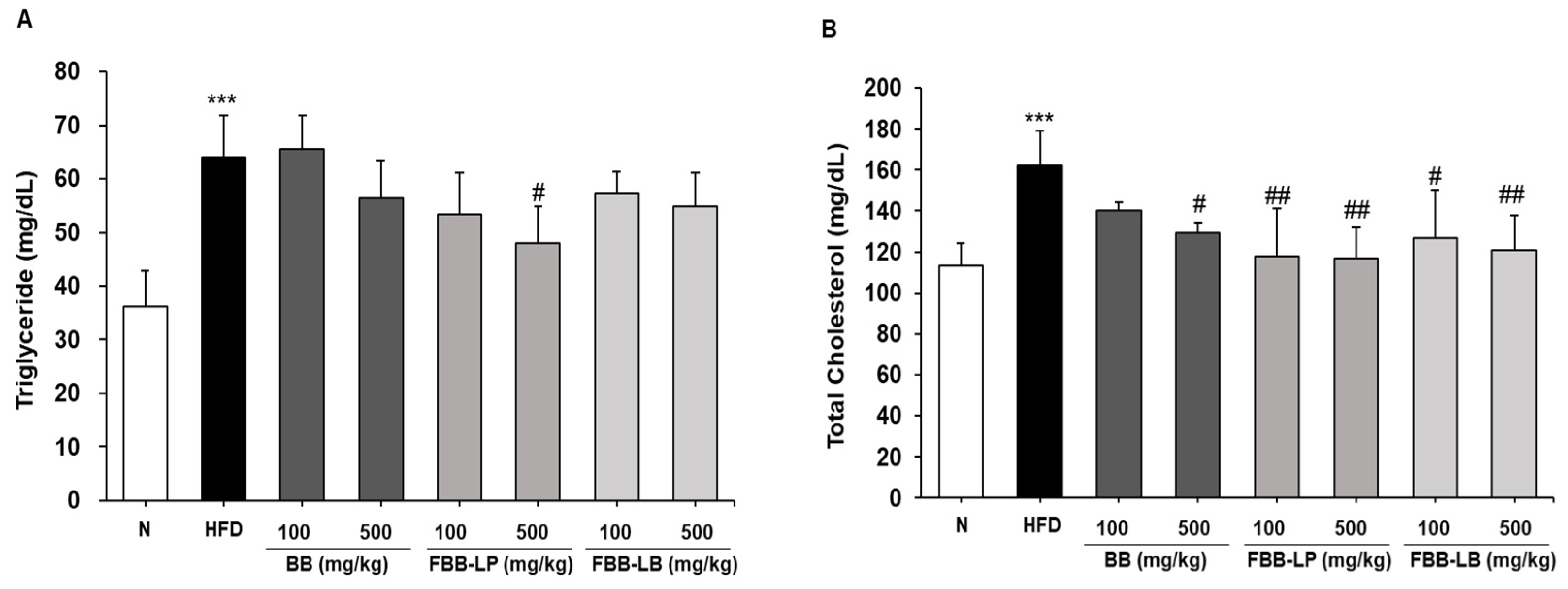
2.5. Effects of Fermented Blackberries on Hyperlipidemia in HFD-Fed Mice
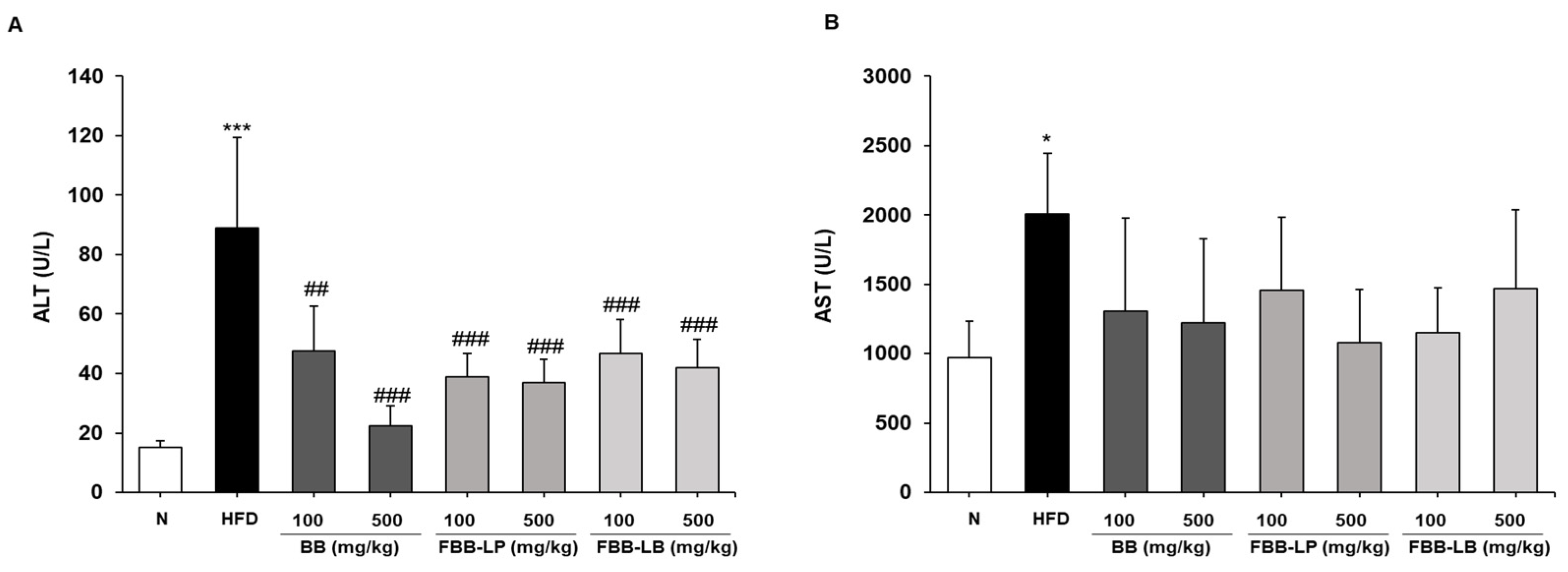
2.6. Effects of Fermented Blackberries on Liver Toxicity in HFD-Fed Mice
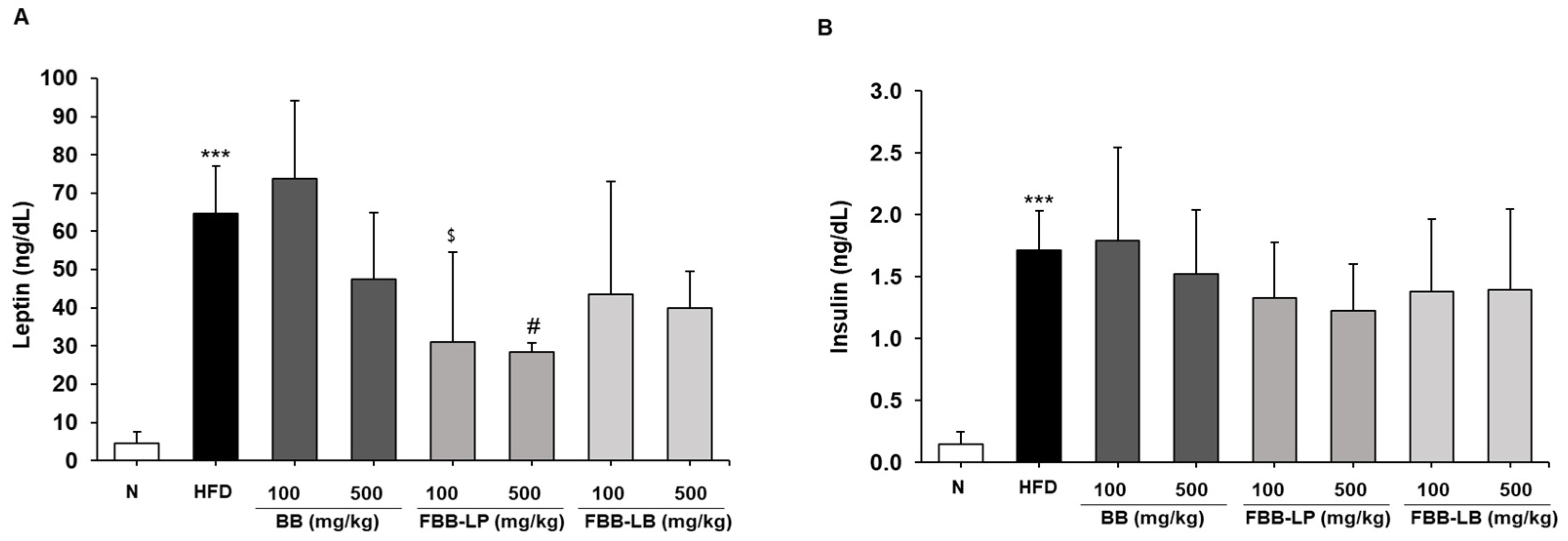
2.7. Effects of Fermented Blackberries on HFD-Induced Insulin Resistance
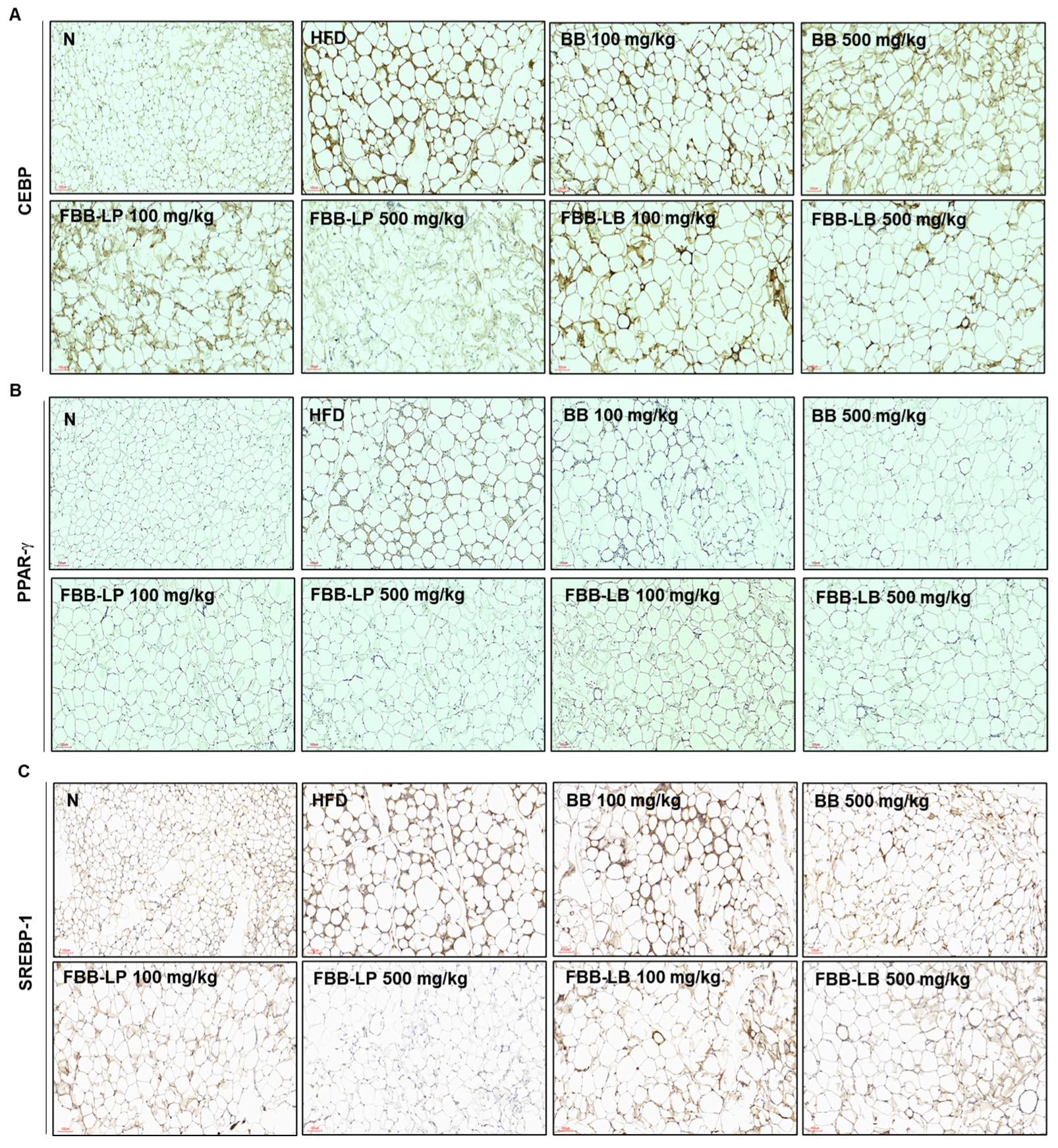
2.8. Effects of Fermented Blackberries on Adipocyte Differentiation in HFD-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of Fermented Blackberries
4.2. Cell Viability
4.3. 3T3-L1 Preadipocytes Adipogenesis
4.4. Oil Red O Staining
4.5. Antibodies
4.6. Immunoblotting
4.7. Animals
4.8. Experimental Groups
4.9. Analysis of Biomarkers in Serum (ELISA)
4.10. Histological Analysis
4.11. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bulik, C.M.; Hardaway, J.A. Turning the tide on obesity? Science 2023, 381, 463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, A.; Qi, W. The Potential to fight obesity with adipogenesis modulating compounds. Int. J. Mol. Sci. 2022, 23, 2299. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, S.; Zhang, C. The related metabolic diseases and treatments of obesity. Healthcare 2022, 10, 1616. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: Advances and challenges. Nat. Rev. Drug Discov. 2022, 21, 201–223. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, L.; Peña, S.; Ugidos, A.; Sayas-Barberá, E.; Pérez-Álvarez, J.; Sendra, E. Food ingredients as anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Gan, R.-Y.; Xu, X.-Y.; Mao, Q.-Q.; Zhang, P.-Z.; Li, H.-B. Effects and mechanisms of edible and medicinal plants on obesity: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 61, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Noor, S.; Lysiuk, R.; Darmohray, R.; Piscopo, S.; Lenchyk, L.; Antonyak, H.; Dehtiarova, K.; Shanaida, M. Polyphenols in metabolic diseases. Molecules 2022, 27, 6280. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.P.A.d.; Conte-Junior, C.A. Health and Bioactive Compounds of Fermented Foods and By-Products. Fermentation 2023, 10, 13. [Google Scholar] [CrossRef]
- Deveci, G.; Çelik, E.; Ağagündüz, D.; Bartkiene, E.; Rocha, J.M.F.; Özogul, F. Certain Fermented Foods and Their Possible Health Effects with a Focus on Bioactive Compounds and Microorganisms. Fermentation 2023, 9, 923. [Google Scholar] [CrossRef]
- Kim, I.-S.; Hwang, C.-W.; Yang, W.-S.; Kim, C.-H. Current perspectives on the physiological activities of fermented soybean-derived cheonggukjang. Int. J. Mol. Sci. 2021, 22, 5746. [Google Scholar] [CrossRef]
- Do Prado, F.G.; Pagnoncelli, M.G.B.; De Melo Pereira, G.V.; Karp, S.G.; Soccol, C.R. Fermented soy products and their potential health benefits: A review. Microorganisms 2022, 10, 1606. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 396129. [Google Scholar] [CrossRef]
- Sadh, P.K.; Kumar, S.; Chawla, P.; Duhan, J.S. Fermentation: A boon for production of bioactive compounds by processing of food industries wastes (by-products). Molecules 2018, 23, 2560. [Google Scholar] [CrossRef]
- Baruah, R.; Ray, M.; Halami, P.M. Preventive and therapeutic aspects of fermented foods. J. Appl. Microbiol. 2022, 132, 3476–3489. [Google Scholar] [CrossRef]
- Grigoras, A.G. Drug delivery systems using pullulan, a biocompatible polysaccharide produced by fungal fermentation of starch. Environ. Chem. Lett. 2019, 17, 1209–1223. [Google Scholar] [CrossRef]
- Jalili, M.; Nazari, M.; Magkos, F. Fermented foods in the management of obesity: Mechanisms of action and future challenges. Int. J. Mol. Sci. 2023, 24, 2665. [Google Scholar] [CrossRef] [PubMed]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented foods, health and the gut microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef] [PubMed]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Roshanravan, N.; Bastani, S.; Tutunchi, H.; Kafil, B.; Nikpayam, O.; Mesri Alamdari, N.; Hadi, A.; Sotoudeh, S.; Ghaffari, S.; Ostadrahimi, A. A comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch. Physiol. Biochem. 2023, 129, 741–751. [Google Scholar] [CrossRef]
- Albert, C.; Codină, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of antioxidant activity of garden blackberries (Rubus fruticosus L.) extracts obtained with different extraction solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Gowd, V.; Bao, T.; Wang, L.; Huang, Y.; Chen, S.; Zheng, X.; Cui, S.; Chen, W. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem. 2018, 269, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Land Lail, H.; Feresin, R.G.; Hicks, D.; Stone, B.; Price, E.; Wanders, D. Berries as a treatment for obesity-induced inflammation: Evidence from preclinical models. Nutrients 2021, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Bao, T.; Chen, W. Antioxidant potential and phenolic profile of blackberry anthocyanin extract followed by human gut microbiota fermentation. Food Res. Int. 2019, 120, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Jeong, D.-H.; Kim, S.; Lee, S.-W.; Sin, H.-S.; Yu, K.-Y.; Jeong, S.-I.; Kim, S.-Y. Fermentation of blackberry with L. plantarum JBMI F5 enhance the protection effect on UVB-mediated photoaging in human foreskin fibroblast and hairless mice through regulation of MAPK/NF-κB signaling. Nutrients 2019, 11, 2429. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Sasaki, Y.; Norikura, T.; Hosokawa, Y.; Kasano, M.; Matsui-Yuasa, I.; Huang, X.; Kobayashi, Y.; Kojima-Yuasa, A. The suppression of the differentiation of adipocytes with Mallotus furetianus is regulated through the posttranslational modifications of C/EBPβ. Food Sci. Nutr. 2023, 11, 6151–6163. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.R.; Kemp, B.E.; Watt, M.J. Adipocyte triglyceride lipase expression in human obesity. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E958–E964. [Google Scholar] [CrossRef] [PubMed]
- Ntambi, J.M.; Young-Cheul, K. Adipocyte differentiation and gene expression. J. Nutr. 2000, 130, 3122S–3126S. [Google Scholar] [CrossRef]
- Xiao, X.; Li, S.; Zhou, X.; Li, M.; Zhang, Y.; Ye, H. The anti-obesogenic effects and underpinning mechanisms of fermented plant-based foods: A review. Trends Food Sci. Technol. 2023, 136, 1–10. [Google Scholar] [CrossRef]
- Knez, E.; Kadac-Czapska, K.; Grembecka, M. Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Life 2023, 13, 655. [Google Scholar] [CrossRef]
- Jung, H.; Yun, Y.-R.; Hong, S.W.; Shin, S. Association between kimchi consumption and obesity based on BMI and abdominal obesity in Korean adults: A cross-sectional analysis of the Health Examinees study. BMJ Open 2024, 14, e076650. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the treatment of overweight and obesity in humans—A review of clinical trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, R.; Schneider, E.; Gunnigle, E.; Cotter, P.D.; Cryan, J.F. Fermented Foods: Harnessing Their Potential to Modulate The Microbiota-Gut-Brain Axis for Mental Health. Neurosci. Biobehav. Rev. 2024, 158, 105562. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Bogatyreva, A.I.; Tolstik, T.V.; Varaeva, Y.R.; Starodubova, A.V. The role of adipokines in inflammatory mechanisms of obesity. Int. J. Mol. Sci. 2022, 23, 14982. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Patil, I.Y.; Jiang, T.; Sancheti, H.; Walsh, J.P.; Stiles, B.L.; Yin, F.; Cadenas, E. High-fat diet induces hepatic insulin resistance and impairment of synaptic plasticity. PLoS ONE 2015, 10, e0128274. [Google Scholar] [CrossRef]
- Micek, A.; Owczarek, M.; Jurek, J.; Guerrera, I.; Torrisi, S.A.; Grosso, G.; Alshatwi, A.A.; Godos, J. Anthocyanin-rich fruits and mental health outcomes in an Italian cohort. J. Berry Res. 2022, 12, 551–564. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Zhao, F.; Cui, R.; Xie, W.; Liu, Q.; Yang, W. Shared biological mechanisms of depression and obesity: Focus on adipokines and lipokines. Aging 2023, 15, 5917–5950. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.Y.; Kim, H.-R.; Lee, S.-H.; Lee, S.-W.; Sin, H.-S.; Lim, T.-G.; Kim, S.-Y.; Park, M.H. Anti-Obesity Properties of Blackberries Fermented with L. plantarum JBMI F5 via Suppression of Adipogenesis Signaling Mechanisms. Int. J. Mol. Sci. 2024, 25, 6164. https://doi.org/10.3390/ijms25116164
Park JY, Kim H-R, Lee S-H, Lee S-W, Sin H-S, Lim T-G, Kim S-Y, Park MH. Anti-Obesity Properties of Blackberries Fermented with L. plantarum JBMI F5 via Suppression of Adipogenesis Signaling Mechanisms. International Journal of Molecular Sciences. 2024; 25(11):6164. https://doi.org/10.3390/ijms25116164
Chicago/Turabian StylePark, Jae Young, Ha-Rim Kim, Seung-Hyeon Lee, Sang-Wang Lee, Hong-Sig Sin, Tae-Gyu Lim, Seon-Young Kim, and Mi Hee Park. 2024. "Anti-Obesity Properties of Blackberries Fermented with L. plantarum JBMI F5 via Suppression of Adipogenesis Signaling Mechanisms" International Journal of Molecular Sciences 25, no. 11: 6164. https://doi.org/10.3390/ijms25116164
APA StylePark, J. Y., Kim, H.-R., Lee, S.-H., Lee, S.-W., Sin, H.-S., Lim, T.-G., Kim, S.-Y., & Park, M. H. (2024). Anti-Obesity Properties of Blackberries Fermented with L. plantarum JBMI F5 via Suppression of Adipogenesis Signaling Mechanisms. International Journal of Molecular Sciences, 25(11), 6164. https://doi.org/10.3390/ijms25116164







