Substance P’s Impact on Chronic Pain and Psychiatric Conditions—A Narrative Review
Abstract
1. Introduction
2. Substance P’s History and Molecular Pathway
2.1. History
2.2. Synthesis and Release
2.3. Tachykinin Receptors
2.4. Metabolic Cascade of Substance P
2.5. Anatomic Localization of Metabolic Effects
3. Animal Models
4. Understanding Substance P: Molecular Mechanisms and Physiological Pathways
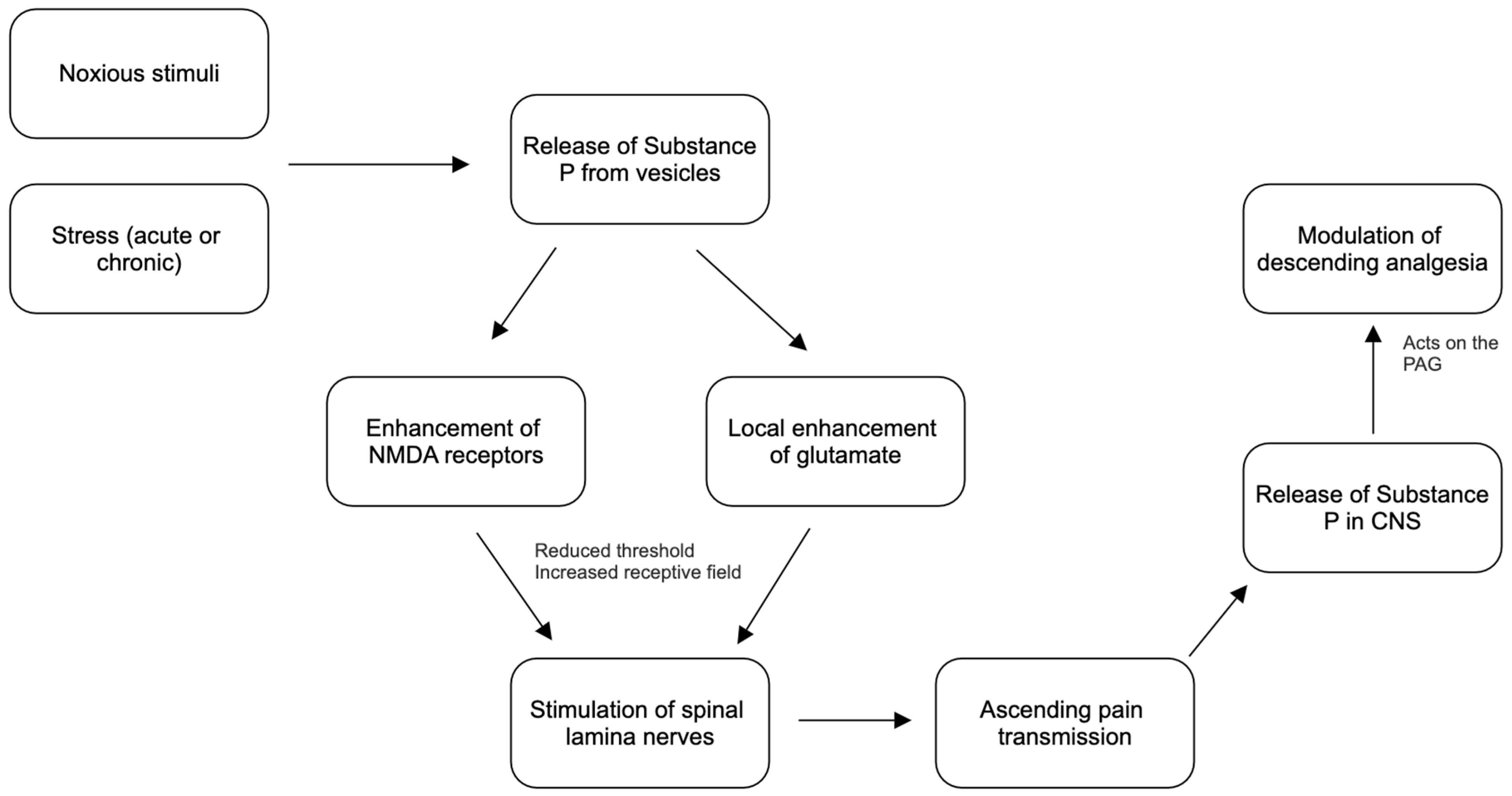
4.1. Pain Transmission and Substance P
4.2. Substance P and Glutamate Enhancement
4.3. Descending Pathways and Substance P
5. Acute, Chronic, and Neuropathic Pain
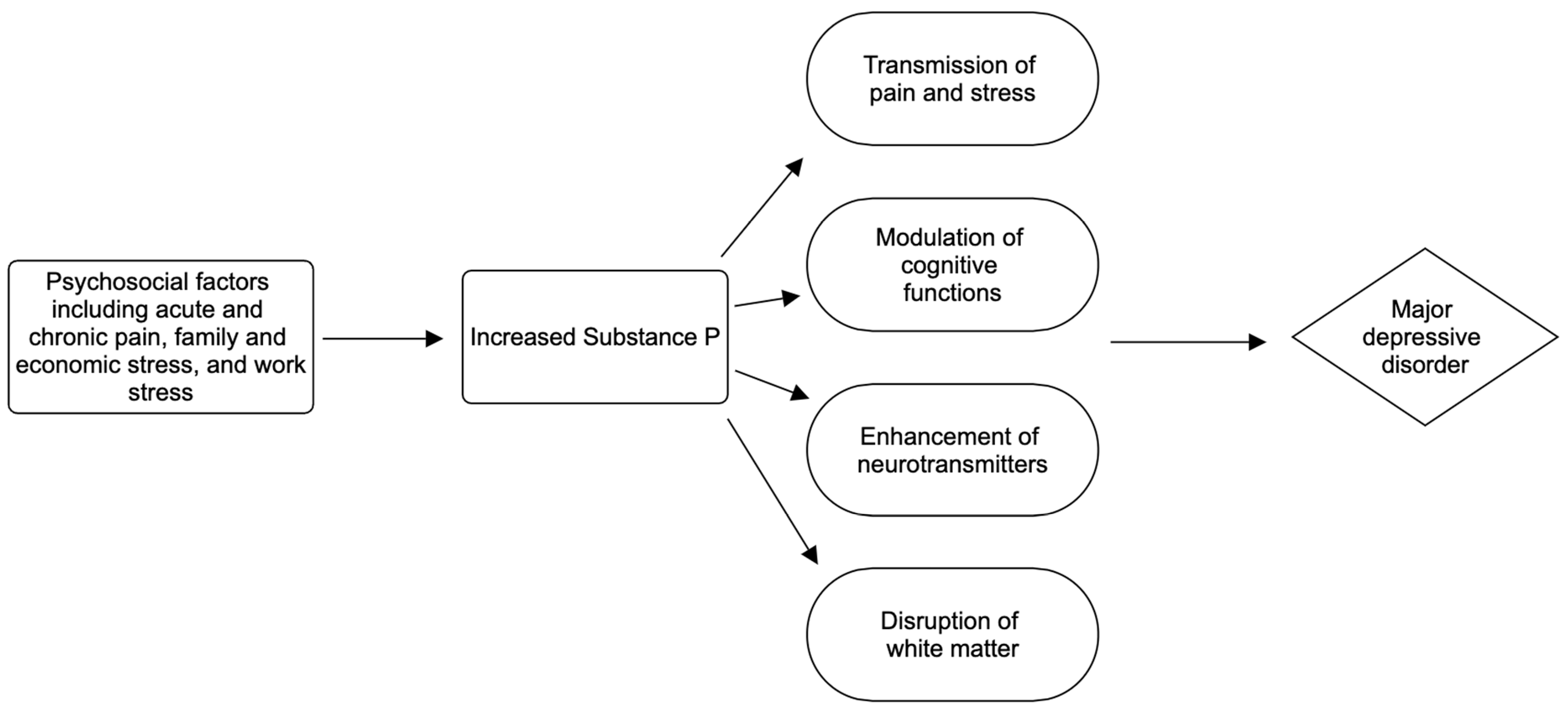
6. Major Depressive Disorder
| Trial | Subjects | Methods | Measure of Outcome | Results |
|---|---|---|---|---|
| Orvepitant in Adult Post Traumatic Stress Disorder [68]. | Male and female outpatients between the ages of 18 and 64 with a diagnosis of non-combative PTSD | Double-blind, placebo-controlled, fixed-dose administration; Placebo vs. Orvepitant 60 mg/day | Change in baseline in the Clinician-Administered PTSD Symptom Severity Scale from day 1 (predose) to week 12 | A decrease in the mean difference of change on the PTSD Symptom Scale was seen in patients who were given Orvepitant in comparison to the placebo. |
| Substance P Antagonist in the Treatment of Posttraumatic Stress Disorder [69]. | Male and female patients between the ages of 18 and 65 with a diagnosis of PTSD | Double-blind, placebo-controlled; placebo vs. vofopitant | Change in baseline in the Clinician-Administered PTSD Symptom Severity Scale from day 1 (predose) to week 8 | A decrease in the mean difference of change on the PTSD Symptom Scale was seen in patients who were given vofopitant in comparison to the placebo. |
| Effect of LY686017 [70]. | Male and female patients between 21 and 65 who meet the criteria for alcohol dependence and who have an elevated score on the general test of anxiety | Double-blind, placebo-controlled; placebo vs. 50 mg tradipitant | Change in baseline in the Alcohol Urges Questionnaire at day 1 to week 8 and biweekly assessment using the Comprehensive Psychiatric Rating Scale | A decrease in the mean difference of change in the Alcohol Urges Questionnaire in patients given tradipitant; a decrease in the mean difference of change in the Comprehensive Psychiatric Rating Scale in patients given tradipitant |
| A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled, Fixed Dose Study Evaluating the Efficacy and Safety of Orvepitant in Subjects with MDD [71]. | Male and female outpatients between 18 and 64 who have a primary diagnosis of MDD | Randomized, double-blind, parallel-group, placebo-controlled, fixed-dose; placebo vs. orvepitant | Change in baseline in the Hamilton Depression Rating Scale from day 1 to week 6 | A decrease in the mean difference of change on the Depression Rating Scale in those patients given orvepitant |
7. Anxiety Disorders
8. Post-Traumatic Stress Disorder
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoyer, D.; Bartfai, T. Neuropeptides and neuropeptide receptors: Drug targets, and peptide and non-peptide ligands: A tribute to Prof. Dieter Seebach. Chem. Biodivers. 2012, 9, 2367–2387. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.E.; Chirgwin, J.M.; Carter, M.S.; Xu, Z.S.; Hershey, A.D. Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A. Proc. Natl. Acad. Sci. USA 1987, 84, 881–885. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saria, A. The tachykinin NK1 receptor in the brain: Pharmacology and putative functions. Eur. J. Pharmacol. 1999, 375, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ganjiwale, A.; Cowsik, S.M. Molecular recognition of tachykinin receptor selective agonists: Insights from structural studies. Mini Rev. Med. Chem. 2013, 13, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Yoshioka, K. Neurotransmitter functions of mammalian tachykinins. Physiol Rev. 1993, 73, 229–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Wei, L.C.; Liu, H.L.; Rao, Z.R. Noradrenergic neurons expressing substance P receptor (NK1) in the locus coeruleus complex: A double immunofluorescence study in the rat. Brain Res. 2000, 873, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, K.; Siddiq, A.; Baig, S.G.; Zehra, S. Substance P: A neuropeptide involved in the psychopathology of anxiety disorders. Neuropeptides 2020, 79, 101993. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A.; Faust, B.; Gondin, A.B.; Damgen, M.A.; Suomivuori, C.-M.; Veldhuis, N.A.; Cheng, Y.; Dror, R.O.; Thal, D.M.; Manglik, A. Selective G protein signaling driven by substance P–neurokinin receptor dynamics. Nat. Chem. Biol. 2022, 18, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Okine, B.N.; Gaspar, J.C.; Finn, D.P. PPARs and pain. Br. J. Pharmacol. 2019, 176, 1421–1442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barde, S.; Aguila, J.; Zhong, W.; Solarz, A.; Mei, I.; Prud’Homme, J.; Palkovits, M.; Turecki, G.; Mulder, J.; Uhlén, M.; et al. Substance P, NPY, CCK and their receptors in five brain regions in major depressive disorder with transcriptomic analysis of locus coeruleus neurons. Eur. Neuropsychopharmacol. 2024, 78, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Bradesi, S.; Svensson, C.I.; Steinauer, J.; Pothoulakis, C.; Yaksh, T.L.; Mayer, E.A. Role of spinal microglia in visceral hyperalgesia and NK1R up-regulation in a rat model of chronic stress. Gastroenterology 2009, 136, 1339–1348. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aguiar, M.S.; Brandão, M.L. Effects of microinjections of the neuropeptide substance P in the dorsal periaqueductal gray on the behaviour of rats in the plus-maze test. Physiol. Behav. 1996, 60, 1183–1186. [Google Scholar] [CrossRef] [PubMed]
- Bilkei-Gorzo, A.; Racz, I.; Michel, K.; Zimmer, A. Diminished anxiety- and depression-related behaviors in mice with selective deletion of the Tac1 gene. J. Neurosci. 2002, 22, 10046–10052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebner, K.; Rupniak, N.M.; Saria, A.; Singewald, N. Substance P in the medial amygdala: Emotional stress-sensitive release and modulation of anxiety-related behavior in rats. Proc. Natl. Acad. Sci. USA 2004, 101, 4280–4285. [Google Scholar] [CrossRef] [PubMed]
- Drew, G.M.; Mitchell, V.A.; Vaughan, C.W. Postsynaptic actions of substance P on rat periaqueductal grey neurons in vitro. Neuropharmacology 2005, 49, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.H.; Ding, W.Q.; Sun, Y.G. Spinal ascending pathways for somatosensory information processing. Trends Neurosci. 2022, 45, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and Physiology. Clin. Plast Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Todd, A.J. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp. Physiol. 2002, 87, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Du, L.; Kim, J.J.; Zhu, F.; He, H.; Dai, N. NMDA and AMPA receptor physiology and role in visceral hypersensitivity: A review. Eur. J. Gastroenterol. Hepatol. 2022, 34, 471–477. [Google Scholar] [CrossRef] [PubMed]
- DeVane, C.L. Substance P: A new era, a new role. Pharmacotherapy 2001, 21, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Randić, M.; Hećimović, H.; Ryu, P.D. Substance P modulates glutamate-induced currents in acutely isolated rat spinal dorsal horn neurones. Neurosci Lett. 1990, 117, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.; Goudet, C. Emerging Trends in Pain Modulation by Metabotropic Glutamate Receptors. Front. Mol. Neurosci. 2019, 11, 464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bleakman, D.; Alt, A.; Nisenbaum, E.S. Glutamate receptors and pain. Semin. Cell Dev. Biol. 2006, 17, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willis, W.D.; Westlund, K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997, 14, 2–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef] [PubMed]
- Drew, G.M.; Lau, B.K.; Vaughan, C.W. Substance P drives endocannabinoid-mediated disinhibition in a midbrain descending analgesic pathway. J. Neurosci. 2009, 29, 7220–7229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mantyh, P.W.; Hunt, S.P. Setting the tone: Superficial dorsal horn projection neurons regulate pain sensitivity. Trends Neurosci. 2004, 27, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Zhu, B.F.; Wang, L.K.; Song, Y.; Zhao, J.G.; Guo, Y.; Zhao, L.; Chen, S. Electroacupuncture alleviates inflammatory pain via adenosine suppression and its mediated substance P expression. Arq. Neuropsiquiatr. 2020, 78, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Schadrack, J.; Zieglgänsberger, W. Activity-dependent changes in the pain matrix. Scand. J. Rheumatol. Suppl. 2000, 113, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Amaro-Díaz, L.; Montoro, C.I.; Fischer-Jbali, L.R.; Galvez-Sánchez, C.M. Chronic Pain and Emotional Stroop: A Systematic Review. J. Clin. Med. 2022, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Tinnirello, A.; Mazzoleni, S.; Santi, C. Chronic Pain in the Elderly: Mechanisms and Distinctive Features. Biomolecules 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Trewern, L.; Jackman, J.; McCartney, D.; Soni, A. Chronic pain: Definitions and diagnosis. BMJ 2023, 381, e076036. [Google Scholar] [CrossRef] [PubMed]
- Ray-Griffith, S.L.; Morrison, B.; Stowe, Z.N. Chronic Pain Prevalence and Exposures during Pregnancy. Pain Res. Manag. 2019, 2019, 6985164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kemp, H.I.; Corner, E.; Colvin, L.A. Chronic pain after COVID-19: Implications for rehabilitation. Br. J. Anaesth. 2020, 125, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Hartung, D.; Turner, J.; Blazina, I.; Chan, B.; Levander, X.; McDonagh, M.; Selph, S.; Fu, R.; Pappas, M. Opioid Treatments for Chronic Pain; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2020. [PubMed]
- Humo, M.; Lu, H.; Yalcin, I. The molecular neurobiology of chronic pain-induced depression. Cell Tissue Res. 2019, 377, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.J.; Gandhi, W.; Salomons, T. Reward processing as a common diathesis for chronic pain and depression. Neurosci. Biobehav. Rev. 2021, 127, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Arango-Dávila, C.A.; Rincón-Hoyos, H.G. Depressive Disorder, Anxiety Disorder and Chronic Pain: Multiple Manifestations of a Common Clinical and Pathophysiological Core. Rev. Colomb. Psiquiatr. (Engl. Ed). 2018, 47, 46–55, (In English and Spanish). [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.C.; Gajsar, H.; Schlüter, M.C.; Bremer, Y.I. Cognitive Biases Toward Pain: Implications for a Neurocognitive Processing Perspective in Chronic Pain and its Interaction With Depression. Clin. J. Pain. 2019, 35, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Mullins, P.M.; Yong, R.J.; Bhattacharyya, N. Associations between chronic pain, anxiety, and depression among adults in the United States. Pain Pract. 2023, 23, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Massart, R.; Mongeau, R.; Lanfumey, L. Beyond the monoaminergic hypothesis: Neuroplasticity and epigenetic changes in a transgenic mouse model of depression. Philos. Trans. R. Soc. Lond B Biol Sci. 2012, 367, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Geracioti, T.D., Jr.; Carpenter, L.; Owens, M.; Baker, D.; Ekhator, N.; Horn, P.; Strawn, J.; Sanacora, G.; Kinkead, B.; Price, L.; et al. Elevated cerebrospinal fluid substance p concentrations in posttraumatic stress disorder and major depression. Am. J. Psychiatry 2006, 163, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Bondy, B.; Baghai, T.C.; Minov, C.; Schüle, C.; Schwarz, M.J.; Zwanzger, P.; Rupprecht, R.; Möller, H.-J. Substance P serum levels are increased in major depression: Preliminary results. Biol. Psychiatry 2003, 53, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural. Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Szok, D.; Tajti, J.; Nyári, A.; Vécsei, L. Therapeutic Approaches for Peripheral and Central Neuropathic Pain. Behav. Neurol. 2019, 2019, 8685954. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouhassira, D. Neuropathic pain: Definition, assessment and epidemiology. Rev. Neurol. 2019, 175, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Masuda, T.; Kohno, K. Microglial diversity in neuropathic pain. Trends Neurosci. 2023, 46, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Gurba, K.N.; Chaudhry, R.; Haroutounian, S. Central Neuropathic Pain Syndromes: Current and Emerging Pharmacological Strategies. CNS Drugs 2022, 36, 483–516. [Google Scholar] [CrossRef] [PubMed]
- Rosner, J.; de Andrade, D.C.; Davis, K.D.; Gustin, S.M.; Kramer, J.L.K.; Seal, R.P.; Finnerup, N.B. Central neuropathic pain. Nat. Rev. Dis. Primers 2023, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Michaelides, A.; Zis, P. Depression, anxiety and acute pain: Links and management challenges. Postgrad. Med. 2019, 131, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rojas, L.; Porras-Segovia, A.; Dunne, H.; Andrade-González, N.; Cervilla, J.A. Prevalence and correlates of major depressive disorder: A systematic review. Braz. J. Psychiatry 2020, 42, 657–672. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shetty, P.A.; Ayari, L.; Madry, J.; Betts, C.; Robinson, D.M.; Kirmani, B.F. The Relationship Between COVID-19 and the Development of Depression: Implications on Mental Health. Neurosci. Insights 2023, 18, 26331055231191513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otte, C.; Gold, S.M.; Penninx, B.W.; Pariante, C.M.; Etkin, A.; Fava, M.; Mohr, D.C.; Schatzberg, A.F. Major depressive disorder. Nat. Rev. Dis. Primers 2016, 2, 16065. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Lim, D.Y.; Chee, K.T. Reimagining the spectrum of affective disorders. Bipolar. Disord. 2020, 22, 638–639. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, D.J.; Frank, E.; Phillips, M.L. Major depressive disorder: New clinical, neurobiological, and treatment perspectives. Lancet 2012, 379, 1045–1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vadivelu, N.; Kai, A.M.; Kodumudi, G.; Babayan, K.; Fontes, M.; Burg, M.M. Pain and Psychology—A Reciprocal Relationship. Ochsner J. 2017, 17, 173–180. [Google Scholar] [PubMed] [PubMed Central]
- Cheeseman, H.J.; Pinnock, R.D.; Henderson, G. Substance P excitation of rat locus coeruleus neurones. Eur. J. Pharmacol. 1983, 94, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Schäble, S.; Topic, B.; Buddenberg, T.; Petri, D.; Huston, J.P.; de Souza Silva, M.A. Neurokinin3-R agonism in aged rats has anxiolytic-, antidepressant-, and promnestic-like effects and stimulates ACh release in frontal cortex, amygdala and hippocampus. Eur. Neuropsychopharmacol. 2011, 21, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.J.; Ackenheil, M. The role of substance P in depression: Therapeutic implications. Dialogues Clin. Neurosci. 2002, 4, 21–29. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Won, E.; Kang, J.; Choi, S.; Kim, A.; Han, K.M.; Yoon, H.K.; Cho, S.H.; Tae, W.S.; Lee, M.S.; Joe, S.H.; et al. The association between substance P and white matter integrity in medication-naive patients with major depressive disorder. Sci. Rep. 2017, 7, 9707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blier, P.; Gobbi, G.; Haddjeri, N.; Santarelli, L.; Mathew, G.; Hen, R. Impact of substance P receptor antagonism on the serotonin and norepinephrine systems: Relevance to the antidepressant/anxiolytic response. J. Psychiatry Neurosci. 2004, 29, 208–218. [Google Scholar] [PubMed] [PubMed Central]
- Mihailescu-Marin, M.M.; Mosoiu, D.V.; Burtea, V.; Sechel, G.; Rogozea, L.M.; Ciurescu, D. Common Pathways for Pain and Depression-Implications for Practice. Am. J. Ther. 2020, 27, e468–e476. [Google Scholar] [CrossRef] [PubMed]
- Dableh, L.J.; Yashpal, K.; Rochford, J.; Henry, J.L. Antidepressant-like effects of neurokinin receptor antagonists in the forced swim test in the rat. Eur. J. Pharmacol. 2005, 507, 99–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Orvepitant (GW823296) in Adult Post Traumatic Stress Disorder. 2010. Available online: https://clinicaltrials.gov/study/NCT01000493?intr=NK1+Antagonist&rank=8 (accessed on 10 May 2024).
- Substance P Antagonist in the Treatment of Posttraumatic Stress Disorder. 2009. Available online: https://clinicaltrials.gov/study/NCT00383786?intr=NK1+Antagonist&page=1&rank=10 (accessed on 10 May 2024).
- Effect of LY686017 on Alcohol Craving. 2008. Available online: https://clinicaltrials.gov/study/NCT00310427?intr=NK1+Antagonist&page=1&rank=4 (accessed on 10 May 2024).
- A Randomized, Double-Blind, Parallel-Group, Placebo-Controlled, Fixed Dose Study Evaluating the Efficacy and Safety of Orvepitant in Subjects with Major Depressive Disorder. 2010. Available online: https://clinicaltrials.gov/study/NCT00880399?intr=NK1+Antagonist&page=2&rank=14 (accessed on 10 May 2024).
- Ibrahim, M.A.; Pellegrini, M.V.; Preuss, C.V. Antiemetic Neurokinin-1 Receptor Blockers. [Updated 11 January 2024]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024; Available online: https://www.ncbi.nlm.nih.gov/books/NBK470394/ (accessed on 20 April 2024).
- Remes, O.; Wainwright, N.; Surtees, P.; Lafortune, L.; Khaw, K.T.; Brayne, C. Generalised anxiety disorder and hospital admissions: Findings from a large, population cohort study. BMJ Open 2018, 8, e018539. [Google Scholar] [CrossRef]
- Gómez Penedo, J.M.; Rubel, J.A.; Blättler, L.; Schmidt, S.J.; Stewart, J.; Egloff, N.; Grosse Holtforth, M. The Complex Interplay of Pain, Depression, and Anxiety Symptoms in Patients With Chronic Pain: A Network Approach. Clin. J. Pain 2020, 36, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Simon, N.M. Anxiety Disorders: A Review. JAMA 2022, 328, 2431–2445. [Google Scholar] [CrossRef] [PubMed]
- Besteher, B.; Gaser, C.; Nenadić, I. Brain Structure and Subclinical Symptoms: A Dimensional Perspective of Psychopathology in the Depression and Anxiety Spectrum. Neuropsychobiology 2020, 79, 270–283. [Google Scholar] [CrossRef] [PubMed]
- Ebner, K.; Singewald, N. The role of substance P in stress and anxiety responses. Amino Acids 2006, 31, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Forkus, S.R.; Raudales, A.M.; Rafiuddin, H.S.; Weiss, N.H.; Messman, B.A.; Contractor, A.A. The Posttraumatic Stress Disorder (PTSD) Checklist for DSM–5: A systematic review of existing psychometric evidence. Clin. Psychol. Sci. Pract. 2023, 30, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Kim, Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 2019, 73, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.K.; Wang, L.; FTsoi, K.K.; Rutovic, S.; Kim, J.S. Post-Traumatic Stress Disorder after Stroke: A Systematic Review. Neurol. India 2022, 70, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.U.; Ebrahimi, O.V.; Hoffart, A. PTSD symptoms among health workers and public service providers during the COVID-19 outbreak. PLoS ONE 2020, 15, e0241032. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N.; Davoodi, K.; Keem, M.; Das, S. Assessment tools for complex post traumatic stress disorder: A systematic review. Int. J. Psychiatry Clin. Pract. 2023, 27, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Spoont, M.; Arbisi, P.; Fu, S.; Greer, N.; Kehle-Forbes, S.; Meis, L.; Rutks, I.; Wilt, T.J. Screening for Post-Traumatic Stress Disorder (PTSD) in Primary Care: A Systematic Review; Department of Veterans Affairs (US): Washington, DC, USA, 2013. [PubMed]
- Mughal, A.Y.; Devadas, J.; Ardman, E.; Levis, B.; Go, V.F.; Gaynes, B.N. A systematic review of validated screening tools for anxiety disorders and PTSD in low to middle income countries. BMC Psychiatry 2020, 20, 338. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roviš, D.; Vasiljev, V.; Jenko-Pražnikar, Z.; Petelin, A.; Drevenšek, G.; Peruč, D.; Černelič-Bizjak, M. Mental health and drug use severity: The role of substance P, neuropeptide Y, self-reported childhood history of trauma, parental bonding and current resiliency. J. Ment. Health 2021, 30, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Uppsala University. Posttraumatic stress disorder reveals an imbalance between signaling systems in the brain. ScienceDaily. 1 December 2015. Available online: www.sciencedaily.com/releases/2015/12/151201093515.htm (accessed on 18 April 2024).
- Vink, R.; Nimmo, A. Identification of an Intravenous Injectable NK1 Receptor Antagonist for Use in Traumatic Brain Injury. Int. J. Mol. Sci. 2024, 25, 3535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, Q.X.; Soh, A.Y.S.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Yeo, W.S. Systematic review with meta-analysis: The association between post-traumatic stress disorder and irritable bowel syndrome. J. Gastroenterol. Hepatol. 2019, 34, 68–73. [Google Scholar] [CrossRef] [PubMed]
| Study | Animal Models | Experimental Conditions | Measurement of Outcome | Conclusions |
|---|---|---|---|---|
| Bilkei-Gorzo et al. (2002) [14]. | Tac1 mutant mice and wild-type mice | Exposure to stress with a forced-swimming test, tail-suspension test, bulbectomy, social interaction test, open-field test | Video recordings of animal behavior with an observer measuring immobility, hyperactivity, distance traveled in an open field, and social interaction with other animals | Mice that did not have the tac1 gene that encodes for Substance P displayed less fear and anxiety and were also more active in depression-related paradigms |
| Ebner et al. (2004) [15]. | Adult male Sprague-Dawley rats | Microinjections of Substance P and NK antagonists as well as immobilization with stress exposure | Measurement of Substance P concentrations by in vivo micro push–pull superfusion and microdialysis; behavior was measured by activity in different arms of a maze | Significantly increased Substance P release in rats exposed to stress in comparison to rats that were not, as well as NK1 antagonist application leading to decreased stress-induced anxiolytic effects |
| Bradesi et al. (2009) [12]. | Male Wister rats | Application of water and sham stress | Western blotting with antibodies for NK1 receptors | Upregulation of NK1 receptors and hyperalgesia in mice exposed to stress |
| Drew et al. (2005) [16]. | Adult male and female Sprague-Dawley rats | Application of drug solutions containing Substance P on neurons within the PAG and RVM | Measurement of the IPSCs and EPSCs of neurons | Increased Substance P levels in the PAG led to modulation of descending pain pathways and analgesia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humes, C.; Sic, A.; Knezevic, N.N. Substance P’s Impact on Chronic Pain and Psychiatric Conditions—A Narrative Review. Int. J. Mol. Sci. 2024, 25, 5905. https://doi.org/10.3390/ijms25115905
Humes C, Sic A, Knezevic NN. Substance P’s Impact on Chronic Pain and Psychiatric Conditions—A Narrative Review. International Journal of Molecular Sciences. 2024; 25(11):5905. https://doi.org/10.3390/ijms25115905
Chicago/Turabian StyleHumes, Charles, Aleksandar Sic, and Nebojsa Nick Knezevic. 2024. "Substance P’s Impact on Chronic Pain and Psychiatric Conditions—A Narrative Review" International Journal of Molecular Sciences 25, no. 11: 5905. https://doi.org/10.3390/ijms25115905
APA StyleHumes, C., Sic, A., & Knezevic, N. N. (2024). Substance P’s Impact on Chronic Pain and Psychiatric Conditions—A Narrative Review. International Journal of Molecular Sciences, 25(11), 5905. https://doi.org/10.3390/ijms25115905