SIRT6 Inhibits Anoikis of Colorectal Cancer Cells by Down-Regulating NDRG1
Abstract
1. Introduction
2. Results
2.1. The Protein Level of SIRT6 Is Decreased during the Anoikis of CRC Cells
2.2. The Protein Level of SIRT6 Is Higher in Anoikis-Resistant CRC Cells
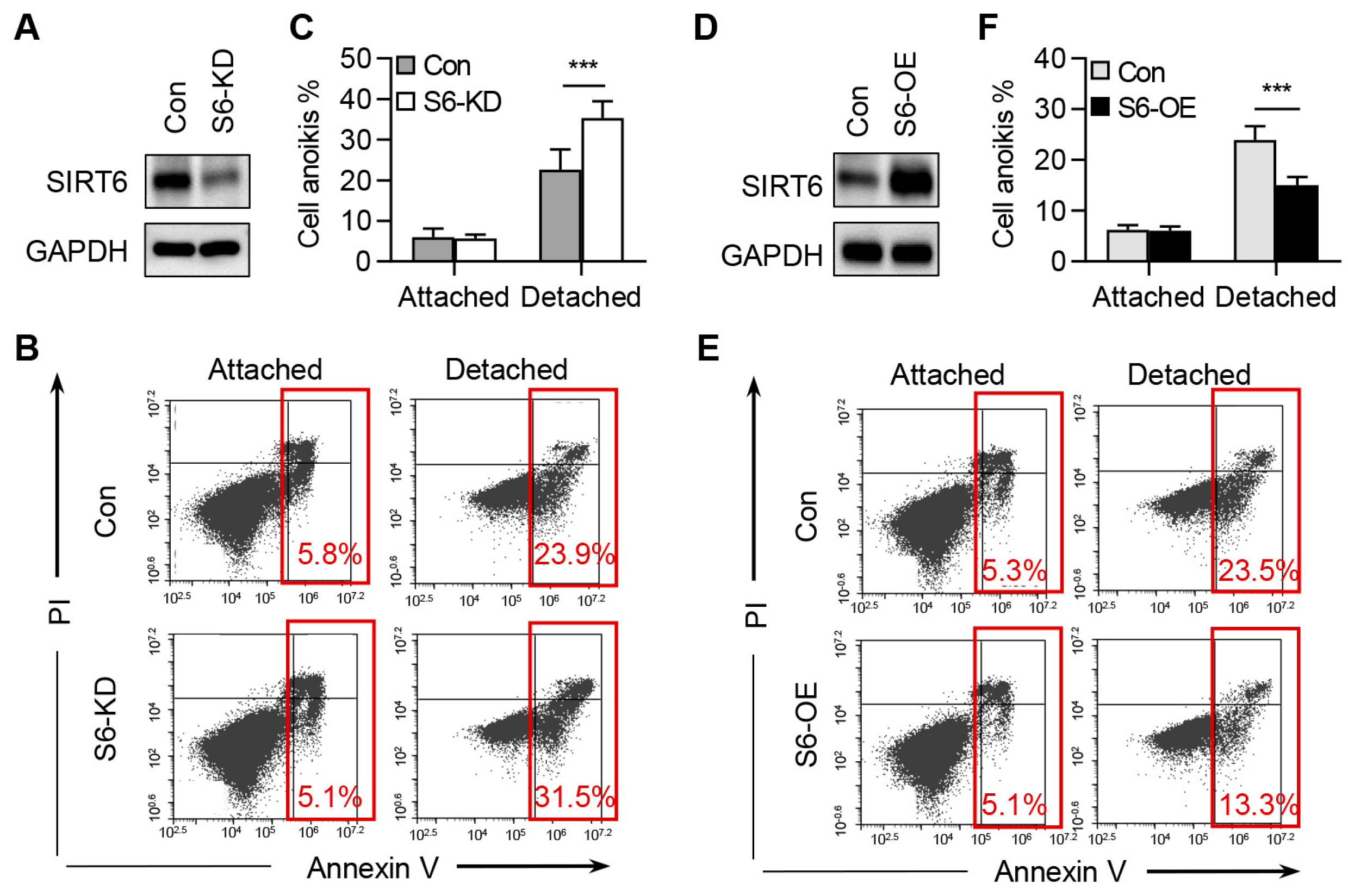
2.3. SIRT6 Inhibits the Anoikis of CRC Cells
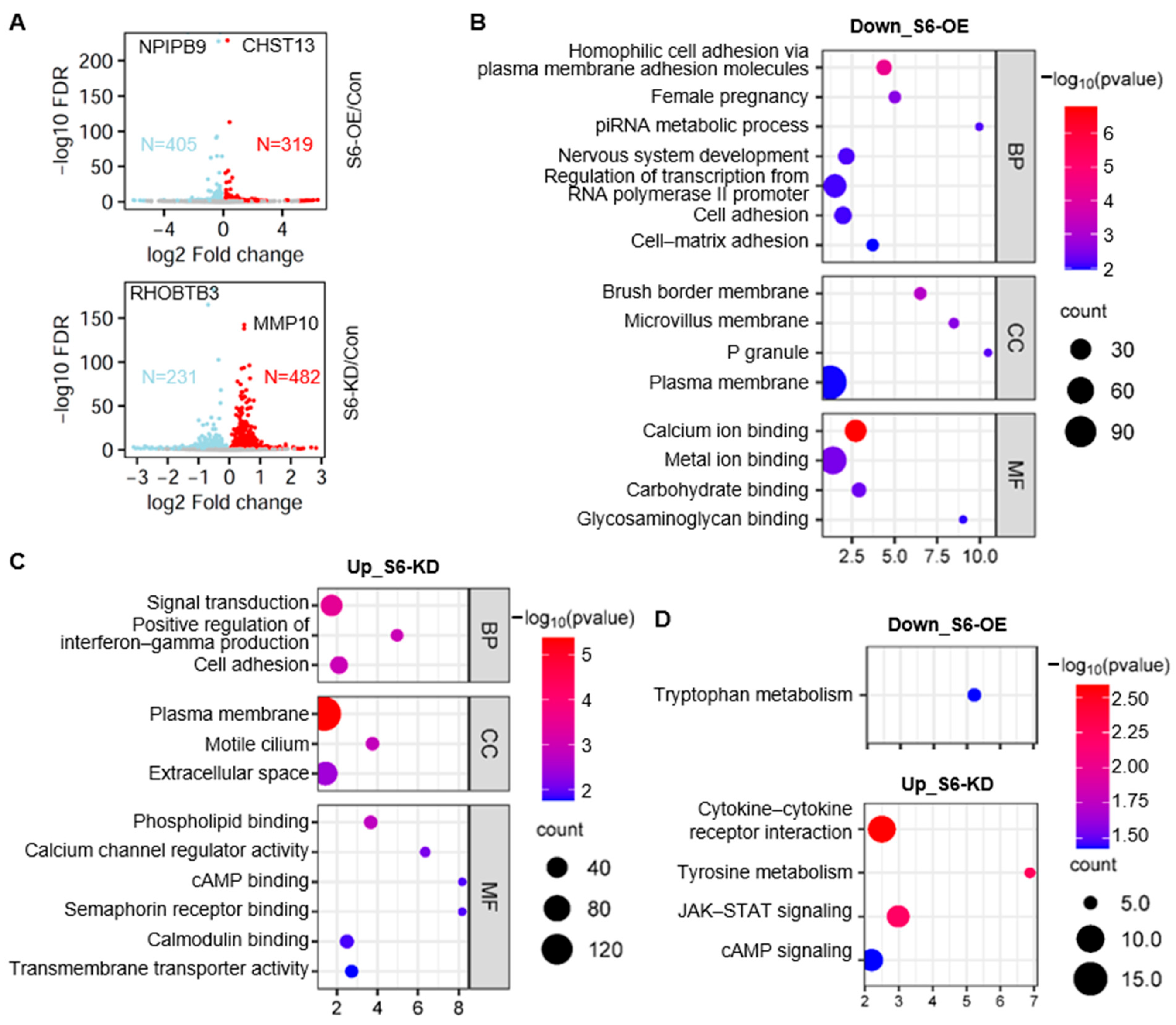
2.4. Identification of SIRT6-Regulated Gene Expression
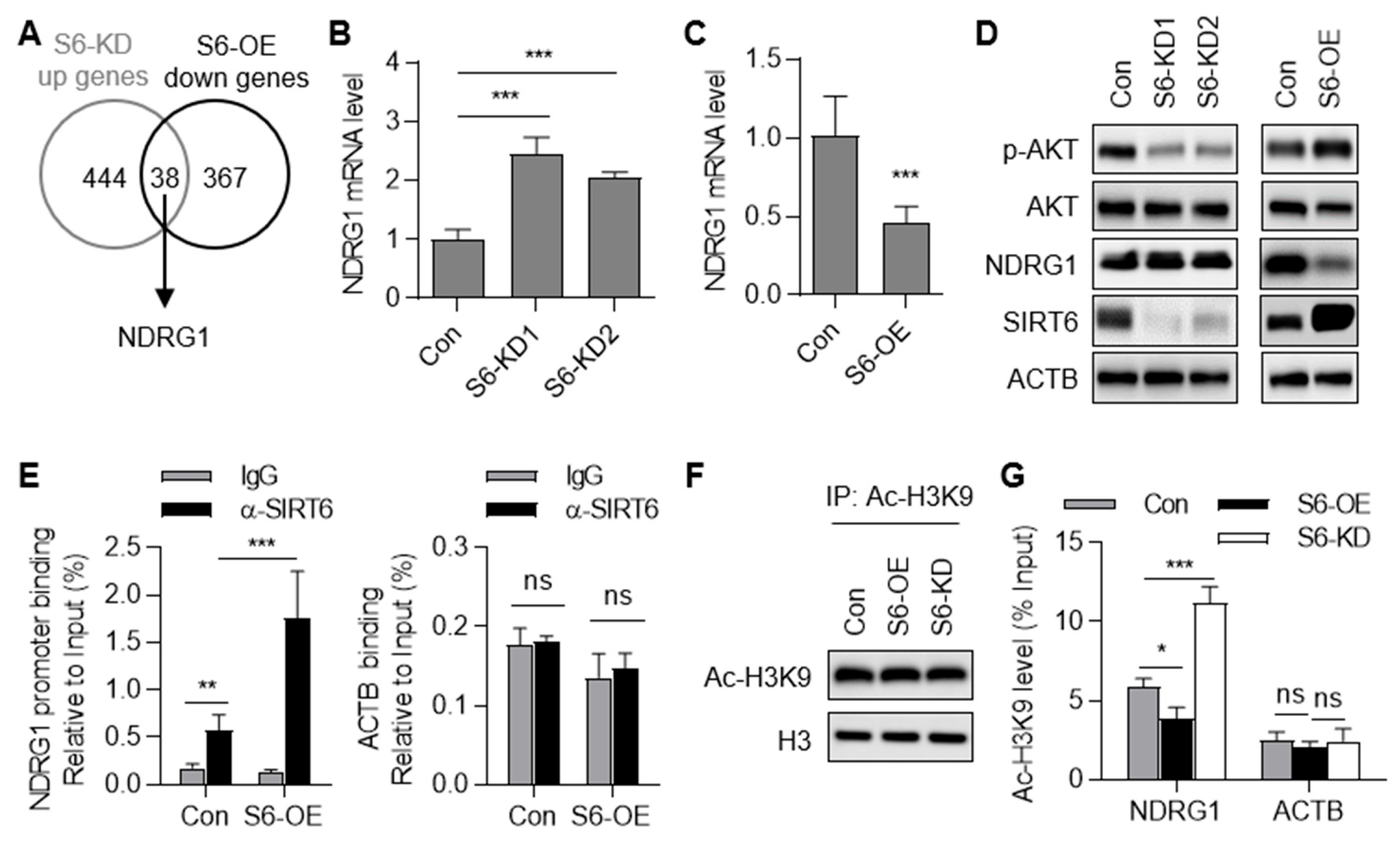
2.5. SIRT6 Represses the Transcription of the NDRG1 Gene
2.6. Down-Regulation of NDRG1 Contributes to SIRT6-Inhibited Anoikis
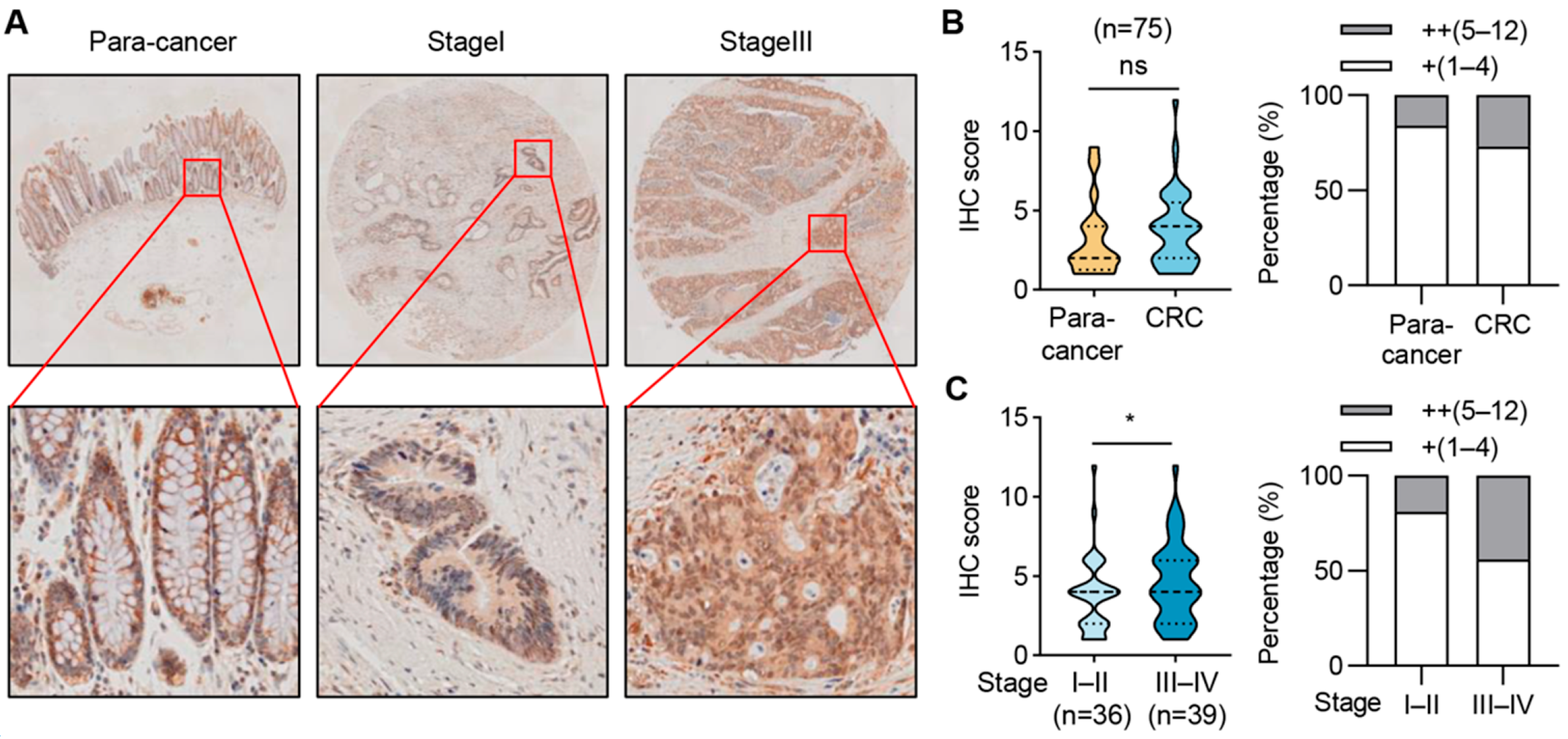
2.7. SIRT6 Expression Is Elevated in Advanced-Stage CRC Samples
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Quantitative Polymerase Chain Reaction (PCR) Method (qPCR)
4.3. Immunoblotting
4.4. SIRT6 Protein Degradation Assay
4.5. SIRT6 Knockdown or Overexpression
4.6. Apoptosis Analysis
4.7. RNA Sequencing (RNA-Seq) Analysis
4.8. Chromatin Immunoprecipitation (ChIP)
4.9. Immunohistochemistry (IHC)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Sattari Fard, F.; Jalilzadeh, N.; Mehdizadeh, A.; Sajjadian, F.; Velaei, K. Understanding and targeting anoikis in metastasis for cancer therapies. Cell Biol. Int. 2023, 47, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, C.L.; Weigel, K.J.; Schafer, Z.T. Cancer cell survival during detachment from the ECM: Multiple barriers to tumour progression. Nat. Rev. Cancer 2014, 14, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Davison-Versagli, C.A.; Leliaert, A.K.; Pape, D.J.; McCallister, C.; Zuo, J.; Durbin, S.M.; Buchheit, C.L.; Zhang, S.; Schafer, Z.T. Oncogenic Ras differentially regulates metabolism and anoikis in extracellular matrix-detached cells. Cell Death Differ. 2016, 23, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Shestov, A.A.; Swain, P.; Yang, C.; Parker, S.J.; Wang, Q.A.; Terada, L.S.; Adams, N.D.; McCabe, M.T.; Pietrak, B.; et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 2016, 532, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Weems, A.D.; Welf, E.S.; Driscoll, M.K.; Zhou, F.Y.; Mazloom-Farsibaf, H.; Chang, B.J.; Murali, V.S.; Gihana, G.M.; Weiss, B.G.; Chi, J.; et al. Blebs promote cell survival by assembling oncogenic signalling hubs. Nature 2023, 615, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Markouli, M.; Strepkos, D.; Basdra, E.K.; Papavassiliou, A.G.; Piperi, C. Prominent Role of Histone Modifications in the Regulation of Tumor Metastasis. Int. J. Mol. Sci. 2021, 22, 2778. [Google Scholar] [CrossRef] [PubMed]
- Pedanou, V.E.; Gobeil, S.; Tabaries, S.; Simone, T.M.; Zhu, L.J.; Siegel, P.M.; Green, M.R. The histone H3K9 demethylase KDM3A promotes anoikis by transcriptionally activating pro-apoptotic genes BNIP3 and BNIP3L. Elife 2016, 5, e16844. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Deng, C.X.; Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 2009, 460, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Sluczanowska-Glabowska, S.; Salmanowicz, M.; Staniszewska, M.; Pawlik, A. The Role of Sirtuins in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 10782. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liang, W. SIRT1 and SIRT6: The role in aging-related diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166815. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Qu, Y.; Xu, S.; Wang, Y.; Liu, X.; Ma, D. SIRT6: A potential therapeutic target for diabetic cardiomyopathy. FASEB J. 2023, 37, e23099. [Google Scholar] [CrossRef] [PubMed]
- Dzidek, A.; Czerwinska-Ledwig, O.; Zychowska, M.; Pilch, W.; Piotrowska, A. The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms. Int. J. Mol. Sci. 2023, 24, 9655. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C.; Zwaans, B.M.; Silberman, D.M.; Gymrek, M.; Goren, A.; Zhong, L.; Ram, O.; Truelove, J.; Guimaraes, A.R.; Toiber, D.; et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell 2012, 151, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, J.U.; Fischer, K.; Baus, K.; Kashyap, A.; Ma, S.; Krupp, M.; Linke, M.; Teufel, A.; Zechner, U.; Strand, D.; et al. Sirtuin-6-dependent genetic and epigenetic alterations are associated with poor clinical outcome in hepatocellular carcinoma patients. Hepatology 2013, 58, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.J.; Yan, Y.J.; Shao, B.Y.; Tian, L.W.; Zhou, W.H. Downregulation of SIRT6 is associated with poor prognosis in patients with non-small cell lung cancer. J. Int. Med. Res. 2018, 46, 1517–1527. [Google Scholar] [CrossRef]
- Kugel, S.; Sebastian, C.; Fitamant, J.; Ross, K.N.; Saha, S.K.; Jain, E.; Gladden, A.; Arora, K.S.; Kato, Y.; Rivera, M.N.; et al. SIRT6 Suppresses Pancreatic Cancer through Control of Lin28b. Cell 2016, 165, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Ming, M.; Han, W.; Zhao, B.; Sundaresan, N.R.; Deng, C.X.; Gupta, M.P.; He, Y.Y. SIRT6 promotes COX-2 expression and acts as an oncogene in skin cancer. Cancer Res. 2014, 74, 5925–5933. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Xie, Q.R.; Li, F.; Cheng, Y.; Wu, T.; Zhang, Y.; Lu, X.; Wong, A.S.T.; Sha, J.; Xia, W. Targeted inhibition of SIRT6 via engineered exosomes impairs tumorigenesis and metastasis in prostate cancer. Theranostics 2021, 11, 6526–6541. [Google Scholar] [CrossRef]
- Andreani, C.; Bartolacci, C.; Persico, G.; Casciaro, F.; Amatori, S.; Fanelli, M.; Giorgio, M.; Galie, M.; Tomassoni, D.; Wang, J.; et al. SIRT6 promotes metastasis and relapse in HER2-positive breast cancer. Sci. Rep. 2023, 13, 22000. [Google Scholar] [CrossRef]
- Abbotto, E.; Miro, C.; Piacente, F.; Salis, A.; Murolo, M.; Nappi, A.; Millo, E.; Russo, E.; Cichero, E.; Sturla, L.; et al. SIRT6 pharmacological inhibition delays skin cancer progression in the squamous cell carcinoma. Biomed. Pharmacother. 2023, 166, 115326. [Google Scholar] [CrossRef]
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Toiber, D.; Erdel, F.; Bouazoune, K.; Silberman, D.M.; Zhong, L.; Mulligan, P.; Sebastian, C.; Cosentino, C.; Martinez-Pastor, B.; Giacosa, S.; et al. SIRT6 recruits SNF2H to DNA break sites, preventing genomic instability through chromatin remodeling. Mol. Cell 2013, 51, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Watari, K.; Shibata, T.; Miyamoto, T.; Murakami, Y.; Nakahara, Y.; Izumi, H.; Wakimoto, H.; Kuwano, M.; Abe, T.; et al. Bidirectional Regulation between NDRG1 and GSK3beta Controls Tumor Growth and Is Targeted by Differentiation Inducing Factor-1 in Glioblastoma. Cancer Res. 2020, 80, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Zhu, F.; Yang, X.; Lu, J.; Zheng, Y.; Zhao, Q.; Wen, X.; Lu, A.; Wang, M.; Zheng, M.; et al. The metastatic suppressor NDRG1 inhibits EMT, migration and invasion through interaction and promotion of caveolin-1 ubiquitylation in human colorectal cancer cells. Oncogene 2017, 36, 4323–4335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nie, L.; Xu, K.; Fu, Y.; Zhong, J.; Gu, K.; Zhang, L. SIRT6, a novel direct transcriptional target of FoxO3a, mediates colon cancer therapy. Theranostics 2019, 9, 2380–2394. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.H.; Zhang, C.L.; Zhang, J.Y.; Gao, P.; He, M.; Li, Y.L. Overexpression of Sirt6 is a novel biomarker of malignant human colon carcinoma. J. Cell Biochem. 2018, 119, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Seoane, J.; Gomis, R.R. TGF-beta Family Signaling in Tumor Suppression and Cancer Progression. Cold Spring Harb. Perspect. Biol. 2017, 9, a022277. [Google Scholar] [CrossRef] [PubMed]
- Thirumurthi, U.; Shen, J.; Xia, W.; LaBaff, A.M.; Wei, Y.; Li, C.W.; Chang, W.C.; Chen, C.H.; Lin, H.K.; Yu, D.; et al. MDM2-mediated degradation of SIRT6 phosphorylated by AKT1 promotes tumorigenesis and trastuzumab resistance in breast cancer. Sci. Signal 2014, 7, ra71. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Yang, H.; Tan, C.; Li, J.; Liu, Z.; Quan, Q.; Kong, S.; Ye, J.; Gao, B.; Fang, D. USP10 antagonizes c-Myc transcriptional activation through SIRT6 stabilization to suppress tumor formation. Cell Rep. 2013, 5, 1639–1649. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Huang, L.; Jia, H.; Aikemu, B.; Zhang, S.; Shao, Y.; Hong, H.; Yesseyeva, G.; Wang, C.; Li, S.; et al. NDRG1 enhances the sensitivity of cetuximab by modulating EGFR trafficking in colorectal cancer. Oncogene 2021, 40, 5993–6006. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Yu, W.; Zhou, X.; Hou, J.; Gao, Y.; Zhang, J.; Gao, X. SIRT6 Inhibits Anoikis of Colorectal Cancer Cells by Down-Regulating NDRG1. Int. J. Mol. Sci. 2024, 25, 5585. https://doi.org/10.3390/ijms25115585
Li F, Yu W, Zhou X, Hou J, Gao Y, Zhang J, Gao X. SIRT6 Inhibits Anoikis of Colorectal Cancer Cells by Down-Regulating NDRG1. International Journal of Molecular Sciences. 2024; 25(11):5585. https://doi.org/10.3390/ijms25115585
Chicago/Turabian StyleLi, Fengying, Wentao Yu, Xiaoling Zhou, Jingyu Hou, Yunyi Gao, Jun Zhang, and Xiangwei Gao. 2024. "SIRT6 Inhibits Anoikis of Colorectal Cancer Cells by Down-Regulating NDRG1" International Journal of Molecular Sciences 25, no. 11: 5585. https://doi.org/10.3390/ijms25115585
APA StyleLi, F., Yu, W., Zhou, X., Hou, J., Gao, Y., Zhang, J., & Gao, X. (2024). SIRT6 Inhibits Anoikis of Colorectal Cancer Cells by Down-Regulating NDRG1. International Journal of Molecular Sciences, 25(11), 5585. https://doi.org/10.3390/ijms25115585





