Systemic Inflammation in Oncologic Patients Undergoing Systemic Treatment and Receiving Whey Protein-Based Nutritional Support
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Patients
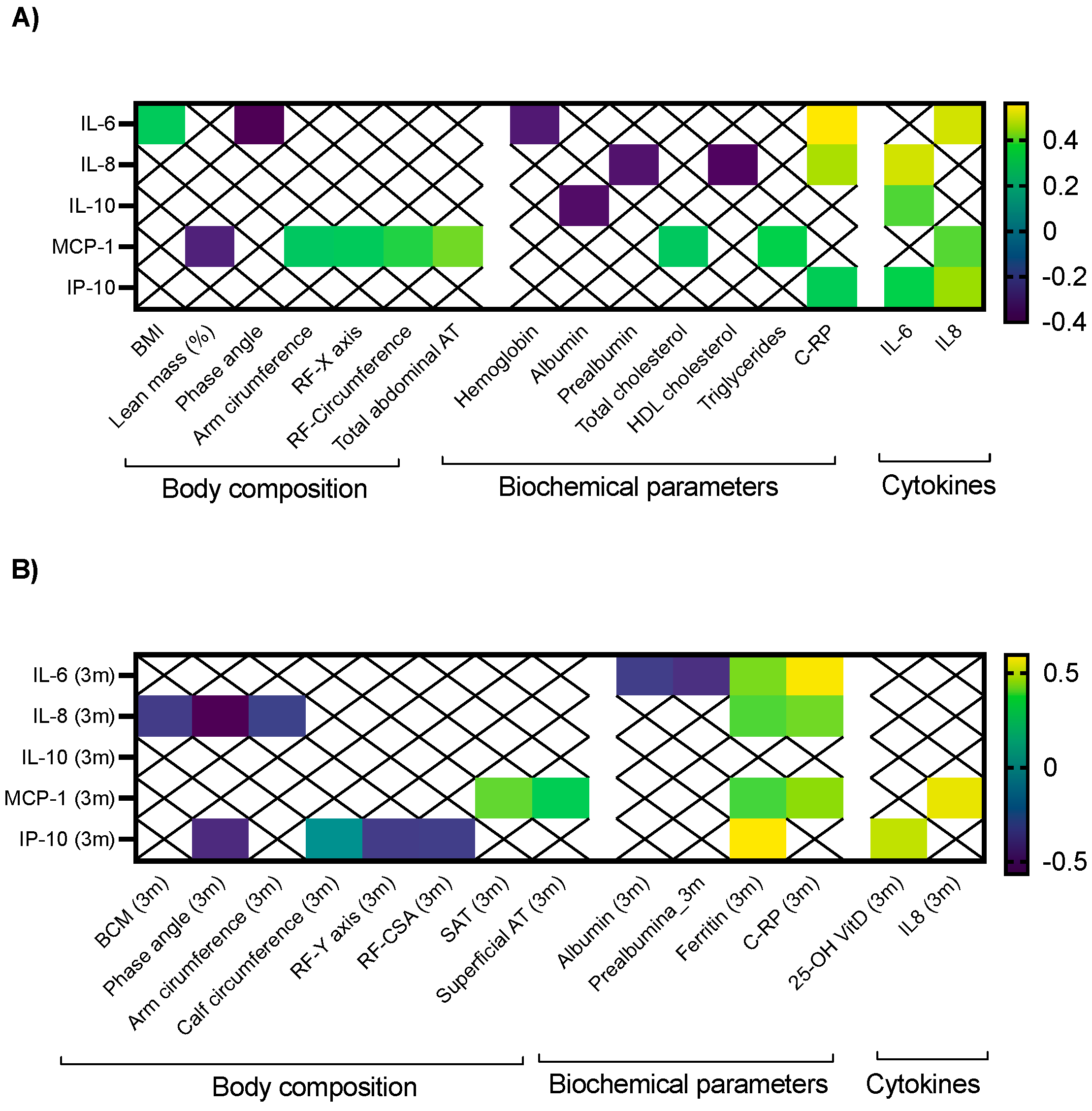
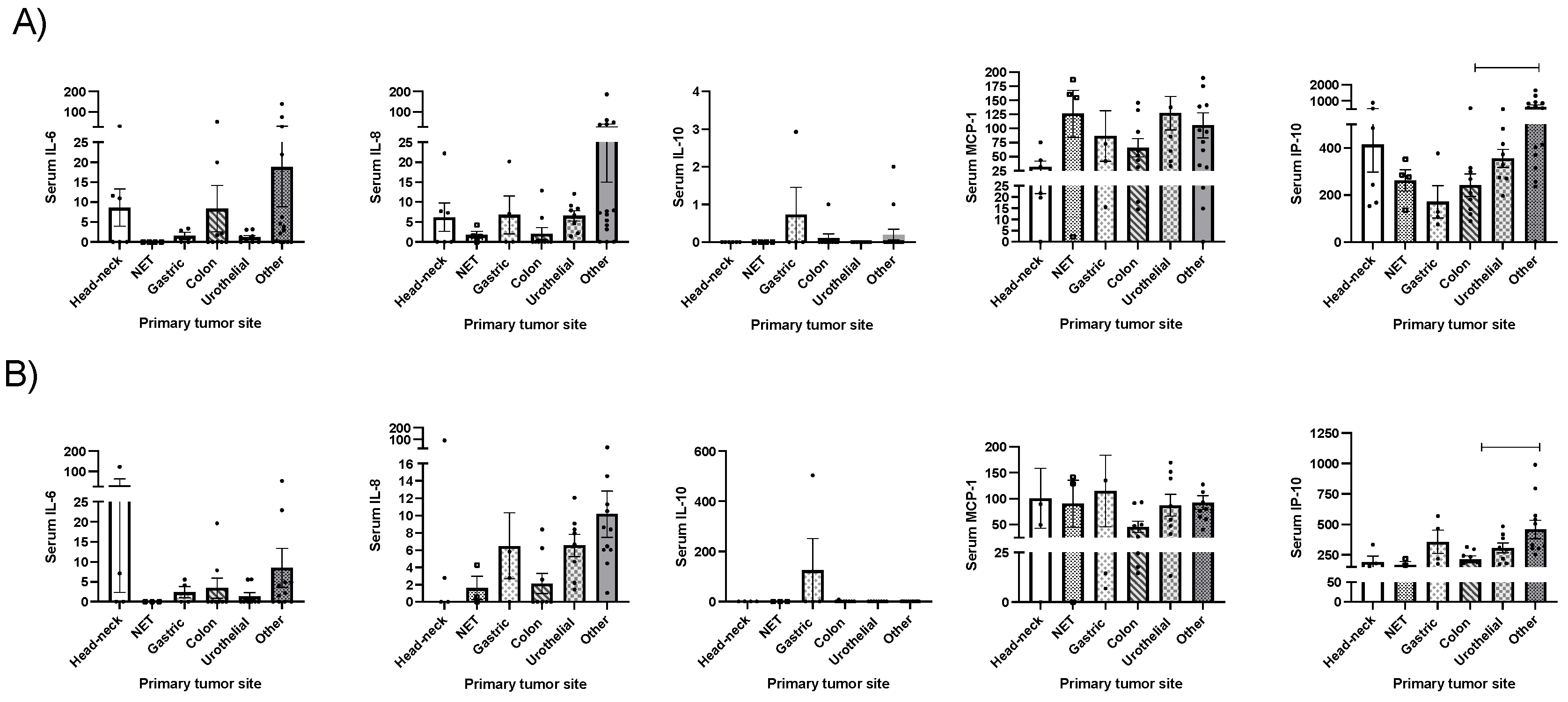
2.2. Clinical Correlations between Cytokines and Morphofunctional Nutritional Evaluation in Cancer Patients
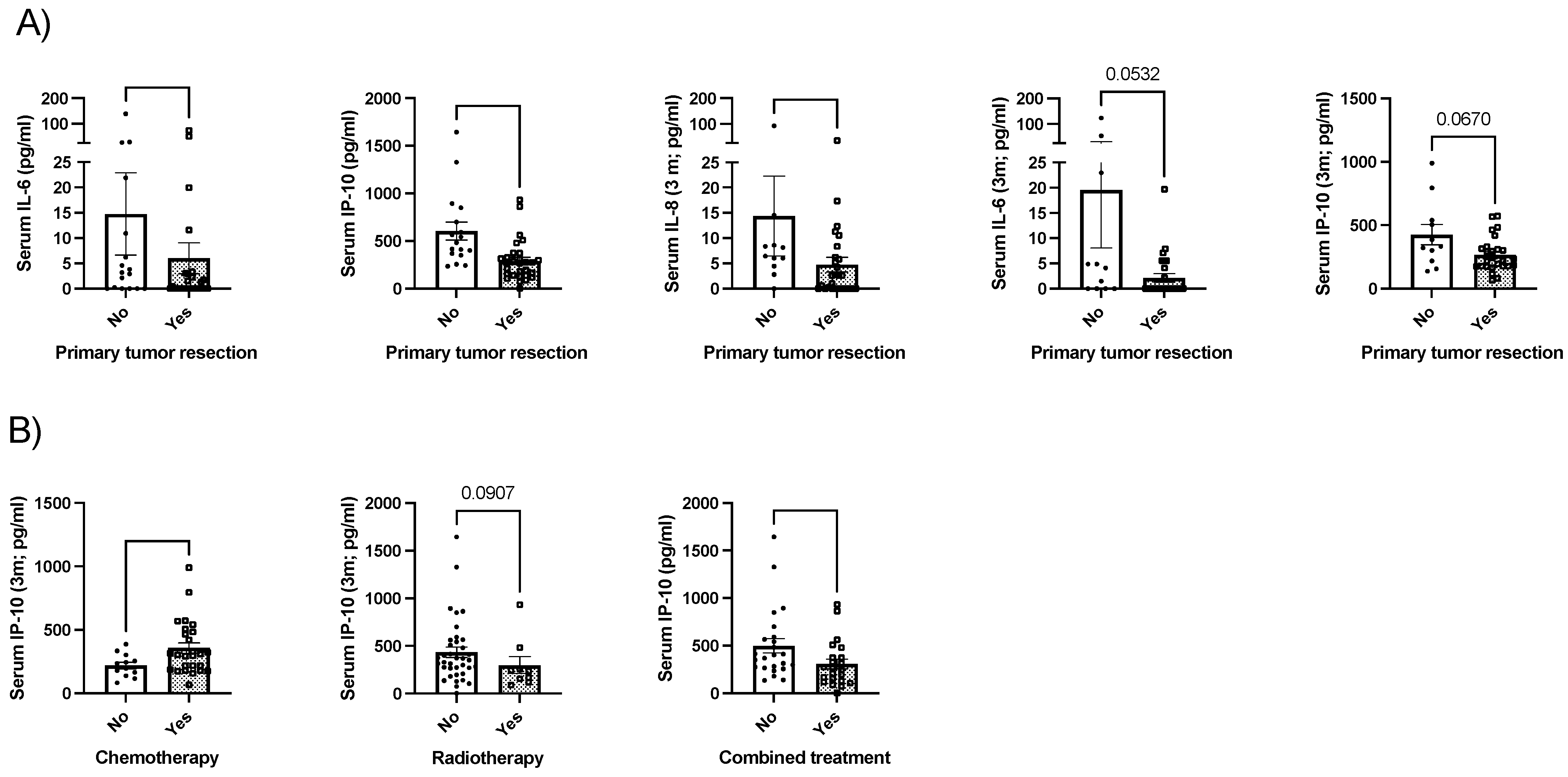
2.3. Whey Protein-Based Nutritional Support and Clinical Outcomes
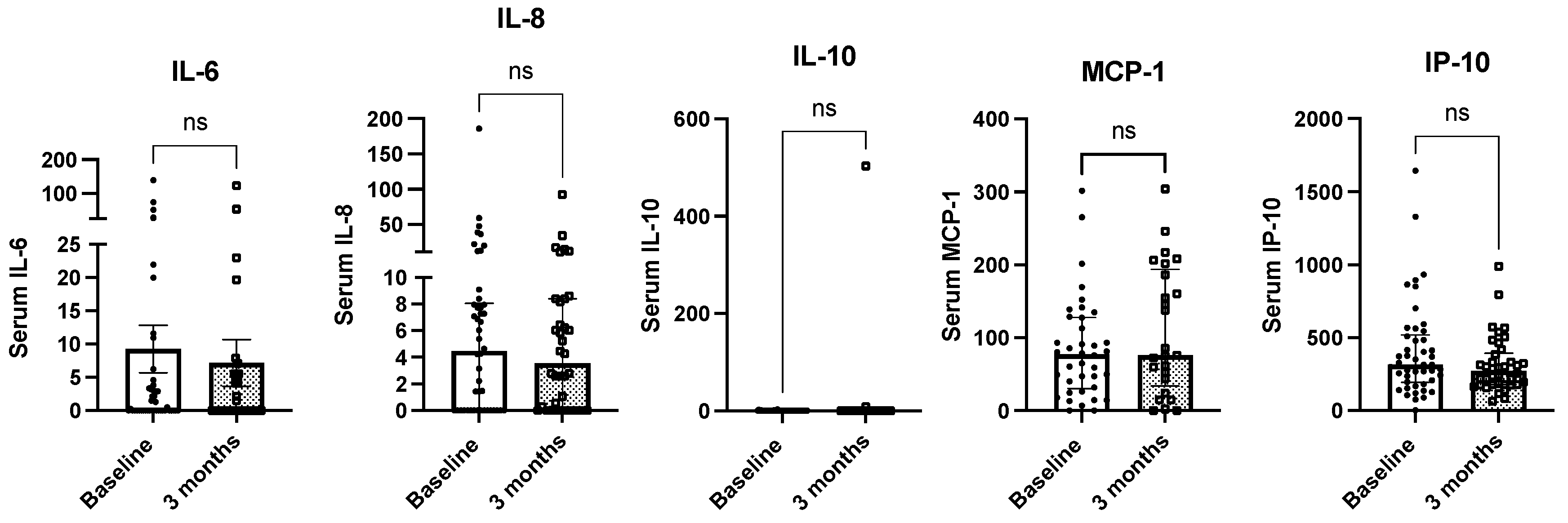
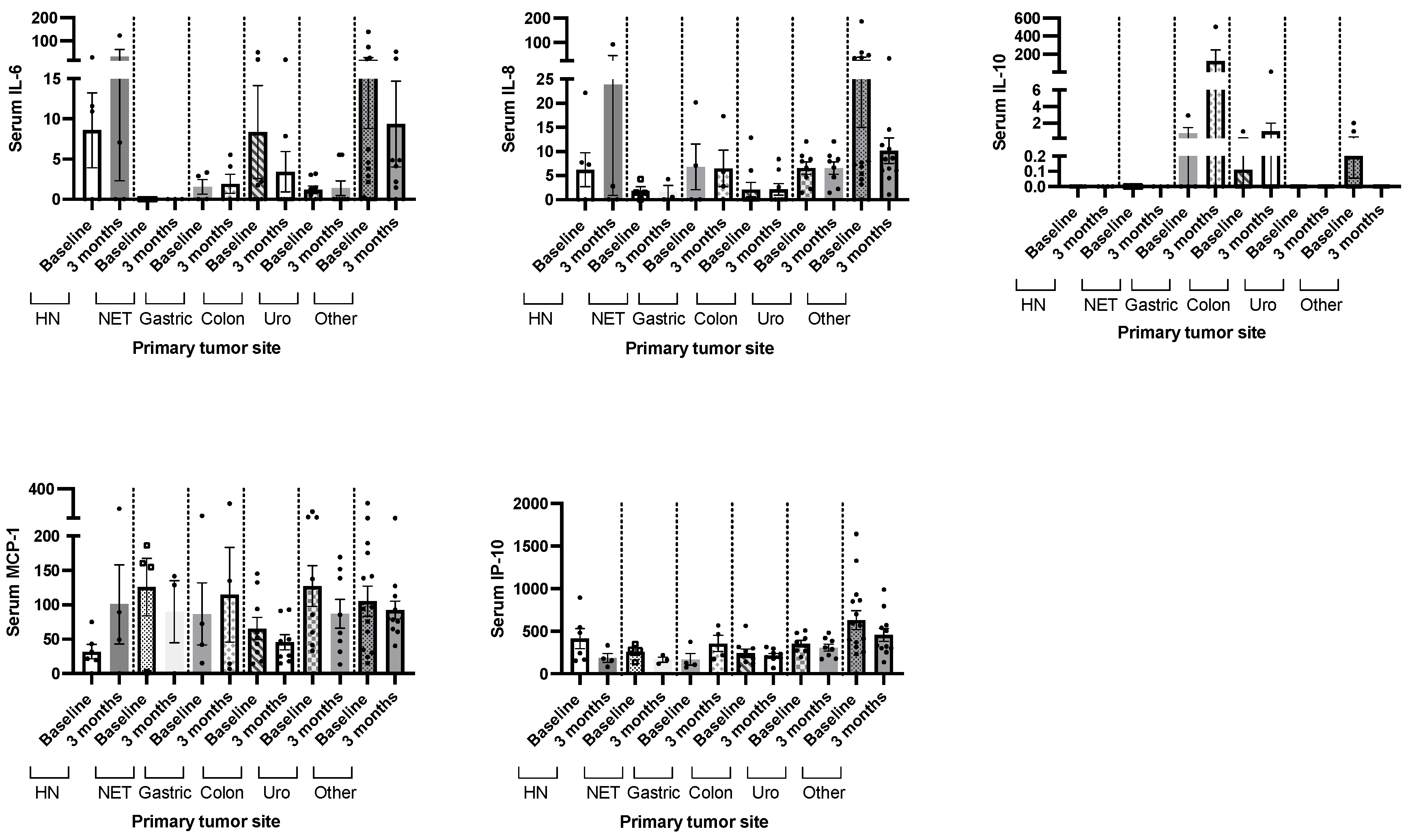
2.4. Whey Protein-Based Nutritional Support and Circulating Cytokine Levels
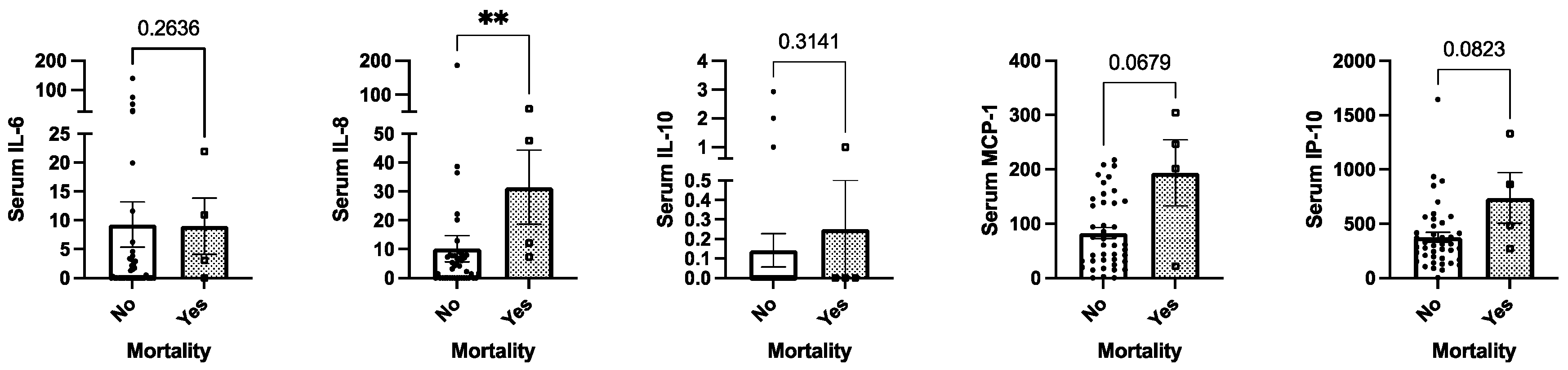
2.5. Mortality and Circulating Cytokine Levels
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Study Protocol, Chronogram and Nutritional Support
4.3. Nutritional and Anthropometric Evaluation
4.4. Cytokine and Chemokine Measurement
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Il’yasova, D.; Colbert, L.H.; Harris, T.B.; Newman, A.B.; Bauer, D.C.; Satterfield, S.; Kritchevsky, S.B. Circulating levels of inflammatory markers and cancer risk in the health aging and body composition cohort. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2413–2418. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Mecca, M.; Picerno, S.; Cortellino, S. The Killer’s Web: Interconnection between Inflammation, Epigenetics and Nutrition in Cancer. Int. J. Mol. Sci. 2024, 25, 2750. [Google Scholar] [CrossRef] [PubMed]
- León-Idougourram, S.; Pérez-Gómez, J.M.; Muñoz Jiménez, C.; L-López, F.; Manzano García, G.; Molina Puertas, M.J.; Herman-Sánchez, N.; Alonso-Echague, R.; Calañas Continente, A.; Gálvez Moreno, M.Á.; et al. Morphofunctional and Molecular Assessment of Nutritional Status in Head and Neck Cancer Patients Undergoing Systemic Treatment: Role of Inflammasome in Clinical Nutrition. Cancers 2022, 14, 494. [Google Scholar] [CrossRef] [PubMed]
- Edwardson, D.W.; Parissenti, A.M.; Kovala, A.T. Chemotherapy and Inflammatory Cytokine Signalling in Cancer Cells and the Tumour Microenvironment. Adv. Exp. Med. Biol. 2019, 1152, 173–215. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Kaluza, J.; Håkansson, N.; Harris, H.R.; Orsini, N.; Michaëlsson, K.; Wolk, A. Influence of anti-inflammatory diet and smoking on mortality and survival in men and women: Two prospective cohort studies. J. Intern. Med. 2019, 285, 75–91. [Google Scholar] [CrossRef] [PubMed]
- van Ommen, B.; van den Broek, T.; de Hoogh, I.; van Erk, M.; van Someren, E.; Rouhani-Rankouhi, T.; Anthony, J.C.; Hogenelst, K.; Pasman, W.; Boorsma, A.; et al. Systems biology of personalized nutrition. Nutr. Rev. 2017, 75, 579–599. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, V.; Lepore, E.; Basciani, S.; Artale, S.; Nordio, M.; Bizzarri, M.; Unfer, V. Positive Effects of α-Lactalbumin in the Management of Symptoms of Polycystic Ovary Syndrome. Nutrients 2022, 14, 3220. [Google Scholar] [CrossRef] [PubMed]
- Foisy-Sauvé, M.; Ahmarani, L.; Delvin, E.; Sané, A.T.; Spahis, S.; Levy, E. Glycomacropeptide Prevents Iron/Ascorbate-Induced Oxidative Stress, Inflammation and Insulin Sensitivity with an Impact on Lipoprotein Production in Intestinal Caco-2/15 Cells. Nutrients 2020, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Mazidi, M.; Sankaranarayanan, R.; Tajik, B.; McArdle, A.; Isanejad, M. Effects of whey and soy protein supplementation on inflammatory cytokines in older adults: A systematic review and meta-analysis. Br. J. Nutr. 2023, 129, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Ma, J.; Khan, M.Z.; Alugongo, G.M.; Chen, T.; Liu, S.; Li, S.; Cao, Z. Unlocking the potential of milk whey protein components in colorectal cancer prevention and therapy. Crit. Rev. Food Sci. Nutr. 2023, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Martínez, A.D.; León Idougourram, S.; Muñoz Jiménez, C.; Rodríguez-Alonso, R.; Alonso Echague, R.; Chica Palomino, S.; Sanz Sanz, A.; Manzano García, G.; Gálvez Moreno, M.; Calañas Continente, A.; et al. Standard Hypercaloric, Hyperproteic vs. Leucine-Enriched Oral Supplements in Patients with Cancer-Induced Sarcopenia, a Randomized Clinical Trial. Nutrients 2023, 15, 2726. [Google Scholar] [CrossRef]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-induced muscle wasting: Latest findings in prevention and treatment. Ther. Adv. Med. Oncol. 2017, 9, 369–382. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Lieffers, J.R.; Bathe, O.F.; Fassbender, K.; Winget, M.; Baracos, V.E. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br. J. Cancer 2012, 107, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, H.; Sun, Y. Effect of acute inflammatory reaction induced by biopsy on tumor microenvironment. J. Cancer Res. Clin. Oncol. 2024, 150, 177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.M.; Wang, Q.; Tran, H.T.; Mitchell, K.G.; Antonoff, M.B.; Hofstetter, W.L.; Mehran, R.J.; Rice, D.C.; Roth, J.A.; Swisher, S.G.; et al. Peripheral cytokines are not influenced by the type of surgical approach for non-small cell lung cancer by four weeks postoperatively. Lung Cancer 2020, 146, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lopez, V.; Gómez Ruiz, A.; Pelegrin, P.; Abellán, B.; Lopez-Conesa, A.; Brusadin, R.; Cayuela, V.; García, A.; Robles Campos, R. Impact of Immune Response in Short-term and Long-term Outcomes after Minimally Invasive Surgery for Colorectal Liver Metastases: Results from a Randomized Study. Surg. Laparosc. Endosc. Percutan Tech. 2021, 31, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Vyas, D.; Laput, G.; Vyas, A.K. Chemotherapy-enhanced inflammation may lead to the failure of therapy and metastasis. Onco Targets Ther. 2014, 7, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Song, H.; Shen, J.; Wang, J.; Yang, Y.; Cao, J.; Xue, L.; Zhao, F.; Xiao, T.; Lin, R. Functional role of skeletal muscle-derived interleukin-6 and its effects on lipid metabolism. Front. Physiol. 2023, 14, 1110926. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Sameem, S.; Dwivedi, V.; Kumar, V.; Dwivedi, A.R.; Pathak, P.; Singh, B.; Bhat, M.A.; Verma, A. Phyto-fabrication of Moringa oleifera peel-sourced silver nanoparticles: A promising approach for combating hepatic cancer by targeting proinflammatory cytokines and mitigating cytokine storms. Chem. Biodivers. 2024, e202400059. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Physiological effects of modulating the interleukin-6 axis. Rheumatology 2018, 57 (Suppl. 2), ii43–ii50. [Google Scholar] [CrossRef]
- Fousek, K.; Horn, L.A.; Palena, C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol. Ther. 2021, 219, 107692. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.C.; Wang, C.J.; Chao, Y.J.; Chen, H.Y.; Wang, H.C.; Tung, H.L.; Lin, J.T.; Shan, Y.S. Elevated Serum Interleukin-8 Level Correlates with Cancer-Related Cachexia and Sarcopenia: An Indicator for Pancreatic Cancer Outcomes. J. Clin. Med. 2018, 7, 502. [Google Scholar] [CrossRef]
- D’Esposito, V.; Di Tolla, M.F.; Lecce, M.; Cavalli, F.; Libutti, M.; Misso, S.; Cabaro, S.; Ambrosio, M.R.; Parascandolo, A.; Covelli, B.; et al. Lifestyle and Dietary Habits Affect Plasma Levels of Specific Cytokines in Healthy Subjects. Front. Nutr. 2022, 9, 913176. [Google Scholar] [CrossRef] [PubMed]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Cranford, T.L.; Enos, R.T.; Velázquez, K.T.; McClellan, J.L.; Davis, J.M.; Singh, U.P.; Nagarkatti, M.; Nagarkatti, P.S.; Robinson, C.M.; Murphy, E.A. Role of MCP-1 on inflammatory processes and metabolic dysfunction following high-fat feedings in the FVB/N strain. Int. J. Obes. 2016, 40, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Mumick, S.; Zhang, C.; Lamb, J.; Dai, H.; Weingarth, D.; Mudgett, J.; Chen, H.; MacNeil, D.J.; Reitman, M.L.; et al. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes. Res. 2005, 13, 1311–1320. [Google Scholar] [CrossRef]
- Wang, L.; Lan, J.; Tang, J.; Luo, N. MCP-1 targeting: Shutting off an engine for tumor development. Oncol. Lett. 2022, 23, 26. [Google Scholar] [CrossRef]
- Li, Y.T.; Wang, Y.C.; Lee, H.L.; Tsao, S.C.; Lu, M.C.; Yang, S.F. Monocyte Chemoattractant Protein-1, a Possible Biomarker of Multiorgan Failure and Mortality in Ventilator-Associated Pneumonia. Int. J. Mol. Sci. 2019, 20, 2218. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Liao, X.; Feng, T.; Wu, Q.; Zhang, J.; Cao, X.; Li, H. Plasma Monocyte Chemoattractant Protein 1 as a Predictive Marker for Sepsis Prognosis: A Prospective Cohort Study. Tohoku J. Exp. Med. 2017, 241, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Su, D.; Li, X.; Li, Z.; Wang, Y.; Qiu, J.; Lin, P.; Zhang, Y.; Guo, P.; Xia, M.; et al. Serum levels of monocyte chemoattractant protein-1 and all-cause and cardiovascular mortality among patients with coronary artery disease. PLoS ONE 2015, 10, e0120633. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.; Nishimura, N.; Sciullo, E.; Wong, P.; Li, W.; Matsumura, F. Modulation of the chemokines KC and MCP-1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Arch. Biochem. Biophys. 2007, 461, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Billottet, C.; Quemener, C.; Bikfalvi, A. CXCR3, a double-edged sword in tumor progression and angiogenesis. Biochim. Biophys. Acta 2013, 1836, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Calvani, R.; Picca, A.; Marini, F.; Biancolillo, A.; Gervasoni, J.; Persichilli, S.; Primiano, A.; Coelho-Junior, H.J.; Cesari, M.; Bossola, M.; et al. Identification of biomarkers for physical frailty and sarcopenia through a new multi-marker approach: Results from the BIOSPHERE study. Geroscience 2021, 43, 727–740. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Zhang, Z.; Zhang, H.; Wang, N.; Chen, X.; Han, X.; Lu, Q.; Chi, S. Effects of Dietary Intervention on Inflammatory Markers in Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 846591. [Google Scholar] [CrossRef]
- Chang, K.V.; Wu, W.T.; Chen, Y.H.; Chen, L.R.; Hsu, W.H.; Lin, Y.L.; Han, D.S. Enhanced serum levels of tumor necrosis factor-α, interleukin-1β, and -6 in sarcopenia: Alleviation through exercise and nutrition intervention. Aging 2023, 15, 13471–13485. [Google Scholar] [CrossRef] [PubMed]
- Trøseid, M.; Arnesen, H.; Hjerkinn, E.M.; Seljeflot, I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism 2009, 58, 1543–1549. [Google Scholar] [CrossRef]
- MacFie, J. Ethical and legal considerations in the provision of nutritional support to the perioperative patient. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 23–29. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee ErSoPaENE. ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Aloy Dos Santos, T.; Luft, V.C.; Souza, G.C.; de Albuquerque Santos, Z.; Keller Jochims, A.M.; Carnevale de Almeida, J. Malnutrition screening tool and malnutrition universal screening tool as a predictors of prolonged hospital stay and hospital mortality: A cohort study. Clin. Nutr. ESPEN 2023, 54, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Alhambra Exposito, M.R.; Herrera-Martinez, A.D.; Manzano Garcia, G.; Espinosa Calvo, M.; Bueno Serrano, C.M.; Galvez Moreno, M.A. Early nutrition support therapy in patients with head-neck cancer. Nutr. Hosp. 2018, 35, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Garcia Almeida, J.M.; Garcia Garcia, C.; Vegas Aguilar, I.M.; Bellido Castaneda, V.; Bellido Guerrero, D. Morphofunctional assessment of patient s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 46) |
|---|---|
| Sex (♂/♀) | 45.7/54.3% (21/25) |
| Age at diagnosis (years) | 74.5 (71–78) |
| Tobacco exposure | |
| No | 50% (21/42) |
| Active | 28.6% (12/42) |
| Previous exposure | 21.4% (9/42) |
| Previous other neoplasms | 19.7% (9/46) |
| Primary tumor location | |
| Head/neck | 13% (6/46) |
| Gastrointestinal NET | 8.7% (4/46) |
| Gastric cancer | 8.7% (4/46) |
| Colorectal cancer | 19.6% (9/46) |
| Urothelial | 32.6% (15/46) |
| Others | 17.4% (8/46) |
| Treatment | |
| Surgery | 63% (29/46) |
| Chemotherapy | 56.5% (26/46) |
| Radiotherapy | 19.6% (9/46) |
| Chemo and radiotherapy | 23.9% (11/46) |
| Combined treatment (surgery plus systemic treatment) | 47.8% (22/46) |
| Symptoms | |
| Weight loss (3 months) | 84.4% (38/45) |
| Weight loss in Kg (3 months) | 5 (4–6) |
| Weight loss (6 months) | 71.7% (33/46) |
| Weight loss in Kg (6 months) | 6.5 (6–7) |
| Gastrointestinal symptoms | 43.5% (20/46) |
| Abdominal pain | 32.6% (15/46) |
| Nauseas/vomits | 22.2% (10/45) |
| Diarrhea | 15.2% (7/46) |
| Dyspnea | 17.4% (8/46) |
| Mucositis | 8.7% (4/46) |
| Quality of life | |
| Any level of dependency | 43.5% (20/46) |
| Self-rated health score | 65 (0–80) |
| ECOG | |
| ECOG 0 | 60.9% (28/46) |
| ECOG 1 | 28.3% (13/46) |
| ECOG 2 | 10.9% (5/46) |
| Mortality | 8.7% (4/46) |
| Variable | OR | CI | p | |
|---|---|---|---|---|
| Mortality | Baseline IL-6 | 0.98 | 0.94–1.03 | 0.62 |
| Baseline IL-8 | 1.02 | 0.99–1.04 | 0.18 | |
| Baseline IL-10 | 1.32 | 0.21–8.00 | 0.76 | |
| Baseline MCP-1 | 1.03 | 1.001–1.05 | 0.03 | |
| Baseline IP-10 | 1.00 | 1.00–1.01 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Martínez, A.D.; Navas Romo, A.; León-Idougourram, S.; Muñoz-Jiménez, C.; Rodríguez-Alonso, R.; Manzano García, G.; Camacho-Cardenosa, M.; Casado-Diaz, A.; Gálvez-Moreno, M.Á.; Molina Puertas, M.J.; et al. Systemic Inflammation in Oncologic Patients Undergoing Systemic Treatment and Receiving Whey Protein-Based Nutritional Support. Int. J. Mol. Sci. 2024, 25, 5821. https://doi.org/10.3390/ijms25115821
Herrera-Martínez AD, Navas Romo A, León-Idougourram S, Muñoz-Jiménez C, Rodríguez-Alonso R, Manzano García G, Camacho-Cardenosa M, Casado-Diaz A, Gálvez-Moreno MÁ, Molina Puertas MJ, et al. Systemic Inflammation in Oncologic Patients Undergoing Systemic Treatment and Receiving Whey Protein-Based Nutritional Support. International Journal of Molecular Sciences. 2024; 25(11):5821. https://doi.org/10.3390/ijms25115821
Chicago/Turabian StyleHerrera-Martínez, Aura D., Ana Navas Romo, Soraya León-Idougourram, Concepción Muñoz-Jiménez, Rosa Rodríguez-Alonso, Gregorio Manzano García, Marta Camacho-Cardenosa, Antonio Casado-Diaz, María Ángeles Gálvez-Moreno, María José Molina Puertas, and et al. 2024. "Systemic Inflammation in Oncologic Patients Undergoing Systemic Treatment and Receiving Whey Protein-Based Nutritional Support" International Journal of Molecular Sciences 25, no. 11: 5821. https://doi.org/10.3390/ijms25115821
APA StyleHerrera-Martínez, A. D., Navas Romo, A., León-Idougourram, S., Muñoz-Jiménez, C., Rodríguez-Alonso, R., Manzano García, G., Camacho-Cardenosa, M., Casado-Diaz, A., Gálvez-Moreno, M. Á., Molina Puertas, M. J., & Jurado Roger, A. (2024). Systemic Inflammation in Oncologic Patients Undergoing Systemic Treatment and Receiving Whey Protein-Based Nutritional Support. International Journal of Molecular Sciences, 25(11), 5821. https://doi.org/10.3390/ijms25115821









