Preclinical Evaluation of Biodistribution and Toxicity of [211At]PSMA-5 in Mice and Primates for the Targeted Alpha Therapy against Prostate Cancer
Abstract
1. Introduction
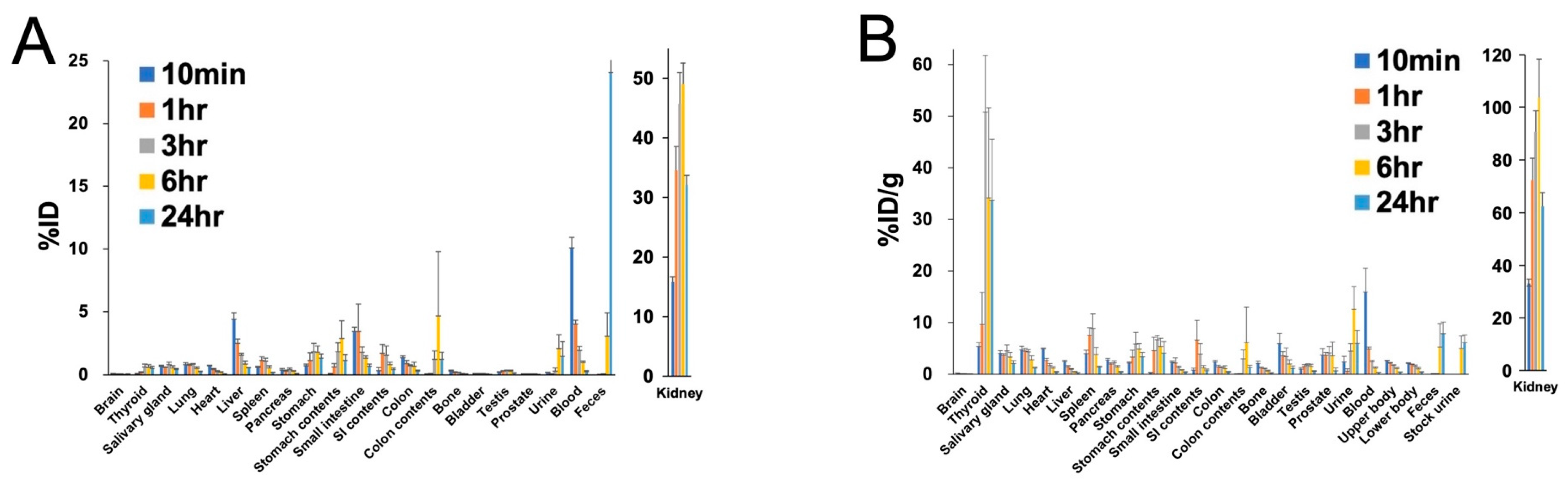
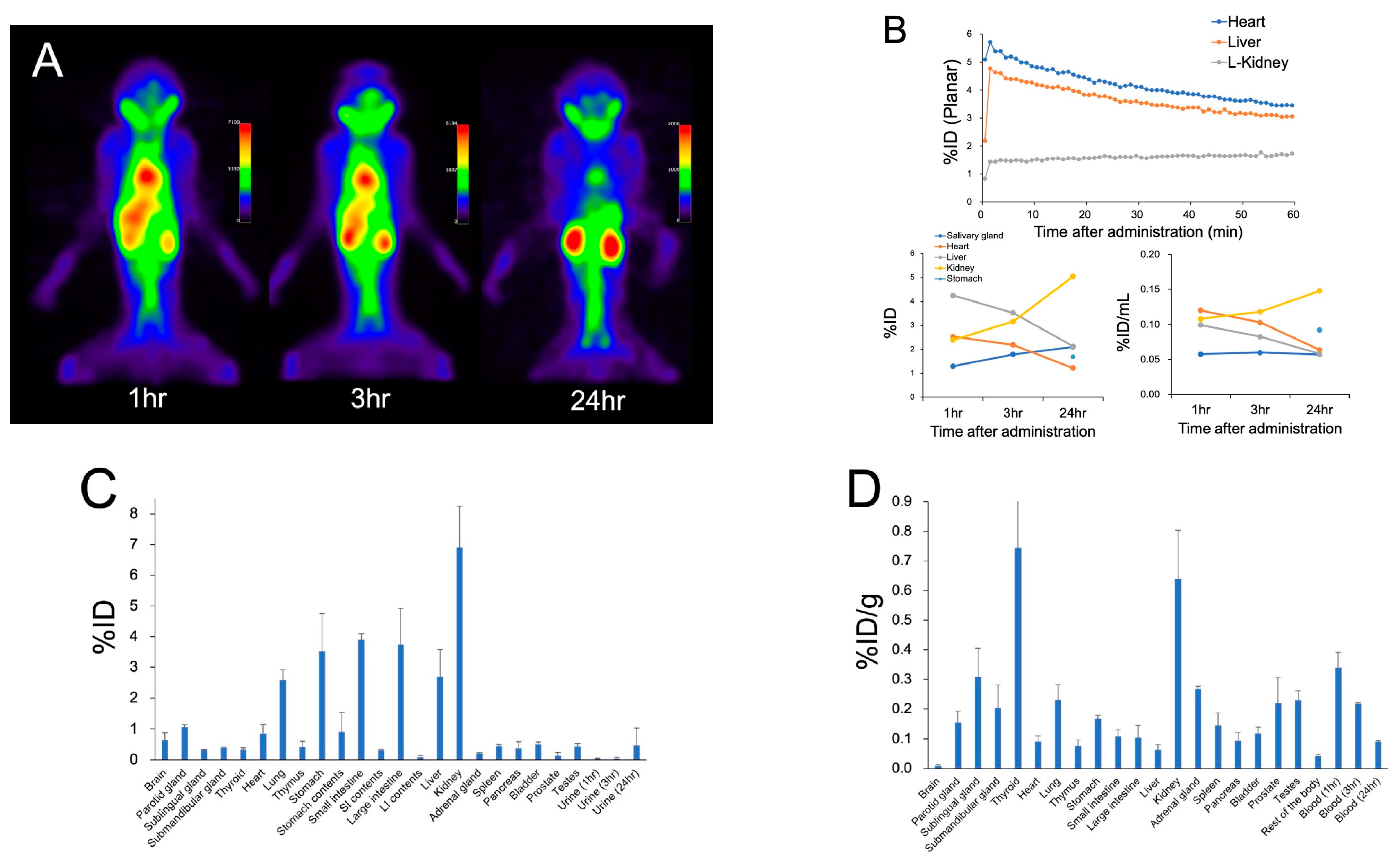
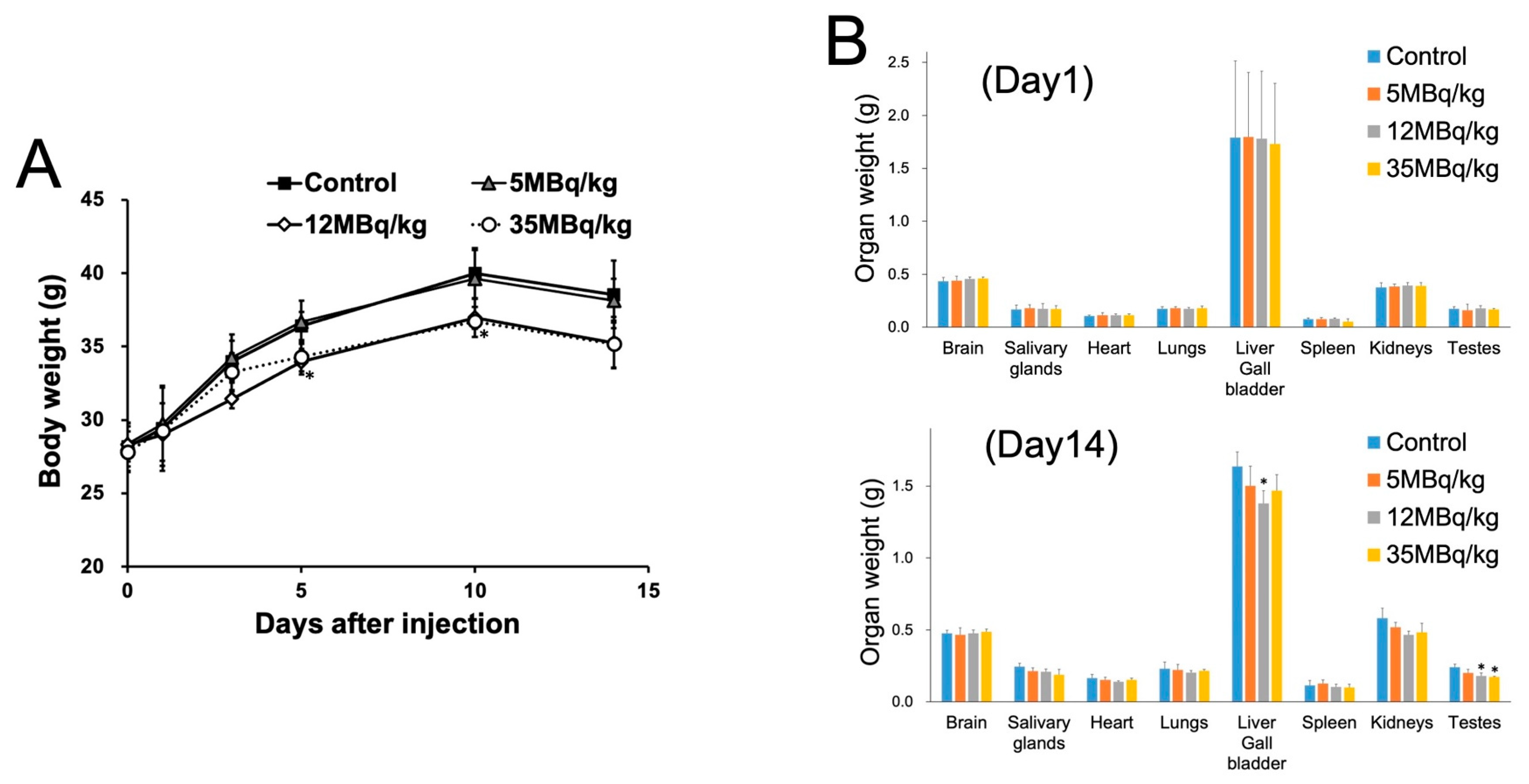
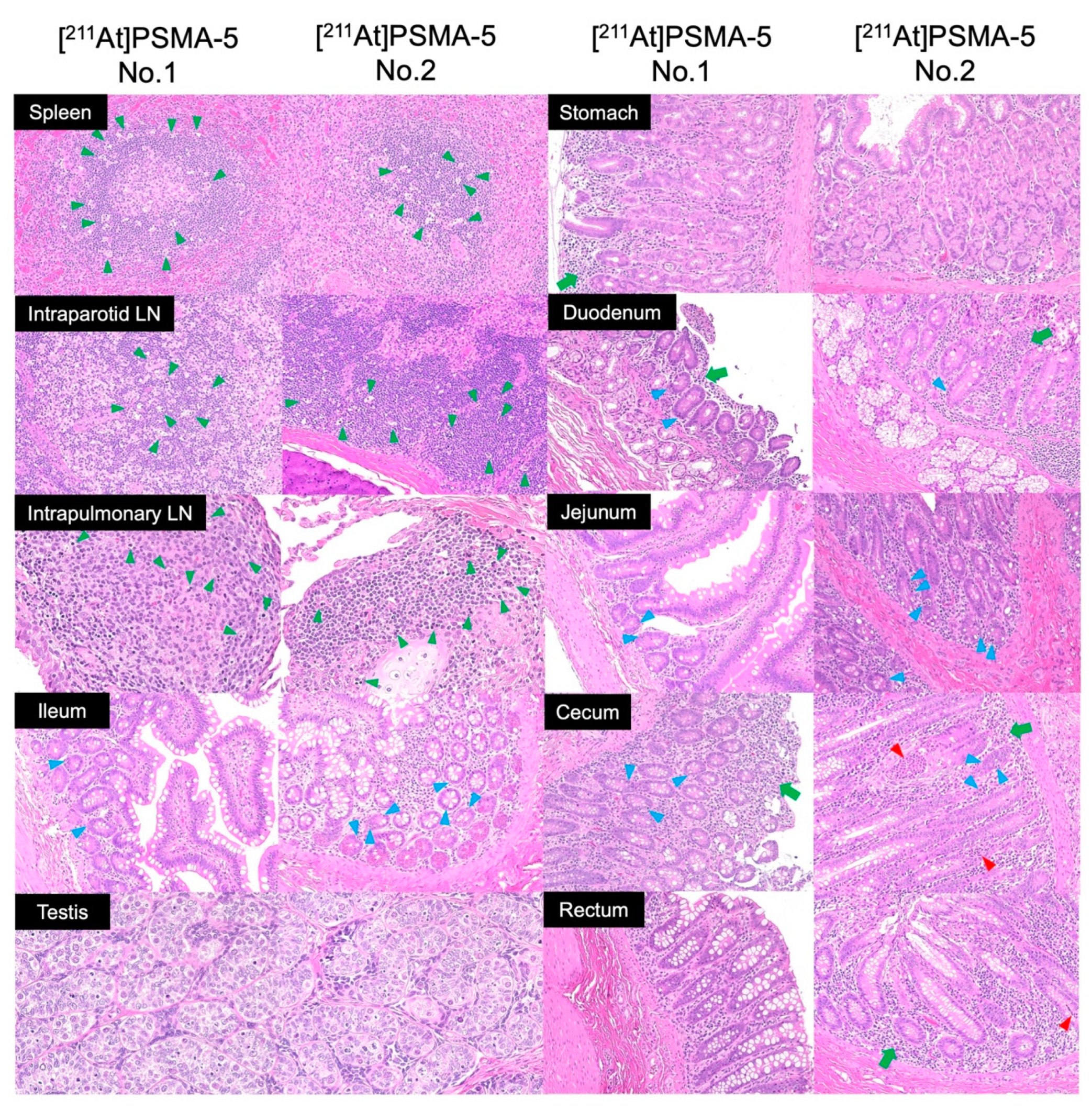
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of [211At]PSMA-5
4.2. Animal Preparation and Administration of [211At]PSMA-5
4.3. Biodistribution Study, Metabolite Study, and Estimation of Absorbed Dose in Human
4.4. Blood Examination
4.5. Histological Evaluation and Organ Weight Measurement
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Chow, K.M.; So, W.Z.; Lee, H.J.; Lee, A.; Yap, D.W.T.; Takwoingi, Y.; Tay, K.J.; Tuan, J.; Thang, S.P.; Lam, W.; et al. Head-to-head Comparison of the Diagnostic Accuracy of Prostate-specific Membrane Antigen Positron Emission Tomography and Conventional Imaging Modalities for Initial Staging of Intermediate- to High-risk Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2023, 84, 36–48. [Google Scholar]
- Mattana, F.; Muraglia, L.; Barone, A.; Colandrea, M.; Diffalah, Y.S.; Provera, S.; Cascio, A.S.; Salè, E.O.; Ceci, F. Prostate-Specific Membrane Antigen-Targeted Therapy in Prostate Cancer: History, Combination Therapies, Trials, and Future Perspective. Cancers 2024, 16, 1643. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Hofman, M.S.; Emmett, L.; Calais, J.; Osborne, J.R.; Iravani, A.; Koo, P.; Lindenberg, L.; et al. Joint EANM/SNMMI procedure guideline for the use of 177Lu-labeled PSMA-targeted radioligand-therapy (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2830–2845. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Keam, S.J. Lutetium Lu 177 Vipivotide Tetraxetan: First Approval. Mol. Diagn. Ther. 2022, 26, 467–475. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Sartor, A.O.; Morris, M.J.; Chi, K.N.; De Bono, J.S.; Shore, N.D.; Crosby, M.; Kreisl, T.N.; Fizazi, K. PSMAfore: A phase 3 study to compare 177Lu-PSMA-617 treatment with a change in androgen receptor pathway inhibitor in taxane-naïve patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2022, 40, TPS211. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef]
- Sathekge, M.M.; O Lawal, I.; Bal, C.; Bruchertseifer, F.; Ballal, S.; Cardaci, G.; Davis, C.; Eiber, M.; Hekimsoy, T.; Knoesen, O.; et al. Actinium-225-PSMA radioligand therapy of metastatic castration-resistant prostate cancer (WARMTH Act): A multicentre, retrospective study. Lancet Oncol. 2024, 25, 175–183. [Google Scholar] [CrossRef]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Böning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted α-Therapy Using 225Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar]
- Parida, G.K.; Panda, R.A.; Bishnoi, K.; Agrawal, K. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med. Princ. Pract. 2023, 32, 178–191. [Google Scholar] [CrossRef]
- Kratochwil, C.; Haberkorn, U.; Giesel, F.L. 225Ac-PSMA-617 for Therapy of Prostate Cancer. Semin. Nucl. Med. 2020, 50, 133–140. [Google Scholar] [CrossRef]
- Ndlovu, H.; Mokoala, K.M.G.; Lawal, I.; Emmett, L.; Sathekge, M.M. Prostate-specific Membrane Antigen: Alpha-labeled Radiopharmaceuticals. PET Clin. 2024, S1556-8598(24)00018-X. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef]
- Ostuni, E.; Taylor, M.R.G. Commercial and business aspects of alpha radioligand therapeutics. Front. Med. 2022, 9, 1070497. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of 211At uptake via the sodium iodide symporter by the addition of ascorbic acid in targeted α-therapy of thyroid cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef]
- Zalutsky, M.R.; Pruszynski, M. Astatine-211: Production and availability. Curr. Radiopharm. 2011, 4, 177–185. [Google Scholar] [CrossRef]
- Rabiei, M.; Asadi, M.; Yousefnia, H. Astatine-211 Radiopharmaceuticals; Status, Trends, and the Future. Curr. Radiopharm. 2023, 17, 7–13. [Google Scholar] [CrossRef]
- Feng, Y.; Zalutsky, M.R. Production, purification and availability of 211At: Near term steps towards global access. Nucl. Med. Biol. 2021, 100–101, 12–23. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, L.A.; Burns, J.D.; Tereshatov, E.E.; Muzzioli, R.; Hagel, K.; Jinadu, N.A.; McCann, L.A.; Picayo, G.A.; Pisaneschi, F.; Piwnica-Worms, D.; et al. Production, isolation, and shipment of clinically relevant quantities of astatine-211: A simple and efficient approach to increasing supply. Nucl. Med. Biol. 2023, 126–127, 108387. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Kadonaga, Y.; Ooe, K.; Wang, Y.; Haba, H.; Toyoshima, A.; Cardinale, J.; Giesel, F.L.; et al. Targeted α-therapy using astatine (211At)-labeled PSMA1, 5, and 6: A preclinical evaluation as a novel compound. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 849–858. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Ooe, K.; Liu, Y.; Kurimoto, K.; Murai, T.; Shidahara, Y.; Okuma, K.; Takeuchi, M.; Nishide, M.; et al. Extended single-dose toxicity study of [211At]NaAt in mice for the first-in-human clinical trial of targeted alpha therapy for differentiated thyroid cancer. Ann. Nucl. Med. 2021, 35, 702–718. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Baccala, A.; Sercia, L.; Li, J.; Heston, W.; Zhou, M. Expression of prostate-specific membrane antigen in tumor-associated neovasculature of renal neoplasms. Urology 2007, 70, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Ooe, K.; Shirakami, Y.; Toyoshima, A.; Shimosegawa, E.; Nakano, T.; Shinohara, A.; Hatazawa, J. Preclinical evaluation of radiation-induced toxicity in targeted alpha therapy using [211At] NaAt in mice : A Revisit. Transl. Oncol. 2020, 13, 100757. [Google Scholar] [CrossRef]
- Kiess, A.P.; Minn, I.; Vaidyanathan, G.; Hobbs, R.F.; Josefsson, A.; Shen, C.; Brummet, M.; Chen, Y.; Choi, J.; Koumarianou, E.; et al. (2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido)Pentyl)Ureido)-Pentanedioic Acid for PSMA-Targeted α-Particle Radiopharmaceutical Therapy. J. Nucl. Med. 2016, 57, 1569–1575. [Google Scholar] [CrossRef]
- Xiao, S.; Zhou, L. Gastric Stem Cells: Physiological and pathological perspectives. Front. Cell Dev. Biol. 2020, 8, 571536. [Google Scholar] [CrossRef]
- Andreu, P.; Colnot, S.; Godard, C.; Gad, S.; Chafey, P.; Niwa-Kawakita, M.; Laurent-Puig, P.; Kahn, A.; Robine, S.; Perret, C.; et al. Godard1. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development 2005, 132, 1443–1451. [Google Scholar] [CrossRef]
- Grootveld, A.K.; Kyaw, W.; Panova, V.; Lau, A.W.; Ashwin, E.; Seuzaret, G.; Dhenni, R.; Bhattacharyya, N.D.; Khoo, W.H.; Biro, M.; et al. Apoptotic cell fragments locally activate tingible body macrophages in the germinal center. Cell 2023, 186, 1144–1161.e18. [Google Scholar] [CrossRef] [PubMed]
- Zlotoff, D.A.; Bhandoola, A. Hematopoietic progenitor migration to the adult thymus. Ann. N. Y. Acad. Sci. 2011, 1217, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Uemura, M.; Soeda, F.; Naka, S.; Ujike, T.; Hatano, K.; Sasaki, H.; Kamiya, T.; Shimosegawa, E.; Kato, H.; et al. High detection rate in [18F]PSMA-1007 PET: Interim results focusing on biochemical recurrence in prostate cancer patients. Ann. Nucl. Med. 2021, 35, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 labelled PSMA-1007: Biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Sprute, K.; Kramer, V.; Koerber, S.A.; Meneses, M.; Fernandez, R.; Soza-Ried, C.; Eiber, M.; Weber, W.A.; Rauscher, I.; Rahbar, K.; et al. Diagnostic Accuracy of 18F-PSMA-1007 PET/CT Imaging for Lymph Node Staging of Prostate Carcinoma in Primary and Biochemical Recurrence. J. Nucl. Med. 2021, 62, 208–213. [Google Scholar] [CrossRef]
- Giesel, F.L.; Knorr, K.; Spohn, F.; Will, L.; Maurer, T.; Flechsig, P.; Neels, O.; Schiller, K.; Amaral, H.; Weber, W.A.; et al. Detection Efficacy of 18F-PSMA-1007 PET/CT in 251 Patients with Biochemical Recurrence of Prostate Cancer After Radical Prostatectomy. J. Nucl. Med. 2019, 60, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Shirakami, Y.; Watabe, T.; Obata, H.; Kaneda, K.; Ooe, K.; Liu, Y.; Teramoto, T.; Toyoshima, A.; Shinohara, A.; Shimosegawa, E.; et al. Synthesis of [211At]4-astato-L-phenylalanine by dihydroxyboryl-astatine substitution reaction in aqueous solution. Sci. Rep. 2021, 11, 12982. [Google Scholar] [CrossRef] [PubMed]
- Valentin, J. Basic anatomical and physiological data for use in radiological protection: Reference values: ICRP Publication 89: Approved by the Commission in September 2001. Ann. ICRP 2002, 32, 5–265. [Google Scholar] [CrossRef]
- Andersson, M.; Johansson, L.; Eckerman, K.; Mattsson, S. IDAC-Dose 2.1, an internal dosimetry program for diagnostic nuclear medicine based on the ICRP adult reference voxel phantoms. EJNMMI Res. 2017, 7, 88. [Google Scholar] [CrossRef]
- ICH Harmonized Tripartite Guideline: Duration of Chronic Toxicity Testing in Animals (Rodent and Non Rodent Toxicity Testing) S4. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 1998. Available online: https://database.ich.org/sites/default/files/S4_Guideline.pdf (accessed on 22 May 2024).

| Organ | Estimated Absorbed Dose (mGy/MBq) |
|---|---|
| Brain | 0.0123 |
| Thyroid | 1.82 |
| Salivary glands | 0.218 |
| Heart | 0.232 |
| Lung | 0.0478 |
| Liver | 0.0708 |
| Stomach | 0.258 |
| Small intestine | 0.089 |
| Colon | 0.087 |
| Kidney | 4.05 |
| Pancreas | 0.105 |
| Spleen | 0.242 |
| Testes | 0.087 |
| Prostate | 0.17 |
| Bladder | 0.147 |
| Red bone marrow | 0.055 |
| (Day 1) | Control | 5 MBq/kg | 12 MBq/kg | 35 MBq/kg |
| RBC (106 cell/µL) | 7.6 ± 0.3 | 7.8 ± 0.3 | 8.0 ± 0.5 | 7.9 ± 0.3 |
| Hb (g/dL) | 12.8 ± 0.7 | 12.9 ± 0.4 | 13.0 ± 0.8 | 12.8 ± 0.7 |
| Ht (%) | 41.8 ± 1.9 | 42.5 ± 1.4 | 42.6 ± 2.3 | 42.3 ± 2 |
| WBC (103 cell/µL) | 8.0 ± 3.7 | 6.3 ± 4.1 | 8.3 ± 4.9 | 8.0 ± 5.2 |
| Platelet (103 cell/µL) | 915 ± 343 | 976 ± 524 | 1329 ± 845 | 952 ± 444 |
| %Lymph (%) | 59.4 ± 16.4 | 66.3 ± 15.8 | 62.6 ± 14.2 | 53.5 ± 16.9 |
| (Day 14) | Control | 5 MBq/kg | 12 MBq/kg | 35 MBq/kg |
| RBC (106 cell/µL) | 8.7 ± 0.5 | 8.7 ± 0.2 | 8.3 ± 0.6 | 8.4 ± 0.4 |
| Hb (g/dL) | 14.2 ± 1 | 14.4 ± 0.4 | 13.8 ± 0.9 | 13.6 ± 0.7 |
| Ht (%) | 45.8 ± 3.1 | 45.4 ± 1 | 43.9 ± 3 | 43.1 ± 2.4 |
| WBC (103 cell/µL) | 9.6 ± 5.4 | 6.9 ± 3.3 | 6.9 ± 1.9 | 5.4 ± 2.8 |
| Platelet (103 cell/µL) | 777 ± 381 | 1120 ± 555 | 922 ± 608 | 1331 ± 1037 |
| %Lymph (%) | 63.9 ± 11.6 | 70 ± 8.8 | 59.9 ± 12.1 | 53.9 ± 6.4 |
| Pre-Administration | 1 h | 3 h | 24 h | |
|---|---|---|---|---|
| RBC (106 cell/µL) | 4.80 ± 0.10 | 4.30 ± 0.30 | 4.70 ± 0.00 | 4.20 ± 0.10 |
| Hb (g/dL) | 11.2 ± 0.8 | 10.3 ± 1.4 | 11.2 ± 0.8 | 9.9 ± 0.5 |
| Ht (%) | 35.9 ± 2.0 | 32.9 ± 4.8 | 35.4 ± 2.5 | 31.8 ± 1.5 |
| WBC (103 cell/µL) | 14.3 ± 3.9 | 10.1 ± 0.7 | 6.7 ± 1.4 | 5.3 ± 0.8 |
| Platelet (103 cell/µL) | 134.2 ± 56.8 | 263.7 ± 33.0 | 306.8 ± 41.2 | 269.7 ± 60.8 |
| %Lymph (%) | 57.0 ± 8.0 | 44.0 ± 16.0 | 52.0 ± 4.5 | 23.7 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watabe, T.; Kaneda-Nakashima, K.; Kadonaga, Y.; Ooe, K.; Sampunta, T.; Hirose, N.; Yin, X.; Haba, H.; Kon, Y.; Toyoshima, A.; et al. Preclinical Evaluation of Biodistribution and Toxicity of [211At]PSMA-5 in Mice and Primates for the Targeted Alpha Therapy against Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 5667. https://doi.org/10.3390/ijms25115667
Watabe T, Kaneda-Nakashima K, Kadonaga Y, Ooe K, Sampunta T, Hirose N, Yin X, Haba H, Kon Y, Toyoshima A, et al. Preclinical Evaluation of Biodistribution and Toxicity of [211At]PSMA-5 in Mice and Primates for the Targeted Alpha Therapy against Prostate Cancer. International Journal of Molecular Sciences. 2024; 25(11):5667. https://doi.org/10.3390/ijms25115667
Chicago/Turabian StyleWatabe, Tadashi, Kazuko Kaneda-Nakashima, Yuichiro Kadonaga, Kazuhiro Ooe, Thosapol Sampunta, Naoki Hirose, Xiaojie Yin, Hiromitsu Haba, Yukiyoshi Kon, Atsushi Toyoshima, and et al. 2024. "Preclinical Evaluation of Biodistribution and Toxicity of [211At]PSMA-5 in Mice and Primates for the Targeted Alpha Therapy against Prostate Cancer" International Journal of Molecular Sciences 25, no. 11: 5667. https://doi.org/10.3390/ijms25115667
APA StyleWatabe, T., Kaneda-Nakashima, K., Kadonaga, Y., Ooe, K., Sampunta, T., Hirose, N., Yin, X., Haba, H., Kon, Y., Toyoshima, A., Cardinale, J., Giesel, F. L., Fukase, K., Tomiyama, N., & Shirakami, Y. (2024). Preclinical Evaluation of Biodistribution and Toxicity of [211At]PSMA-5 in Mice and Primates for the Targeted Alpha Therapy against Prostate Cancer. International Journal of Molecular Sciences, 25(11), 5667. https://doi.org/10.3390/ijms25115667








