Clinical Significance of the Plasma Biomarker Panels in Amyloid-Negative and Tau PET-Positive Amnestic Patients: Comparisons with Alzheimer’s Disease and Unimpaired Cognitive Controls
Abstract
1. Introduction
2. Results
2.1. Cohort Demographics
2.2. Age Effects on Plasma Biomarkers
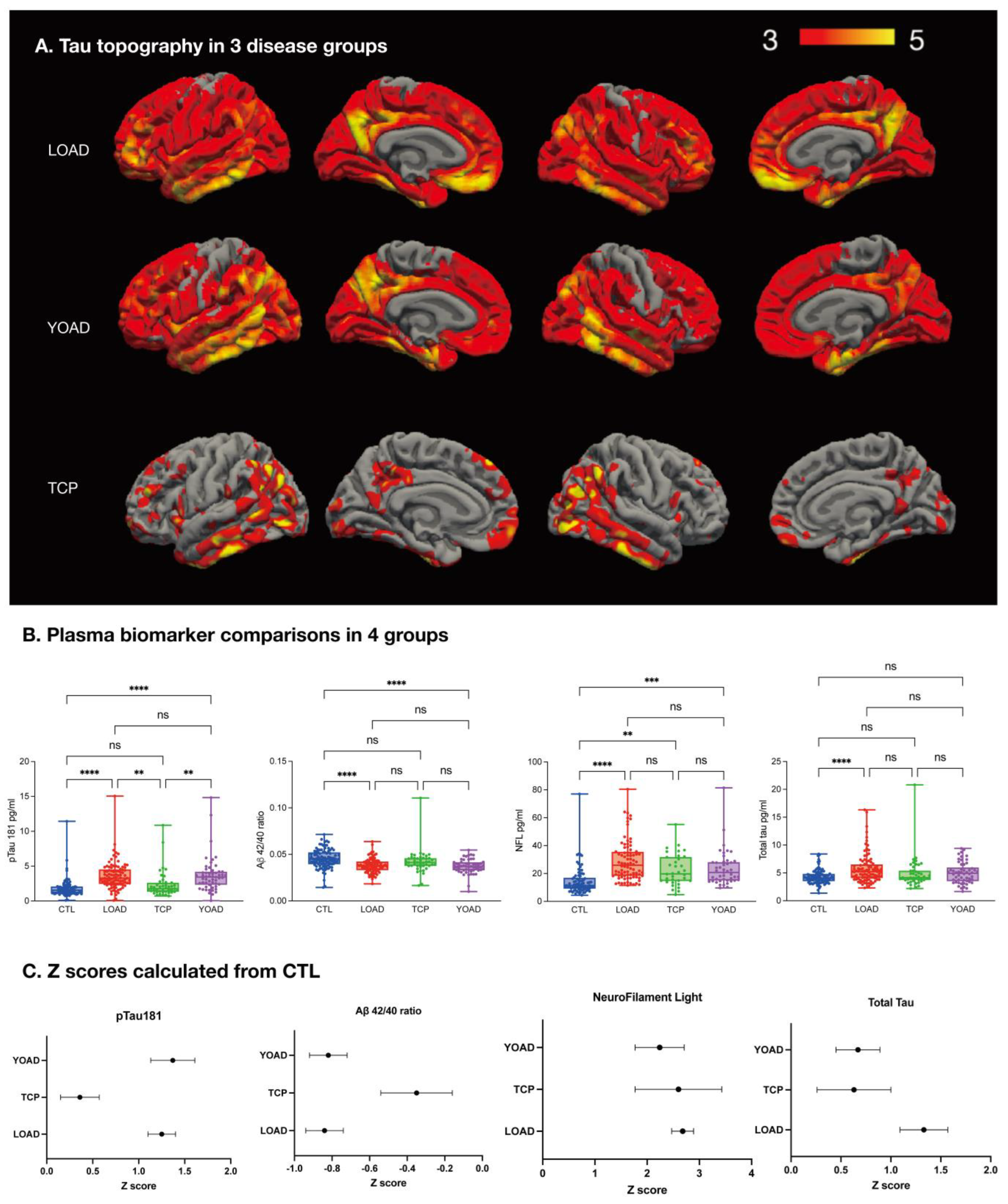
2.3. Tau-PET Distribution
2.4. YOAD Was Associated with Faster Neurodegeneration Than LOAD
2.5. Diagnostic Value of Plasma pTau181 in TCP and AD
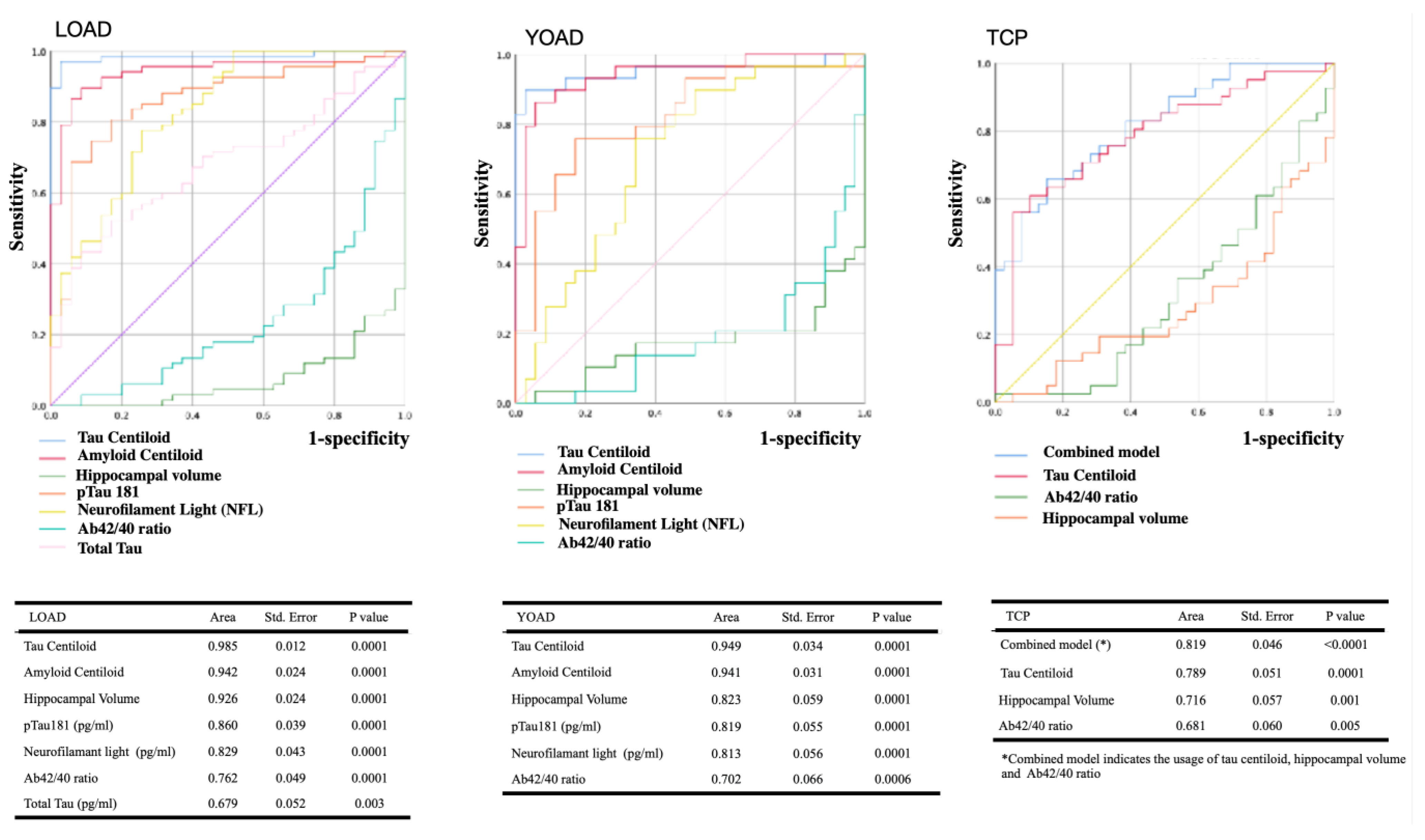
2.6. ROC Curves of Image- or Plasma-Biomarker
2.7. Stepwise Logistic Regression Model Using Hippocampal Volume and Four Plasma Biomarkers for Diagnosis or Differential Diagnosis
2.8. Associations of Plasma Biomarkers with Amyloid and Tau PET Centiloid
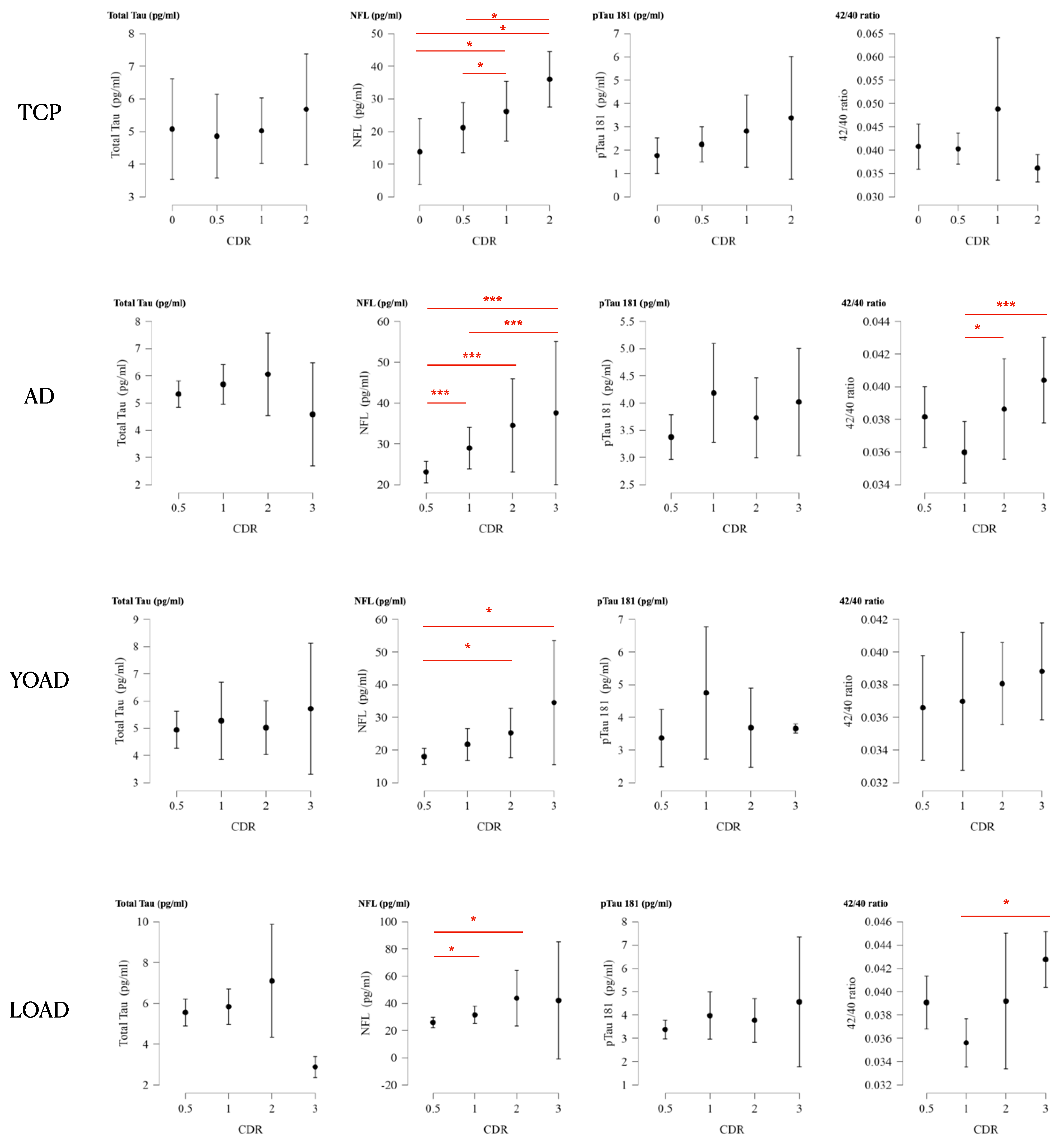
2.9. Plasma Biomarkers for Clinical Staging
2.10. Cognitive Prediction Model
3. Discussion
4. Materials and Methods
4.1. Patient Enrollment
4.2. Diagnosis of AD
4.3. Diagnosis of TCP
4.4. Cognitively Unimpaired CTL
4.5. Demographic and Cognitive Evaluations
4.6. Blood Sample Collection
4.7. Single-Molecule Array Analysis of Plasma Aβ42/40, pTau181, NFL, and T-Tau
4.8. Image Acquisition and Processing
4.9. Amyloid and Tau PET Acquisition
4.10. Surface-Based Topography in Tau-PET
4.11. Assessing the Neurodegenerative Process
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Lelental, N.; Spitzer, P.; Maler, J.M.; Kornhuber, J. Amyloid-beta 42/40 cerebrospinal fluid concentration ratio in the diagnostics of Alzheimer’s disease: Validation of two novel assays. J. Alzheimer’s Dis. 2015, 43, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Benedet, A.L.; Pascoal, T.A.; Karikari, T.K.; Lantero-Rodriguez, J.; Brum, W.S.; Mathotaarachchi, S.; Therriault, J.; Savard, M.; Chamoun, M.; et al. Cerebrospinal fluid p-tau231 as an early indicator of emerging pathology in Alzheimer’s disease. EBioMedicine 2022, 76, 103836. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Ohrfelt, A.; Portelius, E.; Bjerke, M.; Holtta, M.; Rosen, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Bridel, C.; van Wieringen, W.N.; Zetterberg, H.; Tijms, B.M.; Teunissen, C.E.; The NFL Group; Alvarez-Cermeno, J.C.; Andreasson, U.; Axelsson, M.; Backstrom, D.C.; et al. Diagnostic Value of Cerebrospinal Fluid Neurofilament Light Protein in Neurology: A Systematic Review and Meta-analysis. JAMA Neurol. 2019, 76, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Janelidze, S.; Al Khleifat, A.; Leuzy, A.; van der Ende, E.L.; Karikari, T.K.; Benedet, A.L.; Pascoal, T.A.; Lleo, A.; Parnetti, L.; et al. A multicentre validation study of the diagnostic value of plasma neurofilament light. Nat. Commun. 2021, 12, 3400. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Hye, A.; Rajkumar, A.P.; Leuzy, A.; Snowden, S.; Suarez-Calvet, M.; Karikari, T.K.; Scholl, M.; La Joie, R.; Rabinovici, G.D.; et al. An update on blood-based biomarkers for non-Alzheimer neurodegenerative disorders. Nat. Rev. Neurol. 2020, 16, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Ashton, N.J.; Brinkmalm, G.; Brum, W.S.; Benedet, A.L.; Montoliu-Gaya, L.; Lantero-Rodriguez, J.; Pascoal, T.A.; Suarez-Calvet, M.; Rosa-Neto, P.; et al. Blood phospho-tau in Alzheimer disease: Analysis, interpretation, and clinical utility. Nat. Rev. Neurol. 2022, 18, 400–418. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Verberk, I.M.W.; Thijssen, E.H.; Vermunt, L.; Hansson, O.; Zetterberg, H.; van der Flier, W.M.; Mielke, M.M.; Del Campo, M. Blood-based biomarkers for Alzheimer’s disease: Towards clinical implementation. Lancet Neurol. 2022, 21, 66–77. [Google Scholar] [CrossRef]
- Ashton, N.J.; Leuzy, A.; Karikari, T.K.; Mattsson-Carlgren, N.; Dodich, A.; Boccardi, M.; Corre, J.; Drzezga, A.; Nordberg, A.; Ossenkoppele, R.; et al. The validation status of blood biomarkers of amyloid and phospho-tau assessed with the 5-phase development framework for AD biomarkers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2140–2156. [Google Scholar] [CrossRef]
- Bell, W.R.; An, Y.; Kageyama, Y.; English, C.; Rudow, G.L.; Pletnikova, O.; Thambisetty, M.; O’Brien, R.; Moghekar, A.R.; Albert, M.S.; et al. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.O.; Martindale, R.; Pendlebury, W.W.; DeWitt, J.C. Primary age-related tauopathy (PART) in the general autopsy setting: Not just a disease of the elderly. Brain Pathol. 2021, 31, 381–384. [Google Scholar] [CrossRef]
- Crary, J.F.; Trojanowski, J.Q.; Schneider, J.A.; Abisambra, J.F.; Abner, E.L.; Alafuzoff, I.; Arnold, S.E.; Attems, J.; Beach, T.G.; Bigio, E.H.; et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol. 2014, 128, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Braak, H.; Brion, J.P.; Buee, L.; Del Tredici, K.; Goedert, M.; Halliday, G.; Neumann, M.; Spillantini, M.G.; Tolnay, M.; et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015, 129, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Elobeid, A.; Libard, S.; Leino, M.; Popova, S.N.; Alafuzoff, I. Altered Proteins in the Aging Brain. J. Neuropathol. Exp. Neurol. 2016, 75, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Boyle, P.A.; Janelidze, S.; Petyuk, V.A.; Wang, T.; Bennett, D.A.; Hansson, O.; Schneider, J.A. Plasma p-tau181 and p-tau217 in discriminating PART, AD and other key neuropathologies in older adults. Acta Neuropathol. 2023, 146, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Chetelat, G.; Dickson, D.; Fagan, A.M.; Frisoni, G.B.; Jagust, W.; Mormino, E.C.; Petersen, R.C.; Sperling, R.A.; et al. Suspected non-Alzheimer disease pathophysiology--concept and controversy. Nat. Rev. Neurol. 2016, 12, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Small, S.A.; Duff, K. Linking Abeta and tau in late-onset Alzheimer’s disease: A dual pathway hypothesis. Neuron 2008, 60, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wuestefeld, A.; Pichet Binette, A.; Berron, D.; Spotorno, N.; van Westen, D.; Stomrud, E.; Mattsson-Carlgren, N.; Strandberg, O.; Smith, R.; Palmqvist, S.; et al. Age-related and amyloid-beta-independent tau deposition and its downstream effects. Brain 2023, 146, 3192–3205. [Google Scholar] [CrossRef]
- van der Kant, R.; Goldstein, L.S.B.; Ossenkoppele, R. Amyloid-beta-independent regulators of tau pathology in Alzheimer disease. Nat. Rev. Neurosci. 2020, 21, 21–35. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.T.; Li, M.; Lu, J.Y.; Sun, Y.M.; Liang, X.; Bao, W.; Chen, Q.S.; Li, X.Y.; Zhou, X.Y.; et al. Clinical Utility of (18) F-APN-1607 Tau PET Imaging in Patients with Progressive Supranuclear Palsy. Mov. Disord. 2021, 36, 2314–2323. [Google Scholar] [CrossRef]
- Tagai, K.; Ono, M.; Kubota, M.; Kitamura, S.; Takahata, K.; Seki, C.; Takado, Y.; Shinotoh, H.; Sano, Y.; Yamamoto, Y.; et al. High-Contrast In Vivo Imaging of Tau Pathologies in Alzheimer’s and Non-Alzheimer’s Disease Tauopathies. Neuron 2021, 109, 42–58.e48. [Google Scholar] [CrossRef] [PubMed]
- Quintas-Neves, M.; Teylan, M.A.; Besser, L.; Soares-Fernandes, J.; Mock, C.N.; Kukull, W.A.; Crary, J.F.; Oliveira, T.G. Magnetic resonance imaging brain atrophy assessment in primary age-related tauopathy (PART). Acta Neuropathol. Commun. 2019, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Jefferson-George, K.S.; Wolk, D.A.; Lee, E.B.; McMillan, C.T. Cognitive decline associated with pathological burden in primary age-related tauopathy. Alzheimer’s Dement. 2017, 13, 1048–1053. [Google Scholar] [CrossRef]
- Shi, Y.; Murzin, A.G.; Falcon, B.; Epstein, A.; Machin, J.; Tempest, P.; Newell, K.L.; Vidal, R.; Garringer, H.J.; Sahara, N.; et al. Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol. 2021, 141, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Gogola, A.; Minhas, D.S.; Villemagne, V.L.; Cohen, A.D.; Mountz, J.M.; Pascoal, T.A.; Laymon, C.M.; Mason, N.S.; Ikonomovic, M.D.; Mathis, C.A.; et al. Direct Comparison of the Tau PET Tracers (18)F-Flortaucipir and (18)F-MK-6240 in Human Subjects. J. Nucl. Med. 2022, 63, 108–116. [Google Scholar] [CrossRef]
- Therriault, J.; Grothe, M.J. Towards in vivo PET imaging of primary age-related tauopathy. Brain 2023, 146, 3106–3109. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.S.; Choi, S.M.; Kim, B.C.; Lee, M.C.; Lee, K.H.; Lee, J.H. Primary Age-Related Tauopathy: An Elderly Brain Pathology Frequently Encountered during Autopsy. J. Pathol. Transl. Med. 2019, 53, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Teylan, M.; Mock, C.; Gauthreaux, K.; Chen, Y.C.; Chan, K.C.G.; Hassenstab, J.; Besser, L.M.; Kukull, W.A.; Crary, J.F. Cognitive trajectory in mild cognitive impairment due to primary age-related tauopathy. Brain 2020, 143, 611–621. [Google Scholar] [CrossRef]
- Besser, L.M.; Mock, C.; Teylan, M.A.; Hassenstab, J.; Kukull, W.A.; Crary, J.F. Differences in Cognitive Impairment in Primary Age-Related Tauopathy Versus Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2019, 78, 219–228. [Google Scholar] [CrossRef]
- Teylan, M.; Besser, L.M.; Crary, J.F.; Mock, C.; Gauthreaux, K.; Thomas, N.M.; Chen, Y.C.; Kukull, W.A. Clinical diagnoses among individuals with primary age-related tauopathy versus Alzheimer’s neuropathology. Lab. Investig. 2019, 99, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Josephs, K.A.; Murray, M.E.; Tosakulwong, N.; Weigand, S.D.; Knopman, D.S.; Petersen, R.C.; Jack, C.R., Jr.; Whitwell, J.L.; Dickson, D.W. Brain atrophy in primary age-related tauopathy is linked to transactive response DNA-binding protein of 43 kDa. Alzheimer’s Dement. 2019, 15, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Guo, T.; Provost, K.; Korman, D.; Ward, T.J.; Landau, S.M.; Jagust, W.J.; Alzheimer’s Disease Neuroimaging, I. Abnormal tau in amyloid PET negative individuals. Neurobiol. Aging 2022, 109, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-beta and tau in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Delaby, C.; Estelles, T.; Zhu, N.; Arranz, J.; Barroeta, I.; Carmona-Iragui, M.; Illan-Gala, I.; Santos-Santos, M.A.; Altuna, M.; Sala, I.; et al. The Abeta1-42/Abeta1-40 ratio in CSF is more strongly associated to tau markers and clinical progression than Abeta1-42 alone. Alzheimer’s Res. Ther. 2022, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Xie, L.; Wisse, L.E.M.; Vergnet, N.; Ittyerah, R.; Cui, S.; Yushkevich, P.A.; Wolk, D.A.; Alzheimer’s Disease Neuroimaging, I. In vivo measures of tau burden are associated with atrophy in early Braak stage medial temporal lobe regions in amyloid-negative individuals. Alzheimer’s Dement. 2019, 15, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Weigand, A.J.; Bangen, K.J.; Thomas, K.R.; Delano-Wood, L.; Gilbert, P.E.; Brickman, A.M.; Bondi, M.W.; Alzheimer’s Disease Neuroimaging, I. Is tau in the absence of amyloid on the Alzheimer’s continuum?: A study of discordant PET positivity. Brain Commun. 2020, 2, fcz046. [Google Scholar] [CrossRef]
- Lantero Rodriguez, J.; Karikari, T.K.; Suarez-Calvet, M.; Troakes, C.; King, A.; Emersic, A.; Aarsland, D.; Hye, A.; Zetterberg, H.; Blennow, K.; et al. Plasma p-tau181 accurately predicts Alzheimer’s disease pathology at least 8 years prior to post-mortem and improves the clinical characterisation of cognitive decline. Acta Neuropathol. 2020, 140, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Ashton, N.J.; Janelidze, S.; Mattsson-Carlgren, N.; Binette, A.P.; Strandberg, O.; Brum, W.S.; Karikari, T.K.; Gonzalez-Ortiz, F.; Di Molfetta, G.; Meda, F.J.; et al. Differential roles of Abeta42/40, p-tau231 and p-tau217 for Alzheimer’s trial selection and disease monitoring. Nat. Med. 2022, 28, 2555–2562. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Ashton, N.J.; Tegg, M.; Goozee, K.; Singh, A.K.; Karikari, T.K.; Simren, J.; Vanmechelen, E.; Armstrong, N.J.; et al. Diagnostic and prognostic plasma biomarkers for preclinical Alzheimer’s disease. Alzheimer’s Dement. 2022, 18, 1141–1154. [Google Scholar] [CrossRef]
- Mielke, M.M.; Frank, R.D.; Dage, J.L.; Jeromin, A.; Ashton, N.J.; Blennow, K.; Karikari, T.K.; Vanmechelen, E.; Zetterberg, H.; Algeciras-Schimnich, A.; et al. Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes. JAMA Neurol. 2021, 78, 1108–1117. [Google Scholar] [CrossRef]
- O’Bryant, S.; Petersen, M.; Hall, J.; Johnson, L.; Yaffe, K.; Braskie, M.; Toga, A.W.; Rissman, R.A. Characterizing plasma NfL in a community-dwelling multi-ethnic cohort: Results from the HABLE study. Alzheimer’s Dement. 2022, 18, 240–250. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Zhang, F.; Petersen, M.; Hall, J.R.; Johnson, L.A.; Yaffe, K.; Mason, D.; Braskie, M.; Barber, R.A.; Rissman, R.A.; et al. A blood screening tool for detecting mild cognitive impairment and Alzheimer’s disease among community-dwelling Mexican Americans and non-Hispanic Whites: A method for increasing representation of diverse populations in clinical research. Alzheimer’s Dement. 2022, 18, 77–87. [Google Scholar] [CrossRef]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma p-tau181, p-tau217, and other blood-based Alzheimer’s disease biomarkers in a multi-ethnic, community study. Alzheimer’s Dement. 2021, 17, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Zimmer, J.A.; Evans, C.D.; Lu, M.; Ardayfio, P.; Sparks, J.; Wessels, A.M.; Shcherbinin, S.; Wang, H.; Monkul Nery, E.S.; et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA 2023, 330, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Pontecorvo, M.J.; Lu, M.; Burnham, S.C.; Schade, A.E.; Dage, J.L.; Shcherbinin, S.; Collins, E.C.; Sims, J.R.; Mintun, M.A. Association of Donanemab Treatment With Exploratory Plasma Biomarkers in Early Symptomatic Alzheimer Disease: A Secondary Analysis of the TRAILBLAZER-ALZ Randomized Clinical Trial. JAMA Neurol. 2022, 79, 1250–1259. [Google Scholar] [CrossRef]
- Chang, H.I.; Chang, Y.T.; Huang, C.W.; Huang, K.L.; Hsu, J.L.; Hsu, S.W.; Tsai, S.J.; Chang, W.N.; Lee, C.C.; Huang, S.H.; et al. Structural Covariance Network as an Endophenotype in Alzheimer’s Disease-Susceptible Single-Nucleotide Polymorphisms and the Correlations With Cognitive Outcomes. Front. Aging Neurosci. 2021, 13, 721217. [Google Scholar] [CrossRef]
- Chang, H.I.; Hsu, S.W.; Kao, Z.K.; Lee, C.C.; Huang, S.H.; Lin, C.H.; Liu, M.N.; Chang, C.C. Impact of Amyloid Pathology in Mild Cognitive Impairment Subjects: The Longitudinal Cognition and Surface Morphometry Data. Int. J. Mol. Sci. 2022, 23, 14635. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Frisoni, G.B.; Rabinovici, G.D.; Sabbagh, M.; Cappa, S.; Bejanin, A.; Bombois, S.; Epelbaum, S.; Teichmann, M.; et al. Clinical diagnosis of Alzheimer’s disease: Recommendations of the International Working Group. Lancet Neurol. 2021, 20, 484–496. [Google Scholar] [CrossRef]
- Chang, C.C.; Kramer, J.H.; Lin, K.N.; Chang, W.N.; Wang, Y.L.; Huang, C.W.; Lin, Y.T.; Chen, C.; Wang, P.N. Validating the Chinese version of the Verbal Learning Test for screening Alzheimer’s disease. J. Int. Neuropsychol. Soc. 2010, 16, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F.; Perryman, K.M. Neuropsychiatric features of frontotemporal dementia: Evaluation of consensus criteria and review. J. Neuropsychiatry Clin. Neurosci. 2002, 14, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic criteria for Parkinson disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hoglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Muller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Durr, A.; Fowler, C.J.; et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Lin, K.N.; Wang, P.N.; Liu, H.C.; Teng, E.L. Cognitive Abilities Screening Instrument, Chinese Version 2.0 (CASI C-2.0): Administration and clinical application. Acta Neurol. Taiwan. 2012, 21, 180–189. [Google Scholar] [PubMed]
- Greve, D.N.; Fischl, B. False positive rates in surface-based anatomical analysis. Neuroimage 2018, 171, 6–14. [Google Scholar] [CrossRef]
- Reuter, M.; Schmansky, N.J.; Rosas, H.D.; Fischl, B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012, 61, 1402–1418. [Google Scholar] [CrossRef]
| CTL | LOAD | TCP | YOAD | |
|---|---|---|---|---|
| Case numbers | 90 | 96 | 44 | 55 |
| Age at plasma, median (range) | 67 (25~88) | 77 (67~94) * | 75.5 (51~87) * | 66 (46~72) † |
| Disease durations at plasma (year), median (range) | N.A. | 4 (1~14) | 3 (2~13) | 4 (1~17) |
| Estimated year of onset, median (range) | N.A. | 72 (65~85) | 70.5 (49~85) | 60 (43~65) |
| Sex, female (%) | 45 (50) | 62 (65) | 19 (43) | 34 (62) |
| ApoE4 carrier, n (%) | 12, (13) | 43, (45) *† | 9, (21) * | 26, (47) *† |
| Educational years | 12 (0–21) | 6 (0–18) *† | 10.5 (2–16) | 9 (0–16) *† |
| Mini-mental State Examination, median (range) | 28 (25~30) | 20 (0–28) *† | 24 (1–28) * | 21 (0–28) *† |
| CDR, median (range) | 0 | 0.5 (0.5–3) * | 0.5 (0–2) * | 0.5 (0.5–3) * |
| Amyloid centiloid, mean (SD) | 6.12 (17.2) | 76.83 (47.0)*† | 9.2 (27.31) | 65.32 (36.9) *† |
| Tau centiloid, mean (SD) | 2.81 (13.4) | 87.24 (33.22) *† | 28.19 (15.8) * | 99.23 (50.69) *† |
| Gray matter volume (mL), mean (SD) | 588.8 (60.54) | 523.5 (53.03) *† | 556.5 (55.87) * | 537.0 (72.59) *† |
| Hippocampal GM volume fraction (%), mean (SD) | 0.35 (0.041) | 0.26 (0.048) *† | 0.31 (0.045) * | 0.28 (0.048) * |
| Diagnosis | Reference | Accuracy | Parameters | Estimate | z | p |
|---|---|---|---|---|---|---|
| LOAD | CTL | 0.8295 | Hippocampal volume | −7.6634 | −5.8789 | 4.1311 × 10−9 |
| pTau181 | 0.4259 | 2.6842 | 0.0073 | |||
| YOAD | CTL | 0.8456 | Hippocampal volume | −4.4059 | −4.6756 | 2.9311 × 10−6 |
| pTau181 | 0.4110 | 2.5612 | 0.0104 | |||
| Aβ42/40 | 27.7192 | −2.0677 | 0.0387 | |||
| TCP | CTL | 0.7236 | Hippocampal volume | −3.0325 | −3.4372 | 0.0006 |
| Differential Diagnosis | Reference | Accuracy | Parameters | Estimate | z | p |
| LOAD | YOAD | 0.6620 | NFL | 0.0393 | 2.5169 | 0.0118 |
| TCP | LOAD | 0.8062 | Hippocampal volume | 2.6983 | 3.2147 | 0.0013 |
| pTau181 | −0.7275 | −3.2535 | 0.0011 | |||
| TCP | YOAD | 0.7753 | pTau181 | −0.9556 | −3.9956 | 6.4535 × 10−5 |
| Model | Parameters | Unstandardized β | t | p | 95% Confident Intervals (Lower~Upper) |
|---|---|---|---|---|---|
| AD | Hippocampal volume | 6.6297 | 4.6585 | 1.0831 × 10−5 | 3.8028~9.4566 |
| Tau centiloid | −0.0382 | −3.0302 | 0.0032 | −0.0632~−0.0132 | |
| Educational year | 0.2440 | 2.2417 | 0.0274 | 0.0278~0.4603 | |
| YOAD | pTau 181 | −1.3113 | −2.9105 | 0.0075 | −2.2393~−0.3834 |
| Hippocampal volume | 4.2538 | 2.3709 | 0.0258 | 0.5587~7.9489 | |
| LOAD | Hippocampal volume | 8.3245 | 4.5280 | 2.7079 × 10−5 | 4.6506~11.9984 |
| Educational year | 0.3338 | 2.4123 | 0.0188 | 0.0573~0.6104 | |
| Tau Centiloid | −0.0417 | −2.3161 | 0.0238 | −0.0777~−0.0057 | |
| TCP | Hippocampal volume | 4.9901 | 2.6964 | 0.0107 | 1.2330~8.7471 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-I.; Huang, K.-L.; Huang, C.-G.; Huang, C.-W.; Huang, S.-H.; Lin, K.-J.; Chang, C.-C. Clinical Significance of the Plasma Biomarker Panels in Amyloid-Negative and Tau PET-Positive Amnestic Patients: Comparisons with Alzheimer’s Disease and Unimpaired Cognitive Controls. Int. J. Mol. Sci. 2024, 25, 5607. https://doi.org/10.3390/ijms25115607
Chang H-I, Huang K-L, Huang C-G, Huang C-W, Huang S-H, Lin K-J, Chang C-C. Clinical Significance of the Plasma Biomarker Panels in Amyloid-Negative and Tau PET-Positive Amnestic Patients: Comparisons with Alzheimer’s Disease and Unimpaired Cognitive Controls. International Journal of Molecular Sciences. 2024; 25(11):5607. https://doi.org/10.3390/ijms25115607
Chicago/Turabian StyleChang, Hsin-I, Kuo-Lun Huang, Chung-Gue Huang, Chi-Wei Huang, Shu-Hua Huang, Kun-Ju Lin, and Chiung-Chih Chang. 2024. "Clinical Significance of the Plasma Biomarker Panels in Amyloid-Negative and Tau PET-Positive Amnestic Patients: Comparisons with Alzheimer’s Disease and Unimpaired Cognitive Controls" International Journal of Molecular Sciences 25, no. 11: 5607. https://doi.org/10.3390/ijms25115607
APA StyleChang, H.-I., Huang, K.-L., Huang, C.-G., Huang, C.-W., Huang, S.-H., Lin, K.-J., & Chang, C.-C. (2024). Clinical Significance of the Plasma Biomarker Panels in Amyloid-Negative and Tau PET-Positive Amnestic Patients: Comparisons with Alzheimer’s Disease and Unimpaired Cognitive Controls. International Journal of Molecular Sciences, 25(11), 5607. https://doi.org/10.3390/ijms25115607






