A Novel Minimally Invasive Surgically Induced Skeletal Muscle Injury Model in Sheep
Abstract
1. Introduction
2. Results
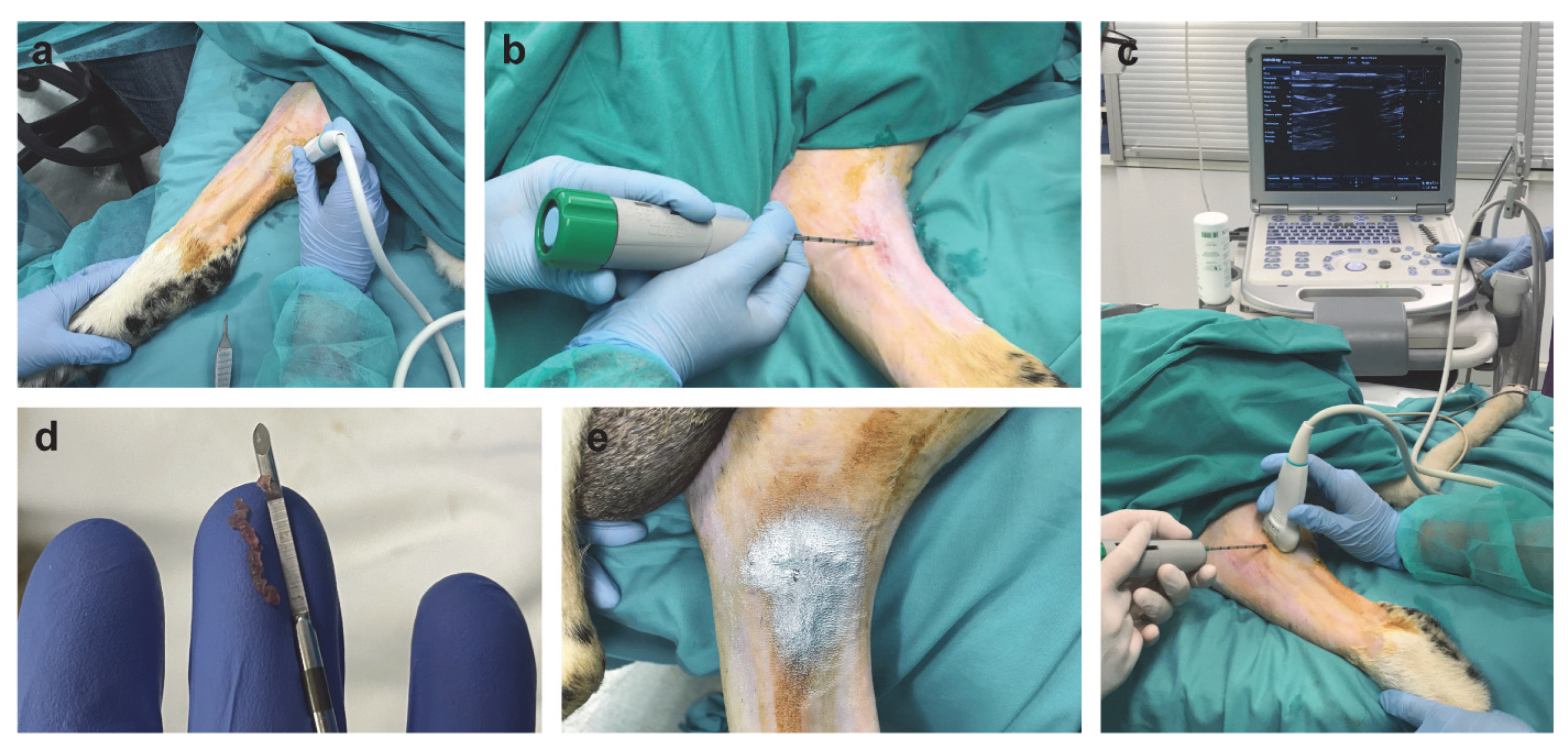
2.1. Surgically Induced Skeletal Muscle Injury in a Sheep Model
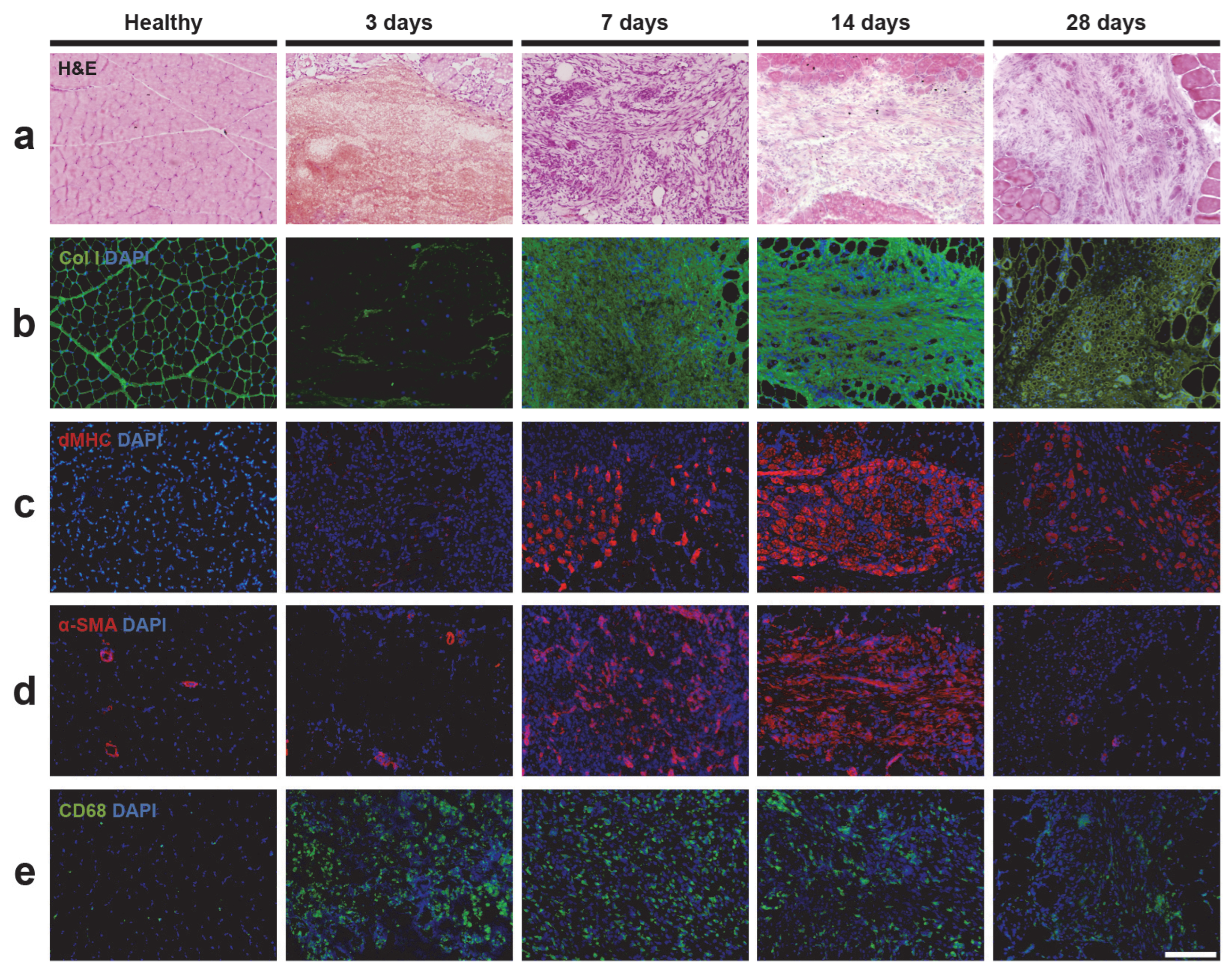
2.2. Histology Analysis of Skeletal Muscle Injury
2.3. Immunofluorescence Analysis of Skeletal Muscle Injury
2.3.1. Extracellular Matrix and Fibrosis
2.3.2. Muscle Regeneration
2.3.3. Vascularization
2.3.4. Inflammation
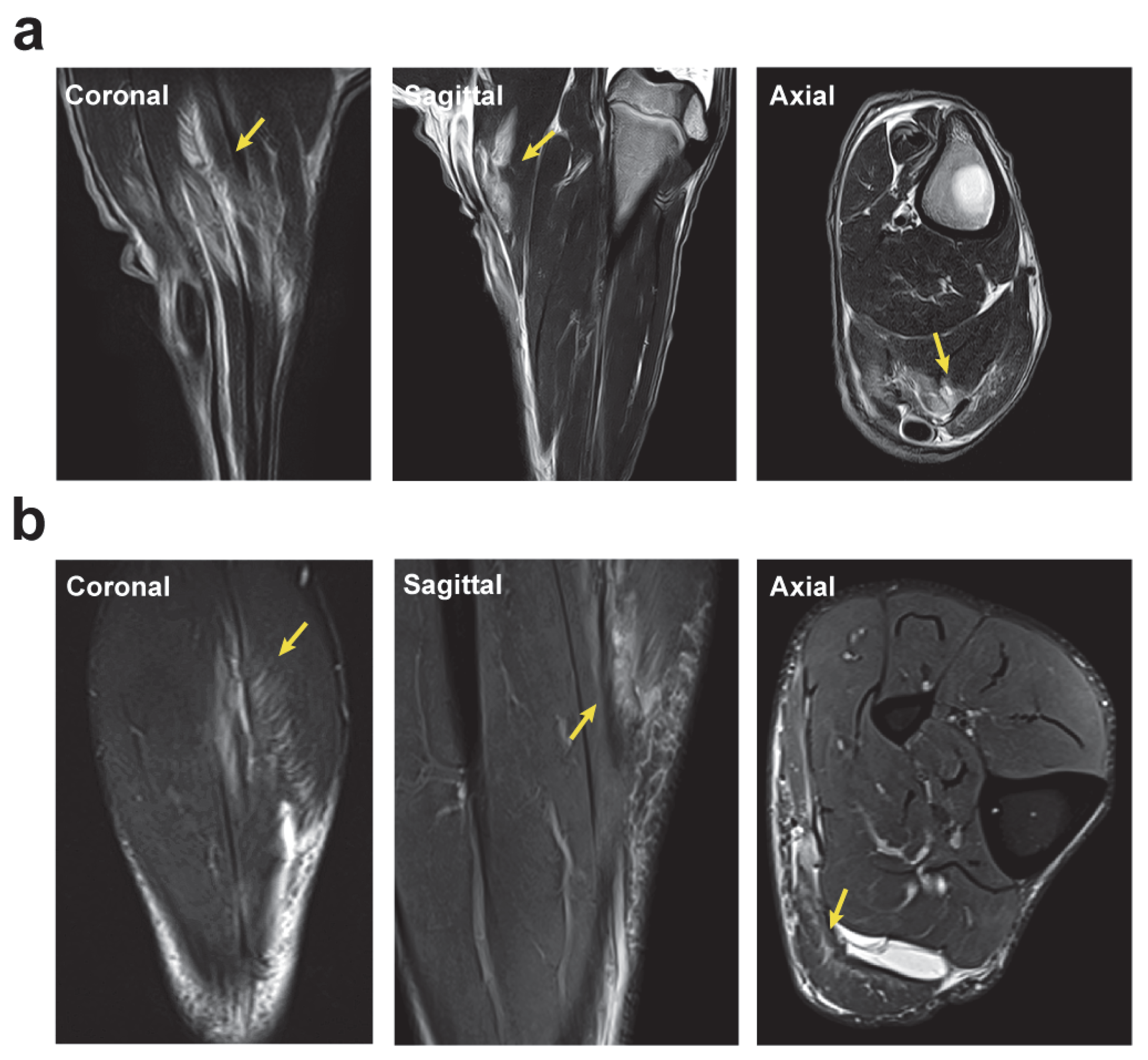
2.4. Magnetic Resonance Imaging Analysis of the Longitudinal Evolution of Skeletal Muscle Injury
2.5. Surgically Induced Skeletal Muscle Injury in a Sheep Model Mimics Muscle Lesions Observed in Human Athletes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Surgically Induced Skeletal Muscle Injury in a Sheep Model
4.3. Magnetic Resonance Imaging Analysis of the Longitudinal Evolution of Skeletal Muscle Injury
4.4. Histology and Immunofluorescence Analysis of Skeletal Muscle Injury
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- SantAnna, J.P.C.; Pedrinelli, A.; Hernandez, A.J.; Fernandes, T.L. Muscle Injury: Pathophysiology, Diagnosis, and Treatment. Rev. Bras. Ortop. 2022, 57, 1–13. [Google Scholar] [CrossRef]
- Laumonier, T.; Menetrey, J. Muscle Injuries and Strategies for Improving Their Repair. J. Exp. Orthop. 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Qazi, T.H.; Duda, G.N.; Ort, M.J.; Perka, C.; Geissler, S.; Winkler, T. Cell Therapy to Improve Regeneration of Skeletal Muscle Injuries. J. Cachexia Sarcopenia Muscle 2019, 10, 501–516. [Google Scholar] [CrossRef]
- Valle, X.; Alentorn-Geli, E.; Tol, J.L.; Hamilton, B.; Garrett, W.E.J.; Pruna, R.; Til, L.; Gutierrez, J.A.; Alomar, X.; Balius, R.; et al. Muscle Injuries in Sports: A New Evidence-Informed and Expert Consensus-Based Classification with Clinical Application. Sports Med. 2017, 47, 1241–1253. [Google Scholar] [CrossRef]
- Prieto-González, P.; Martínez-Castillo, J.L.; Fernández-Galván, L.M.; Casado, A.; Soporki, S.; Sánchez-Infante, J. Epidemiology of Sports-Related Injuries and Associated Risk Factors in Adolescent Athletes: An Injury Surveillance. Int. J. Environ. Res. Public. Health 2021, 18, 4857. [Google Scholar] [CrossRef]
- Green, B.; Pizzari, T. Calf Muscle Strain Injuries in Sport: A Systematic Review of Risk Factors for Injury. Br. J. Sports Med. 2017, 51, 1189–1194. [Google Scholar] [CrossRef]
- Kääriäinen, M.; Järvinen, T.; Järvinen, M.; Rantanen, J.; Kalimo, H. Relation between Myofibers and Connective Tissue during Muscle Injury Repair. Scand. J. Med. Sci. Sports 2000, 10, 332–337. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Aärimaa, V.; Vaittinen, S.; Kalimo, H.; Järvinen, M. Muscle Injuries: Optimising Recovery. Best. Pract. Res. Clin. Rheumatol. 2007, 21, 317–331. [Google Scholar] [CrossRef]
- Baker, B.A. An Old Problem: Aging and Skeletal-Muscle-Strain Injury. J. Sport Rehabil. 2017, 26, 180–188. [Google Scholar] [CrossRef]
- Huard, J.; Li, Y.; Fu, F.H. Muscle Injuries and Repair: Current Trends in Research. JBJS 2002, 84, 822. [Google Scholar] [CrossRef]
- Hamilton, B.; Valle, X.; Rodas, G.; Til, L.; Grive, R.P.; Rincon, J.A.G.; Tol, J.L. Classification and Grading of Muscle Injuries: A Narrative Review. Br. J. Sports Med. 2015, 49, 306. [Google Scholar] [CrossRef]
- Chan, O.; Del Buono, A.; Best, T.M.; Maffulli, N. Acute Muscle Strain Injuries: A Proposed New Classification System. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 2356–2362. [Google Scholar] [CrossRef]
- Mueller-Wohlfahrt, H.-W.; Haensel, L.; Mithoefer, K.; Ekstrand, J.; English, B.; McNally, S.; Orchard, J.; van Dijk, C.N.; Kerkhoffs, G.M.; Schamasch, P.; et al. Terminology and Classification of Muscle Injuries in Sport: The Munich Consensus Statement. Br. J. Sports Med. 2013, 47, 342–350. [Google Scholar] [CrossRef]
- Pollock, N.; James, S.L.J.; Lee, J.C.; Chakraverty, R. British Athletics Muscle Injury Classification: A New Grading System. Br. J. Sports Med. 2014, 48, 1347–1351. [Google Scholar] [CrossRef]
- Valle, X.; Mechó, S.; Pruna, R.; Pedret, C.; Isern-Kebschull, J.; Monllau, J.C.; Rodas, G. The MLG-R Muscle Injury Classification for Hamstrings. Examples and Guidelines for Its Use. Apunts Med. Esport 2019, 54, 73–79. [Google Scholar] [CrossRef]
- Pedret, C.; Balius, R.; Blasi, M.; Dávila, F.; Aramendi, J.F.; Masci, L.; de la Fuente, J. Ultrasound Classification of Medial Gastrocnemious Injuries. Scand. J. Med. Sci. Sports 2020, 30, 2456–2465. [Google Scholar] [CrossRef]
- Järvinen, T.A.H.; Järvinen, T.L.N.; Kääriäinen, M.; Kalimo, H.; Järvinen, M. Muscle Injuries: Biology and Treatment. Am. J. Sports Med. 2005, 33, 745–764. [Google Scholar] [CrossRef]
- Sicherer, S.T.; Venkatarama, R.S.; Grasman, J.M. Recent Trends in Injury Models to Study Skeletal Muscle Regeneration and Repair. Bioengineering 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, Y.; Ikemoto-Uezumi, M.; Hitachi, K.; Fukada, S.-I.; Uezumi, A. Methods for Accurate Assessment of Myofiber Maturity During Skeletal Muscle Regeneration. Front. Cell Dev. Biol. 2020, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-W.; Choi, J.-H.; Jo, T.; Shin, H.; Suh, J.M. Systemic and Local Phenotypes of Barium Chloride Induced Skeletal Muscle Injury in Mice. Ann. Geriatr. Med. Res. 2019, 23, 83–89. [Google Scholar] [CrossRef]
- Pratt, S.J.P.; Lawlor, M.W.; Shah, S.B.; Lovering, R.M. An in Vivo Rodent Model of Contraction-Induced Injury in the Quadriceps Muscle. Injury 2012, 43, 788–793. [Google Scholar] [CrossRef]
- Le, G.; Lowe, D.A.; Kyba, M. Freeze Injury of the Tibialis Anterior Muscle. Methods Mol. Biol. 2016, 1460, 33–41. [Google Scholar] [CrossRef]
- Almeida, C.F.; Vainzof, M. Skeletal Muscle Injury by Electroporation: A Model to Study Degeneration/Regeneration Pathways in Muscle. Methods Mol. Biol. 2020, 2063, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liao, Z.; Wang, J.; Xiang, H.; Zhu, X.; Che, X.; Tang, Y.; Xie, J.; Mao, C.; Zhao, H.; et al. Research on Skeletal Muscle Impact Injury Using a New Rat Model from a Bioimpact Machine. Front. Bioeng. Biotechnol. 2022, 10, 1055668. [Google Scholar] [CrossRef] [PubMed]
- Langendorf, E.K.; Klein, A.; Drees, P.; Rommens, P.M.; Mattyasovszky, S.G.; Ritz, U. Exposure to Radial Extracorporeal Shockwaves Induces Muscle Regeneration after Muscle Injury in a Surgical Rat Model. J. Orthop. Res. 2020, 38, 1386–1397. [Google Scholar] [CrossRef]
- Banstola, A.; Reynolds, J.N.J. The Sheep as a Large Animal Model for the Investigation and Treatment of Human Disorders. Biology 2022, 11, 1251. [Google Scholar] [CrossRef]
- Novakova, S.S.; Rodriguez, B.L.; Vega-Soto, E.E.; Nutter, G.P.; Armstrong, R.E.; Macpherson, P.C.D.; Larkin, L.M. Repairing Volumetric Muscle Loss in the Ovine Peroneus Tertius Following a 3-Month Recovery. Tissue Eng. Part. A 2020, 26, 837–851. [Google Scholar] [CrossRef]
- Aguilar-García, D.; Fernández-Sarmiento, J.A.; Del Mar Granados Machuca, M.; Rodríguez, J.M.; Rascón, P.M.; Calvo, R.N.; Ruiz, Y.M.; Poveda, J.M.C.; Castañeda, J.M.; Bertomeu, R.C.; et al. Histological and Biochemical Evaluation of Plasma Rich in Growth Factors Treatment for Grade II Muscle Injuries in Sheep. BMC Vet. Res. 2022, 18, 400. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Muñoz, P.; Fernández-Martín, A.; Torrella, R.; Serres, X.; la Varga, M.D.; Viscor, G.; Järvinen, T.; Martínez-Ibáñez, V.; Peiró, J.; Rodas, G.; et al. A New Surgical Model of Skeletal Muscle Injuries in Rats Reproduces Human Sports Lesions. Int. J. Sports Med. 2015, 37, 183–190. [Google Scholar] [CrossRef]
- Schiaffino, S.; Rossi, A.C.; Smerdu, V.; Leinwand, L.A.; Reggiani, C. Developmental Myosins: Expression Patterns and Functional Significance. Skelet. Muscle 2015, 5, 22. [Google Scholar] [CrossRef]
- Klinge, U.; Dievernich, A.; Tolba, R.; Klosterhalfen, B.; Davies, L. CD68+ Macrophages as Crucial Components of the Foreign Body Reaction Demonstrate an Unconventional Pattern of Functional Markers Quantified by Analysis with Double Fluorescence Staining. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2020, 108, 3134–3146. [Google Scholar] [CrossRef]
- Hayashi, D.; Hamilton, B.; Guermazi, A.; de Villiers, R.; Crema, M.D.; Roemer, F.W. Traumatic Injuries of Thigh and Calf Muscles in Athletes: Role and Clinical Relevance of MR Imaging and Ultrasound. Insights Imaging 2012, 3, 591–601. [Google Scholar] [CrossRef]
- Armfield, D.R.; Kim, D.H.-M.; Towers, J.D.; Bradley, J.P.; Robertson, D.D. Sports-Related Muscle Injury in the Lower Extremity. Clin. Sports Med. 2006, 25, 803–842. [Google Scholar] [CrossRef]
- Chu, S.K.; Rho, M.E. Hamstring Injuries in the Athlete: Diagnosis, Treatment, and Return to Play. Curr. Sports Med. Rep. 2016, 15, 184–190. [Google Scholar] [CrossRef]
- Green, B.; McClelland, J.A.; Semciw, A.I.; Schache, A.G.; McCall, A.; Pizzari, T. The Assessment, Management and Prevention of Calf Muscle Strain Injuries: A Qualitative Study of the Practices and Perspectives of 20 Expert Sports Clinicians. Sports Med. Open 2022, 8, 10. [Google Scholar] [CrossRef]
- Bersini, S.; Gilardi, M.; Mora, M.; Krol, S.; Arrigoni, C.; Candrian, C.; Zanotti, S.; Moretti, M. Tackling Muscle Fibrosis: From Molecular Mechanisms to next Generation Engineered Models to Predict Drug Delivery. Adv. Drug Deliv. Rev. 2018, 129, 64–77. [Google Scholar] [CrossRef]
- Gardner, T.; Kenter, K.; Li, Y. Fibrosis Following Acute Skeletal Muscle Injury: Mitigation and Reversal Potential in the Clinic. J. Sports Med. 2020, 2020, 7059057. [Google Scholar] [CrossRef]
- Li, Y.; Foster, W.; Deasy, B.M.; Chan, Y.; Prisk, V.; Tang, Y.; Cummins, J.; Huard, J. Transforming Growth Factor-Beta1 Induces the Differentiation of Myogenic Cells into Fibrotic Cells in Injured Skeletal Muscle: A Key Event in Muscle Fibrogenesis. Am. J. Pathol. 2004, 164, 1007–1019. [Google Scholar] [CrossRef]
- Mahdy, M.A.A. Skeletal Muscle Fibrosis: An Overview. Cell Tissue Res. 2019, 375, 575–588. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant Repair and Fibrosis Development in Skeletal Muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.-F.; Chen, C.P.-C.; Tsui, P.-H.; Chen, C.-N.; Hsu, C.-C. Stretch-Induced Healing of Injured Muscles Is Associated With Myogenesis and Decreased Fibrosis. Am. J. Sports Med. 2022, 50, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Agata, N.; Itoh, Y.; Inoue-Miyazu, M.; Mizumura, K.; Sokabe, M.; Taguchi, T.; Kawakami, K. Post-Injury Stretch Promotes Recovery in a Rat Model of Muscle Damage Induced by Lengthening Contractions. J. Physiol. Sci. 2018, 68, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Ra, Y.-J.; Lee, K.M.; Lee, J.Y.; Ghil, S.H. Therapeutic Effect of Passive Mobilization Exercise on Improvement of Muscle Regeneration and Prevention of Fibrosis after Laceration Injury of Rat. Arch. Phys. Med. Rehabil. 2006, 87, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Uehara, K.; Ota, S.; Tobita, K.; Ambrosio, F.; Cummins, J.H.; Terada, S.; Fu, F.H.; Huard, J. The Timing of Administration of a Clinically Relevant Dose of Losartan Influences the Healing Process after Contusion Induced Muscle Injury. J. Appl. Physiol. 2013, 114, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Bedair, H.S.; Karthikeyan, T.; Quintero, A.; Li, Y.; Huard, J. Angiotensin II Receptor Blockade Administered after Injury Improves Muscle Regeneration and Decreases Fibrosis in Normal Skeletal Muscle. Am. J. Sports Med. 2008, 36, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Hilliard, B.A.; Amin, M.; Harris, M.Y.; Hobson, L.J.; Cruz, G.E.; Popoff, S.N. Blocking CTGF/CCN2 Reduces Established Skeletal Muscle Fibrosis in a Rat Model of Overuse Injury. FASEB J. 2020, 34, 6554–6569. [Google Scholar] [CrossRef] [PubMed]
- Bourzac, C.; Bensidhoum, M.; Pallu, S.; Portier, H. Use of Adult Mesenchymal Stromal Cells in Tissue Repair: Impact of Physical Exercise. Am. J. Physiol. Cell Physiol. 2019, 317, C642–C654. [Google Scholar] [CrossRef] [PubMed]
- Valero, M.C.; Huntsman, H.D.; Liu, J.; Zou, K.; Boppart, M.D. Eccentric Exercise Facilitates Mesenchymal Stem Cell Appearance in Skeletal Muscle. PLoS ONE 2012, 7, e29760. [Google Scholar] [CrossRef]
- Contreras-Muñoz, P.; Torrella, J.R.; Venegas, V.; Serres, X.; Vidal, L.; Vila, I.; Lahtinen, I.; Viscor, G.; Martínez-Ibáñez, V.; Peiró, J.L.; et al. Muscle Precursor Cells Enhance Functional Muscle Recovery and Show Synergistic Effects With Postinjury Treadmill Exercise in a Muscle Injury Model in Rats. Am. J. Sports Med. 2021, 49, 1073–1085. [Google Scholar] [CrossRef]
- Minari, A.L.A.; Oyama, L.M.; dos Santos, R.V.T. Downhill Exercise-Induced Changes in Gene Expression Related with Macrophage Polarization and Myogenic Cells in the Triceps Long Head of Rats. Inflammation 2015, 38, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Marotta, M.; Sarria, Y.; Ruiz-Roig, C.; Munell, F.; Roig-Quilis, M. Laser Microdissection-Based Expression Analysis of Key Genes Involved in Muscle Regeneration in Mdx Mice. Neuromuscul. Disord. 2007, 17, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Guiraud, S.; Edwards, B.; Squire, S.E.; Moir, L.; Berg, A.; Babbs, A.; Ramadan, N.; Wood, M.J.; Davies, K.E. Embryonic Myosin Is a Regeneration Marker to Monitor Utrophin-Based Therapies for DMD. Hum. Mol. Genet. 2019, 28, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Ichii, O.; Otsuka-Kanazawa, S.; Nakamura, T.; Elewa, Y.H.A.; Kon, Y. Degenerative and Regenerative Features of Myofibers Differ among Skeletal Muscles in a Murine Model of Muscular Dystrophy. J. Muscle Res. Cell Motil. 2016, 37, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Huard, J. Stem Cells, Blood Vessels, and Angiogenesis as Major Determinants for Musculoskeletal Tissue Repair. J. Orthop. Res. 2019, 37, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Hu, P. Skeletal Muscle Regeneration Is Modulated by Inflammation. J. Orthop. Translat 2018, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chazaud, B.; Brigitte, M.; Yacoub-Youssef, H.; Arnold, L.; Gherardi, R.; Sonnet, C.; Lafuste, P.; Chretien, F. Dual and Beneficial Roles of Macrophages during Skeletal Muscle Regeneration. Exerc. Sport. Sci. Rev. 2009, 37, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sathe, A.A.; Smith, G.R.; Ruf-Zamojski, F.; Nair, V.; Lavine, K.J.; Xing, C.; Sealfon, S.C.; Zhou, L. Heterogeneous Origins and Functions of Mouse Skeletal Muscle-Resident Macrophages. Proc. Natl. Acad. Sci. USA 2020, 117, 20729–20740. [Google Scholar] [CrossRef] [PubMed]
- Rybalko, V.; Hsieh, P.-L.; Merscham-Banda, M.; Suggs, L.J.; Farrar, R.P. The Development of Macrophage-Mediated Cell Therapy to Improve Skeletal Muscle Function after Injury. PLoS ONE 2015, 10, e0145550. [Google Scholar] [CrossRef]
- Martins, L.; Gallo, C.C.; Honda, T.S.B.; Alves, P.T.; Stilhano, R.S.; Rosa, D.S.; Koh, T.J.; Han, S.W. Skeletal Muscle Healing by M1-like Macrophages Produced by Transient Expression of Exogenous GM-CSF. Stem Cell Res. Ther. 2020, 11, 473. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L. The Many Roles of Macrophages in Skeletal Muscle Injury and Repair. Front. Cell Dev. Biol. 2022, 10, 952249. [Google Scholar] [CrossRef] [PubMed]
- Bordalo, M.; Arnaiz, J.; Yamashiro, E.; Al-Naimi, M.R. Imaging of Muscle Injuries: MR Imaging-Ultrasound Correlation. Magn. Reson. Imaging Clin. N. Am. 2023, 31, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Roemer, F.W.; Robinson, P.; Tol, J.L.; Regatte, R.R.; Crema, M.D. Imaging of Muscle Injuries in Sports Medicine: Sports Imaging Series. Radiology 2017, 282, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Valentin, S.; Yeates, T.D.; Licka, T.; Elliott, J. In Vivo MRI Features of Spinal Muscles in the Ovine Model. J. Orthop. Translat 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Isern-Kebschull, J.; Mechó, S.; Pruna, R.; Kassarjian, A.; Valle, X.; Yanguas, X.; Alomar, X.; Martinez, J.; Pomés, J.; Rodas, G. Sports-Related Lower Limb Muscle Injuries: Pattern Recognition Approach and MRI Review. Insights Imaging 2020, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, Z.; Li, J.; Guo, Z.; Zhang, F.; Wang, K.; Bai, X.; Wang, Q.; Guan, Y.; Wang, Y.; et al. Platelet-Rich Plasma Promotes Skeletal Muscle Regeneration and Neuromuscular Functional Reconstitution in a Concentration-Dependent Manner in a Rat Laceration Model. Biochem. Biophys. Res. Commun. 2023, 672, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Menetrey, J.; Kasemkijwattana, C.; Day, C.S.; Bosch, P.; Vogt, M.; Fu, F.H.; Moreland, M.S.; Huard, J. Growth Factors Improve Muscle Healing in Vivo. J. Bone Jt. Surg. Br. 2000, 82, 131–137. [Google Scholar] [CrossRef]
- Muraine, L.; Bensalah, M.; Butler-Browne, G.; Bigot, A.; Trollet, C.; Mouly, V.; Negroni, E. Update on Anti-Fibrotic Pharmacotherapies in Skeletal Muscle Disease. Curr. Opin. Pharmacol. 2023, 68, 102332. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidal, L.; Vila, I.; Venegas, V.; Sacristán, A.; Contreras-Muñoz, P.; Lopez-Garzon, M.; Giné, C.; Rodas, G.; Marotta, M. A Novel Minimally Invasive Surgically Induced Skeletal Muscle Injury Model in Sheep. Int. J. Mol. Sci. 2024, 25, 5612. https://doi.org/10.3390/ijms25115612
Vidal L, Vila I, Venegas V, Sacristán A, Contreras-Muñoz P, Lopez-Garzon M, Giné C, Rodas G, Marotta M. A Novel Minimally Invasive Surgically Induced Skeletal Muscle Injury Model in Sheep. International Journal of Molecular Sciences. 2024; 25(11):5612. https://doi.org/10.3390/ijms25115612
Chicago/Turabian StyleVidal, Laura, Ingrid Vila, Vanesa Venegas, Anabel Sacristán, Paola Contreras-Muñoz, Maria Lopez-Garzon, Carles Giné, Gil Rodas, and Mario Marotta. 2024. "A Novel Minimally Invasive Surgically Induced Skeletal Muscle Injury Model in Sheep" International Journal of Molecular Sciences 25, no. 11: 5612. https://doi.org/10.3390/ijms25115612
APA StyleVidal, L., Vila, I., Venegas, V., Sacristán, A., Contreras-Muñoz, P., Lopez-Garzon, M., Giné, C., Rodas, G., & Marotta, M. (2024). A Novel Minimally Invasive Surgically Induced Skeletal Muscle Injury Model in Sheep. International Journal of Molecular Sciences, 25(11), 5612. https://doi.org/10.3390/ijms25115612






