Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability
Abstract
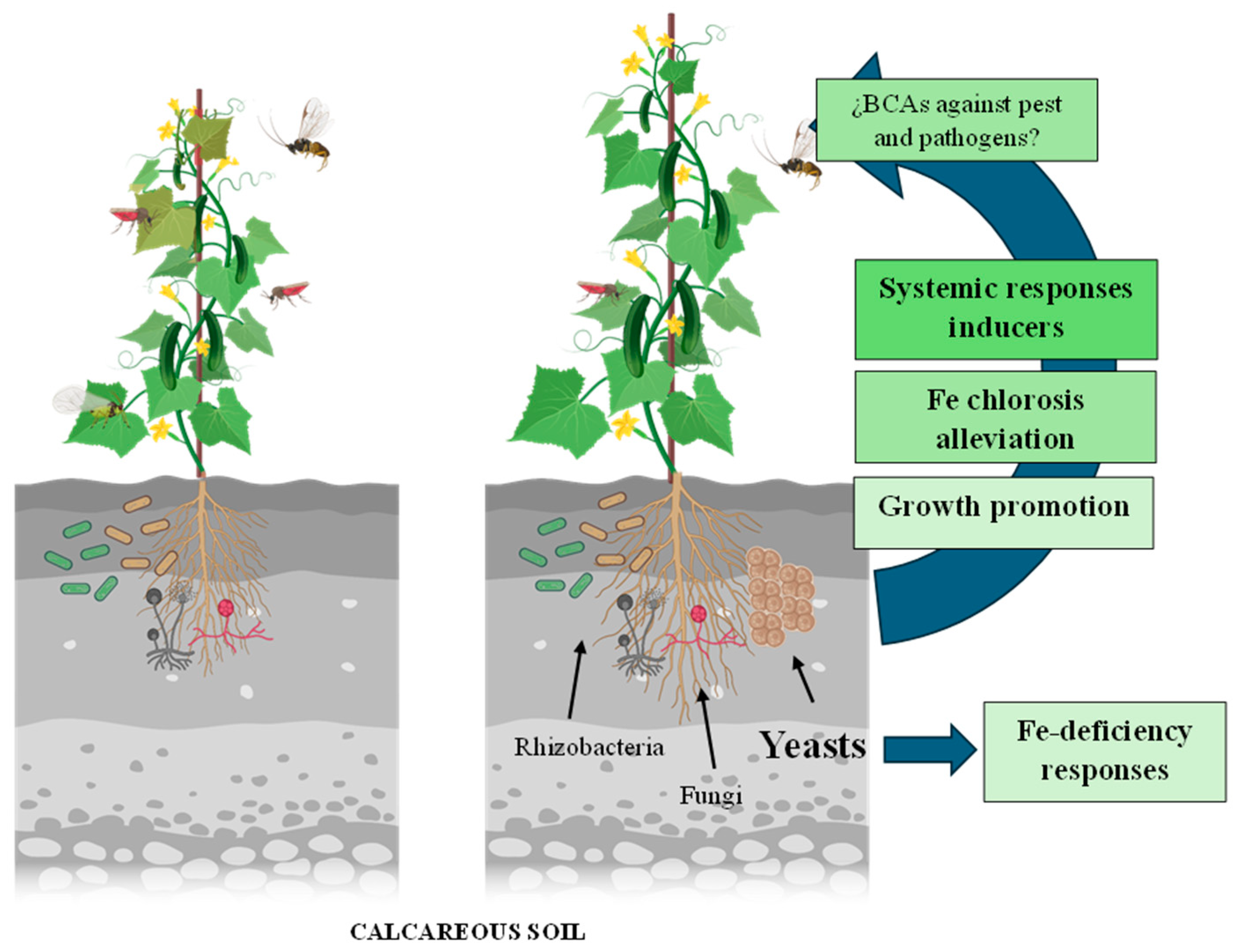
1. Introduction
2. Results
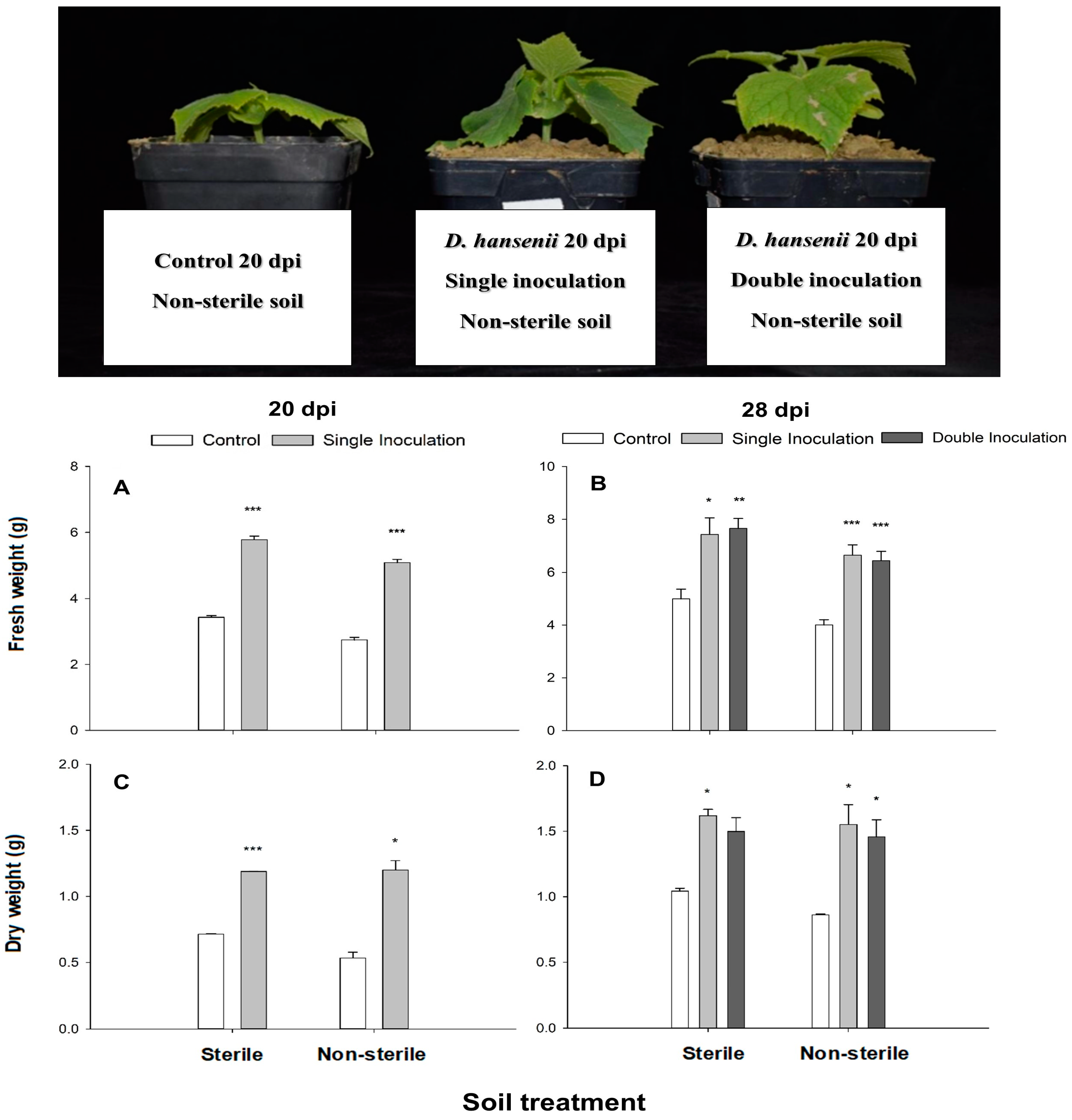
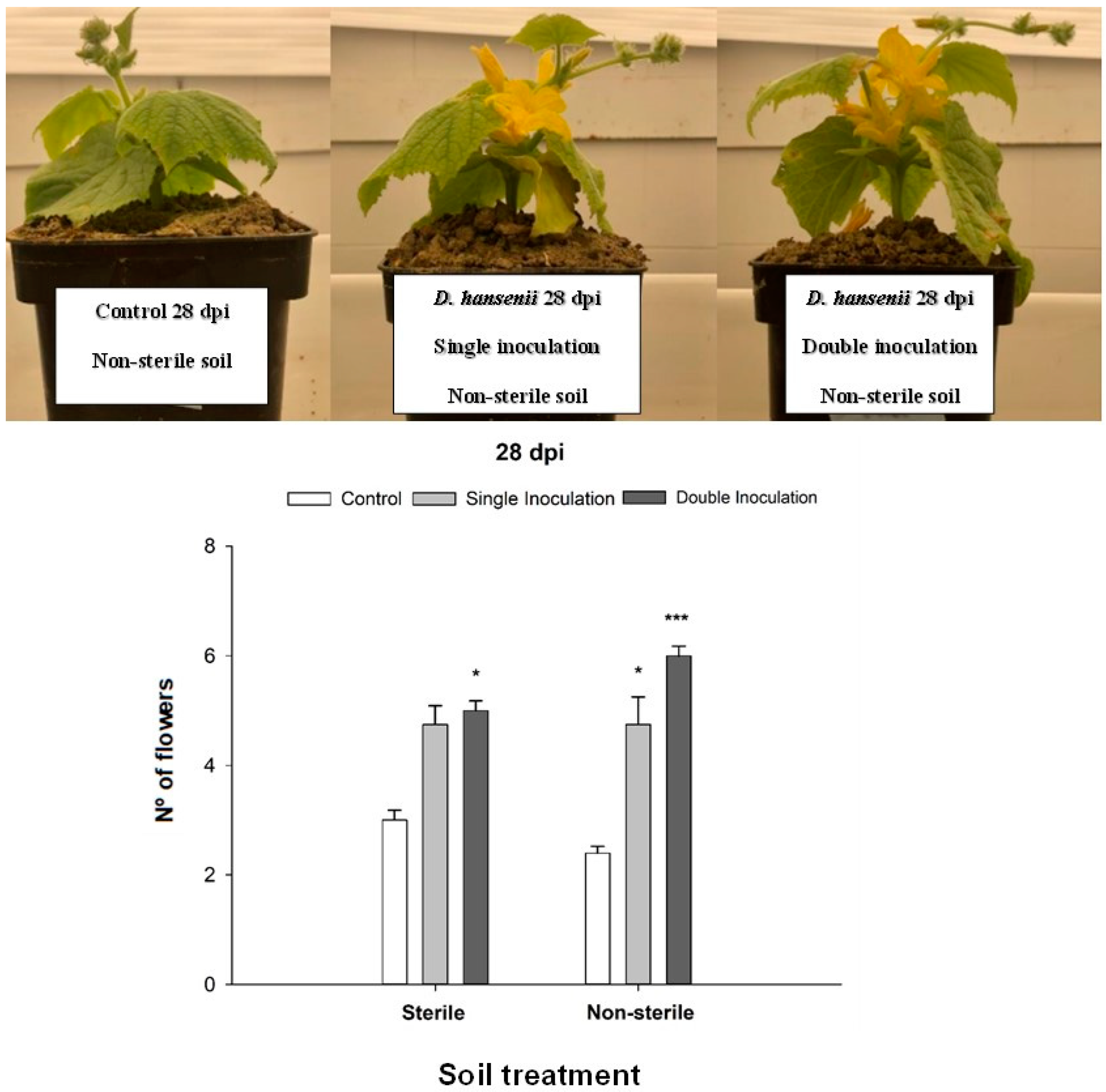
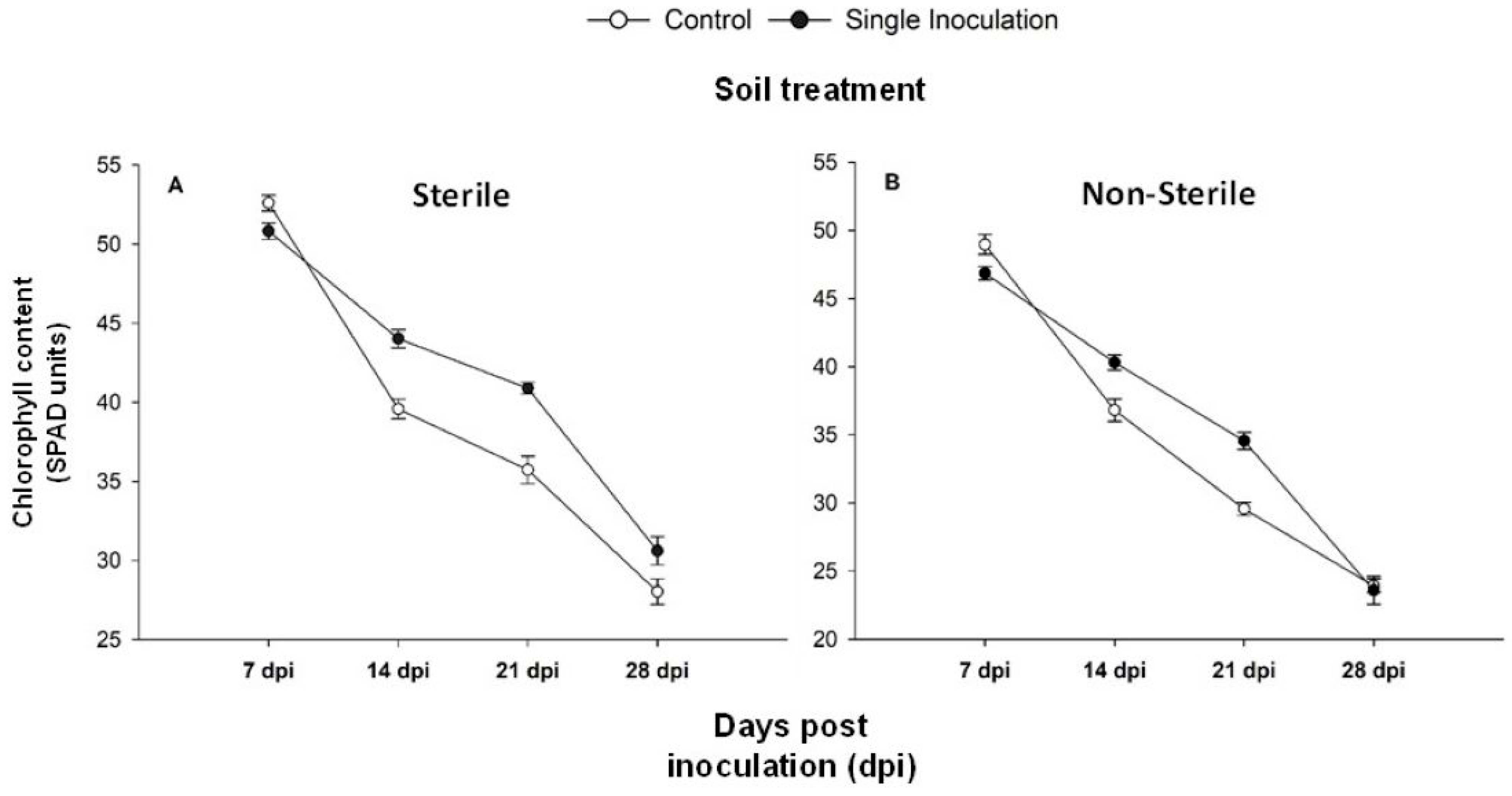
2.1. Debaryomices hansenii Effect on Plant Growth Promotion, Flowers Development and Chlorophyll Content
2.2. Debaryomyces hansenii Effect on Mineral Total Uptake
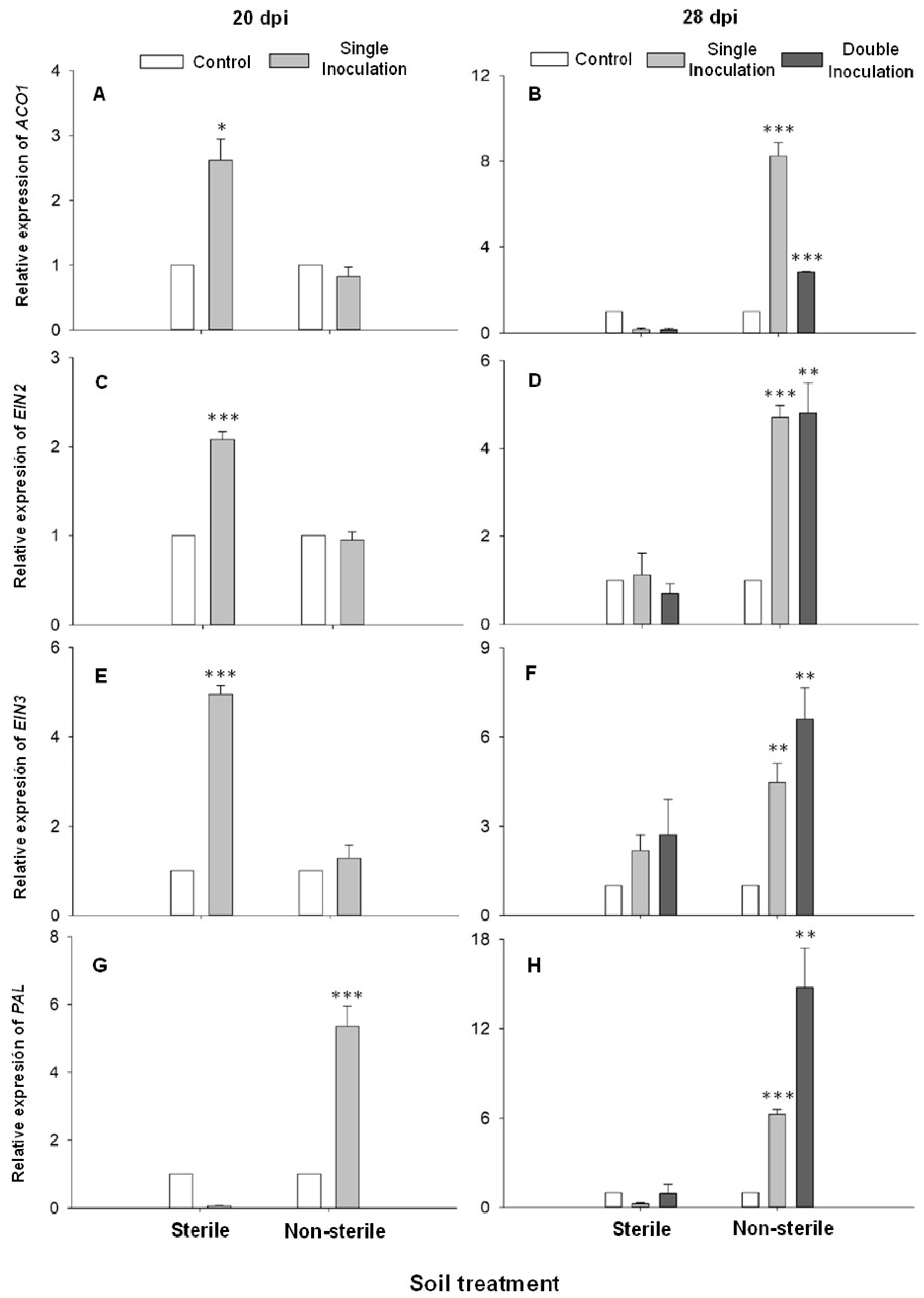
2.3. Debaryomyces hansenii Effect on Gene Expression
3. Discussion
4. Materials and Methods
4.1. Yeast Strain and Inoculum Preparation
4.2. Calcareous Soil Bioassay
4.2.1. Plant Culture, Inoculation, Experimental Design, and Sampling
4.2.2. Assessments and Data Analysis
Mineral Total Uptake (MTU)
Measurement of Relative Chlorophyll Contents (SPAD Values)
qRT-PCR Analysis
Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romera, F.J.; García, M.J.; Lucena, C.; Martínez-Medina, A.; Aparicio, M.A.; Ramos, J.; Alcántara, E.; Angulo, M.; Pérez-Vicente, R. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front. Plant Sci. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Lucena, C.; Alcalá-Jiménez, M.T.; Romera, F.J.; Ramos, J. Several Yeast Species Induce Iron Deficiency Responses in Cucumber Plants (Cucumis sativus L.). Microorganisms 2021, 9, 2603. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal Endophytes: Beyond Herbivore Management. Front. Microbiol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Branine, M.; Bazzicalupo, A.; Branco, S. Biology and Applications of Endophytic Insect-Pathogenic Fungi. PLoS Pathog. 2019, 15, e1007831. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Sultana, F. Application and Mechanisms of Plant Growth Promoting Fungi (PGPF) for Phytostimulation. In Organic Agriculture; Das, S.K., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Kumari, P.; Singh, A.; Kharwar, R.N. Phytostimulation and ISR Responses of Fungi. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 459–473. [Google Scholar]
- Aparicio, M.A.; Lucena, C.; García, M.J.; Ruiz-Castilla, F.J.; Jiménez-Adrián, P.; López-Berges, M.S.; Prieto, P.; Alcántara, E.; Pérez-Vicente, R.; Ramos, J.; et al. The Nonpathogenic Strain of Fusarium oxysporum FO12 Induces Fe Deficiency Responses in Cucumber (Cucumis sativus L.) Plants. Planta 2023, 257, 50. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Cano, J.; Romera, F.J.; Prieto, P.; García, M.J.; Sevillano-Caño, J.; Agustí-Brisach, C.; Pérez-Vicente, R.; Ramos, J.; Lucena, C. Effect of the Nonpathogenic Strain Fusarium oxysporum FO12 on Fe Acquisition in Rice (Oryza sativa L.) Plants. Plants 2023, 12, 3145. [Google Scholar] [CrossRef] [PubMed]
- Quesada Moraga, E. Entomopathogenic Fungi as Endophytes: Their Broader Contribution to IPM and Crop Production. Biocontrol Sci. Technol. 2020, 30, 864–877. [Google Scholar] [CrossRef]
- García-Espinoza, F.; García, M.J.; Quesada-Moraga, E.; Yousef-Yousef, M. Entomopathogenic Fungus-Related Priming Defense Mechanisms in Cucurbits Impact Spodoptera littoralis (Boisduval) Fitness. Appl. Environ. Microbiol. 2023, 89, e00940-23. [Google Scholar] [CrossRef]
- García-Espinoza, F.; Quesada-Moraga, E.; García del Rosal, M.J.; Yousef-Yousef, M. Entomopathogenic Fungi-Mediated Solubilization and Induction of Fe Related Genes in Melon and Cucumber Plants. J. Fungi 2023, 9, 258. [Google Scholar] [CrossRef]
- Serteyn, L.; Quaghebeur, C.; Ongena, M.; Cabrera, N.; Barrera, A.; Molina-Montenegro, M.A.; Francis, F.; Ramírez, C.C. Induced Systemic Resistance by a Plant Growth-Promoting Rhizobacterium Impacts Development and Feeding Behavior of Aphids. Insects 2020, 11, 234. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, M.; Sharma, A.; Sharma, V. Insights into Plant Beneficial Microorganism-Triggered Induced Systemic Resistance. Plant Stress 2023, 7, 100140. [Google Scholar] [CrossRef]
- Yu, Y.; Gui, Y.; Li, Z.; Jiang, C.; Guo, J.; Niu, D. Induced Systemic Resistance for Improving Plant Immunity by Beneficial Microbes. Plants 2022, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Zehra, A.; Raytekar, N.A.; Meena, M.; Swapnil, P. Efficiency of Microbial Bio-Agents as Elicitors in Plant Defense Mechanism under Biotic Stress: A Review. Curr. Res. Microb. Sci. 2021, 2, 100054. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, J.; Xie, Q.; Jiang, Y.; Yu, N.; Wang, E. Nutrient Exchange and Regulation in Arbuscular Mycorrhizal Symbiosis. Mol. Plant 2017, 10, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Huisman, R.; Geurts, R. A Roadmap toward Engineered Nitrogen-Fixing Nodule Symbiosis. Plant Commun. 2020, 1, 100019. [Google Scholar] [CrossRef] [PubMed]
- Verbon, E.H.; Trapet, P.L.; Stringlis, I.A.; Kruijs, S.; Bakker, P.A.H.M.; Pieterse, C.M.J. Iron and Immunity. Annu. Rev. Phytopathol. 2017, 55, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.W.; Ye, Y.Q.; Zheng, S.J. An Underground Tale: Contribution of Microbial Activity to Plant Iron Acquisition via Ecological Processes. Ann. Bot. 2014, 113, 7–18. [Google Scholar] [CrossRef]
- Zamioudis, C.; Korteland, J.; Van Pelt, J.A.; van Hamersveld, M.; Dombrowski, N.; Bai, Y.; Hanson, J.; Van Verk, M.C.; Ling, H.; Schulze-Lefert, P.; et al. Rhizobacterial Volatiles and Photosynthesis-related Signals Coordinate MYB72 Expression in Arabidopsis Roots during Onset of Induced Systemic Resistance and Iron-deficiency Responses. Plant J. 2015, 84, 309–322. [Google Scholar] [CrossRef]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial Interactions in the Rhizosphere: Beneficial Influences of Plant Growth-Promoting Rhizobacteria on Nutrient Acquisition Process. A Review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Zamioudis, C.; Hanson, J.; Pieterse, C.M.J. Β-Glucosidase BGLU42 Is a MYB72-dependent Key Regulator of Rhizobacteria-induced Systemic Resistance and Modulates Iron Deficiency Responses in Arabidopsis Roots. New Phytol. 2014, 204, 368–379. [Google Scholar] [CrossRef]
- Harbort, C.J.; Hashimoto, M.; Inoue, H.; Niu, Y.; Guan, R.; Rombolà, A.D.; Kopriva, S.; Voges, M.J.E.E.E.; Sattely, E.S.; Garrido-Oter, R.; et al. Root-Secreted Coumarins and the Microbiota Interact to Improve Iron Nutrition in Arabidopsis. Cell Host Microbe 2020, 28, 825–837.e6. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced Systemic Resistance and Promotion of Plant Growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Bakker, P.A.H.M.; Doornbos, R.F.; Zamioudis, C.; Berendsen, R.L.; Pieterse, C.M.J. Induced Systemic Resistance and the Rhizosphere Microbiome. Plant Pathol. J. 2013, 29, 136–143. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Navathe, S.; Chand, R.; Pandey, S.P. A Halotolerant Growth Promoting Rhizobacteria Triggers Induced Systemic Resistance in Plants and Defends against Fungal Infection. Sci. Rep. 2019, 9, 4054. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium That Also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef]
- Fan, D.; Smith, D.L. Characterization of Selected Plant Growth-Promoting Rhizobacteria and Their Non-Host Growth Promotion Effects. Microbiol. Spectr. 2021, 9, e0027921. [Google Scholar] [CrossRef]
- Kashyap, A.S.; Manzar, N.; Rajawat, M.V.S.; Kesharwani, A.K.; Singh, R.P.; Dubey, S.C.; Pattanayak, D.; Dhar, S.; Lal, S.K.; Singh, D. Screening and Biocontrol Potential of Rhizobacteria Native to Gangetic Plains and Hilly Regions to Induce Systemic Resistance and Promote Plant Growth in Chilli against Bacterial Wilt Disease. Plants 2021, 10, 2125. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of Plants with Microbial World: Exploring the Regulatory Networks for PGPR Mediated Defense Signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef]
- Pozo, M.J.; Azcón-Aguilar, C. Unraveling Mycorrhiza-Induced Resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Köllner, T.G.; Chen, F. Colonization by Arbuscular Mycorrhizal and Endophytic Fungi Enhanced Terpene Production in Tomato Plants and Their Defense against a Herbivorous Insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Zitlalpopoca-Hernandez, G.; Pozo, M.J.; Hauser, T.P.; Meyling, N.V. Combined Effects of Root-Associated Entomopathogenic and Mycorrhizal Fungi on the Foliar Pathogen Botrytis cinerea in Tomato. Biol. Control 2022, 175, 105034. [Google Scholar] [CrossRef]
- Alkooranee, J.T.; Yin, Y.; Aledan, T.R.; Jiang, Y.; Lu, G.; Wu, J.; Li, M. Systemic Resistance to Powdery Mildew in Brassica Napus (AACC) and Raphanus alboglabra (RRCC) by Trichoderma harzianum TH12. PLoS ONE 2015, 10, e0142177. [Google Scholar] [CrossRef] [PubMed]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef]
- Di Lelio, I.; Forni, G.; Magoga, G.; Brunetti, M.; Bruno, D.; Becchimanzi, A.; De Luca, M.G.; Sinno, M.; Barra, E.; Bonelli, M.; et al. A Soil Fungus Confers Plant Resistance against a Phytophagous Insect by Disrupting the Symbiotic Role of Its Gut Microbiota. Proc. Natl. Acad. Sci. USA 2023, 120, e2216922120. [Google Scholar] [CrossRef]
- Morán-Diez, M.E.; Martínez de Alba, Á.E.; Rubio, M.B.; Hermosa, R.; Monte, E. Trichoderma and the Plant Heritable Priming Responses. J. Fungi 2021, 7, 318. [Google Scholar] [CrossRef]
- Monte, E. The Sophisticated Evolution of Trichoderma to Control Insect Pests. Proc. Natl. Acad. Sci. USA 2023, 120, e2301971120. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A Multipurpose, Plant-Beneficial Microorganism for Eco-Sustainable Agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef]
- Briat, J.-F.; Dubos, C.; Gaymard, F. Iron Nutrition, Biomass Production, and Plant Product Quality. Trends Plant Sci. 2015, 20, 33–40. [Google Scholar] [CrossRef]
- Vélez-Bermúdez, I.C.; Schmidt, W. Plant Strategies to Mine Iron from Alkaline Substrates. Plant Soil 2023, 483, 1–25. [Google Scholar] [CrossRef]
- Molnár, Z.; Solomon, W.; Mutum, L.; Janda, T. Understanding the Mechanisms of Fe Deficiency in the Rhizosphere to Promote Plant Resilience. Plants 2023, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Nishizawa, N.K. Iron Uptake, Translocation, and Regulation in Higher Plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Brumbarova, T.; Bauer, P.; Ivanov, R. Molecular Mechanisms Governing Arabidopsis Iron Uptake. Trends Plant Sci. 2015, 20, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, R.; Brumbarova, T.; Bauer, P. Fitting into the Harsh Reality: Regulation of Iron-Deficiency Responses in Dicotyledonous Plants. Mol. Plant 2012, 5, 27–42. [Google Scholar] [CrossRef]
- Lucena, C.; Romera, F.J.; García, M.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene Participates in the Regulation of Fe Deficiency Responses in Strategy I Plants and in Rice. Front. Plant Sci. 2015, 6, 166531. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, H.; Wang, N.; Li, J.; Zhao, W.; Du, J.; Wang, D.; Ling, H.-Q. FIT Interacts with AtbHLH38 and AtbHLH39 in Regulating Iron Uptake Gene Expression for Iron Homeostasis in Arabidopsis. Cell Res. 2008, 18, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Cui, Y.; Liu, Y.; Fan, H.; Du, J.; Huang, Z.; Yuan, Y.; Wu, H.; Ling, H.-Q. Requirement and Functional Redundancy of Ib Subgroup BHLH Proteins for Iron Deficiency Responses and Uptake in Arabidopsis thaliana. Mol. Plant 2013, 6, 503–513. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Lucena, C.; Romera, F.J. Ethylene and Nitric Oxide Involvement in the Regulation of Fe and P Deficiency Responses in Dicotyledonous Plants. Int. J. Mol. Sci. 2021, 22, 4904. [Google Scholar] [CrossRef]
- Curie, C.; Mari, S. New Routes for Plant Iron Mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef]
- Tsai, H.; Schmidt, W. One Way. or Another? Iron Uptake in Plants. New Phytol. 2017, 214, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Schmidt, W. Mobilization of Iron by Plant-Borne Coumarins. Trends Plant Sci. 2017, 22, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.B.; Giehl, R.F.H.; Doll, S.; Mock, H.-P.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wiren, N. Feruloyl-CoA 6′-Hydroxylase1-Dependent Coumarins Mediate Iron Acquisition from Alkaline Substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef] [PubMed]
- García, M.J.; Lucena, C.; Romera, F.J.; Alcántara, E.; Pérez-Vicente, R. Ethylene and Nitric Oxide Involvement in the Up-Regulation of Key Genes Related to Iron Acquisition and Homeostasis in Arabidopsis. J. Exp. Bot. 2010, 61, 3885–3899. [Google Scholar] [CrossRef] [PubMed]
- Acharya, K.; Chandra, S.; Chakraborty, N.; Acharya, R. Nitric Oxide Functions as a Signal in Induced Systemic Resistance. Arch. Phytopathol. Plant Prot. 2011, 44, 1335–1342. [Google Scholar] [CrossRef]
- Montejano-Ramírez, V.; Valencia-Cantero, E. Cross-Talk between Iron Deficiency Response and Defense Establishment in Plants. Int. J. Mol. Sci. 2023, 24, 6236. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, J.H.; Long, T.A.; McDowell, J.M. Iron Homeostasis and Plant Immune Responses: Recent Insights and Translational Implications. J. Biol. Chem. 2020, 295, 13444–13457. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; Van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-Dependent Coumarin Exudation Shapes Root Microbiome Assembly to Promote Plant Health. Proc. Natl. Acad. Sci. USA 2018, 115, e5213. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Guo, J.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.; Wang, J. Paenibacillus polymyxa BFKC01 Enhances Plant Iron Absorption via Improved Root Systems and Activated Iron Acquisition Mechanisms. Plant Physiol. Biochem. 2016, 105, 162–173. [Google Scholar] [CrossRef]
- Martínez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne Signals from Trichoderma Fungi Stimulate Iron Uptake Responses in Roots Resulting in Priming of Jasmonic Acid-Dependent Defences in Shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant Cell Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Selection and Evaluation of Micro-Organisms for Biocontrol of Verticillium dahliae in Olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Mulero-Aparicio, A.; Agustí-Brisach, C.; Varo, Á.; López-Escudero, F.J.; Trapero, A. A Non-Pathogenic Strain of Fusarium oxysporum as a Potential Biocontrol Agent against Verticillium Wilt of Olive. Biol. Control 2019, 139, 104045. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Cernava, T.; Turrà, D.; Schaefer, A.; Di Pietro, A.; López-Escudero, F.J.; Trapero, A.; Berg, G. The Role of Volatile Organic Compounds and Rhizosphere Competence in Mode of Action of the Non-Pathogenic Fusarium oxysporum FO12 Toward Verticillium Wilt. Front. Microbiol. 2019, 10, 468375. [Google Scholar] [CrossRef] [PubMed]
- Resquín-Romero, G.; Garrido-Jurado, I.; Quesada-Moraga, E. Combined Use of Entomopathogenic Fungi and Their Extracts for the Control of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Biol. Control 2016, 92, 101–110. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Ruiz-García, A.; Santiago-Álvarez, S. Laboratory Evaluation of Entomopathogenic Fungi Beauveria bassiana and Metarhizium anisopliae against Puparia and Adults of Ceratitis capitata (Diptera: Tephritidae). J. Econ. Entomol. 2006, 99, 1955–1966. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Yousef-Naef, M.; Garrido-Jurado, I. Advances in the Use of Entomopathogenic Fungi as Biopesticides in Suppressing Crop Insect Pests. In Biopesticides for Sustainable Agriculture; Burleigh Dodds Science Publishing: Cambridgeshire, UK, 2020; pp. 63–98. [Google Scholar]
- Romera, F.J.; Alcántara, E.; De La Guardia, M.D. Role of Roots and Shoots in the Regulation of the Fe Efficiency Responses in Sunflower and Cucumber. Physiol. Plant 1992, 85, 141–146. [Google Scholar] [CrossRef]
- Lucena, C.; Porras, R.; Romera, F.J.; Alcántara, E.; García, M.J.; Pérez-Vicente, R. Similarities and Differences in the Acquisition of Fe and P by Dicot Plants. Agronomy 2018, 8, 148. [Google Scholar] [CrossRef]
- Misson, J.; Raghothama, K.G.; Jain, A.; Jouhet, J.; Block, M.A.; Bligny, R.; Ortet, P.; Creff, A.; Somerville, S.; Rolland, N.; et al. A Genome-Wide Transcriptional Analysis Using Arabidopsis thaliana Affymetrix Gene Chips Determined Plant Responses to Phosphate Deprivation. Proc. Natl. Acad. Sci. USA 2005, 102, 11934–11939. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.; Marin, E.; Floriani, M.; Chiarenza, S.; Richaud, P.; Nussaume, L.; Thibaud, M.C. Phosphate Deficiency Promotes Modification of Iron Distribution in Arabidopsis Plants. Biochimie 2006, 88, 1767–1771. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Torrent, J. The Severity of Iron Chlorosis in Sensitive Plants Is Related to Soil Phosphorus Levels. J. Sci. Food Agric. 2014, 94, 2766–2773. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Cañasveras, J.C.; del Campillo, M.C.; Barrón, V.; Torrent, J. Iron Chlorosis in Field Grown Olive as Affected by Phosphorus Fertilization. Eur. J. Agron. 2013, 51, 101–107. [Google Scholar] [CrossRef]
- Ziegler, J.; Schmidt, S.; Chutia, R.; Müller, J.; Böttcher, C.; Strehmel, N.; Scheel, D.; Abel, S. Non-Targeted Profiling of Semi-Polar Metabolites in Arabidopsis Root Exudates Uncovers a Role for Coumarin Secretion and Lignification during the Local Response to Phosphate Limitation. J. Exp. Bot. 2016, 67, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.K.; Liang, G. Fe Deficiency-induced Ethylene Synthesis Confers Resistance to Botrytis cinerea. New Phytol. 2023, 237, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Beckers, G.J.M.; Conrath, U. Priming for Stress Resistance: From the Lab to the Field. Curr. Opin. Plant Biol. 2007, 10, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Pieterse, C.M.J.; Mauch-Mani, B. Priming in Plant–Pathogen Interactions. Trends Plant Sci. 2002, 7, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Langenbach, C.J.G.; Jaskiewicz, M.R. Priming for Enhanced Defense. Annu. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-H.; Li, F.; Duan, Q.-H.; Han, T.; Xu, Z.-H.; Bai, S.-N. Ethylene Perception Is Involved in Female Cucumber Flower Development. Plant J. 2010, 61, 862–872. [Google Scholar] [CrossRef]
- Maric, A. Beyond the Genetics of Flowering: Integration of Ethylene Signaling and Histone Methylation Controls Flowering Time. Plant Physiol. 2023, 192, 2224–2226. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 235913. [Google Scholar] [CrossRef]
- Ramos-Moreno, L.; Ruiz-Castilla, F.J.; Bravo, C.; Martínez, E.; Menéndez, M.; Dios-Palomares, R.; Ramos, J. Inoculation with a Terroir Selected Debaryomyces hansenii Strain Changes Physico-Chemical Characteristics of Iberian Cured Pork Loin. Meat Sci. 2019, 157, 107875. [Google Scholar] [CrossRef] [PubMed]
- Romheld, V.; Marschner, H. Iron Deficiency Stress Induced Morphological and Physiological Changes in Root Tips of Sunflower. Physiol. Plant 1981, 53, 354–360. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A Rapid Nitric-perchloric Acid Digestion Method for Multi-element Tissue Analysis. Commun. Soil Sci Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- UNE EN 13804:2013; Foodstuffs—Determination of Elements and Their Chemical Species—General Considerations and Specific Requirements. UNE: Madrid, Spain, 2013.
- García, M.J.; Corpas, F.J.; Lucena, C.; Alcántara, E.; Pérez-Vicente, R.; Zamarreño, Á.M.; Bacaicoa, E.; García-Mina, J.M.; Bauer, P.; Romera, F.J. A Shoot Fe Signaling Pathway Requiring the OPT3 Transporter Controls GSNO Reductase and Ethylene in Arabidopsis thaliana Roots. Front. Plant Sci. 2018, 9, 375982. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, e445. [Google Scholar] [CrossRef]

| Mineral Concentration (mg kg−1 dw) | Mineral Concentration (mg kg−1 dw) | Association Coefficient Value (r) | p-Value |
|---|---|---|---|
| Zn | Fe | −0.0480 | 0.7746 |
| Mn | −0.2779 | 0.0912 | |
| Cu | 0.5188 | ≤0.001 | |
| P | −0.1269 | 0.4476 | |
| Fe | Zn | −0.0480 | 0.7746 |
| Mn | 0.4102 | ≤0.05 | |
| Cu | 0.3453 | ≤0.05 | |
| P | −0.3972 | ≤0.05 | |
| Mn | Zn | −0.2779 | 0.0912 |
| Fe | 0.4102 | ≤0.05 | |
| Cu | −0.1287 | 0.4411 | |
| P | −0.3807 | ≤0.05 | |
| Cu | Zn | 0.5188 | ≤0.001 |
| Fe | 0.3453 | ≤0.05 | |
| Mn | −0.1287 | 0.4411 | |
| P | −0.4250 | ≤0.01 | |
| P | Zn | −0.1269 | 0.4476 |
| Fe | −0.3972 | ≤0.05 | |
| Mn | −0.3807 | ≤0.05 | |
| Cu | −0.4250 | ≤0.01 |
| Clay g kg−1 | Organic Carbon g kg−1 | CaCO3 g kg−1 | pH1:2.5 | EC1:5 dS m−1 | CEC cmol kg−1 | POlsen mg kg−1 | FeDTPA mg kg−1 |
|---|---|---|---|---|---|---|---|
| 370 | 9.3 | 338 | 7.9 | 1.50 | 31.3 | 13.4 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sevillano-Caño, J.; García, M.J.; Córdoba-Galván, C.; Luque-Cruz, C.; Agustí-Brisach, C.; Lucena, C.; Ramos, J.; Pérez-Vicente, R.; Romera, F.J. Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability. Int. J. Mol. Sci. 2024, 25, 5729. https://doi.org/10.3390/ijms25115729
Sevillano-Caño J, García MJ, Córdoba-Galván C, Luque-Cruz C, Agustí-Brisach C, Lucena C, Ramos J, Pérez-Vicente R, Romera FJ. Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability. International Journal of Molecular Sciences. 2024; 25(11):5729. https://doi.org/10.3390/ijms25115729
Chicago/Turabian StyleSevillano-Caño, Jesús, María José García, Clara Córdoba-Galván, Carmen Luque-Cruz, Carlos Agustí-Brisach, Carlos Lucena, José Ramos, Rafael Pérez-Vicente, and Francisco Javier Romera. 2024. "Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability" International Journal of Molecular Sciences 25, no. 11: 5729. https://doi.org/10.3390/ijms25115729
APA StyleSevillano-Caño, J., García, M. J., Córdoba-Galván, C., Luque-Cruz, C., Agustí-Brisach, C., Lucena, C., Ramos, J., Pérez-Vicente, R., & Romera, F. J. (2024). Exploring the Role of Debaryomyces hansenii as Biofertilizer in Iron-Deficient Environments to Enhance Plant Nutrition and Crop Production Sustainability. International Journal of Molecular Sciences, 25(11), 5729. https://doi.org/10.3390/ijms25115729









