Could SP-A and SP-D Serum Levels Predict COVID-19 Severity?
Abstract
1. Introduction
2. Results
2.1. Study Participants
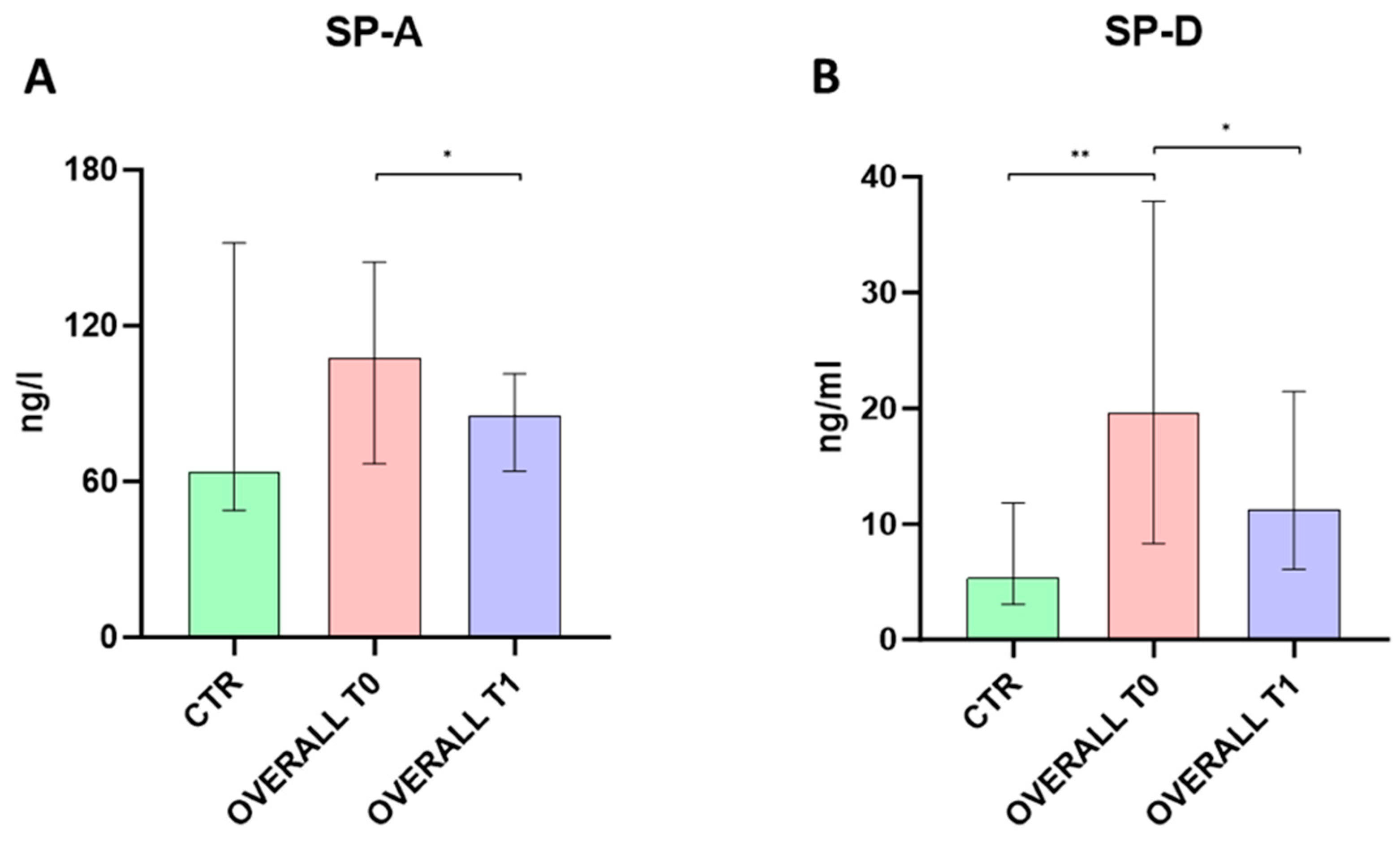
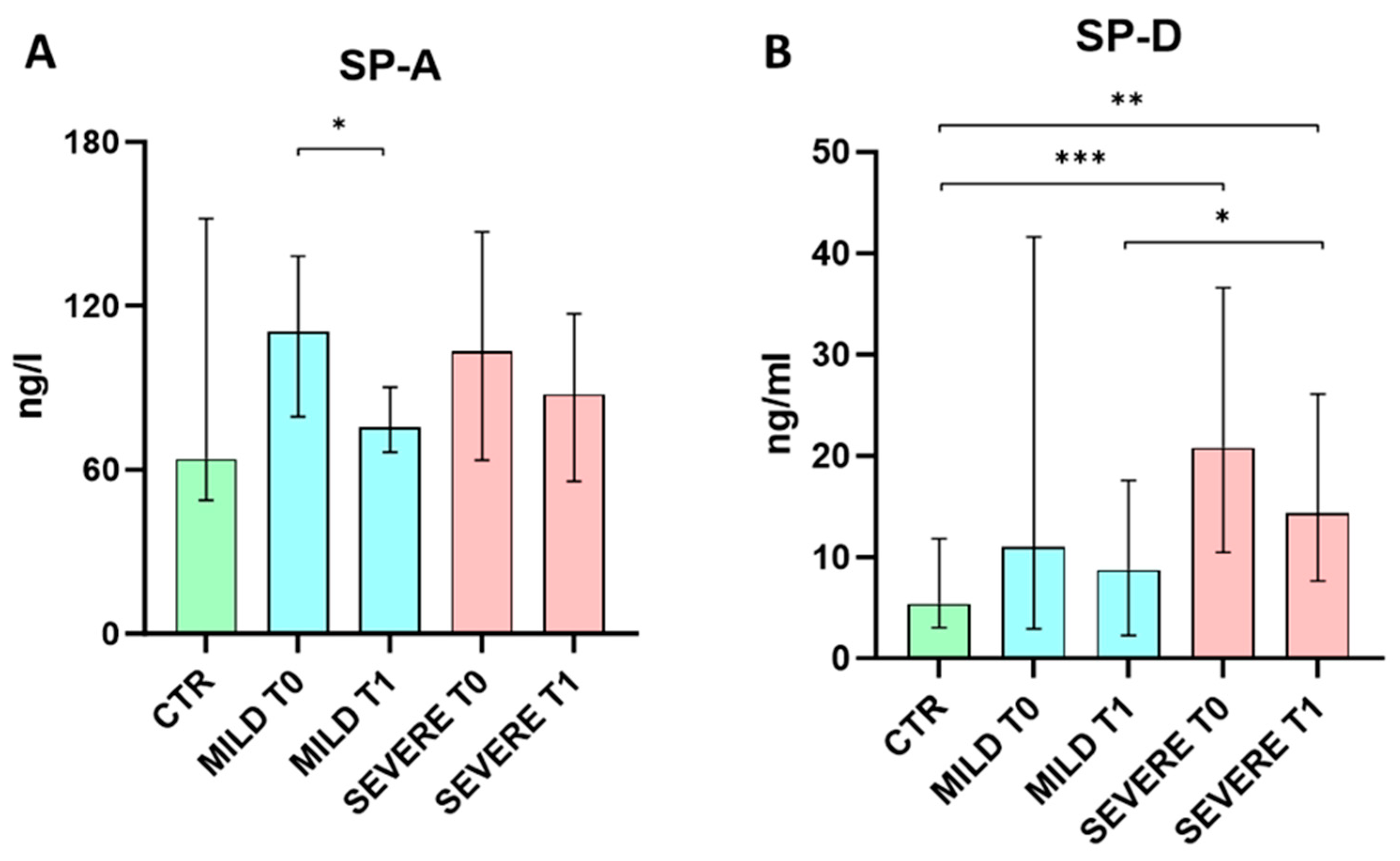
2.2. Serum SP-A and SP-D Levels Evaluation
3. Discussion
4. Materials and Methods
4.1. Study Participants and Ethical Approvals
4.2. Serological Evaluation of SP-A and SP-D Levels
4.3. RT-qPCR Detection of SARS-CoV-2 RNA
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef]
- Barek, M.A.; Aziz, M.A.; Islam, M.S. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: A meta-analysis with 55 studies and 10014 cases. Heliyon 2020, 6, e05684. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020, 55, 2000607. [Google Scholar] [CrossRef]
- Chroneos, Z.; Sever-Chroneos, Z.; Shepherd, V. Pulmonary Surfactant: An Immunological Perspective. Cell. Physiol. Biochem. 2010, 25, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Ghati, A.; Dam, P.; Tasdemir, D.; Kati, A.; Sellami, H.; Sezgin, G.C.; Ildiz, N.; Franco, O.L.; Mandal, A.K.; Ocsoy, I. Exogenous pulmonary surfactant: A review focused on adjunctive therapy for severe acute respiratory syndrome coronavirus 2 including SP-A and SP-D as added clinical marker. Curr. Opin. Colloid Interface Sci. 2021, 51, 101413. [Google Scholar] [CrossRef]
- Han, S.; Mallampalli, R.K. The role of surfactant in lung disease and host defense against pulmonary infections. Ann. Am. Thorac. Soc. 2015, 12, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Jacob, I.B.; Gemmiti, A.; Xiong, W.; Reynolds, E.; Nicholas, B.; Thangamani, S.; Jia, H.; Wang, G. Human surfactant protein A inhibits SARS-CoV-2 infectivity and alleviates lung injury in a mouse infection model. Front. Immunol. 2024, 15, 1370511. [Google Scholar] [CrossRef] [PubMed]
- Noah, T.L.; Murphy, P.C.; Alink, J.J.; Leigh, M.W.; Hull, W.M.; Stahlman, M.T.; Whitsett, J.A. Bronchoalveolar lavage fluid surfactant protein-A and surfactant protein-D are inversely related to inflammation in early cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003, 168, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Griese, M.; Maderlechner, N.; Ahrens, P.; Kitz, R. Surfactant proteins A and D in children with pulmonary disease due to gastroesophageal reflux. Am. J. Respir. Crit. Care Med. 2002, 165, 1546–1550. [Google Scholar] [CrossRef] [PubMed]
- Hartl, D.; Griese Ludwig-Maximilians, M. Surfactant protein D in human lung diseases. Eur. J. Clin. Investig. 2006, 36, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Agustama, A.; Surgean Veterini, A.; Utariani, A. Correlation of Surfactant Protein-D (SP-D) Serum Levels with ARDS Severity and Mortality in COVID-19 Patients in Indonesia. Acta Medica Acad. 2022, 51, 21–28. [Google Scholar] [CrossRef]
- Kerget, B.; Kerget, F.; Koçak, A.O.; Kızıltunç, A.; Araz, Ö.; Uçar, E.Y.; Akgün, M. Are Serum Interleukin 6 and Surfactant Protein D Levels Associated with the Clinical Course of COVID-19? Lung 2020, 198, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ju, Q.; Cao, J.; Tang, W.; Zhang, J. Impact of serum SP-A and SP-D levels on comparison and prognosis of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Medicine 2017, 96, e7083. [Google Scholar] [CrossRef] [PubMed]
- Polak, S.B.; Van Gool, I.C.; Cohen, D.; von der Thüsen, J.H.; van Paassen, J. A systematic review of pathological findings in COVID-19: A pathophysiological timeline and possible mechanisms of disease progression. Mod. Pathol. 2020, 33, 2128–2138. [Google Scholar] [CrossRef] [PubMed]
- Krynytska, I.; Marushchak, M.; Birchenko, I.; Dovgalyuk, A.; Tokarskyy, O. COVID-19-associated acute respiratory distress syndrome versus classical acute respiratory distress syndrome (a narrative review). Iran. J. Microbiol. 2021, 13, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Pabon, M.; Choi, A.M.K.; Siempos, I.I.; Fredenburgh, L.E.; Baron, R.M.; Jeon, K.; Chung, C.R.; Yang, J.H.; Park, C.M.; et al. Plasma surfactant protein-D as a diagnostic biomarker for acute respiratory distress syndrome: Validation in US and Korean cohorts. BMC Pulm. Med. 2017, 17, 204. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Liu, Z.H.; Wei, R.; Pan, S.D.; Mao, N.Y.; Chen, B.; Han, J.J.; Zhang, F.S.; Holmskov, U.; Xia, Z.L.; et al. Elevated plasma surfactant protein D (SP-D) levels and a direct correlation with anti-severe acute respiratory syndrome coronavirus-specific IgG antibody in SARS patients. Scand. J. Immunol. 2009, 69, 508–515. [Google Scholar] [CrossRef]
- Tong, M.; Xiong, Y.; Zhu, C.; Xu, H.; Zheng, Q.; Jiang, Y.; Zou, L.; Xiao, X.; Chen, F.; Yan, X.; et al. Serum surfactant protein D in COVID-19 is elevated and correlated with disease severity. BMC Infect. Dis. 2021, 21, 737. [Google Scholar] [CrossRef] [PubMed]
- Tiezzi, M.; Morra, S.; Seminerio, J.; Van Muylem, A.; Godefroid, A.; Law-Weng-Sam, N.; Van Praet, A.; Corbière, V.; Orte Cano, C.; Karimi, S.; et al. SP-D and CC-16 Pneumoproteins’ Kinetics and Their Predictive Role during SARS-CoV-2 Infection. Front. Med. 2022, 8, 761299. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Testa, F.; Sulejmani, A.; Pepe, F.; Giorgio Lovaglio, P.; Berta, P.; Dominici, R.; Leoni, V.; Prosperi, D.; Vittadini, G.; et al. Surfactant protein D (SP-D) as a biomarker of SARS-CoV-2 infection. Clin. Chim. Acta Int. J. Clin. Chem. 2022, 537, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Jayadi; Airlangga, P.S.; Kusuma, E.; Waloejo, C.S.; Salinding, A.; Lestari, P. Correlation between serum surfactant protein-D level with respiratory compliance and acute respiratory distress syndrome in critically ill COVID-19 Patients: A retrospective observational study. Int. J. Crit. Illn. Inj. Sci. 2022, 12, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, H.; Saito, A.; Kuronuma, K.; Moniwa, K.; Nishikiori, H.; Takahashi, S.; Chiba, H. The Soluble Lectin Families as Novel Biomarkers for COVID-19 Pneumonia. In Vivo 2023, 37, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Kuan, C.C. Vaccination to reduce severe COVID-19 and mortality in COVID-19 patients: A systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1770–1776. [Google Scholar] [PubMed]
- Nomori, H.; Horio, H.; Fuyuno, G.; Kobayashi, R.; Morinaga, S.; Suemasu, K. Serum surfactant protein A levels in healthy individuals are increased in smokers. Lung 1998, 176, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, Y.; Tsutahara, S.; Shijubo, N.; Takahashi, H.; Shiratori, M.; Hattori, A.; Honda, Y.; Abe, S.; Akino, T. Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis. Am. Rev. Respir. Dis. 1993, 147, 723–729. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Case Definition for Coronavirus Disease 2019 (COVID-19). 2020. Available online: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition (accessed on 1 February 2021).
- World Health Organization. Clinical Management of COVID-19; WHO: Geneva, Switzerland, 2020; pp. 1–62. [Google Scholar]
| Parameters | SARS-CoV-2 Infected Patients (n = 51) (A) | Healthy Donors (n = 11) (B) | A vs. B p-Values |
|---|---|---|---|
| Age (years) a | 68 (56.5–78) | 39 (30–52) | <0.0001 |
| Male b | 24 (47%) | 6 (54.5%) | 0.7465 |
| SARS-CoV-2 vaccination (at least 2 doses) b | 14 (27%) | 11 (100%) | <0.0001 |
| Respiratory support b | VMK 12 (23%) A.A. 9 (18%) CPAP 30 (59%) | NA | - |
| D-dimer (μg/L) a | 1092 (631.5–1621) | NA | - |
| PCR (mg/dL) a | 4.17 (1.39–6.5) | NA | - |
| Ferritin (ng/mL) a | 823 (357–1244) | NA | - |
| LDH (Ui/L) a | 242 (209.5–252) | NA | - |
| Fibrinogen (mg/dL) a | 407 (325–440) | NA | - |
| Lymphocytes (103/mL) a | 1285 (815–1795) | NA | - |
| Parameters | Mild SARS-CoV-2 Infected Patients (n = 21) (A) | Severe SARS-CoV-2 Infected Patients (n = 30) (B) | A vs. B p-Values |
|---|---|---|---|
| Age (years) a | 75 (62–83) | 65 (56–74) | 0.0739 |
| Male b | 8 (38%) | 66 (53%) | 0.2955 |
| SARS-CoV-2 vaccination (at least 2 doses) b | 12 (57%) | 2 (7%) | 0.0001 |
| Respiratory support b | VMK 12 (57%) A.A. 9 (43%) | CPAP 30 (100%) | 0.0001 |
| ARDS development b | 0 (0%) | 0 (0%) | - |
| D-dimer (μg/L) a | 1209 (669–1621) | 1007 (535–1920) | 0.6906 |
| PCR (mg/dL) a | 4.63 (0.83–6.5) | 4.17 (1.4–7.35) | 0.6814 |
| Ferritin (ng/mL) a | 821 (239–1199) | 823 (511–1294) | 0.5183 |
| LDH (Ui/L) a | 284 (199–390) | 265 (229–284) | 0.9007 |
| Fibrinogen (mg/dL) a | 514 (384–555) | 444 (362–555) | 0.2244 |
| Lymphocytes (103/mL) a | 910 (630–1345) | 1055 (763–1465) | 0.1622 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddaloni, L.; Zullino, V.; Bugani, G.; Lazzaro, A.; Brisciani, M.; Mastroianni, C.M.; Santinelli, L.; Ruberto, F. Could SP-A and SP-D Serum Levels Predict COVID-19 Severity? Int. J. Mol. Sci. 2024, 25, 5620. https://doi.org/10.3390/ijms25115620
Maddaloni L, Zullino V, Bugani G, Lazzaro A, Brisciani M, Mastroianni CM, Santinelli L, Ruberto F. Could SP-A and SP-D Serum Levels Predict COVID-19 Severity? International Journal of Molecular Sciences. 2024; 25(11):5620. https://doi.org/10.3390/ijms25115620
Chicago/Turabian StyleMaddaloni, Luca, Veronica Zullino, Ginevra Bugani, Alessandro Lazzaro, Matteo Brisciani, Claudio Maria Mastroianni, Letizia Santinelli, and Franco Ruberto. 2024. "Could SP-A and SP-D Serum Levels Predict COVID-19 Severity?" International Journal of Molecular Sciences 25, no. 11: 5620. https://doi.org/10.3390/ijms25115620
APA StyleMaddaloni, L., Zullino, V., Bugani, G., Lazzaro, A., Brisciani, M., Mastroianni, C. M., Santinelli, L., & Ruberto, F. (2024). Could SP-A and SP-D Serum Levels Predict COVID-19 Severity? International Journal of Molecular Sciences, 25(11), 5620. https://doi.org/10.3390/ijms25115620







