Poly (I:C)-Induced microRNA-30b-5p Negatively Regulates the JAK/STAT Signaling Pathway to Mediate the Antiviral Immune Response in Silver Carp (Hypophthalmichthys molitrix) via Targeting CRFB5
Abstract
1. Introduction
2. Results
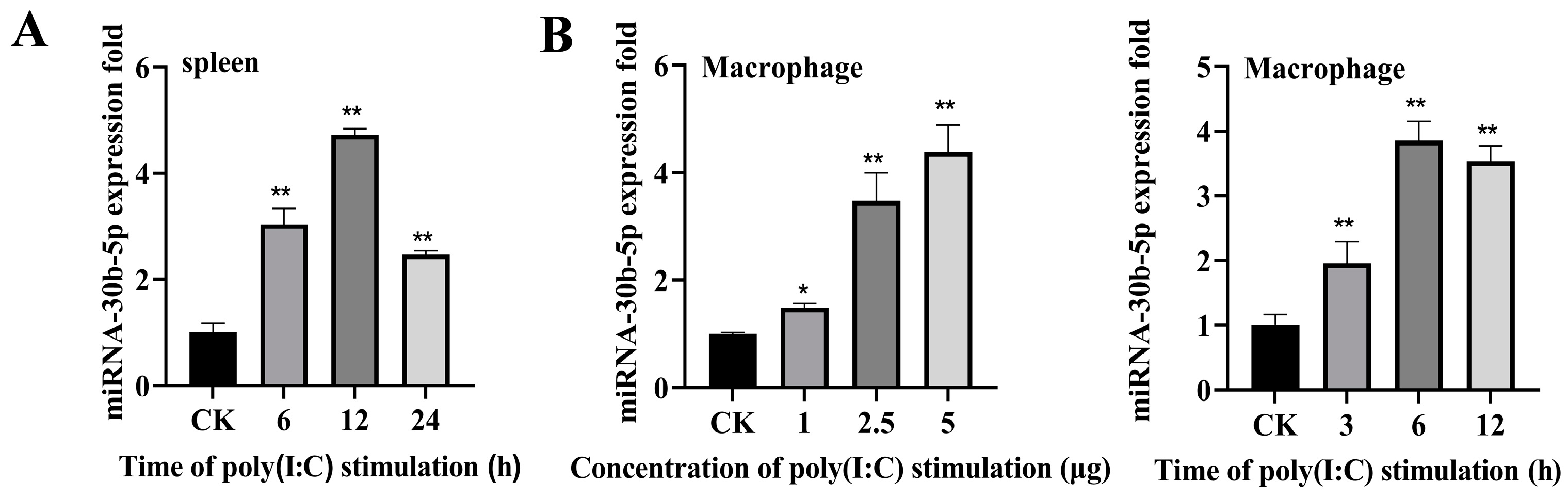
2.1. Poly (I:C) Enhanced miR-30b-5p Expression
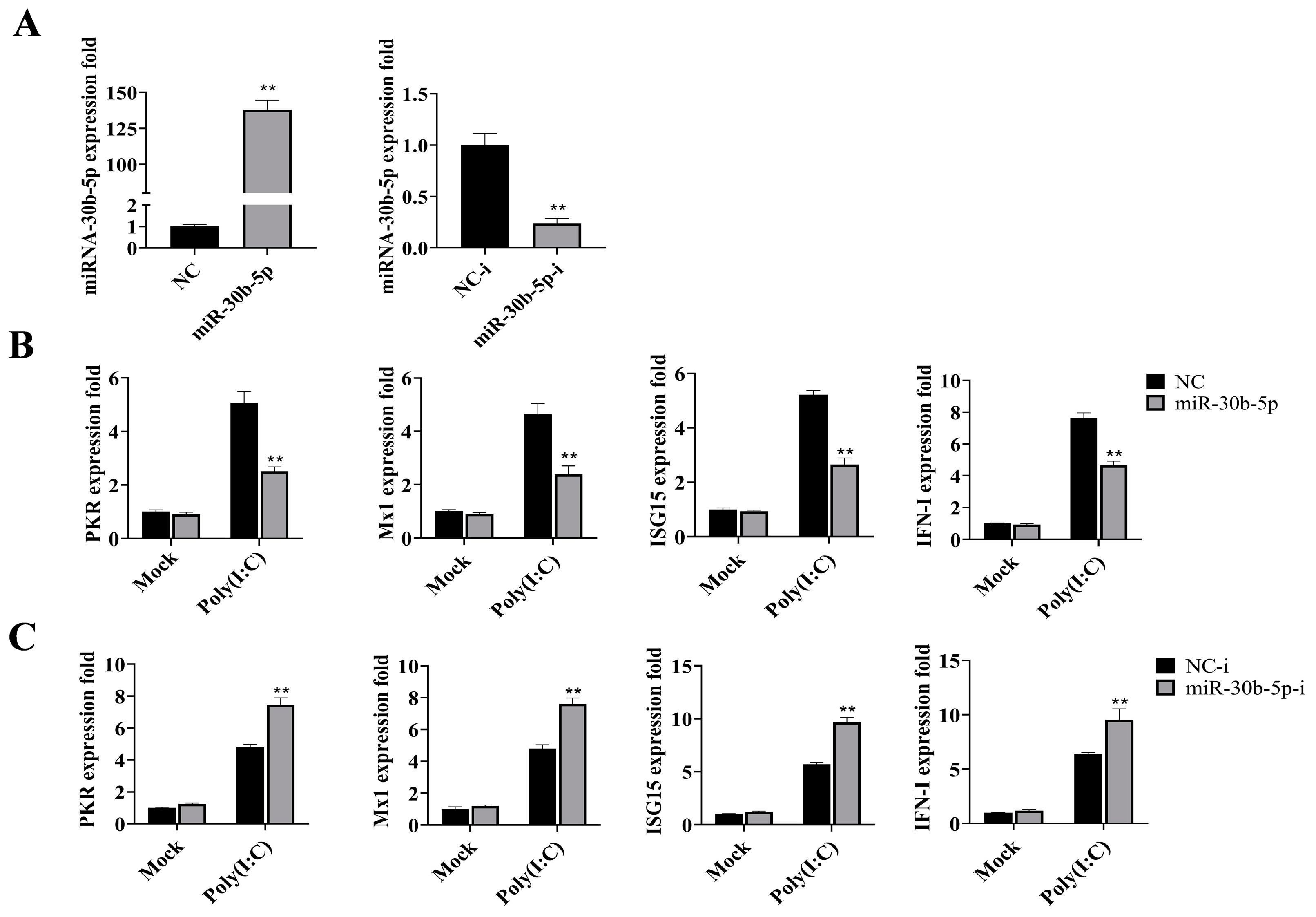
2.2. MiR-30b-5p Inhibited the Production of Antiviral Genes
2.3. MiR-30b-5p Targets CRFB5
2.4. MiR-30b-5p Suppresses the Expression of CRFB5 at the Post-transcriptional Level
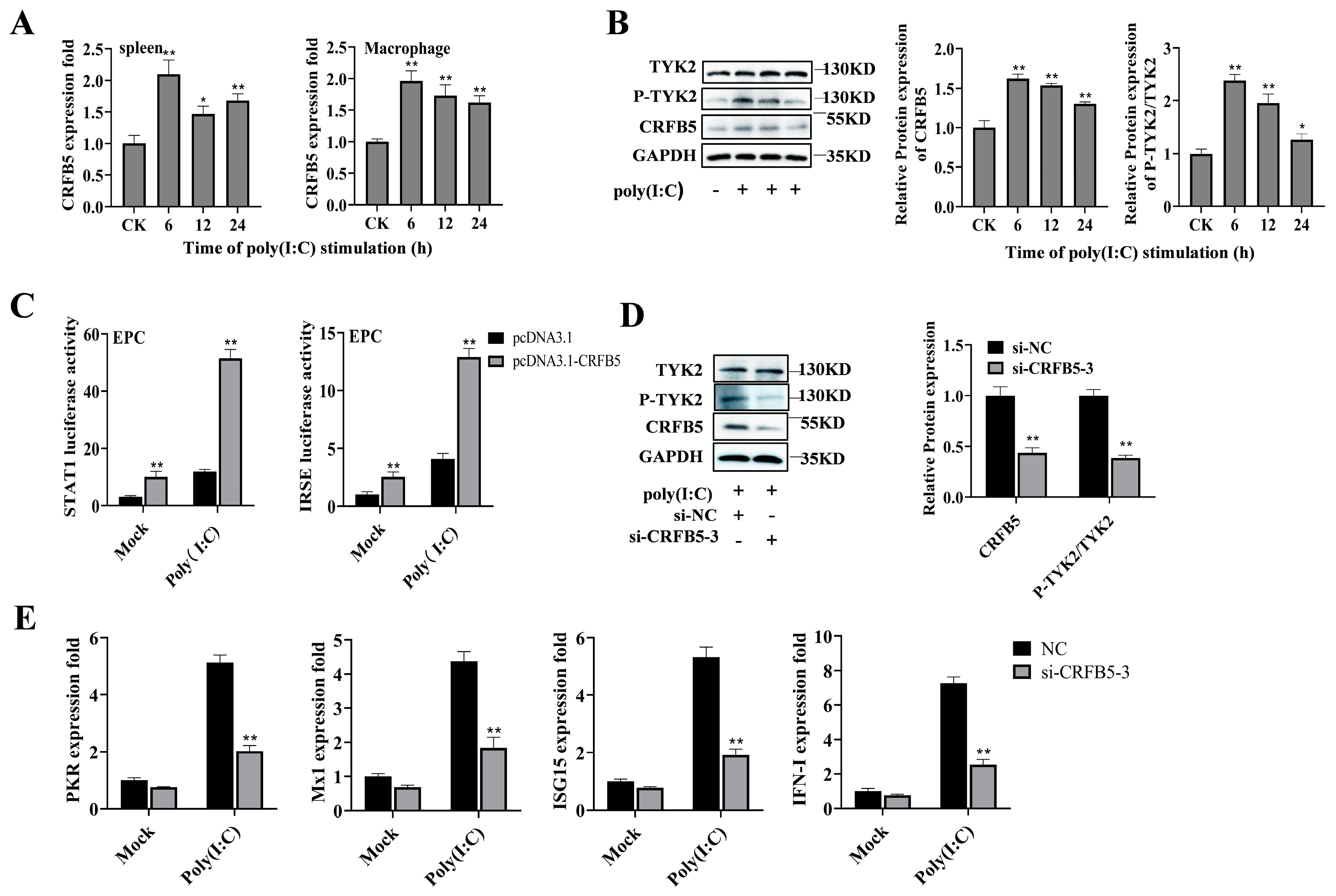
2.5. CRFB5 Is Involved in the Poly (I:C)-Induced Immune Response through the JAK/STAT Signaling Pathway
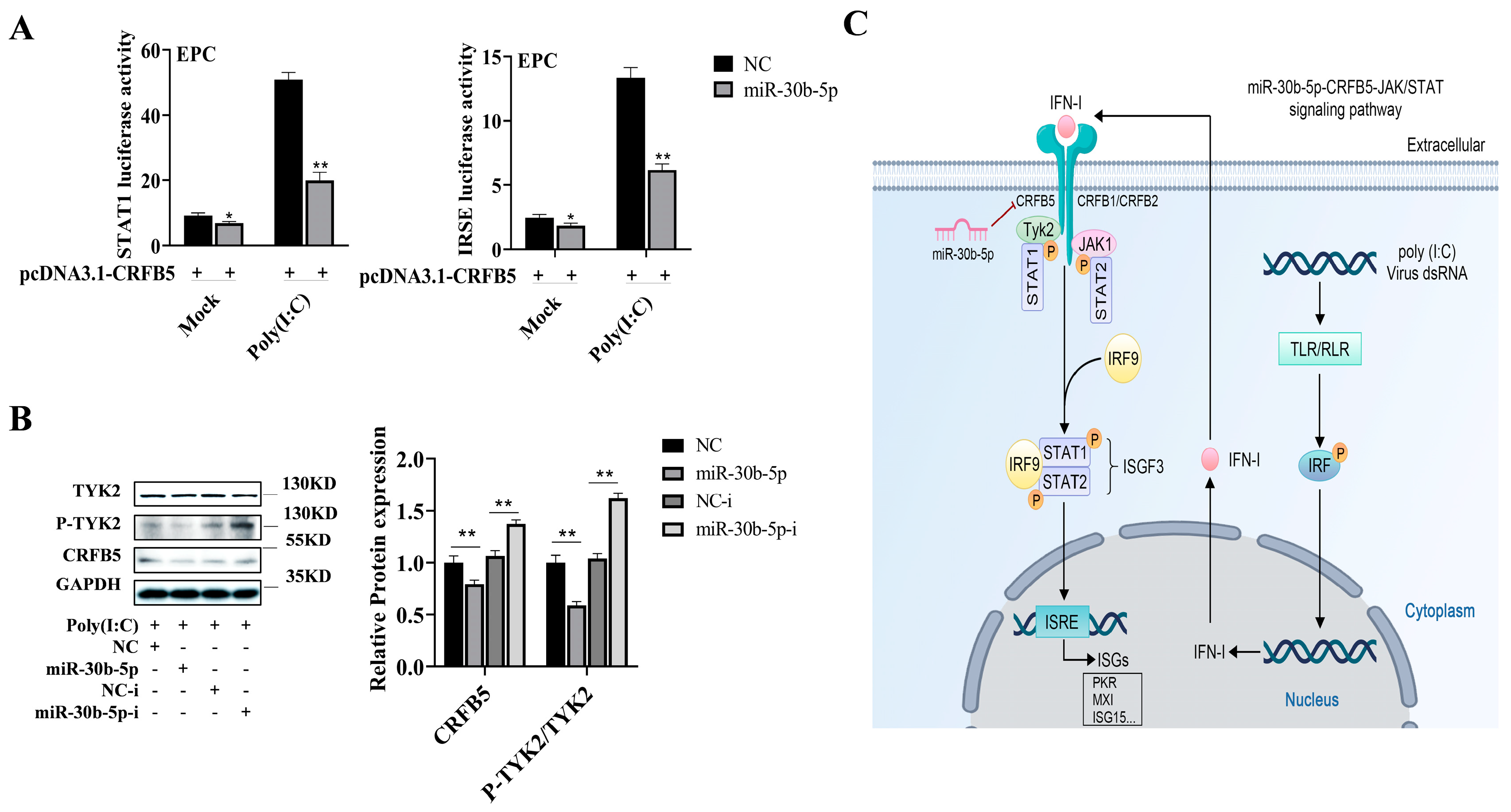
2.6. MiR-30b-5p Regulates the CRFB5-mediated JAK/STAT Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Inducing Infection in Silver Carp
4.2. Cell Culture and Cell Stimulation Experiment
4.3. Plasmid Construction and Transfection
4.4. Prediction of the miR-30b-5p Target Gene
4.5. miRNA Mimics, Inhibitors, and RNA Interference
4.6. Dual Luciferase Reporter Gene Detection Assays
4.7. qPCR Detection
4.8. Western Blotting
4.9. Statistical Analysis of Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharrock, J.; Sun, J.C. Innate immunological memory: From plants to animals. Curr. Opin. Immunol. 2020, 62, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Zhang, Z.; Wu, L.; Zhang, L.; Zhou, F. The Functional Deubiquitinating Enzymes in Control of Innate Antiviral Immunity. Adv. Sci. 2021, 8, 2002484. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.L.; Abendroth, A.; McSharry, B.P.; Slobedman, B. Interferon-Independent Upregulation of Interferon-Stimulated Genes during Human Cytomegalovirus Infection is Dependent on IRF3 Expression. Viruses 2019, 11, 246. [Google Scholar] [CrossRef]
- Tang, Z.Z.; Wang, T.Y.; Chen, Y.M.; Chen, T.Y. Cloning and characterisation of type I interferon receptor 1 in orange-spotted grouper (Epinephelus coioides) for response to nodavirus infection. Fish Shellfish Immunol. 2020, 101, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Aggad, D.; Mazel, M.; Boudinot, P.; Mogensen, K.E.; Hamming, O.J.; Hartmann, R.; Kotenko, S.; Herbomel, P.; Lutfalla, G.; Levraud, J.P. The two groups of zebrafish virus-induced interferons signal via distinct receptors with specific and shared chains. J. Immunol. 2009, 183, 3924–3931. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Cheng, J.; Chen, S.; Hou, J.; Li, N.; Xia, H.; Xia, L.; Lu, Y.; Nie, P. Identification and characterization of tilapia CRFB1, CRFB2 and CRFB5 reveals preferential receptor usage of three IFN subtypes in perciform fishes. Fish Shellfish Immunol. 2020, 107 Pt A, 194–201. [Google Scholar] [CrossRef]
- Laghari, Z.A.; Chen, S.N.; Li, L.; Huang, B.; Gan, Z.; Zhou, Y.; Huo, H.J.; Hou, J.; Nie, P. Functional, signalling and transcriptional differences of three distinct type I IFNs in a perciform fish, the mandarin fish Siniperca chuatsi. Dev. Comp. Immunol. 2018, 84, 94–108. [Google Scholar] [CrossRef]
- Guan, Y.; Chen, J.; Guan, H.; Chen, T.T.; Teng, Y.; Wei, Z.; Li, Z.; Ouyang, S.; Chen, X. Structural and Functional Characterization of a Fish Type I Subgroup d IFN Reveals Its Binding to Receptors. J. Immunol. 2024, 212, 1207–1220. [Google Scholar] [CrossRef]
- Rani, V.; Sengar, R.S. Biogenesis and mechanisms of microRNA-mediated gene regulation. Biotechnol. Bioeng. 2022, 119, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Zheng, W.; Chu, Q.; Yang, L.; Sun, L.; Xu, T. Circular RNA circDtx1 regulates IRF3-mediated antiviral immune responses through suppression of miR-15a-5p-dependent TRIF downregulation in teleost fish. PLoS Pathog. 2021, 17, e1009438. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jin, Y.; Zhang, W.; Xiang, Y.; Jia, P.; Yi, M.; Jia, K. MiR-202-5p Inhibits RIG-I-Dependent Innate Immune Responses to RGNNV Infection by Targeting TRIM25 to Mediate RIG-I Ubiquitination. Viruses 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Wu, S.; Wu, Y.; Liu, T.; Zhao, X.; Li, Y. MicroRNA-196a/-196b regulate the progression of hepatocellular carcinoma through modulating the JAK/STAT pathway via targeting SOCS2. Cell Death Dis. 2019, 10, 333. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; He, X.J.; Zhang, W.; Chen, Y.L.; Yang, J.; Xiang, W.; Ding, Y. MiR-145 participates in the development of lupus nephritis by targeting CSF1 to regulate the JAK/STAT signaling pathway. Cytokine 2022, 154, 155877. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Dong, L.P.; Cui, Y.G.; Lu, H.Y. MiR-520h inhibits viability and facilitates apoptosis of KGN cells through modulating IL6R and the JAK/STAT pathway. Reprod. Biol. 2022, 22, 100607. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Armbruster, J.W. Phylogenetic classification of extant genera of fishes of the order Cypriniformes (Teleostei: Ostariophysi). Zootaxa 2018, 4476, 6–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, H.; Xu, M.; Qiu, T.X.; Chen, J. Azoxystrobin increases the infection of spring viraemia of carp virus in fish. Chemosphere 2021, 285, 131465. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Xu, Y.; Su, M.; Lu, L.; Wang, H. Susceptibility of Goldfish to Cyprinid Herpesvirus 2 (CyHV-2) SH01 Isolated from Cultured Crucian Carp. Viruses 2021, 13, 1761. [Google Scholar] [CrossRef]
- Tamer, C.; Benkaroun, J.; Kurucay, H.N.; Albayrak, H.; Weidmann, M. Development of a recombinase polymerase amplification assay for viral haemorrhagic septicemia virus. J. Fish. Dis. 2022, 45, 1065–1071. [Google Scholar] [CrossRef]
- Chu, Q.; Xu, T. MicroRNA regulation of Toll-like receptor, RIG-I-like receptor and Nod-like receptor pathways in teleost fish. Rev. Aquac. 2020, 12, 2177–2193. [Google Scholar] [CrossRef]
- Xu, T.; Chu, Q.; Cui, J. Rhabdovirus-Inducible MicroRNA-210 Modulates Antiviral Innate Immune Response via Targeting STING/MITA in Fish. J. Immunol. 2018, 201, 982–994. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Li, Y.; Deng, P.; Yang, C.; Li, Y.; Liao, L.; Zhu, Z.; Wang, Y.; Huang, R. miR-2188-5p promotes GCRV replication by the targeted degradation of klf2a in Ctenopharyngodon idellus. Dev. Comp. Immunol. 2023, 138, 104516. [Google Scholar] [CrossRef]
- Qin, X.; Chen, J.; Wu, L.; Liu, Z. MiR-30b-5p acts as a tumor suppressor, repressing cell proliferation and cell cycle in human hepatocellular carcinoma. Biomed. Pharmacother. 2017, 89, 742–750. [Google Scholar] [CrossRef]
- Fan, M.; Ma, X.; Wang, F.; Zhou, Z.; Zhang, J.; Zhou, D.; Hong, Y.; Wang, Y.; Wang, G.; Dong, Q. MicroRNA-30b-5p functions as a metastasis suppressor in colorectal cancer by targeting Rap1b. Cancer Lett. 2020, 477, 144–156. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, S.; Jia, Q.; Zhang, A.; Li, Y.; Zhu, Y.; Lv, S.; Zhang, J. The microRNA in ventricular remodeling: The miR-30 family. Biosci. Rep. 2019, 39, BSR20190788. [Google Scholar] [CrossRef]
- Shwe, A.; Krasnov, A.; Visnovska, T.; Ramberg, S.; Østbye, T.K.; Andreassen, R. Differential Expression of miRNAs and Their Predicted Target Genes Indicates That Gene Expression in Atlantic Salmon Gill Is Post-Transcriptionally Regulated by miRNAs in the Parr-Smolt Transformation and Adaptation to Sea Water. Int. J. Mol. Sci. 2022, 23, 8831. [Google Scholar] [CrossRef]
- Liu, M.; Tang, H.; Gao, K.; Zhang, X.; Yang, Z.; Gao, Y.; Shan, X. Identification and Characterization of Immune-Associated MicroRNAs in Silver Carp (Hypophthalmichthys molitrix) Responding to Aeromonas veronii and LPS Stimulation. Animals 2024, 14, 285. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, X.C.; Jia, R.Y. Toll-Like Receptors and RIG-I-Like Receptors Play Important Roles in Resisting Flavivirus. J. Immunol. Res. 2018, 2018, 6106582. [Google Scholar] [CrossRef]
- Robertsen, B. The role of type I interferons in innate and adaptive immunity against viruses in Atlantic salmon. Dev. Comp. Immunol. 2018, 80, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, Y.B.; Liu, T.K.; Gan, L.; Yu, F.F.; Liu, Y.; Gui, J.F. Characterization of fish IRF3 as an IFN-inducible protein reveals evolving regulation of IFN response in vertebrates. J. Immunol. 2010, 185, 7573–7582. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.F.; Zhang, Y.B.; Liu, T.K.; Liu, Y.; Sun, F.; Jiang, J.; Gui, J.F. Fish virus-induced interferon exerts antiviral function through Stat1 pathway. Mol. Immunol. 2010, 47, 2330–2341. [Google Scholar] [CrossRef]
- Chen, K.; Tian, J.; Shi, Y.; Xie, T.; Huang, W.; Jia, Z.; Zhang, Y.; Yuan, G.; Yan, H.; Wang, J.; et al. Distinct antiviral activities of IFNφ1 and IFNφ4 in zebrafish. Fish Shellfish Immunol. 2024, 146, 109396. [Google Scholar] [CrossRef]
- Chen, J.; Guan, Y.; Guan, H.; Mu, Y.; Ding, Y.; Zou, J.; Ouyang, S.; Chen, X. Molecular and Structural Basis of Receptor Binding and Signaling of a Fish Type I IFN with Three Disulfide Bonds. J. Immunol. 2022, 209, 806–819. [Google Scholar] [CrossRef]
- Chu, Q.; Han, J.; Sun, L.; Cui, J.; Xu, T. Characterization of MDA5 and microRNA-203 negatively regulates the RLR signaling pathway via targeting MDA5 in miiuy croaker. Dev. Comp. Immunol. 2022, 126, 104259. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, W.; Sun, Y.; Xu, T. microRNA-489 negatively modulates RIG-I signaling pathway via targeting TRAF6 in miiuy croaker after poly(I:C) stimulation. Fish Shellfish Immunol. 2021, 113, 61–68. [Google Scholar] [CrossRef]
- Song, X.; Ma, J. SRRM1 promotes the proliferation, migration, and invasion of hepatocellular carcinoma cells by regulating the JAK/STAT signaling pathway. Tissue Cell 2022, 79, 101954. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, M.Y.; Wang, G.X.; Lu, L.L.; Lin, L.; Lei, L.; Wu, T. Ruxolitinib ameliorated coxsackievirus B3-induced acute viral myocarditis by suppressing the JAK-STAT pathway. Int. Immunopharmacol. 2023, 124 Pt A, 110797. [Google Scholar] [CrossRef]
- Zhou, H.; Jia, X.; Hu, K.; Mo, Z.; Xu, W.; Peng, L.; Wang, K.; Zhu, X. TMEM2 binds to CSNK2A3 to inhibit HBV infection via activation of the JAK/STAT pathway. Exp. Cell Res. 2021, 400, 112517. [Google Scholar] [CrossRef]
- Hu, L.; Liu, R.; Zhang, L. Advance in bone destruction participated by JAK/STAT in rheumatoid arthritis and therapeutic effect of JAK/STAT inhibitors. Int. Immunopharmacol. 2022, 111, 109095. [Google Scholar] [CrossRef] [PubMed]
- Clere-Jehl, R.; Mariotte, A.; Meziani, F.; Bahram, S.; Georgel, P.; Helms, J. JAK-STAT Targeting Offers Novel Therapeutic Opportunities in Sepsis. Trends Mol. Med. 2020, 26, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, H.T.; Chyuan, I.T.; Lai, J.H. Targeting the JAK-STAT pathway in autoimmune diseases and cancers: A focus on molecular mechanisms and therapeutic potential. Biochem. Pharmacol. 2021, 193, 114760. [Google Scholar] [CrossRef]
- Bi, D.; Cui, J.; Chu, Q.; Xu, T. MicroRNA-21 contributes to suppress cytokines production by targeting TLR28 in teleost fish. Mol. Immunol. 2017, 83, 107–114. [Google Scholar] [CrossRef]
- Lewis, B.P.; Shih, I.H.; Jones-Rhoades, M.W.; Bartel, D.P.; Burge, C.B. Prediction of mammalian microRNA targets. Cell 2003, 115, 787–798. [Google Scholar] [CrossRef]
- John, B.; Enright, A.J.; Aravin, A.; Tuschl, T.; Sander, C.; Marks, D.S. Human MicroRNA targets. PLoS Biol. 2004, 2, e363. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Primer ID | Sequences (5′ to 3′) |
|---|---|
| miR-30b-5p (sense) | UGUAAACAUCCUACACUCAGCU |
| miR-30b-5p (antisense) | CUGAGUUAGGAUGUUUACAUU |
| NC (sense) | UUCUCCGAACGUGUCACGUTT |
| NC (antisense) | ACGUGACACGUUCGAGAATT |
| miR-30b-5p-i | AGCUGAGUUAGGAUGUUUACA |
| NC-i | CAGUACUUUUUGUGUAGUACAA |
| si-CRFB5-1 (sense) | CGGCUUUCAAUCCCGUAATT |
| si-CRFB5-1 (antisense) | UUACGGAUGAAACGCCGTT |
| si-CRFB5-2 (sense) | CCGACAUUUAUUACCUUAUTT |
| si-CRFB5-2 (antisense) | AUAAGUAAUCAUGUGGTT |
| si-CRFB5-3 (sense) | GCUGCUGUGUUUCUCAGTT |
| si-CRFB5-3 (antisense) | UUGUAGAAGUCAGCAGCTT |
| siRNA-NC (sense) | UUCUCCGAACGUGUCACGUTTTT |
| siRNA-NC (antisense) | ACGUGACACGUUCGGAGAATT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Tang, H.; Gao, K.; Zhang, X.; Ma, Z.; Jia, Y.; Yang, Z.; Inam, M.; Gao, Y.; Wang, G.; et al. Poly (I:C)-Induced microRNA-30b-5p Negatively Regulates the JAK/STAT Signaling Pathway to Mediate the Antiviral Immune Response in Silver Carp (Hypophthalmichthys molitrix) via Targeting CRFB5. Int. J. Mol. Sci. 2024, 25, 5712. https://doi.org/10.3390/ijms25115712
Liu M, Tang H, Gao K, Zhang X, Ma Z, Jia Y, Yang Z, Inam M, Gao Y, Wang G, et al. Poly (I:C)-Induced microRNA-30b-5p Negatively Regulates the JAK/STAT Signaling Pathway to Mediate the Antiviral Immune Response in Silver Carp (Hypophthalmichthys molitrix) via Targeting CRFB5. International Journal of Molecular Sciences. 2024; 25(11):5712. https://doi.org/10.3390/ijms25115712
Chicago/Turabian StyleLiu, Meng, Huan Tang, Kun Gao, Xiqing Zhang, Zhenhua Ma, Yunna Jia, Zihan Yang, Muhammad Inam, Yunhang Gao, Guiqin Wang, and et al. 2024. "Poly (I:C)-Induced microRNA-30b-5p Negatively Regulates the JAK/STAT Signaling Pathway to Mediate the Antiviral Immune Response in Silver Carp (Hypophthalmichthys molitrix) via Targeting CRFB5" International Journal of Molecular Sciences 25, no. 11: 5712. https://doi.org/10.3390/ijms25115712
APA StyleLiu, M., Tang, H., Gao, K., Zhang, X., Ma, Z., Jia, Y., Yang, Z., Inam, M., Gao, Y., Wang, G., & Shan, X. (2024). Poly (I:C)-Induced microRNA-30b-5p Negatively Regulates the JAK/STAT Signaling Pathway to Mediate the Antiviral Immune Response in Silver Carp (Hypophthalmichthys molitrix) via Targeting CRFB5. International Journal of Molecular Sciences, 25(11), 5712. https://doi.org/10.3390/ijms25115712






