Neurotoxicity and Developmental Neurotoxicity of Copper Sulfide Nanoparticles on a Human Neuronal In-Vitro Test System
Abstract
1. Introduction
2. Results
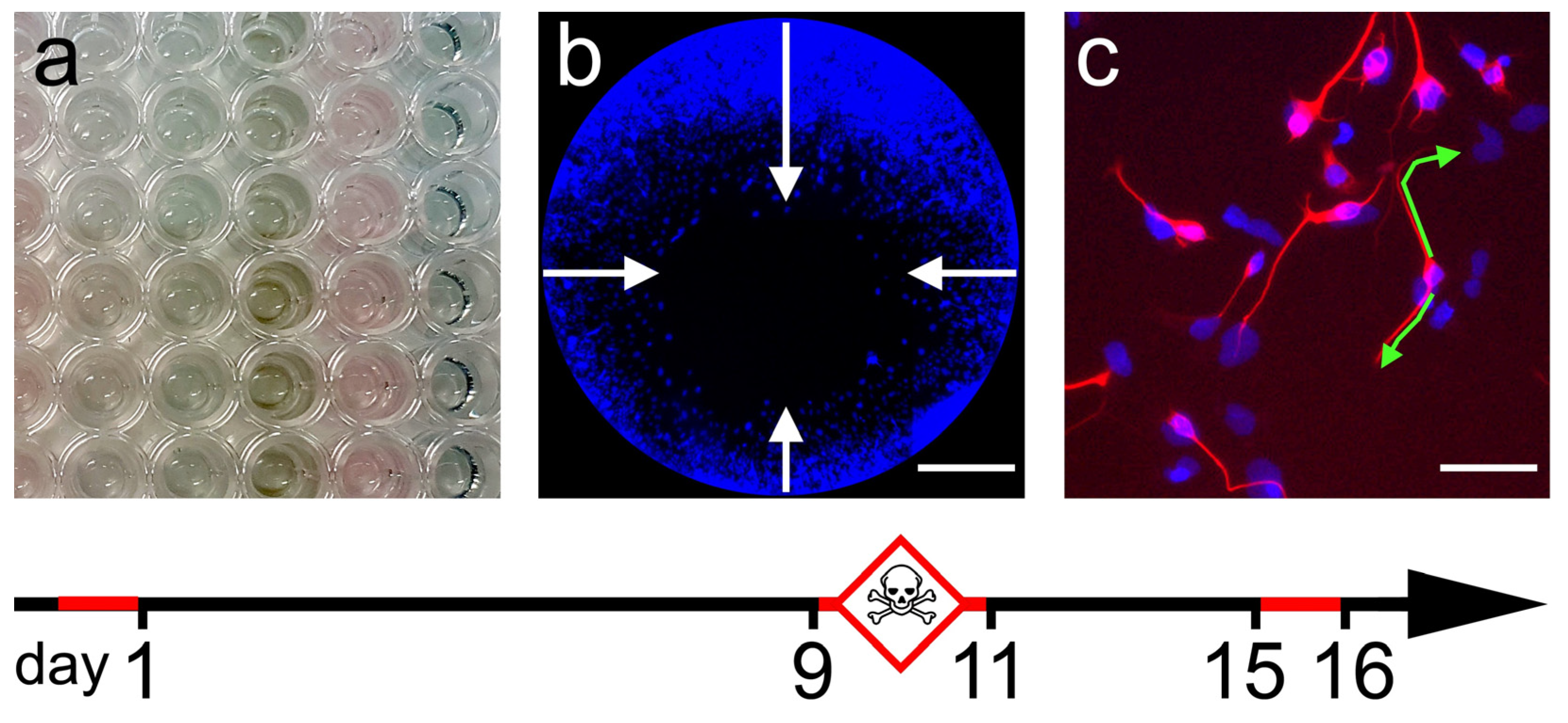
2.1. General Cytotoxicity
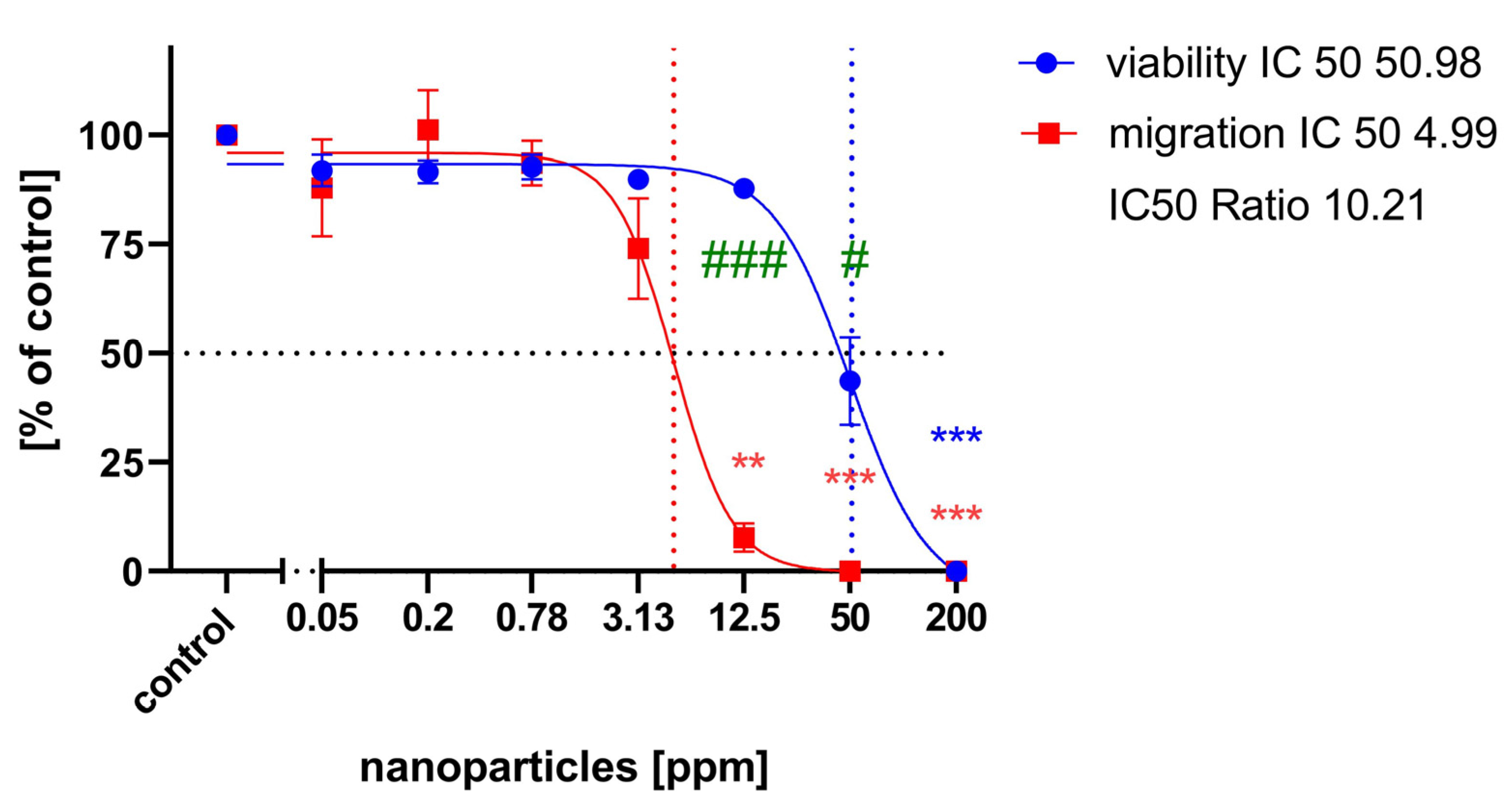
2.2. Cell Migration Assay
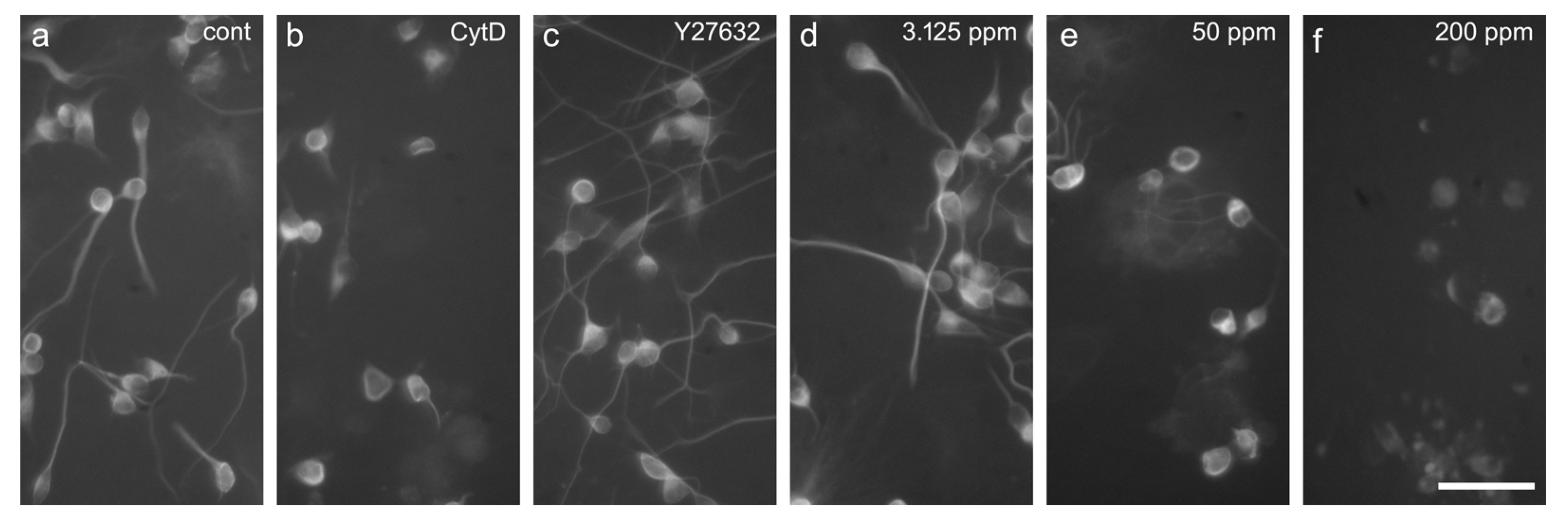
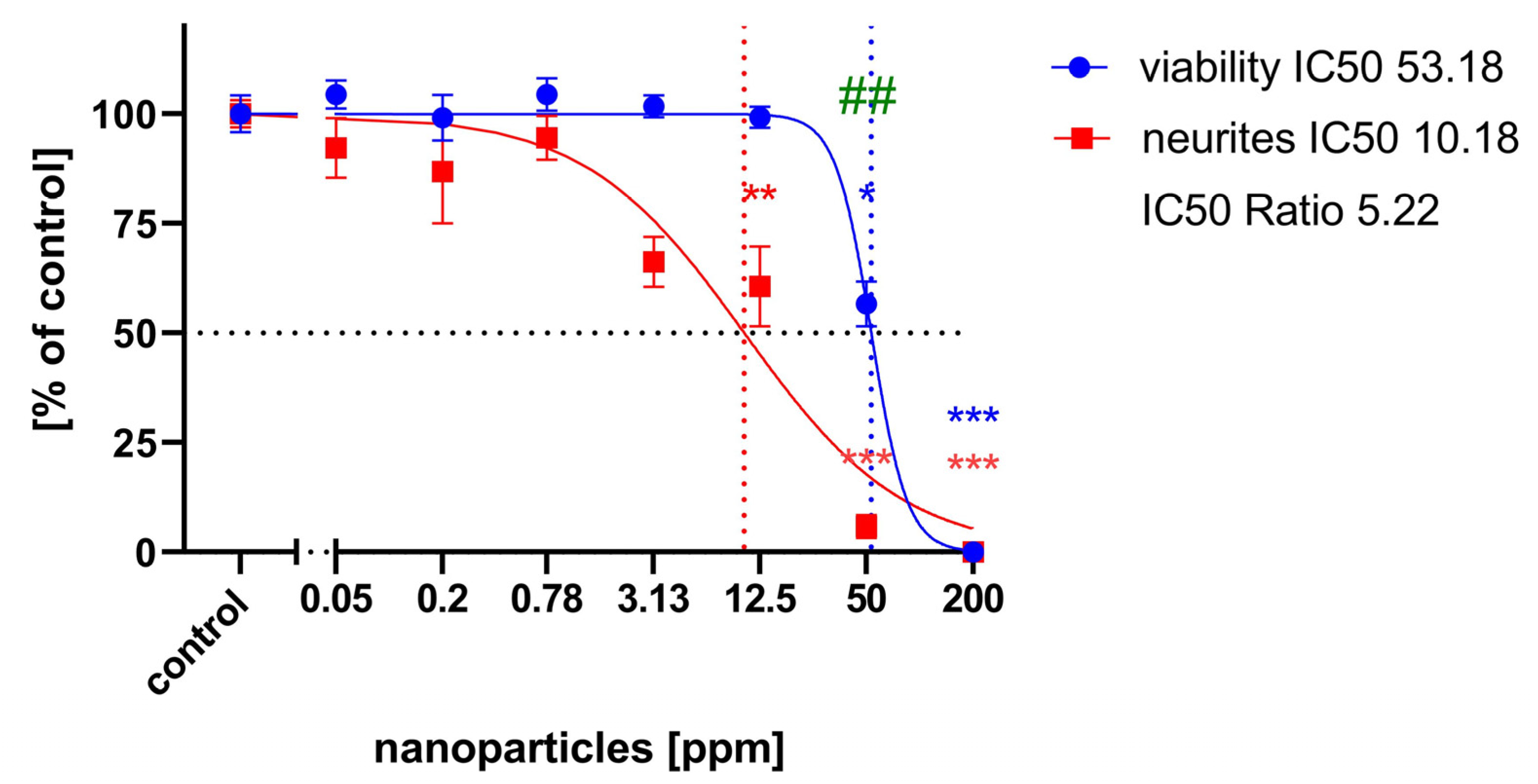
2.3. Neurite Outgrowth Assay
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Nanoparticles
4.3. Cell Culture
4.4. General Cytotoxicity
4.5. Cell Migration Assay
4.6. Neurite Outgrowth Assay
4.7. Statistics and Prediction Model
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xuan, L.; Zhao, J.; Skonieczna, M.; Zhou, P.-K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2020, 14, e327. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.K.; Rajesh, K.M. Green synthesis of nanoparticles using plant extracts: A review. Environ Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Kumari, A.; Mandzhieva, S.S.; Sushkova, S.; Prazdnova, E.V.; Zargar, S.M.; Raza, A.; Minkina, T.; Chung, G. Nanobionics in Crop Production: An Emerging Approach to Modulate Plant Functionalities. Plants 2022, 11, 692. [Google Scholar] [CrossRef]
- Botha, N.L.; Cloete, K.J.; Šmit, Ž.; Isaković, K.; Akbari, M.; Morad, R.; Madiba, I.; David, O.M.; Santos, L.P.M.; Dube, A.; et al. Ionome mapping and amino acid metabolome profiling of Phaseolus vulgaris L. seeds imbibed with computationally informed phytoengineered copper sulphide nanoparticles. Discov. Nano 2024, 19, 8. [Google Scholar] [CrossRef]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Cupaioli, F.A.; Zucca, F.A.; Boraschi, D.; Zecca, L. Engineered nanoparticles. How brain friendly is this new guest? Prog. Neurobiol. 2014, 119–120, 20–38. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.O. Risks from accidental exposures to engineered nanoparticles and neurological health effects: A critical review. Part. Fibre Toxicol. 2010, 7, 42. [Google Scholar] [CrossRef]
- Lucchini, R.G.; Dorman, D.C.; Elder, A.; Veronesi, B. Neurological impacts from inhalation of pollutants and the nose-brain connection. Neurotoxicology 2012, 33, 838–841. [Google Scholar] [CrossRef]
- Love, S.A.; Maurer-Jones, M.A.; Thompson, J.W.; Lin, Y.S.; Haynes, C.L. Assessing nanoparticle toxicity. Annu. Rev. Anal. Chem. 2012, 5, 181–205. [Google Scholar] [CrossRef]
- Meigs, L.; Smirnova, L.; Rovida, C.; Leist, M.; Hartung, T. Animal testing and its alternatives—The most important omics is economics. ALTEX 2018, 35, 275–305. [Google Scholar] [CrossRef]
- Leist, M.; Hartung, T. Inflammatory findings on species extrapolations: Humans are definitely no 70-kg mice. Arch. Toxicol. 2013, 87, 563–567. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnology 2016, 14, 65. [Google Scholar] [CrossRef]
- Hoelting, L.; Scheinhardt, B.; Bondarenko, O.; Schildknecht, S.; Kapitza, M.; Tanavde, V.; Tan, B.; Lee, Q.Y.; Mecking, S.; Leist, M.; et al. A 3-dimensional human embryonic stem cell (hESC)-derived model to detect developmental neurotoxicity of nanoparticles. Arch. Toxicol. 2013, 87, 721–733. [Google Scholar] [CrossRef]
- Stern, M.; Gierse, A.; Tan, S.; Bicker, G. Human Ntera2 cells as a predictive in vitro test system for developmental neurotoxicity. Arch. Toxicol. 2014, 88, 127–136. [Google Scholar] [CrossRef]
- Schmitz, A.; Dempewolf, S.; Tan, S.; Bicker, G.; Stern, M. Developmental Neurotoxicity of Fipronil and Rotenone on a Human Neuronal In Vitro Test System. Neurotox. Res. 2021, 39, 1189–1202. [Google Scholar] [CrossRef]
- Andrews, P.W. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984, 103, 285–293. [Google Scholar] [CrossRef]
- Pleasure, S.J.; Page, C.; Lee, V.M. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J. Neurosci. 1992, 12, 1802–1815. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Bicker, G. Human model neurons in studies of brain cell damage and neural repair. Curr. Mol. Med. 2007, 7, 541–554. [Google Scholar] [CrossRef]
- Rootwelt, T.; Dunn, M.; Yudkoff, M.; Itoh, T.; Almaas, R.; Pleasure, D. Hypoxic cell death in human NT2-N neurons: Involvement of NMDA and non-NMDA glutamate receptors. J. Neurochem. 1998, 71, 1544–1553. [Google Scholar] [CrossRef]
- Paquet-Durand, F.; Gierse, A.; Bicker, G. Diltiazem protects human NT-2 neurons against excitotoxic damage in a model of simulated ischemia. Brain Res. 2006, 1124, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Roloff, F.; Scheiblich, H.; Dewitz, C.; Dempewolf, S.; Stern, M.; Bicker, G. Enhanced neurite outgrowth of human model (NT2) neurons by small-molecule inhibitors of Rho/ROCK signaling. PLoS ONE 2015, 10, e0118536. [Google Scholar] [CrossRef]
- Taylor, M.A.; Kan, H.L.; Gollapudi, B.B.; Marty, M.S. An in vitro developmental neurotoxicity screening assay for retinoic acid-induced neuronal differentiation using the human NT2/D1 cell line. Neurotoxicology 2019, 73, 258–264. [Google Scholar] [CrossRef]
- Küçükdoğru, R.; Türkez, H.; Arslan, M.E.; Tozlu, Ö.Ö.; Sönmez, E.; Mardinoğlu, A.; Cacciatore, I.; Di Stefano, A. Neuroprotective effects of boron nitride nanoparticles in the experimental Parkinson’s disease model against MPP+ induced apoptosis. Metab. Brain Dis. 2020, 35, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Paquet-Durand, F.; Tan, S.; Bicker, G. Turning teratocarcinoma cells into neurons: Rapid differentiation of NT-2 cells in floating spheres. Brain Res. Dev. Brain Res. 2003, 142, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Podrygajlo, G.; Tegenge, M.A.; Gierse, A.; Paquet-Durand, F.; Tan, S.; Bicker, G.; Stern, M. Cellular phenotypes of human model neurons (NT2) after differentiation in aggregate culture. Cell Tissue Res. 2009, 336, 439–452. [Google Scholar] [CrossRef]
- Podrygajlo, G.; Song, Y.; Schlesinger, F.; Krampfl, K.; Bicker, G. Synaptic currents and transmitter responses in human NT2 neurons differentiated in aggregate culture. Neurosci. Lett. 2010, 468, 207–210. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Böhnel, H.; Gessler, F.; Bicker, G. Neurotransmitter vesicle release from human model neurons (NT2) is sensitive to botulinum toxin A. Cell Mol. Neurobiol. 2012, 32, 1021–1029. [Google Scholar] [CrossRef]
- Rakic, P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef]
- Abbott, L.C.; Nigussie, F. Mercury Toxicity and Neurogenesis in the Mammalian Brain. Int. J. Mol. Sci. 2021, 22, 7520. [Google Scholar] [CrossRef]
- Tegenge, M.A.; Bicker, G. Nitric oxide and cGMP signal transduction positively regulates the motility of human neuronal precursor (NT2) cells. J. Neurochem. 2009, 110, 1828–1841. [Google Scholar] [CrossRef]
- Tegenge, T.A.; Rockel, T.D.; Fritsche, E.; Bicker, G. Nitric oxide stimulates human neural progenitor cell migration via cGMP-mediated signal transduction. Cell Mol. Life Sci. 2011, 68, 2089–2099. [Google Scholar] [CrossRef]
- Rai, S.; Khotari, R. Cytotoxic activity and DNA fragmentation study of nano structured assemblies of copper sulfide nanoparticles using single route molecular precursor source of copper. Dig. J. Nanomater. Biostructures 2022, 17, 1011–1028. [Google Scholar] [CrossRef]
- Krug, A.K.; Balmer, N.V.; Matt, F.; Schönenberger, F.; Merhof, D.; Leist, M. Evaluation of a human neurite growth assay as specific screen for developmental neurotoxicants. Arch. Toxicol. 2013, 87, 2215–2231. [Google Scholar] [CrossRef]
- Aschner, M.; Ceccatelli, S.; Daneshian, M.; Fritsche, E.; Hasiwa, N.; Hartung, T.; Hogberg, H.T.; Leist, M.; Li, A.; Mundi, W.R.; et al. Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: Example lists and criteria for their selection and use. ALTEX 2017, 34, 49–74. [Google Scholar] [CrossRef][Green Version]
- Tian, Q.; Tang, M.; Sun, Y.; Zou, R.; Chen, Z.; Zhu, M.; Yang, S.; Wang, J.; Wang, J.; Hu, J. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 2011, 23, 3542–3547. [Google Scholar] [CrossRef]
- Ain, N.U.; Abdul Nasir, J.; Khan, Z.; Butler, I.S.; Rehman, Z. Copper sulfide nanostructures: Synthesis and biological applications. RSC Adv. 2022, 12, 7550–7567. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Liu, Y.; Li, B.; Chen, C.; Wu, G. Oxidative stress and acute changes in murine brain tissues after nasal instillation of copper particles with different sizes. J. Nanosci. Nanotechnol. 2014, 14, 4534–4540. [Google Scholar] [CrossRef]
- OECD. Test No. 426: Developmental Neurotoxicity Study. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2007. [Google Scholar] [CrossRef]
- Contreras, E.G.; Klämbt, C. The Drosophila blood-brain barrier emerges as a model for understanding human brain diseases. Neurobiol. Dis. 2023, 180, 106071. [Google Scholar] [CrossRef]
- Andersson, O.; Hansen, S.H.; Hellman, K.; Olsen, L.R.; Andersson, G.; Badolo, L.; Svenstrup, N.; Nielsen, P.A. The grasshopper: A novel model for assessing vertebrate brain uptake. J. Pharmacol. Exp. Ther. 2013, 346, 211–218. [Google Scholar] [CrossRef]
- Hellman, K.; Nielsen, P.A.; Ek, F.; Olsson, R. An ex Vivo Model for Evaluating Blood-Brain Barrier Permeability, Efflux, and Drug Metabolism. ACS Chem. Neurosci. 2016, 7, 668–680. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Schiøtt, M.; Hansen, S.H.; Nielsen, P.A.; Badolo, L. An invertebrate model for CNS drug discovery: Transcriptomic and functional analysis of a mammalian P-glycoprotein ortholog. Biochim. Biophys. Acta 2015, 1850, 2439–2451. [Google Scholar] [CrossRef]
- Bergmann, G.A.; Froembling, S.; Joseph, N.; Bode, K.; Bicker, G.; Stern, M. An intact insect embryo for developmental neurotoxicity testing of directed axonal elongation. ALTEX 2019, 36, 643–649. [Google Scholar] [CrossRef]
- Bode, K.; Bohn, M.; Reitmeier, J.; Betker, P.; Stern, M.; Bicker, G. A locust embryo as predictive developmental neurotoxicity testing system for pioneer axon pathway formation. Arch. Toxicol. 2020, 94, 4099–4113. [Google Scholar] [CrossRef]
- Luther, E.M.; Petters, C.; Bulcke, F.; Kaltz, A.; Thiel, K.; Bickmeyer, U.; Dringen, R. Endocytotic uptake of iron oxide nanoparticles by cultured brain microglial cells. Acta Biomater. 2013, 9, 8454–8465. [Google Scholar] [CrossRef]
- Lv, Z.; Li, S.; Zeng, G.; Yao, K.; Han, H. Recent progress of nanomedicine in managing dry eye disease. Adv. Ophthalmol. Pract. Res. 2024, 4, 23–31. [Google Scholar] [CrossRef]
- Vighi, E.; Trifunović, D.; Veiga-Crespo, P.; Rentsch, A.; Hoffmann, D.; Sahaboglu, A.; Strasser, T.; Kulkarni, M.; Bertolotti, E.; van den Heuvel, A.; et al. Combination of cGMP analogue and drug delivery system provides functional protection in hereditary retinal degeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E2997–E3006. [Google Scholar] [CrossRef]
- Benelli, G. Mode of action of nanoparticles against insects. Environ. Sci. Pollut. Res. Int. 2018, 25, 12329–12341. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, B.G.; Lalitha, K.; Shivakumar, M.S. Biosynthesis of copper nanoparticles using symbiotic bacterium Xenorhabdus sp, isolated from entomopathogenic nematode and its antimicrobial and insecticidal activity against Spodoptera litura. Inorg. Nano-Metal. Chem. 2022; epub ahead of print. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public. Health 2021, 18, 10536. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Majedi, S.M.; Lee, H.K.; Kelly, B.C. Chemometric analytical approach for the cloud point extraction and inductively coupled plasma mass spectrometric determination of zinc oxide nanoparticles in water samples. Anal. Chem 2012, 84, 6546–6552. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern, M.; Botha, N.; Cloete, K.J.; Maaza, M.; Tan, S.; Bicker, G. Neurotoxicity and Developmental Neurotoxicity of Copper Sulfide Nanoparticles on a Human Neuronal In-Vitro Test System. Int. J. Mol. Sci. 2024, 25, 5650. https://doi.org/10.3390/ijms25115650
Stern M, Botha N, Cloete KJ, Maaza M, Tan S, Bicker G. Neurotoxicity and Developmental Neurotoxicity of Copper Sulfide Nanoparticles on a Human Neuronal In-Vitro Test System. International Journal of Molecular Sciences. 2024; 25(11):5650. https://doi.org/10.3390/ijms25115650
Chicago/Turabian StyleStern, Michael, Nandipha Botha, Karen J. Cloete, Malik Maaza, Saime Tan, and Gerd Bicker. 2024. "Neurotoxicity and Developmental Neurotoxicity of Copper Sulfide Nanoparticles on a Human Neuronal In-Vitro Test System" International Journal of Molecular Sciences 25, no. 11: 5650. https://doi.org/10.3390/ijms25115650
APA StyleStern, M., Botha, N., Cloete, K. J., Maaza, M., Tan, S., & Bicker, G. (2024). Neurotoxicity and Developmental Neurotoxicity of Copper Sulfide Nanoparticles on a Human Neuronal In-Vitro Test System. International Journal of Molecular Sciences, 25(11), 5650. https://doi.org/10.3390/ijms25115650







