Bioinformatic Analysis of Topoisomerase IIα Reveals Interdomain Interdependencies and Critical C-Terminal Domain Residues
Abstract
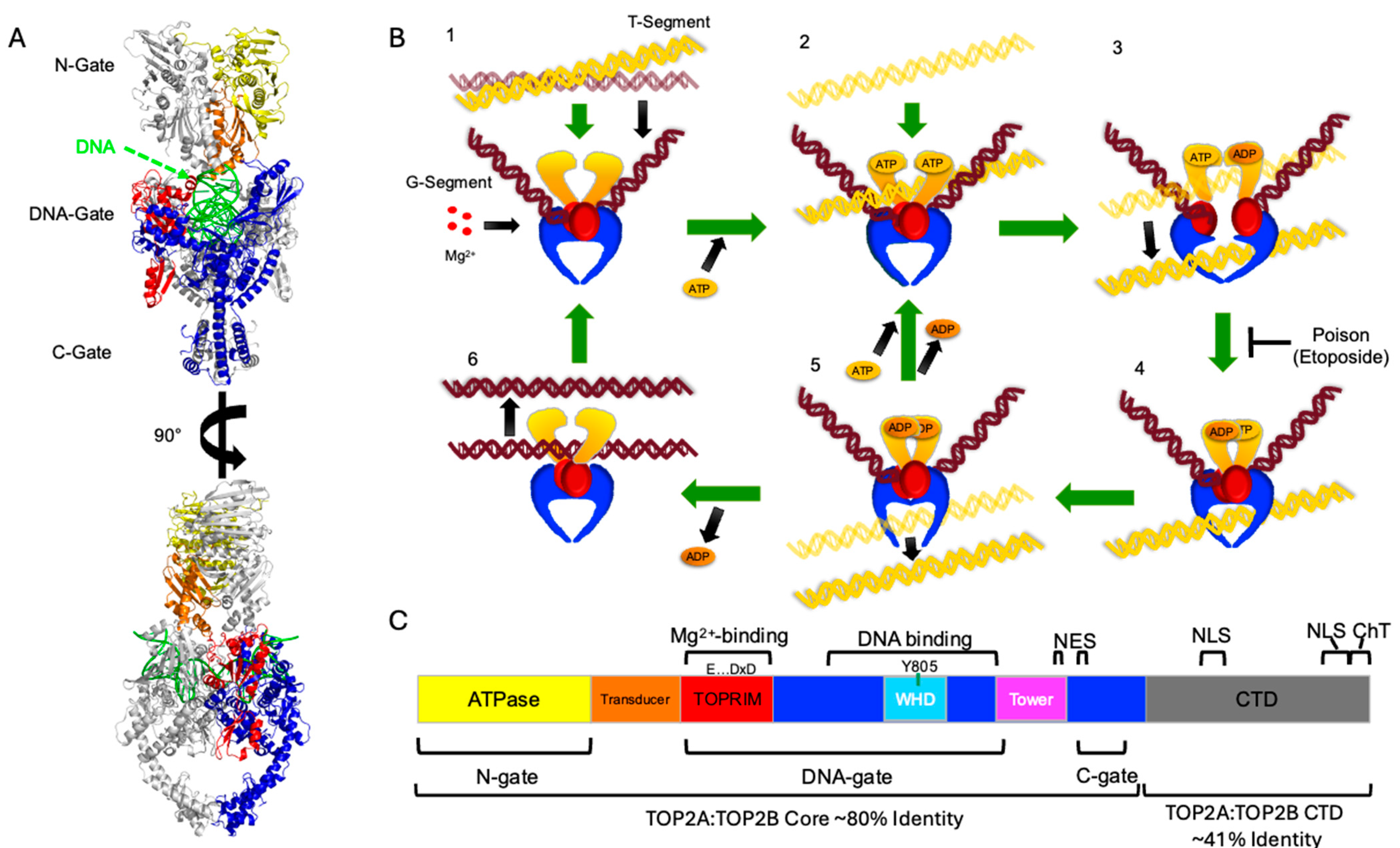
1. Introduction
2. Results
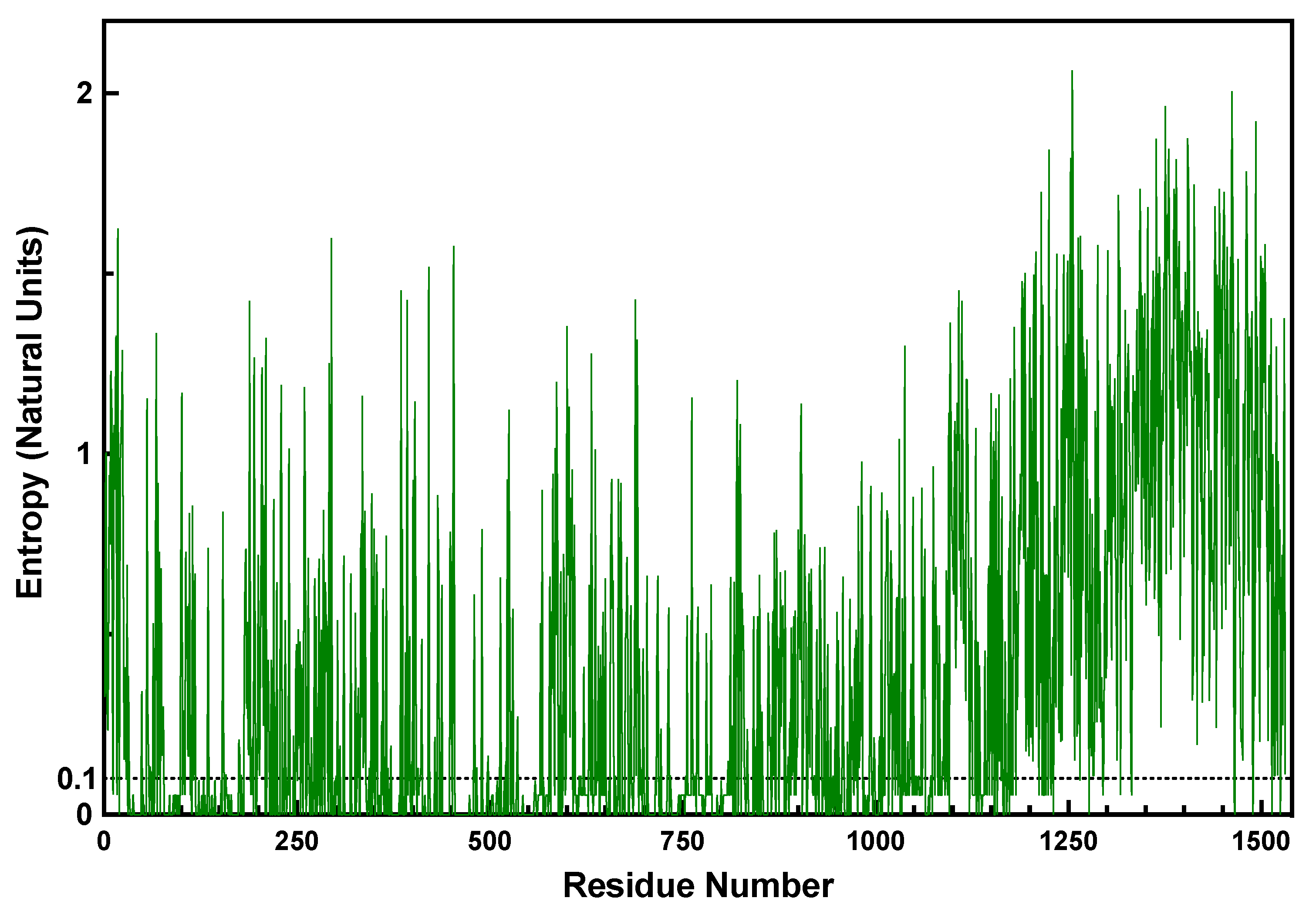
2.1. Improvements Made to the PSICalc Software Tool and Shannon Entropy Filtering
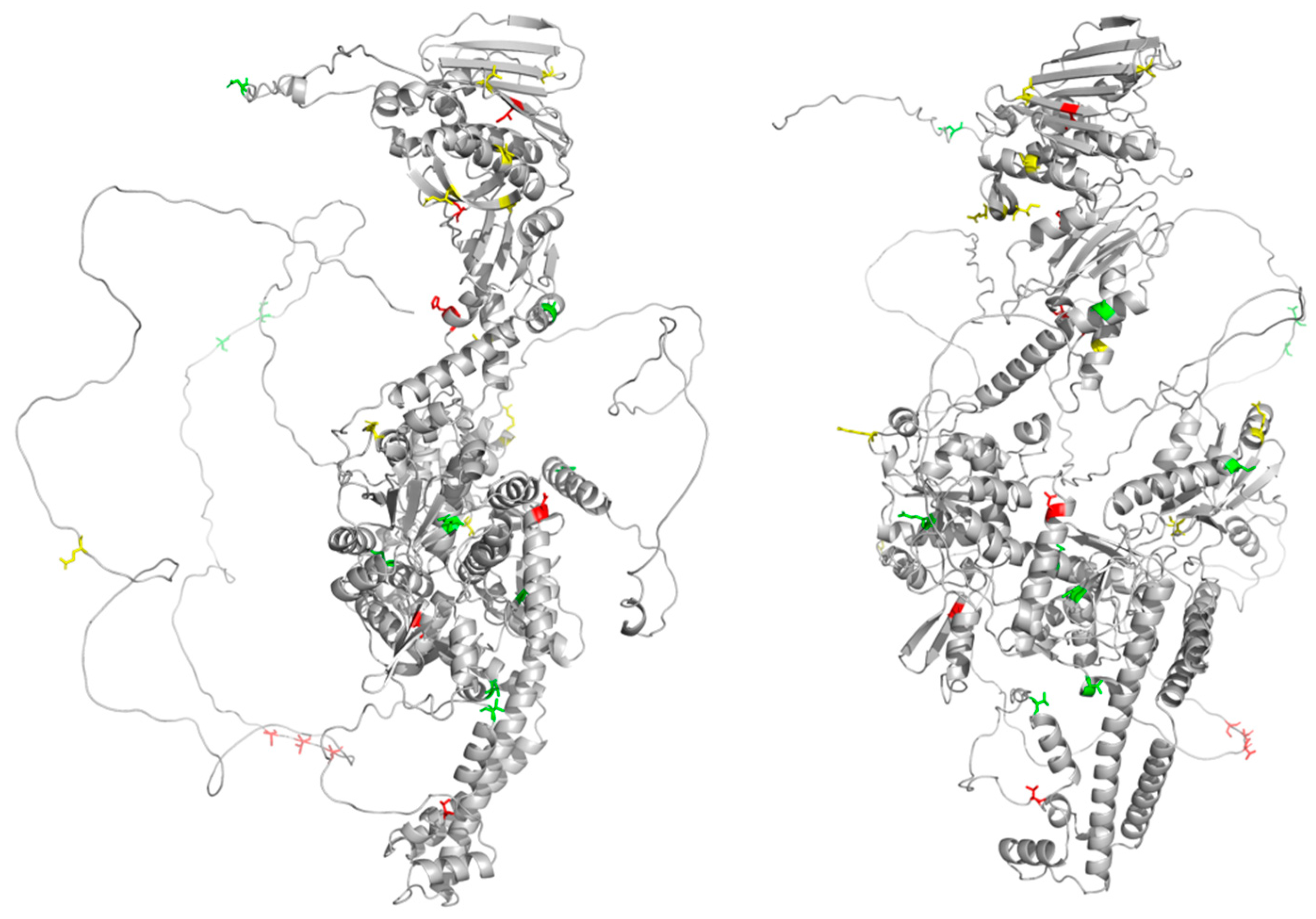
2.2. PSICalc Identifies Complex Interdomain Clusters
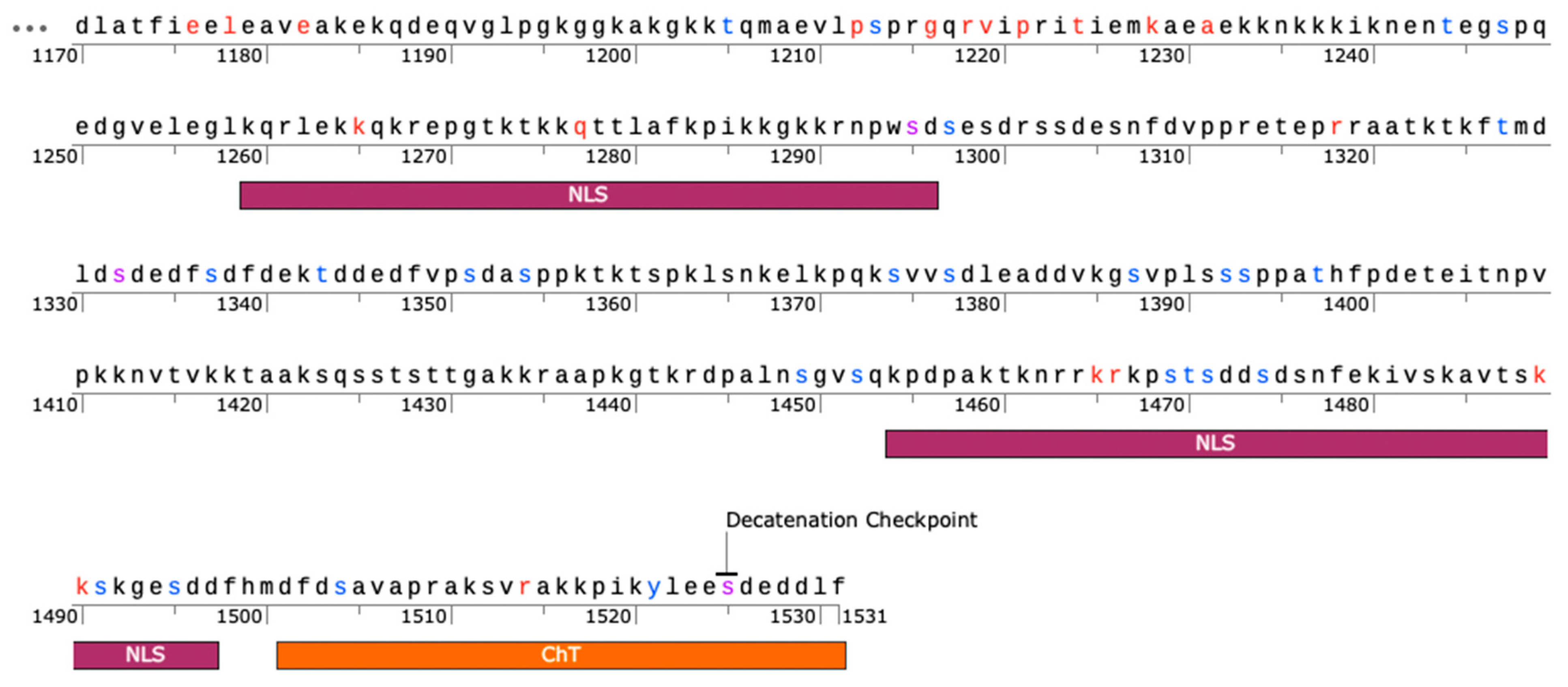
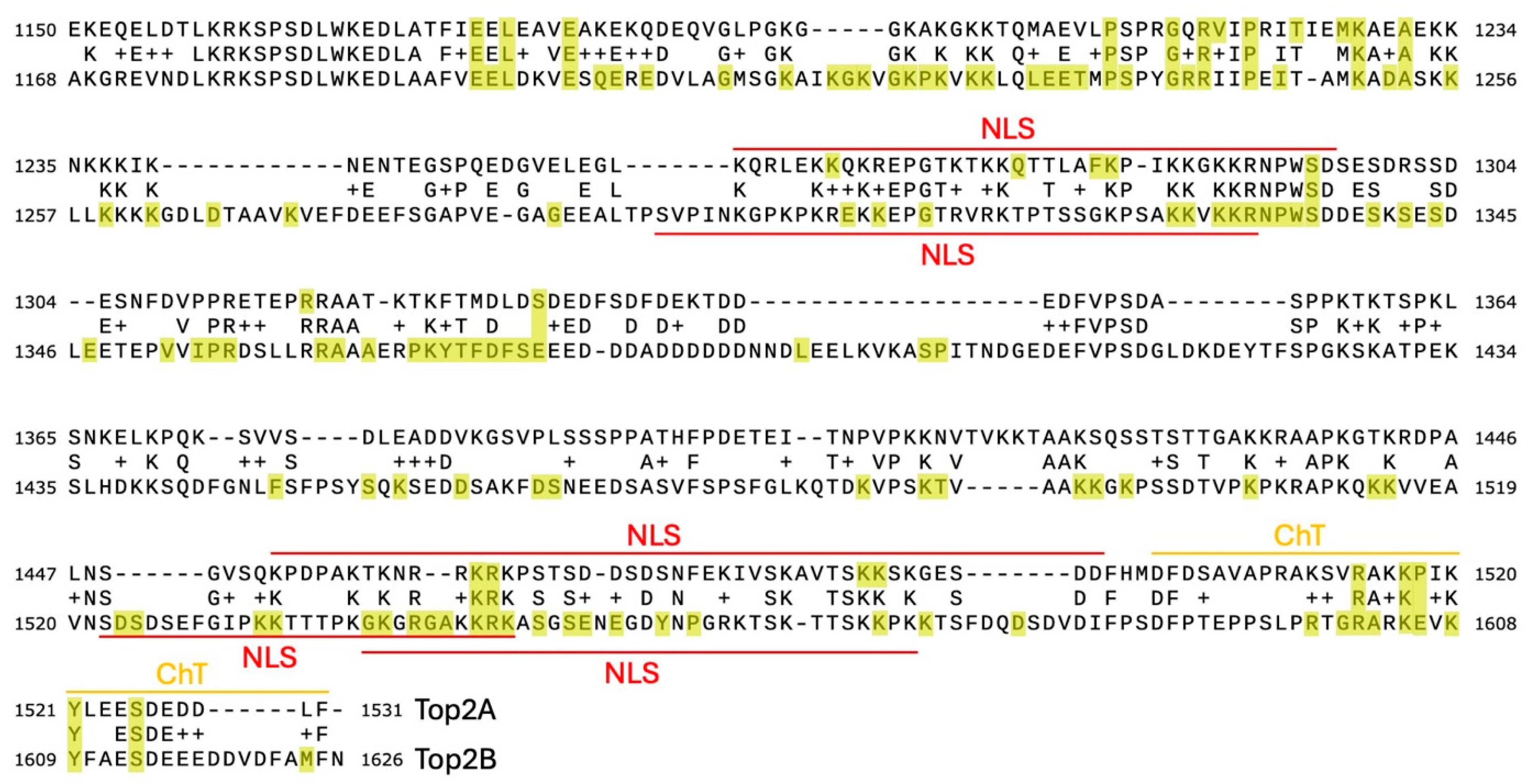
2.3. PSICalc Identifies C-Terminal Domain Clusters
2.4. Shannon Entropy Filtering Identifies Invariant Sites in the CTD
| Position in Top2A | Amino Acid | Notes | Source |
|---|---|---|---|
| 1176 | E | ||
| 1178 | L | ||
| 1182 | E | ||
| 1212 | P | ||
| 1216 | G | ||
| 1218 | R | ||
| 1219 | V | ||
| 1221 | P | ||
| 1224 | T | ||
| 1228 | K | SUMO Site | [59] |
| 1231 | A | ||
| 1265 | K | NLS (1259-1296) | [48,57] |
| 1277 | Q | NLS (1259-1296) | [48,57] |
| 1295 | S | PO4 Site; Mitosis-Associated (PlK-1); NLS (1259-1296) | [48,57,59] |
| 1318 | R | ||
| 1332 | S | PO4 Site; Mitosis-Associated (PlK-1) | [59] |
| 1465 | K | NLS (1454-1497) | [48,57] |
| 1466 | R | NLS (1454-1497) | [48,57] |
| 1489 | K | NLS (1454-1497) | [48,57] |
| 1490 | K | NLS (1454-1497) | [48,57] |
| 1514 | R | ||
| 1525 | S | PO4 Site; Decatenation checkpoint | [47,59,60] |
2.5. Low-Shannon-Entropy Positions Correlate with Charged Positions
2.6. Analysis of Top2B Dataset Reveals Patterns of Interdependency
3. Materials and Methods
3.1. Top2A and Top2B MSA Datasets
3.2. Data Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, A.K.; Liu, T.S.; Wang, C.C. Statistical analysis of residue variability in cytochrome c. J. Mol. Biol. 1976, 102, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Durston, K.K.; Chiu, D.K.; Wong, A.K.; Li, G.C. Statistical discovery of site inter-dependencies in sub-molecular hierarchical protein structuring. EURASIP J. Bioinform. Syst. Biol. 2012, 2012, 8. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Hamacher, K. hoDCA: Higher order direct-coupling analysis. BMC Bioinform. 2018, 19, 546. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Nartey, C.M.; Koo, H.J.; Laurendon, C.; Shaik, H.Z.; O‘Maille, P.; Noel, J.P.; Morcos, F. Coevolutionary Information Captures Catalytic Functions and Reveals Divergent Roles of Terpene Synthase Interdomain Connections. Biochemistry 2024, 63, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Townsley, T.D.; Wilson, J.T.; Akers, H.; Bryant, T.; Cordova, S.; Wallace, T.L.; Durston, K.K.; Deweese, J.E. PSICalc: A novel approach to identifying and ranking critical non-proximal interdependencies within the overall protein structure. Bioinform. Adv. 2022, 2, vbac058. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 2009, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Sun, Y.; Huang, S.N.; Nitiss, J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016, 17, 703–721. [Google Scholar] [CrossRef]
- McKie, S.J.; Neuman, K.C.; Maxwell, A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. Bioessays 2021, e2000286. [Google Scholar] [CrossRef]
- Deweese, J.E.; Osheroff, N. The DNA cleavage reaction of topoisomerase II: Wolf in sheep’s clothing. Nucleic Acids Res. 2009, 37, 738–749. [Google Scholar] [CrossRef]
- Murphy, M.B.; Mercer, S.L.; Deweese, J.E. Inhibitors and Poisons of Mammalian Type II Topoisomerases. In Advances in Molecular Toxicology; Fishbein, J.C., Heilman, J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 11, pp. 203–240. [Google Scholar]
- Capranico, G.; Tinelli, S.; Austin, C.A.; Fisher, M.L.; Zunino, F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim. Biophys. Acta 1992, 1132, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, E.; Sala, E.; Negri, C.; Oliani, C.; Supino, R.; Astraldi Ricotti, G.B.; Bottiroli, G. Topoisomerase II alpha and beta in human tumor cells grown in vitro and in vivo. Anticancer Res. 1992, 12, 2093–2099. [Google Scholar] [PubMed]
- Christensen, M.O.; Larsen, M.K.; Barthelmes, H.U.; Hock, R.; Andersen, C.L.; Kjeldsen, E.; Knudsen, B.R.; Westergaard, O.; Boege, F.; Mielke, C. Dynamics of human DNA topoisomerases IIα and IIβ in living cells. J. Cell. Biol. 2002, 157, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.J.; Porter, A.C. Construction, characterization, and complementation of a conditional-lethal DNA topoisomerase IIalpha mutant human cell line. Mol. Biol. Cell 2004, 15, 5700–5711. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, A.; Kikuchi, A. Functional compatibility between isoform alpha and beta of type II DNA topoisomerase. J. Cell Sci. 2004, 117, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Cowell, I.G.; Casement, J.W.; Austin, C.A. To Break or Not to Break: The Role of TOP2B in Transcription. Int. J. Mol. Sci. 2023, 24, 14806. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Cowell, I.G.; Austin, C.A. Mechanism of Generation of Therapy Related Leukemia in Response to Anti-Topoisomerase II Agents. Int. J. Environ. Res. Public Health 2012, 9, 2075–2091. [Google Scholar] [CrossRef]
- Toyoda, E.; Kagaya, S.; Cowell, I.G.; Kurosawa, A.; Kamoshita, K.; Nishikawa, K.; Iiizumi, S.; Koyama, H.; Austin, C.A.; Adachi, N. NK314, a topoisomerase II inhibitor that specifically targets the alpha isoform. J. Biol. Chem. 2008, 283, 23711–23720. [Google Scholar] [CrossRef]
- Bau, J.T.; Kang, Z.; Austin, C.A.; Kurz, E.U. Salicylate, a catalytic inhibitor of topoisomerase II, inhibits DNA cleavage and is selective for the alpha isoform. Mol. Pharmacol. 2014, 85, 198–207. [Google Scholar] [CrossRef]
- Ortega, J.A.; Arencibia, J.M.; Minniti, E.; Byl, J.A.W.; Franco-Ulloa, S.; Borgogno, M.; Genna, V.; Summa, M.; Bertozzi, S.M.; Bertorelli, R.; et al. Novel, Potent, and Druglike Tetrahydroquinazoline Inhibitor That Is Highly Selective for Human Topoisomerase II α over β. J. Med. Chem. 2020, 63, 12873–12886. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline Chemotherapy and Cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.E.; Cusack, B.J.; Talley, T.T.; Walsh, G.M.; Olson, R.D. Comparative effects of doxorubicin and a doxorubicin analog, 13-deoxy, 5-iminodoxorubicin (GPX-150), on human topoisomerase IIbeta activity and cardiac function in a chronic rabbit model. Investig. New Drugs 2016, 34, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.L.; Kerrigan, J.E.; Lin, C.P.; Azarova, A.M.; Tsai, Y.C.; Ban, Y.; Liu, L.F. Topoisomerase IIbeta mediated DNA double-strand breaks: Implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007, 67, 8839–8846. [Google Scholar] [CrossRef] [PubMed]
- Cowell, I.G.; Sondka, Z.; Smith, K.; Lee, K.C.; Manville, C.M.; Sidorczuk-Lesthuruge, M.; Rance, H.A.; Padget, K.; Jackson, G.H.; Adachi, N.; et al. Model for MLL translocations in therapy-related leukemia involving topoisomerase IIbeta-mediated DNA strand breaks and gene proximity. Proc. Natl. Acad. Sci. USA 2012, 109, 8989–8994. [Google Scholar] [CrossRef] [PubMed]
- Azarova, A.M.; Lyu, Y.L.; Lin, C.P.; Tsai, Y.C.; Lau, J.Y.; Wang, J.C.; Liu, L.F. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc. Natl. Acad. Sci. USA 2007, 104, 11014–11019. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Inouye, M. GHKL, an emergent ATPase/kinase superfamily. Trends Biochem. Sci. 2000, 25, 24–28. [Google Scholar] [CrossRef]
- Classen, S.; Olland, S.; Berger, J.M. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc. Natl. Acad. Sci. USA 2003, 100, 10629–10634. [Google Scholar] [CrossRef]
- Oestergaard, V.H.; Bjergbaek, L.; Skouboe, C.; Giangiacomo, L.; Knudsen, B.R.; Andersen, A.H. The transducer domain is important for clamp operation in human DNA topoisomerase IIalpha. J. Biol. Chem. 2004, 279, 1684–1691. [Google Scholar] [CrossRef]
- Bendsen, S.; Oestergaard, V.H.; Skouboe, C.; Brinch, M.; Knudsen, B.R.; Andersen, A.H. The QTK loop is essential for the communication between the N-terminal atpase domain and the central cleavage--ligation region in human topoisomerase IIalpha. Biochemistry 2009, 48, 6508–6515. [Google Scholar] [CrossRef] [PubMed]
- Aravind, L.; Leipe, D.D.; Koonin, E.V. Toprim--a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 1998, 26, 4205–4213. [Google Scholar] [CrossRef]
- Deweese, J.E.; Osheroff, N. The Use of Divalent Metal Ions by Type II Topoisomerases. Metallomics 2010, 2, 450–459. [Google Scholar] [CrossRef]
- Roca, J. The path of the DNA along the dimer interface of topoisomerase II. J. Biol. Chem. 2004, 279, 25783–25788. [Google Scholar] [CrossRef] [PubMed]
- Shaiu, W.L.; Hu, T.; Hsieh, T.S. The hydrophilic, protease-sensitive terminal domains of eucaryotic DNA topoisomerases have essential intracellular functions. Pac. Symp. Biocomput. 1999, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Linka, R.M.; Porter, A.C.; Volkov, A.; Mielke, C.; Boege, F.; Christensen, M.O. C-terminal regions of topoisomerase IIα and IIβ determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 2007, 35, 3810–3822. [Google Scholar] [CrossRef]
- McClendon, A.K.; Gentry, A.C.; Dickey, J.S.; Brinch, M.; Bendsen, S.; Andersen, A.H.; Osheroff, N. Bimodal recognition of DNA geometry by human topoisomerase II alpha: Preferential relaxation of positively supercoiled DNA requires elements in the C-terminal domain. Biochemistry 2008, 47, 13169–13178. [Google Scholar] [CrossRef]
- Meczes, E.L.; Gilroy, K.L.; West, K.L.; Austin, C.A. The impact of the human DNA topoisomerase II C-terminal domain on activity. PLoS ONE 2008, 3, e1754. [Google Scholar] [CrossRef]
- Gilroy, K.L.; Austin, C.A. The impact of the C-terminal domain on the interaction of human DNA topoisomerase II alpha and beta with DNA. PLoS ONE 2011, 6, e14693. [Google Scholar] [CrossRef]
- Lane, A.B.; Gimenez-Abian, J.F.; Clarke, D.J. A novel chromatin tether domain controls topoisomerase IIalpha dynamics and mitotic chromosome formation. J. Cell Biol. 2013, 203, 471–486. [Google Scholar] [CrossRef]
- Clarke, D.J.; Azuma, Y. Non-Catalytic Roles of the Topoisomerase IIalpha C-Terminal Domain. Int. J. Mol. Sci. 2017, 18, 2438. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.G.; Menzie, R.A.; Rhoades, J.H.; Fief, C.A.; Deweese, J.E. Reviewing the Modification, Interactions, and Regulation of the C-terminal Domain of Topoisomerase IIα as a Prospect for Future Therapeutic Targeting. EC Pharmacol. Toxicol. 2020, 8, 27–43. [Google Scholar]
- Dougherty, A.C.; Hawaz, M.G.; Hoang, K.G.; Trac, J.; Keck, J.M.; Ayes, C.; Deweese, J.E. Exploration of the Role of the C-Terminal Domain of Human DNA Topoisomerase IIalpha in Catalytic Activity. ACS Omega 2021, 6, 25892–25903. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Lee, J.H.; Carcamo, C.C.; Parker, M.W.; Berger, J.M. DNA-Stimulated Liquid-Liquid phase separation by eukaryotic topoisomerase ii modulates catalytic function. Elife 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Mirski, S.E.; Bielawski, J.C.; Cole, S.P. Identification of functional nuclear export sequences in human topoisomerase IIalpha and beta. Biochem. Biophys. Res. Commun. 2003, 306, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yuan, J.; Chen, J.; Lou, Z. Topoisomerase IIalpha controls the decatenation checkpoint. Nat. Cell Biol. 2009, 11, 204–210. [Google Scholar] [CrossRef]
- Mirski, S.E.; Gerlach, J.H.; Cummings, H.J.; Zirngibl, R.; Greer, P.A.; Cole, S.P. Bipartite nuclear localization signals in the C terminus of human topoisomerase IIa. Exp. Cell Res. 1997, 237, 452–455. [Google Scholar] [CrossRef] [PubMed]
- McClendon, A.K.; Osheroff, N. The geometry of DNA supercoils modulates topoisomerase-mediated DNA cleavage and enzyme response to anticancer drugs. Biochemistry 2006, 45, 3040–3050. [Google Scholar] [CrossRef] [PubMed]
- Bedez, C.; Lotz, C.; Batisse, C.; Broeck, A.V.; Stote, R.H.; Howard, E.; Pradeau-Aubreton, K.; Ruff, M.; Lamour, V. Post-translational modifications in DNA topoisomerase 2alpha highlight the role of a eukaryote-specific residue in the ATPase domain. Sci. Rep. 2018, 8, 9272. [Google Scholar] [CrossRef]
- Boija, A.; Klein, I.A.; Sabari, B.R.; Dall’Agnese, A.; Coffey, E.L.; Zamudio, A.V.; Li, C.H.; Shrinivas, K.; Manteiga, J.C.; Hannett, N.M.; et al. Transcription Factors Activate Genes through the Phase-Separation Capacity of Their Activation Domains. Cell 2018, 175, 1842–1855.e1816. [Google Scholar] [CrossRef]
- Cho, W.K.; Spille, J.H.; Hecht, M.; Lee, C.; Li, C.; Grube, V.; Cisse, I.I. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 2018, 361, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Palomba, F.; Niedzialkowska, E.; Digman, M.A.; Gratton, E.; Stukenberg, P.T. The inner centromere is a biomolecular condensate scaffolded by the chromosomal passenger complex. Nat. Cell Biol. 2019, 21, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Zamudio, A.V.; Dall’Agnese, A.; Henninger, J.E.; Manteiga, J.C.; Afeyan, L.K.; Hannett, N.M.; Coffey, E.L.; Li, C.H.; Oksuz, O.; Sabari, B.R.; et al. Mediator Condensates Localize Signaling Factors to Key Cell Identity Genes. Mol. Cell 2019, 76, 753–766.e756. [Google Scholar] [CrossRef] [PubMed]
- Musselman, J.R.; England, D.C.; Fielding, L.A.; Durham, C.T.; Baxter, E.; Jiang, X.; Lisic, E.C.; Deweese, J.E. Topoisomerase IIα C-terminal Domain Mutations and Catalytic Function. bioRxiv 2023, 2023.07.29.551120. [Google Scholar] [CrossRef]
- Moreira, F.; Arenas, M.; Videira, A.; Pereira, F. Evolutionary History of TOPIIA Topoisomerases in Animals. J. Mol. Evol. 2022, 90, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Mirski, S.E.; Gerlach, J.H.; Cole, S.P. Sequence determinants of nuclear localization in the alpha and beta isoforms of human topoisomerase II. Exp. Cell Res. 1999, 251, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Lotz, C.; Lamour, V. The interplay between DNA topoisomerase 2α post-translational modifications and drug resistance. Cancer Drug Resist. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Liu, X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase IIalpha in cell cycle progression. J. Biol. Chem. 2008, 283, 6209–6221. [Google Scholar] [CrossRef]
- Wyles, J.P.; Wu, Z.; Mirski, S.E.; Cole, S.P. Nuclear interactions of topoisomerase II alpha and beta with phospholipid scramblase 1. Nucleic Acids Res. 2007, 35, 4076–4085. [Google Scholar] [CrossRef]
- Vanden Broeck, A.; Lotz, C.; Drillien, R.; Haas, L.; Bedez, C.; Lamour, V. Structural basis for allosteric regulation of Human Topoisomerase IIalpha. Nat. Commun. 2021, 12, 2962. [Google Scholar] [CrossRef]
- Santamaria, A.; Wang, B.; Elowe, S.; Malik, R.; Zhang, F.; Bauer, M.; Schmidt, A.; Sillje, H.H.; Korner, R.; Nigg, E.A. The Plk1-dependent phosphoproteome of the early mitotic spindle. Mol. Cell. Proteom. 2011, 10, M110.004457. [Google Scholar] [CrossRef]
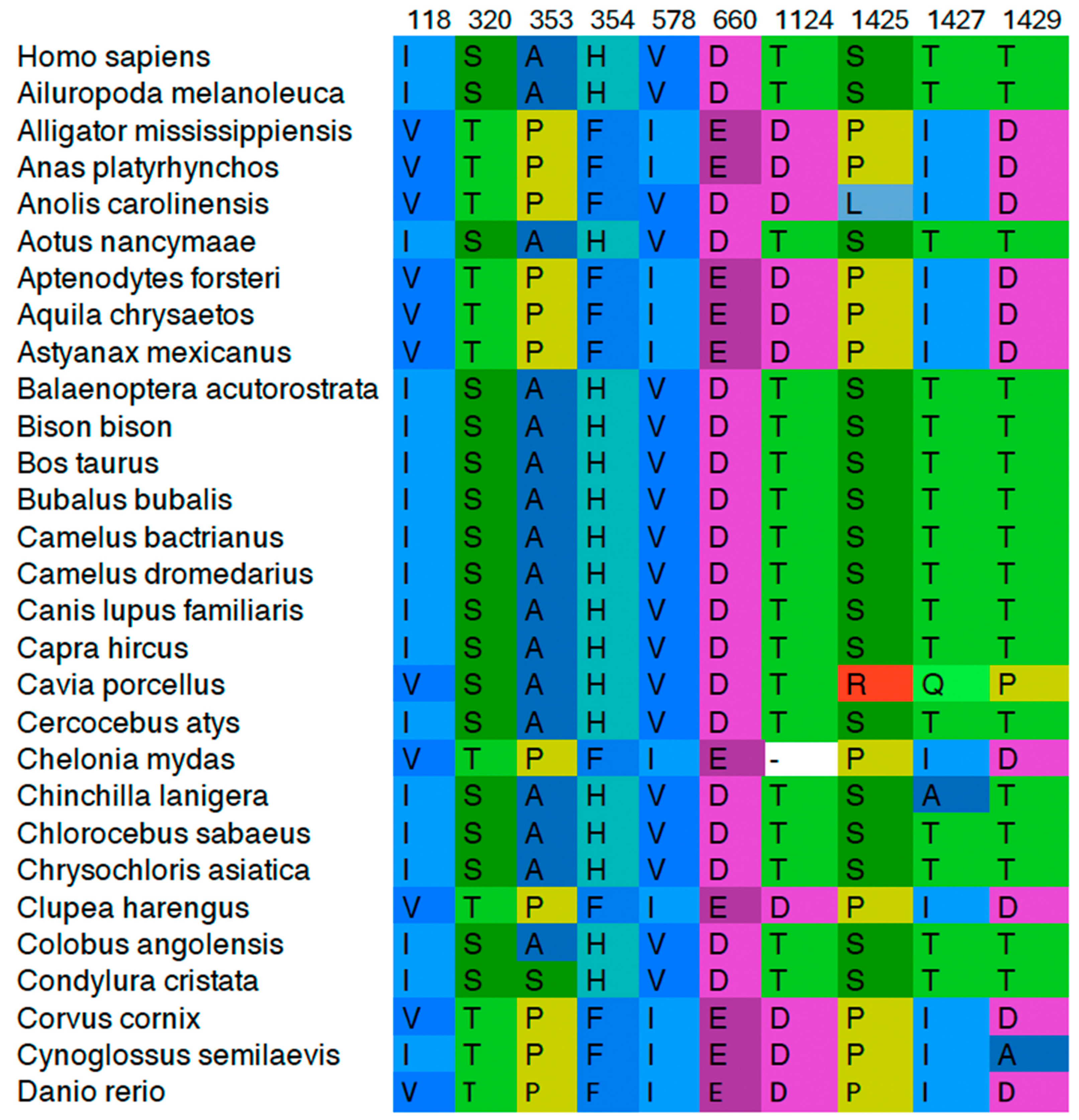


| Cluster (AA and Position Numbers) 1 | SRMode | Domain/Fold |
|---|---|---|
| I118, S320, A353, H354, V578, D660, T1124, S1425, T1427, T1429 | 0.164548 | 118—ATPase domain 320, 353, 354—Transducer 578, 660—Active Site 1124—C-Gate 1425, 1427, 1429—CTD |
| D23, A401, Q637, M762, T825, C1008, V1097, I1225, D1378, A1381 | 0.147561 | 23—ATPase domain 401—Transducer 637, 762, 825, 1008—Active Site 1097—C-Gate 1225, 1378, 1381—CTD |
| I70, E74, L115, S183, V240, V338, R450, V928, R982, E1479 | 0.153242 | 70, 74, 115, 183, 240—ATPse 338—Transducer 450—TOPRIM 928, 982—Active Site 1479—CTD |
| Cluster A | SRMode B | Previously Mutated C | Biochemical Effect C |
|---|---|---|---|
| 3rd Order | Max: 0.66 | ||
| 1189, 1232, 1272 | 0.514864 | T1272A, T1274A, T1278A, T1279A | Inc Relaxation |
| 1244, 1256, 1374 | 0.471158 | S1374G, S1377A | Inc Relaxation |
| 1217, 1364, 1482 | 0.434758 | S1351A, S1354G, T1358A, S1361A, S1365G | Dec Relaxation; Slower Decatenation |
| 1206, 1357, 1404 | 0.43775 | ND | |
| 1247, 1249, 1338 | 0.408826 | T1324A, T1327A, S1332A, S1337A, T1343A | Dec Relaxation |
| 1317, 1358, 1499 | 0.398492 | S1351A, S1354G, T1358A, S1361A, S1365G | Dec Relaxation; Slower Decatenation |
| 1394, 1397, 1399 | 0.359155 | S1387I, S1391G, S1392A, S1393A, T1397G | Inc Relaxation; Weaker binding |
| 1390, 1405, 1456 | 0.379833 | Same as above | |
| 1209, 1214, 1411 | 0.351671 | ND | |
| 1336, 1353, 1413 | 0.344962 | S1351A, S1354G, T1358A, S1361A, S1365G | Dec Relaxation; Slower Decatenation |
| 1172, 1306, 1377 | 0.277244 | S1374G, S1377A | None |
| 1227, 1237, 1303 | 0.168657 | S1295A, S1297A, S1302G, S1303G, S1306G | Dec Relaxation |
| 1273, 1476, 1531 | 0.390435 | S1469A, T1470I, S1471A, S1474A, S1476A | Strong Dec Relaxation |
| 4th Order | Max: 0.50 | ||
| 1190, 1316, 1480, 1512 | 0.331816 | S1469A, T1470I, S1471A, S1474A, S1476A And T1487A, S1488I, S1491A, S1495I | Strong Dec Relaxation Inc Relaxation |
| 1198, 1213, 1236, 1383 | 0.179402 | S1387I, S1391G, S1392A, S1393A, T1397G | Inc Relaxation |
| 1247, 1249, 1338, 1485 | 0.307347 | T1324A, T1327A, S1332A, S1337A, T1343A And T1487A, S1488I, S1491A, S1495I | Dec Relaxation Inc Relaxation |
| 1200, 1227, 1237, 1303 | 0.117365 | S1295A, S1297A, S1302G, S1303G, S1306G | Dec Relaxation |
| 5th Order | Max: 0.40 | ||
| 1217, 1304, 1344, 1364, 1482 | 0.249352 | S1295A, S1297A, S1302G, S1303G, S1306G And S1351A, S1354G, T1358A, S1361A, S1365G | Dec Relaxation Dec Relaxation; Slower Decatenation |
| 1189, 1232, 1272, 1321, 1513 | 0.259725 | T1272A, T1274A, T1278A, T1279A | Inc Relaxation |
| 1334, 1336, 1353, 1366, 1413 | 0.191835 | T1324A, T1327A, S1332A, S1337A, T1343A And S1351A, S1354G, T1358A, S1361A, S1365G | Dec Relaxation Dec Relaxation; Slower Decatenation |
| 1323, 1390, 1405, 1453, 1456 | 0.205813 | T1324A, T1327A, S1332A, S1337A, T1343A And S1449A, S1452A | Dec Relaxation None |
| 1200, 1215, 1227, 1237, 1303 | 0.087985 | S1295A, S1297A, S1302G, S1303G, S1306G | Dec Relaxation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endsley, C.E.; Moore, K.A.; Townsley, T.D.; Durston, K.K.; Deweese, J.E. Bioinformatic Analysis of Topoisomerase IIα Reveals Interdomain Interdependencies and Critical C-Terminal Domain Residues. Int. J. Mol. Sci. 2024, 25, 5674. https://doi.org/10.3390/ijms25115674
Endsley CE, Moore KA, Townsley TD, Durston KK, Deweese JE. Bioinformatic Analysis of Topoisomerase IIα Reveals Interdomain Interdependencies and Critical C-Terminal Domain Residues. International Journal of Molecular Sciences. 2024; 25(11):5674. https://doi.org/10.3390/ijms25115674
Chicago/Turabian StyleEndsley, Clark E., Kori A. Moore, Thomas D. Townsley, Kirk K. Durston, and Joseph E. Deweese. 2024. "Bioinformatic Analysis of Topoisomerase IIα Reveals Interdomain Interdependencies and Critical C-Terminal Domain Residues" International Journal of Molecular Sciences 25, no. 11: 5674. https://doi.org/10.3390/ijms25115674
APA StyleEndsley, C. E., Moore, K. A., Townsley, T. D., Durston, K. K., & Deweese, J. E. (2024). Bioinformatic Analysis of Topoisomerase IIα Reveals Interdomain Interdependencies and Critical C-Terminal Domain Residues. International Journal of Molecular Sciences, 25(11), 5674. https://doi.org/10.3390/ijms25115674








