Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment
Abstract
1. Introduction
2. Results
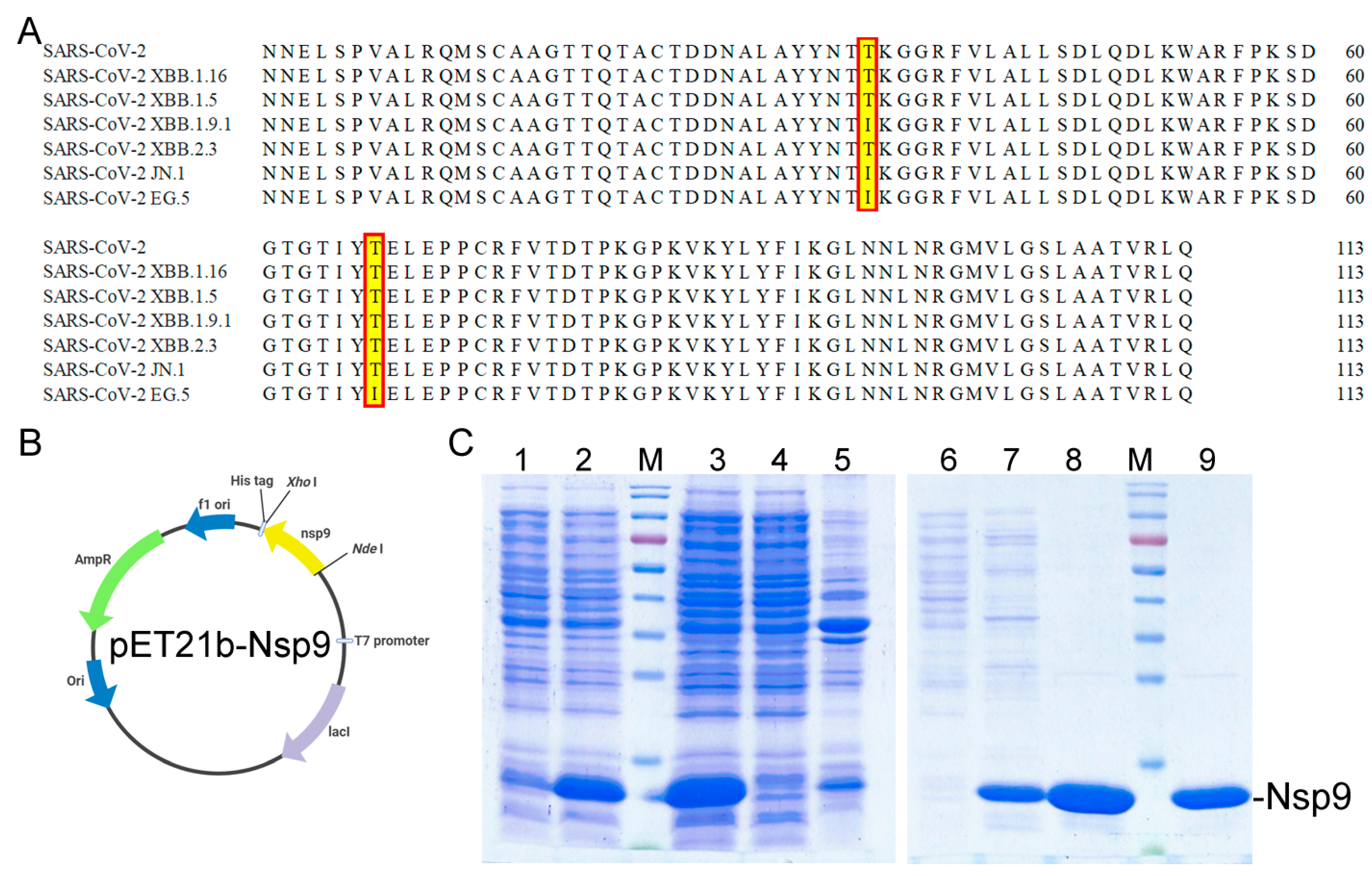
2.1. Cloning, Expression, and Purification of the Recombinant Nsp9 Protein from SARS-CoV-2
2.2. ssDNA-Binding Activity of Nsp9 and Inhibition Tests Using Myricetin and Oridonin
2.3. Exploring Nsp9 Inhibition with N. miranda Plant Extracts
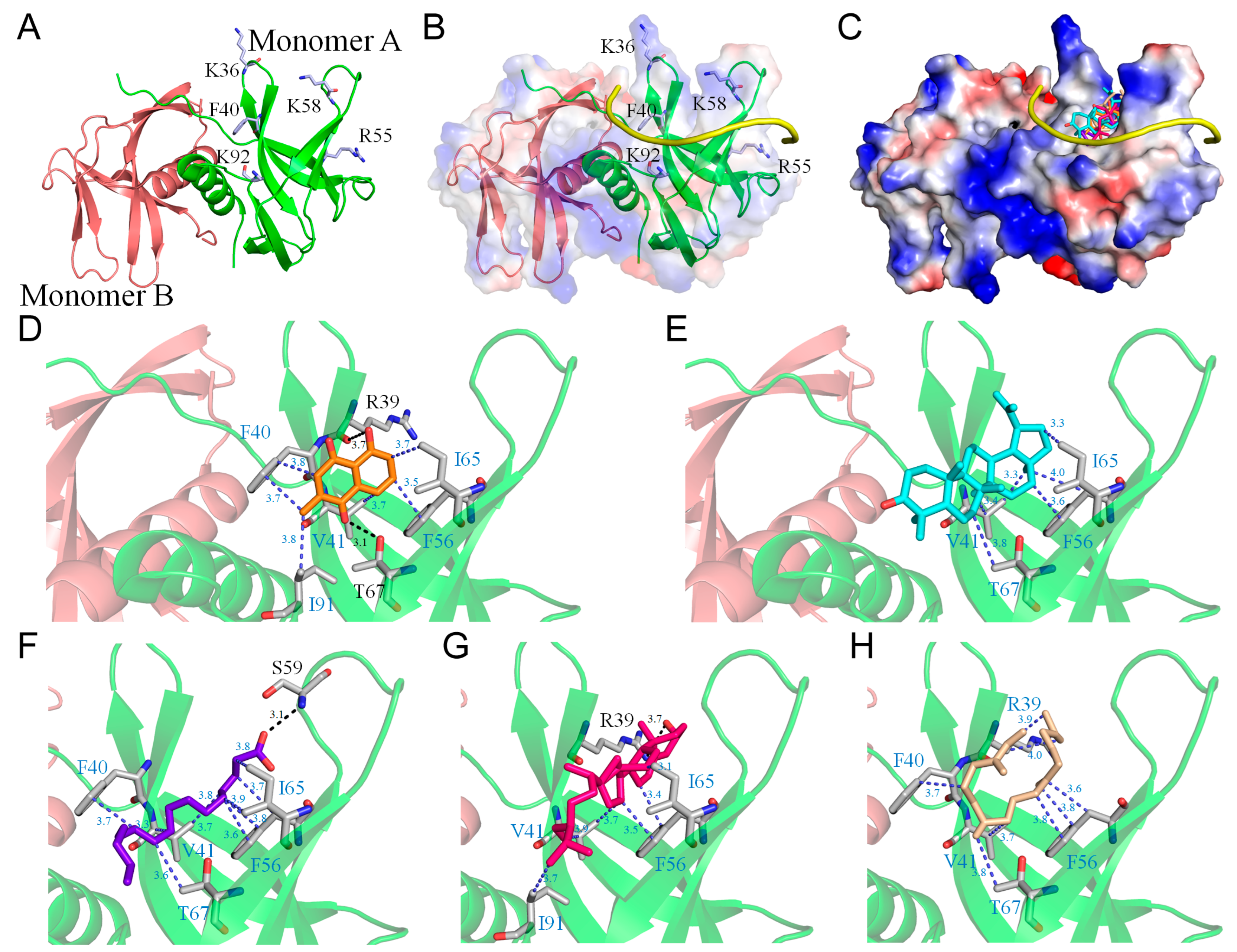
2.4. Molecular Docking of Nsp9
2.5. Cytotoxic Effects of N. miranda Extract on H1975 Lung Carcinoma Cells
2.6. Inhibition of H1975 Cell Migration by N. miranda Extract
2.7. Induction of Apoptosis in H1975 Cells by N. miranda Extract
2.8. G2 Cell-Cycle Arrest in H1975 Cells by N. miranda Leaf Extract
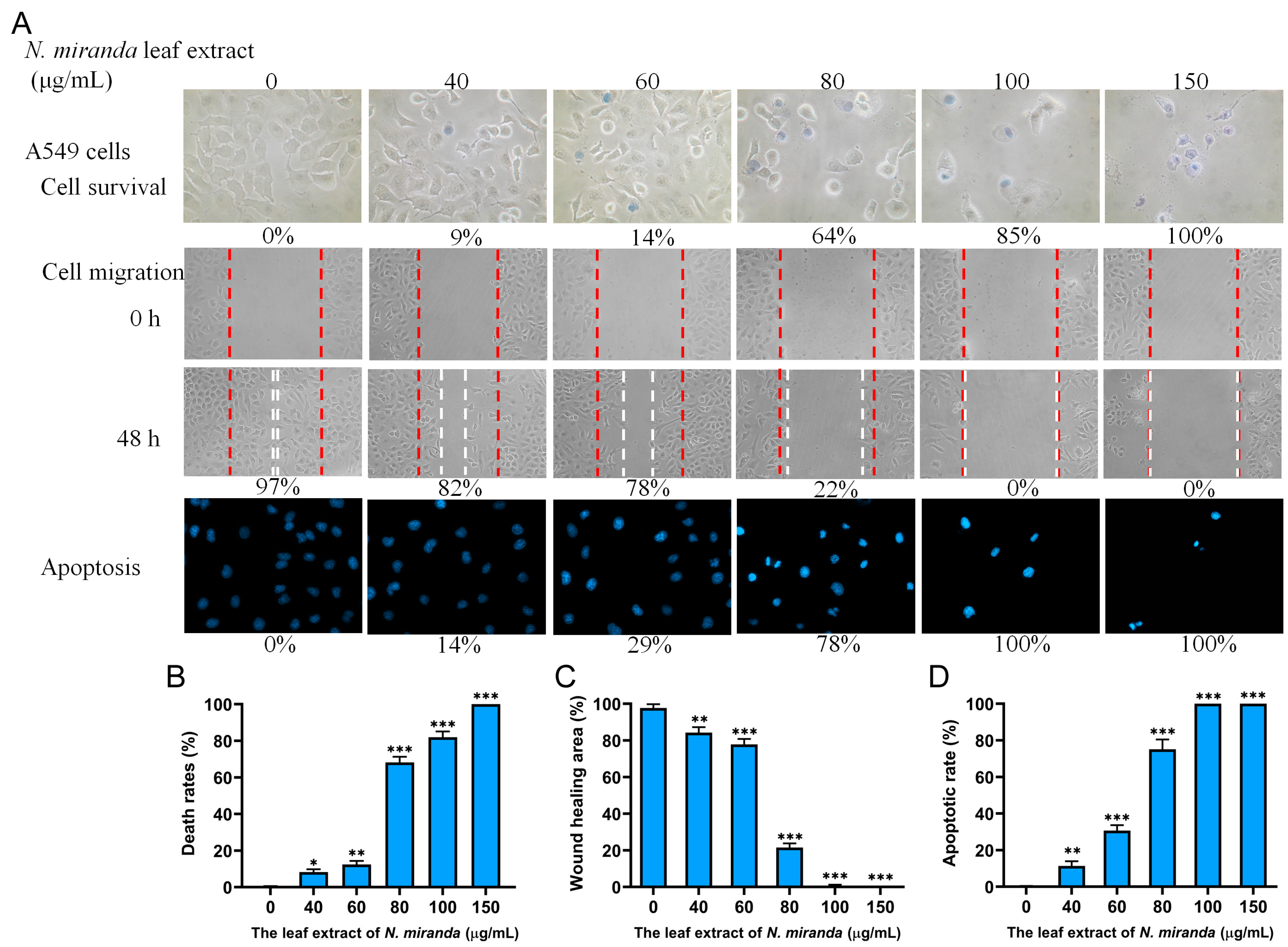
2.9. Anticancer Potential of N. miranda Extract on A549 Lung Carcinoma Cells
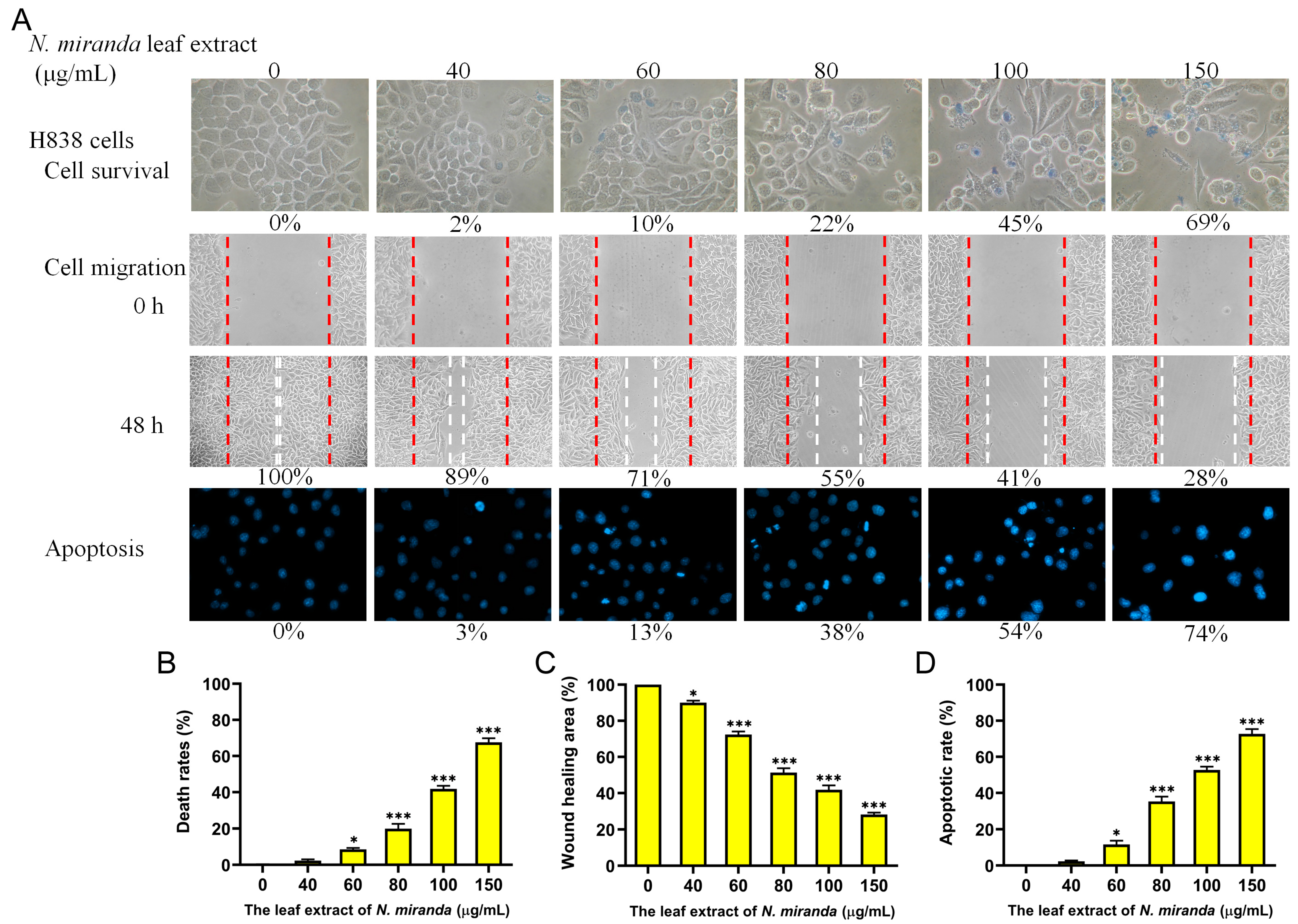
2.10. Anticancer Potential of N. miranda Extract on H838 Lung Carcinoma Cells
2.11. Comparative Anticancer Effects on Lung Carcinoma Cells
2.12. Comparative Analysis of Anticancer Potentials of N. miranda Extracts Obtained Using Various Solvents
2.13. Combined Effect of N. miranda Leaf Extract and the Clinical Anticancer Drug Afatinib on H1975 Cells
2.14. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Expression and Purification of the Recombinant Protein
4.3. Electrophoretic Mobility Shift Analysis (EMSA)
4.4. Nsp9 Inhibition
4.5. Plant Materials and Extract Preparations
4.6. Trypan Blue Cytotoxicity Assay
4.7. Chromatin Condensation Assay
4.8. Wound-Healing Assay
4.9. Flow Analysis
4.10. Binding Analysis Using AutoDock Vina
4.11. GC-MS Analysis
4.12. MTT Cell Viability Assay
4.13. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wójciak, M.; Feldo, M.; Stolarczyk, P.; Płachno, B.J. Carnivorous Plants from Nepenthaceae and Droseraceae as a Source of Secondary Metabolites. Molecules 2023, 28, 2155. [Google Scholar] [CrossRef]
- Miclea, I. Secondary Metabolites with Biomedical Applications from Plants of the Sarraceniaceae Family. Int. J. Mol. Sci. 2022, 23, 9877. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Panda, M.; Biswal, B.K. Emerging role of plumbagin: Cytotoxic potential and pharmaceutical relevance towards cancer therapy. Food Chem. Toxicol. 2019, 125, 566–582. [Google Scholar] [CrossRef]
- Miguel, S.; Hehn, A.; Bourgaud, F. Nepenthes: State of the art of an inspiring plant for biotechnologists. J. Biotechnol. 2018, 265, 109–115. [Google Scholar] [CrossRef]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Plumbagin, a Natural Product with Potent Anticancer Activities, Binds to and Inhibits Dihydroorotase, a Key Enzyme in Pyrimidine Biosynthesis. Int. J. Mol. Sci. 2021, 22, 6861. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Lien, Y.; Chen, J.-H.; Lin, E.-S.; Huang, C.-Y. Identification and characterization of dihydropyrimidinase inhibited by plumbagin isolated from Nepenthes miranda extract. Biochimie 2020, 171, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Luyen, B.T.; Koo, J.E.; Kim, S.; Koh, Y.S.; Thanh, N.V.; Cuong, N.X.; Kiem, P.V.; Minh, C.V.; Kim, Y.H. In vitro anti-inflammatory components isolated from the carnivorous plant Nepenthes mirabilis (Lour. ) Rafarin. Pharm. Biol. 2016, 54, 588–594. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q. Surface hydrophobicity of slippery zones in the pitchers of two Nepenthes species and a hybrid. Sci. Rep. 2016, 6, 19907. [Google Scholar] [CrossRef]
- Steiner, S.; Kratzel, A.; Barut, G.T.; Lang, R.M.; Aguiar Moreira, E.; Thomann, L.; Kelly, J.N.; Thiel, V. SARS-CoV-2 biology and host interactions. Nat. Rev. Microbiol. 2024, 22, 206–225. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Jiang, S. SARS-CoV-2 evolution from the BA.2.86 to JN.1 variants: Unexpected consequences. Trends Immunol. 2024, 45, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Planas, D.; Staropoli, I.; Michel, V.; Lemoine, F.; Donati, F.; Prot, M.; Porrot, F.; Guivel-Benhassine, F.; Jeyarajah, B.; Brisebarre, A.; et al. Distinct evolution of SARS-CoV-2 Omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat. Commun. 2024, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- von Delft, A.; Hall, M.D.; Kwong, A.D.; Purcell, L.A.; Saikatendu, K.S.; Schmitz, U.; Tallarico, J.A.; Lee, A.A. Accelerating antiviral drug discovery: Lessons from COVID-19. Nat. Rev. Drug Discov. 2023, 22, 585–603. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Hilgenfeld, R.; Whitley, R.; De Clercq, E. Therapeutic strategies for COVID-19: Progress and lessons learned. Nat. Rev. Drug Discov. 2023, 22, 449–475. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Chen, X.; Wu, J.; Duan, X.; Men, K. Small molecules in the treatment of COVID-19. Signal Transduct. Target. Ther. 2022, 7, 387. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Bad news for Paxlovid? Resistance may be coming. Science 2022, 377, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M. Why scientists are racing to develop more COVID antivirals. Nature 2022, 601, 496. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.; Xu, Z.; Hong, B.; Zhao, B.; Tian, Y.; Wang, C.; Yang, L.; Zou, Z.; Li, L.; Liu, K.; et al. Cepharanthine analogs mining and genomes of Stephania accelerate anti-coronavirus drug discovery. Nat. Commun. 2024, 15, 1537. [Google Scholar] [CrossRef]
- Chen, X.; Huang, X.; Ma, Q.; Kuzmič, P.; Zhou, B.; Zhang, S.; Chen, J.; Xu, J.; Liu, B.; Jiang, H.; et al. Preclinical evaluation of the SARS-CoV-2 M(pro) inhibitor RAY1216 shows improved pharmacokinetics compared with nirmatrelvir. Nat. Microbiol. 2024, 9, 1075–1088. [Google Scholar] [CrossRef]
- Small, G.I.; Fedorova, O.; Olinares, P.D.B.; Chandanani, J.; Banerjee, A.; Choi, Y.J.; Molina, H.; Chait, B.T.; Darst, S.A.; Campbell, E.A. Structural and functional insights into the enzymatic plasticity of the SARS-CoV-2 NiRAN domain. Mol. Cell 2023, 83, 3921–3930.e7. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.; Ganskih, S.; Wei, Y.; Gabel, A.; Zielinski, S.; Keshishian, H.; Lareau, C.A.; Zimmermann, L.; Makroczyova, J.; Pearce, C.; et al. SND1 binds SARS-CoV-2 negative-sense RNA and promotes viral RNA synthesis through NSP9. Cell 2023, 186, 4834–4850.e23. [Google Scholar] [CrossRef] [PubMed]
- Malone, B.F.; Perry, J.K.; Olinares, P.D.B.; Lee, H.W.; Chen, J.; Appleby, T.C.; Feng, J.Y.; Bilello, J.P.; Ng, H.; Sotiris, J.; et al. Structural basis for substrate selection by the SARS-CoV-2 replicase. Nature 2023, 614, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Huang, Y.; Ge, J.; Liu, Z.; Lu, P.; Huang, B.; Gao, S.; Wang, J.; Tan, L.; Ye, S.; et al. A mechanism for SARS-CoV-2 RNA capping and its inhibition by nucleotide analog inhibitors. Cell 2022, 185, 4347–4360.e17. [Google Scholar] [CrossRef] [PubMed]
- Park, G.J.; Osinski, A.; Hernandez, G.; Eitson, J.L.; Majumdar, A.; Tonelli, M.; Henzler-Wildman, K.; Pawłowski, K.; Chen, Z.; Li, Y.; et al. The mechanism of RNA capping by SARS-CoV-2. Nature 2022, 609, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.R.; Gully, B.S.; Colson, R.N.; Rossjohn, J. Crystal Structure of the SARS-CoV-2 Non-structural Protein 9, Nsp9. iScience 2020, 23, 101258. [Google Scholar] [CrossRef] [PubMed]
- Littler, D.R.; Liu, M.; McAuley, J.L.; Lowery, S.A.; Illing, P.T.; Gully, B.S.; Purcell, A.W.; Chandrashekaran, I.R.; Perlman, S.; Purcell, D.F.J.; et al. A natural product compound inhibits coronaviral replication in vitro by binding to the conserved Nsp9 SARS-CoV-2 protein. J. Biol. Chem. 2021, 297, 101362. [Google Scholar] [CrossRef] [PubMed]
- Murzin, A. OB(oligonucleotide/oligosaccharide binding)-fold: Common structural and functional solution for non-homologous sequences. EMBO J. 1993, 12, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-S.; Luo, R.-H.; Huang, C.-Y. A Complexed Crystal Structure of a Single-Stranded DNA-Binding Protein with Quercetin and the Structural Basis of Flavonol Inhibition Specificity. Int. J. Mol. Sci. 2022, 23, 588. [Google Scholar] [CrossRef]
- Lin, E.-S.; Huang, Y.-H.; Luo, R.-H.; Basharat, Z.; Huang, C.-Y. Crystal Structure of an SSB Protein from Salmonella enterica and Its Inhibition by Flavanonol Taxifolin. Int. J. Mol. Sci. 2022, 23, 4399. [Google Scholar] [CrossRef]
- Lin, E.S.; Huang, Y.H.; Huang, C.Y. Characterization of the Chimeric PriB-SSBc Protein. Int. J. Mol. Sci. 2021, 22, 10854. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-S.; Huang, C.-Y. Crystal structure of the single-stranded DNA-binding protein SsbB in complex with the anticancer drug 5-fluorouracil: Extension of the 5-fluorouracil interactome to include the oligonucleotide/oligosaccharide-binding fold protein. Biochem. Biophys. Res. Commun. 2020, 534, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Lin, E.-S.; Huang, C.-Y. Complexed crystal structure of SSB reveals a novel single-stranded DNA binding mode (SSB)3:1: Phe60 is not crucial for defining binding paths. Biochem. Biophys. Res. Commun. 2019, 520, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Chen, I.C.; Huang, C.Y. Characterization of an SSB-dT25 complex: Structural insights into the S-shaped ssDNA binding conformation. RSC Adv. 2019, 9, 40388–40396. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Huang, C.-Y. The glycine-rich flexible region in SSB is crucial for PriA stimulation. RSC Adv. 2018, 8, 35280–35288. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y. Crystal structure of SSB complexed with inhibitor myricetin. Biochem. Biophys. Res. Commun. 2018, 504, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-S.; Huang, Y.-H.; Chung, J.-C.; Su, H.-H.; Huang, C.-Y. The Inhibitory Effects and Cytotoxic Activities of the Stem Extract of Nepenthes miranda against Single-Stranded DNA-Binding Protein and Oral Carcinoma Cells. Plants 2023, 12, 2188. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLO-BOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, G.; Singh, M.; Vredenburgh, J.J. Leptomeningeal Metastasis from Non–Small Cell Lung Cancer and Current Landscape of Treatments. Clin. Cancer Res. 2022, 29, 11–29. [Google Scholar] [CrossRef]
- Baydoun, A.; Lee, V.L.; Biswas, T. Oligometastatic Non-Small Cell Lung Cancer: A Practical Review of Prospective Trials. Cancers 2022, 14, 5339. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, H.; Wang, S.; Yang, H.; Kong, F.-M. Treatment-Related Pneumonitis of EGFR Tyrosine Kinase Inhibitors Plus Thoracic Radiation Therapy in Patients With Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. 2024, 118, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, A.; Luciani, A. Topotecan or other agents as second-line therapy for relapsed small-cell lung cancer: A meta-analysis of randomized studies. Mol. Clin. Oncol. 2021, 15, 218. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Huang, Z.; Li, P.; Sun, Z.; Hou, X.; Li, Z.; Sang, R.; Guo, Z.; Wu, S.; Cao, Y. Investigating the efficacy and mechanisms of Jinfu’an decoction in treating non-small cell lung cancer using network pharmacology and in vitro and in vivo experiments. J. Ethnopharmacol. 2023, 321, 117518. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Liu, B.; Chen, M.; Qin, Y.; Li, J.; Li, S.; Xu, X. Treatment of non-small cell lung cancer with Yiqi Buxue prescriptions combined with adjuvant chemotherapy on the cancer therapy-related cardiovascular toxicity: A systematic review and meta-analysis. J. Ethnopharmacol. 2024, 323, 117665. [Google Scholar] [CrossRef] [PubMed]
- González, A.S.C.; Valencia, M.G.; Cervantes-Villagrana, R.D.; Zapata, A.B.; Cervantes-Villagrana, A.R. Cytotoxic and Antitumor Effects of the Hydroalcoholic Extract of Tagetes erecta in Lung Cancer Cells. Molecules 2023, 28, 7055. [Google Scholar] [CrossRef] [PubMed]
- Dushkov, A.; Vosáhlová, Z.; Tzintzarov, A.; Kalíková, K.; Křížek, T.; Ugrinova, I. Analysis of the Ibotenic Acid, Muscimol, and Ergosterol Content of an Amanita Muscaria Hydroalcoholic Extract with an Evaluation of Its Cytotoxic Effect against a Panel of Lung Cell Lines In Vitro. Molecules 2023, 28, 6824. [Google Scholar] [CrossRef]
- Mia, A.R.; Dey, D.; Sakib, M.R.; Biswas, Y.; Prottay, A.A.S.; Paul, N.; Rimti, F.H.; Abdullah, Y.; Biswas, P.; Iftehimul, M.; et al. The efficacy of natural bioactive compounds against prostate cancer: Molecular targets and synergistic activities. Phytotherapy Res. 2023, 37, 5724–5754. [Google Scholar] [CrossRef]
- Melfi, F.; Carradori, S.; Mencarelli, N.; Campestre, C.; Gallorini, M.; Di Giacomo, S.; Di Sotto, A. Natural products as a source of new anticancer chemotypes. Expert Opin. Ther. Patents 2023, 33, 721–744. [Google Scholar] [CrossRef]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An analysis of FDA-approved drugs: Natural products and their derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Ghafari, M.; Beer, M.; Lythgoe, K.; Simmonds, P.; Stilianakis, N.I.; Katzourakis, A. The evolution of SARS-CoV-2. Nat. Rev. Microbiol. 2023, 21, 361–379. [Google Scholar] [CrossRef]
- Ruiz-Aravena, M.; McKee, C.; Gamble, A.; Lunn, T.; Morris, A.; Snedden, C.E.; Yinda, C.K.; Port, J.R.; Buchholz, D.W.; Yeo, Y.Y.; et al. Ecology, evolution and spillover of coronaviruses from bats. Nat. Rev. Microbiol. 2021, 20, 299–314. [Google Scholar] [CrossRef]
- Abbasi, J. What to Know About EG.5, the Latest SARS-CoV-2 “Variant of Interest”. JAMA 2023, 330, 900–901. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, Y.; Xu, Y.; Jian, F.; Song, W.; Yisimayi, A.; Wang, P.; Wang, J.; Liu, J.; Yu, L.; et al. Fast evolution of SARS-CoV-2 BA.2·86 to JN.1 under heavy immune pressure. Lancet Infect. Dis. 2023, 24, e70–e72. [Google Scholar] [CrossRef] [PubMed]
- Looi, M.K. COVID-19: WHO adds JN.1 as new variant of interest. BMJ 2023, 383, p2975. [Google Scholar] [CrossRef]
- Lin, E.-S.; Huang, C.-Y. Cytotoxic Activities and the Allantoinase Inhibitory Effect of the Leaf Extract of the Carnivorous Pitcher Plant Nepenthes miranda. Plants 2022, 11, 2265. [Google Scholar] [CrossRef]
- El-Kamand, S.; Du Plessis, M.D.; Breen, N.; Johnson, L.; Beard, S.; Kwan, A.H.; Richard, D.J.; Cubeddu, L.; Gamsjaeger, R. A distinct ssDNA/RNA binding interface in the Nsp9 protein from SARS-CoV-2. Proteins 2022, 90, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Ji, H.; Yuza, Y.; Meyerson, M.; Wong, K.-K.; Tenen, D.G.; Halmos, B. An Alternative Inhibitor Overcomes Resistance Caused by a Mutation of the Epidermal Growth Factor Receptor. Cancer Res. 2005, 65, 7096–7101. [Google Scholar] [CrossRef] [PubMed]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In Vitro Cultivation of Human Tumors: Establishment of Cell Lines Derived From a Series of Solid Tumors2. JNCI J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Zia, F.; Draoui, M.; E Brenneman, D.; Fridkin, M.; Davidson, A.; Gozes, I. A vasoactive intestinal peptide antagonist inhibits non-small cell lung cancer growth. Proc. Natl. Acad. Sci. USA 1993, 90, 4345–4349. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.K.; Aquilanti, E.; Persky, N.S.; Yang, X.; Kim, E.E.; Brenan, L.; Goodale, A.B.; Alan, D.; Sharpe, T.; Shue, R.E.; et al. Comprehensive mutational scanning of EGFR reveals TKI sensitivities of extracellular domain mutants. Nat. Commun. 2024, 15, 2742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cui, L.; Wang, J.; Zhao, W.; Teng, Y. Aspirin boosts the synergistic effect of EGFR/p53 inhibitors on lung cancer cells by regulating AKT/mTOR and p53 pathways. Cell Biochem. Funct. 2023, 42, e3902. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Fu, Q.; Wang, Y.; Cui, L.; Zhang, W.; Teng, Y.; Yu, P. Synergy between vinorelbine and afatinib in the inhibition of non-small cell lung cancer progression by EGFR and p53 signaling pathways. Biomed. Pharmacother. 2020, 134, 111144. [Google Scholar] [CrossRef]
- Chen, G.; Noor, A.; Kronenberger, P.; Teugels, E.; Umelo, I.A.; De Grève, J. Synergistic Effect of Afatinib with Su11274 in Non-Small Cell Lung Cancer Cells Resistant to Gefitinib or Erlotinib. PLoS ONE 2013, 8, e59708. [Google Scholar] [CrossRef] [PubMed]
- Król, E.; Płachno, B.J.; Adamec, L.; Stolarz, M.; Dziubińska, H.; Trębacz, K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world’. Ann. Bot. 2011, 109, 47–64. [Google Scholar] [CrossRef]
- Monniaux, M.; Hay, A. Cells, walls, and endless forms. Curr. Opin. Plant Biol. 2016, 34, 114–121. [Google Scholar] [CrossRef]
- Schwallier, R.; de Boer, H.J.; Visser, N.; van Vugt, R.R.; Gravendeel, B. Traps as treats: A traditional sticky rice snack persisting in rapidly changing Asian kitchens. J. Ethnobiol. Ethnomed. 2015, 11, 24. [Google Scholar] [CrossRef]
- Klein, C.; Brinkmann, U.; Reichert, J.M.; Kontermann, R.E. The present and future of bispecific antibodies for cancer therapy. Nat. Rev. Drug Discov. 2024, 23, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Abel, G.A.; Hamilton, W.; Pritchard-Jones, K.; Gross, C.P.; Walter, F.M.; Renzi, C.; Johnson, S.; McPhail, S.; Elliss-Brookes, L.; et al. Diagnosis of cancer as an emergency: A critical review of current evidence. Nat. Rev. Clin. Oncol. 2016, 14, 45–56. [Google Scholar] [CrossRef]
- Lee, J.S.; Adler, L.; Karathia, H.; Carmel, N.; Rabinovich, S.; Auslander, N.; Keshet, R.; Stettner, N.; Silberman, A.; Agemy, L.; et al. Urea Cycle Dysregulation Generates Clinically Relevant Genomic and Biochemical Signatures. Cell 2018, 174, 1559–1570.e22. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef]
- Lee, K.W.; Bode, A.M.; Dong, Z. Molecular targets of phytochemicals for cancer prevention. Nat. Rev. Cancer 2011, 11, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Mann, J. Natural products in cancer chemotherapy: Past, present and future. Nat. Rev. Cancer 2002, 2, 143–148. [Google Scholar] [CrossRef]
- Changxing, L.; Galani, S.; Hassan, F.-U.; Rashid, Z.; Naveed, M.; Fang, D.; Ashraf, A.; Qi, W.; Arif, A.; Saeed, M.; et al. Biotechnological approaches to the production of plant-derived promising anticancer agents: An update and overview. Biomed. Pharmacother. 2020, 132, 110918. [Google Scholar] [CrossRef]
- Darwiche, N.; El-Banna, S.; Gali-Muhtasib, H. Cell cycle modulatory and apoptotic effects of plant-derived anticancer drugs in clinical use or development. Expert Opin. Drug Discov. 2007, 2, 361–379. [Google Scholar] [CrossRef]
- Laface, C.; Maselli, F.M.; Santoro, A.N.; Iaia, M.L.; Ambrogio, F.; Laterza, M.; Guarini, C.; De Santis, P.; Perrone, M.; Fedele, P. The Resistance to EGFR-TKIs in Non-Small Cell Lung Cancer: From Molecular Mechanisms to Clinical Application of New Therapeutic Strategies. Pharmaceutics 2023, 15, 1604. [Google Scholar] [CrossRef] [PubMed]
- Robichaux, J.P.; Le, X.; Vijayan, R.S.K.; Hicks, J.K.; Heeke, S.; Elamin, Y.Y.; Lin, H.Y.; Udagawa, H.; Skoulidis, F.; Tran, H.; et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 2021, 597, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Minkovsky, N.; Berezov, A. BIBW-2992, a dual receptor tyrosine kinase inhibitor for the treatment of solid tumors. Curr. Opin. Investig. Drugs 2008, 9, 1336–1346. [Google Scholar] [PubMed]
- De, U.; Son, J.Y.; Jeon, Y.; Ha, S.-Y.; Park, Y.J.; Yoon, S.; Ha, K.-T.; Choi, W.S.; Lee, B.M.; Kim, I.S.; et al. Plumbagin from a tropical pitcher plant (Nepenthes alata Blanco) induces apoptotic cell death via a p53-dependent pathway in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2018, 123, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.G.; Im, E.; Lee, H.-J.; Lee, E.-O. Plumbagin reduces osteopontin-induced invasion through inhibiting the Rho-associated kinase signaling pathway in A549 cells and suppresses osteopontin-induced lung metastasis in BalB/c mice. Bioorganic Med. Chem. Lett. 2017, 27, 1914–1918. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Baba, H.; Phan, N.D.; Kim, M.J.; Maneenet, J.; Sawaki, K.; Kanda, M.; Okumura, T.; Fujii, T.; Okada, T.; et al. Targeting Pancreatic Cancer with Novel Plumbagin Derivatives: Design, Synthesis, Molecular Mechanism, In Vitro and In Vivo Evaluation. J. Med. Chem. 2023, 66, 8054–8065. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, B.B.; Jamal, M.S.; Fischer, J.W.; Mustafa, A.; Verma, A.K. Plumbagin, a plant derived natural agent inhibits the growth of pancreatic cancer cells in in vitro and in vivo via targeting EGFR, Stat3 and NF-κB signaling pathways. Int. J. Cancer 2012, 131, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-S.; Huang, Y.-H.; Yang, P.-C.; Peng, W.-F.; Huang, C.-Y. Complexed Crystal Structure of the Dihydroorotase Domain of Human CAD Protein with the Anticancer Drug 5-Fluorouracil. Biomolecules 2023, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.-H.; Huang, Y.-H.; Lin, E.-S.; Chen, C.-J.; Huang, C.-Y. Structural basis for the interaction modes of dihydroorotase with the anticancer drugs 5-fluorouracil and 5-aminouracil. Biochem. Biophys. Res. Commun. 2021, 551, 33–37. [Google Scholar] [CrossRef]
- Huang, C.Y. Structure, catalytic mechanism, posttranslational lysine carbamylation, and inhibition of dihydropyrimidinases. Adv. Protein Chem. Struct. Biol. 2020, 122, 63–96. [Google Scholar]
- Cheng, J.-H.; Huang, Y.-H.; Lin, J.-J.; Huang, C.-Y. Crystal structures of monometallic dihydropyrimidinase and the human dihydroorotase domain K1556A mutant reveal no lysine carbamylation within the active site. Biochem. Biophys. Res. Commun. 2018, 505, 439–444. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Wu, H.; Wang, X. Beneficial health effects of lupenone triterpene: A review. Biomed. Pharmacother. 2018, 103, 198–203. [Google Scholar] [CrossRef]
- Bednarczyk-Cwynar, B.; Wiȩcaszek, T.; Ruszkowski, P. Cytotoxic Activity of Some Lupeol Derivatives. Nat. Prod. Commun. 2016, 11, 1237–1238. [Google Scholar] [CrossRef]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic acid inhibits prostate cancer cell proliferation and metastasis by suppressing the PI3K/Akt pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Ann, Y.-S.; Ko, C.-I.; Lee, S.-H.; Lee, W.W.; Jeon, Y.-J. Apoptotic and antiproliferative effects of Stigmast-5-en-3-ol from Dendronephthya gigantea on human leukemia HL-60 and human breast cancer MCF-7 cells. Toxicol. Vitr. 2018, 52, 297–305. [Google Scholar] [CrossRef]
- Richardson, J.S.M.; Sethi, G.; Lee, G.S.; Malek, S.N.A. Chalepin: Isolated from Ruta angustifolia L. Pers induces mitochondrial mediated apoptosis in lung carcinoma cells. BMC Complement. Altern. Med. 2016, 16, 389. [Google Scholar] [CrossRef] [PubMed]
- Seebaluck, R.; Gurib-Fakim, A.; Mahomoodally, F. Medicinal plants from the genus Acalypha (Euphorbiaceae)–A review of their ethnopharmacology and phytochemistry. J. Ethnopharmacol. 2015, 159, 137–157. [Google Scholar] [CrossRef] [PubMed]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; Robertson, D.L. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef]
- Wu, S.W.; Chen, Y.J.; Chang, Y.W.; Huang, C.Y.; Liu, B.H.; Yu, F.Y. Novel enzyme-linked aptamer-antibody sandwich assay and hybrid lateral flow strip for SARS-CoV-2 detection. J. Nanobiotechnol. 2024, 22, 5. [Google Scholar] [CrossRef]
- Tam, D.; Lorenzo-Leal, A.C.; Hernández, L.R.; Bach, H. Targeting SARS-CoV-2 Non-Structural Proteins. Int. J. Mol. Sci. 2023, 24, 13002. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, S.C.; Ye, L.; Maimaitiyiming, Y.; Naranmandura, H. Targeting viral proteins for restraining SARS-CoV-2: Focusing lens on viral proteins beyond spike for discovering new drug targets. Expert Opin. Drug Discov. 2023, 18, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.-Y. COVID-19 drug discovery and treatment options. Nat. Rev. Microbiol. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Rouphael, N.G.; Diemert, D.J.; Falsey, A.R.; Losada, C.; Baden, L.R.; Frey, S.E.; Whitaker, J.A.; Little, S.J.; An-derson, E.J.; et al. Comparison of bivalent and monovalent SARS-CoV-2 variant vaccines: The phase 2 randomized open-label COVAIL trial. Nat. Med. 2023, 29, 2334–2346. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Piccoli, L.; Case, J.B.; Park, Y.-J.; Beltramello, M.; Guarino, B.; Dang, H.; de Melo, G.D.; Pinto, D.; Sprouse, K.; et al. Neutralization, effector function and immune imprinting of Omicron variants. Nature 2023, 621, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Svetlov, D.; Artsimovitch, I. NMPylation and de-NMPylation of SARS-CoV-2 nsp9 by the NiRAN domain. Nucleic. Acids Res. 2021, 49, 8822–8835. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yang, Y.; Li, M.; Zhang, Y.; Zheng, L.; Ge, J.; Huang, Y.C.; Liu, Z.; Wang, T.; Gao, S.; et al. Coupling of N7-methyltransferase and 3′-5′ exoribonuclease with SARS-CoV-2 polymerase reveals mechanisms for capping and proof-reading. Cell 2021, 184, 3474–3485.e11. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ge, J.; Zheng, L.; Zhang, Y.; Gao, Y.; Wang, T.; Huang, Y.; Yang, Y.; Gao, S.; Li, M.; et al. Cryo-EM Structure of an Extended SARS-CoV-2 Replication and Transcription Complex Reveals an Intermediate State in Cap Synthesis. Cell 2021, 184, 184–193.e10. [Google Scholar] [CrossRef]
- Slanina, H.; Madhugiri, R.; Bylapudi, G.; Schultheiß, K.; Karl, N.; Gulyaeva, A.; Gorbalenya, A.E.; Linne, U.; Ziebuhr, J. Coronavirus replication-transcription complex: Vital and selective NMPylation of a conserved site in nsp9 by the Ni-RAN-RdRp subunit. Proc. Natl. Acad. Sci. USA 2021, 118, e2022310118. [Google Scholar] [CrossRef]
- Dickey, T.H.; Altschuler, S.E.; Wuttke, D.S. Single-Stranded DNA-Binding Proteins: Multiple Domains for Multiple Functions. Structure 2013, 21, 1074–1084. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Muchiri, R.N. Affinity selection-mass spectrometry in the discovery of anti-SARS-CoV-2 compounds. Mass Spectrom. Rev. 2024, 43, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.J.; Mak, T.; Littler, D.R.; Rossjohn, J.; Liu, M. Discovery of Anti-SARS-CoV-2 Nsp9 Binders from Natural Products by a Native Mass Spectrometry Approach. J. Nat. Prod. 2023, 86, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Littler, D.R.; Rossjohn, J.; Quinn, R.J. Binding Studies of the Prodrug HAO472 to SARS-CoV-2 Nsp9 and Variants. ACS Omega 2022, 7, 7327–7332. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Hsu, C.-H.; Sun, Y.-J.; Wu, H.-N.; Hsiao, C.-D. Complexed crystal structure of replication restart primosome protein PriB reveals a novel single-stranded DNA-binding mode. Nucleic. Acids Res. 2006, 34, 3878–3886. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, Appendix 3, A-3B. [Google Scholar]
- Larsson, R.; Nygren, P. A rapid fluorometric method for semiautomated determination of cytotoxicity and cellular proliferation of human tumor cell lines in microculture. Anticancer Res. 1989, 9, 1111–1119. [Google Scholar] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Dallakyan, S.; Olson, A.J. Small-molecule library screening by docking with pyrx. Methods Mol. Biol. 2015, 1263, 243–250. [Google Scholar] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]






| Inhibitor | IC50 |
|---|---|
| Myricetin | N.D. |
| Oridonin | N.D. |
| Acetone extract of Sinningia bullata leaves | N.D. |
| Water extract of Nepenthes miranda leaves | N.D. |
| Methanol extract of Nepenthes miranda leaves | 103.4 ± 8.2 μg/mL |
| Ethanol extract of Nepenthes miranda leaves | 138.9 ± 12.2 μg/mL |
| Acetone extract of Nepenthes miranda leaves | 72.5 ± 6.0 μg/mL |
| Ethyl acetate extract of Nepenthes miranda leaves | 307.7 ± 20.4 μg/mL |
| n-Hexane extract of Nepenthes miranda leaves | 775.4 ± 30.2 μg/mL |
| Affinity (kcal/mol) | Interaction | Residue (distance, Å) | |
|---|---|---|---|
| Plumbagin | −5.7 | Hydrogen bond | R39 (3.7), T67 (3.1) |
| Hydrophobic | F40 (3.7, 3.7), V41 (3.7), F56 (3.5), I65 (3.7), I91(3.8) | ||
| Lupenone | −7.1 | Hydrophobic | V41 (3.4, 3.3), F56 (4.0, 3.6), I65 (3.3), T67 (3.8) |
| Palmitic acid | −4.9 | Hydrogen bond | S59 (3.1) |
| Hydrophobic | F40 (3.7), V41 (3.4, 3.7), F56 (3.6, 3.7, 3.8), I65 (3.9, 3.8), T67 (3.6) | ||
| Stigmast-5-en-3-ol | −7.9 | Hydrogen bond | R39 (3.7) |
| Hydrophobic | V41 (3.9, 3.7), F56 (3.5, 3.4), I65 (3.1), I91 (3.7) | ||
| Neophytadiene | −5.1 | Hydrophobic | R39 (3.9, 4.0), F40 (3.7), V41 (3.7), F56 (3.8, 3.8, 3.6), T67 (3.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.-H.; Lin, E.-S.; Huang, Y.-H.; Lien, Y.; Huang, C.-Y. Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment. Int. J. Mol. Sci. 2024, 25, 6120. https://doi.org/10.3390/ijms25116120
Su H-H, Lin E-S, Huang Y-H, Lien Y, Huang C-Y. Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment. International Journal of Molecular Sciences. 2024; 25(11):6120. https://doi.org/10.3390/ijms25116120
Chicago/Turabian StyleSu, Hsin-Hui, En-Shyh Lin, Yen-Hua Huang, Yi Lien, and Cheng-Yang Huang. 2024. "Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment" International Journal of Molecular Sciences 25, no. 11: 6120. https://doi.org/10.3390/ijms25116120
APA StyleSu, H.-H., Lin, E.-S., Huang, Y.-H., Lien, Y., & Huang, C.-Y. (2024). Inhibition of SARS-CoV-2 Nsp9 ssDNA-Binding Activity and Cytotoxic Effects on H838, H1975, and A549 Human Non-Small Cell Lung Cancer Cells: Exploring the Potential of Nepenthes miranda Leaf Extract for Pulmonary Disease Treatment. International Journal of Molecular Sciences, 25(11), 6120. https://doi.org/10.3390/ijms25116120







