Complete Mitochondrial Genomes and Phylogenetic Analysis of Genus Henricia (Asteroidea: Spinulosida: Echinasteridae)
Abstract
1. Introduction
2. Results
2.1. Mitogenome Features and AT/GC-Skew
2.2. PCG Characteristics
2.3. Relative Synonymous Codon Usage
2.4. Characteristics of rRNA and tRNA Genes
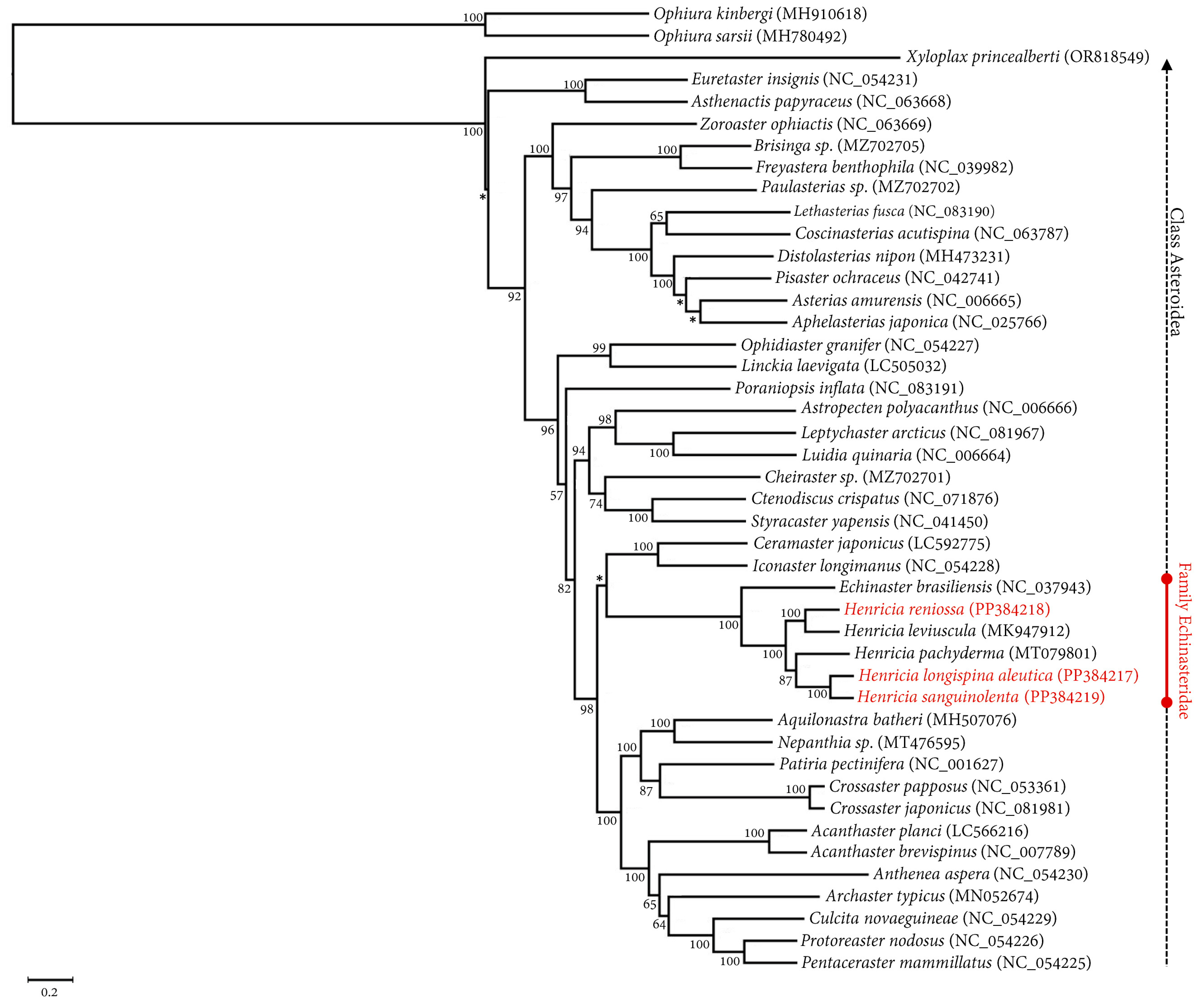
2.5. Phylogenetic Relationships and Gene Arrangement
3. Materials and Methods
3.1. Sample Collection
3.2. Species Identification Based on the Morphological Characteristics
3.3. DNA Extraction and Mitochondrial DNA Amplification
3.4. Mitogenome Annotation and Sequence Analysis
3.5. Phylogenetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed]
- Rubinoff, D.; Cameron, S.; Will, K. A genomic perspective on the shortcomings of mitochondrial DNA for “barcoding” identification. J. Hered. 2006, 97, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Dool, S.E.; Puechmaille, S.J.; Foley, N.M.; Allegrini, B.; Bastian, A.; Mutumi, G.L.; Maluleke, T.G.; Odendaal, L.J.; Teeling, E.C.; Jacobs, D.S. Nuclear introns outperform mitochondrial DNA in inter-specific phylogenetic reconstruction: Lessons from horseshoe bats (Rhinolophidae: Chiroptera). Mol. Phylogenet. Evol. 2016, 97, 196–212. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, C.; Tran, N.T.; Sun, Z.; Wu, J.; Ge, H.; Lu, Z.; Zhong, C.; Zhu, Z.; Yang, Q. Comparison of the complete mitochondrial genome of Phyllophorus liuwutiensis (Echinodermata: Holothuroidea: Phyllophoridae) to that of other sea cucumbers. FEBS Open Bio 2020, 10, 1587–1600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vantomme, L.; Jossart, Q.; Gérard, K.; Danis, B.; Moreau, C. Preliminary Assessment of Sea Star (Echinodermata, Asteroidea) Diversity in the Coastal Magellanic Region (South Chile) and Their Geographical Distribution. Diversity 2023, 15, 1129. [Google Scholar] [CrossRef]
- Balakirev, E.S. Trans-Species Polymorphism in Mitochondrial Genome of Camarodont Sea Urchins. Genes 2019, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.V.; Minale, L.; Riccio, R.; Zollo, F. Steroidal oligoglycosides and polyhydroxysteroids from Echinoderms. Fortschr. Chem. Org. Naturst. 1993, 62, 75–308. [Google Scholar] [CrossRef] [PubMed]
- Ivanchina, N.V.; Kicha, A.A.; Stonik, V.A. Steroid glycosides from marine organisms. Steroids 2011, 76, 425–454. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Downey, M.E. Starfishes of the Atlantic. 1992. Available online: https://api.semanticscholar.org/CorpusID:82206354 (accessed on 1 February 2024).
- Fontanella, F.; Hopkins, T.; Féral, J.; David, B. Preliminary phylogeny of Echinaster (Othilia) from the Gulf of Mexico based on morphological characters (Echinodermata: Asteroidea). Echinoderm Res. 2001, 337, 91–95. [Google Scholar]
- Knott, J.R.; Phillips, F.; Reheis, M.C.; Sada, D.; Jayko, A.; Axen, G. Geologic and hydrologic concerns about pupfish divergence during the last glacial maximum. Proc. R. Soc. B 2018, 285, 20171648. [Google Scholar] [CrossRef] [PubMed Central]
- Mah, C.L.; Blake, D.B. Global diversity and phylogeny of the Asteroidea (Echinodermata). PLoS ONE 2012, 7, e35644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mah, C.L. New Genera, Species, and observations on the biology of Antarctic Valvatida (Asteroidea). Zootaxa 2023, 5310, 1–88. [Google Scholar] [CrossRef] [PubMed]
- Ubagan, M.D.; Lee, J.; Shin, S.; Lee, T. Two newly recorded echinoderms from the mesophotic zone in Korea. J. Species Res. 2023, 12, 180–188. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ward, R.D.; Holmes, B.H.; O’Hara, T.D. DNA barcoding discriminates echinoderm species. Mol. Ecol. Resour. 2008, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Knott, K.E.; Ringvold, H.; Blicher, M.E. Morphological and molecular analysis of Henricia Gray, 1840 (Asteroidea: Echinodermata) from the Northern Atlantic Ocean. Zool. J. Linn. Soc. 2018, 182, 791–807. [Google Scholar] [CrossRef]
- Wakita, D.; Fujita, T.; Kajihara, H. Molecular systematics and morphological analyses of the subgenus Setihenricia (Echinodermata: Asteroidea: Henricia) from Japan. Species Divers. 2019, 24, 119–135. [Google Scholar] [CrossRef][Green Version]
- Gun, L.; Yumiao, R.; Haixian, P.; Liang, Z. Comprehensive analysis and comparison on the codon usage pattern of whole Mycobacterium tuberculosis coding genome from different area. BioMed Res. Int. 2018, 2018, 3574976. [Google Scholar] [CrossRef] [PubMed]
- Ubagan, M.D.; Shin, S. Two newly recorded sea stars of genus Henricia (Asteroidea: Spinulosida: Echinasteridae) from the East Sea, Korea. Korean J. Environ. Biol. 2023, 41, 364–369. [Google Scholar] [CrossRef]
- Ubagan, M.D.; Shin, S. A newly recorded sea star of genus Henricia (Asteroidea: Spinulosida: Echinasteridae) from the East Sea, Korea. J. Species Res. 2019, 8, 109–112. [Google Scholar] [CrossRef]
- Sun, S.; Xiao, N.; Sha, Z. Mitogenomics provides new insights into the phylogenetic relationships and evolutionary history of deep-sea sea stars (Asteroidea). Sci. Rep. 2022, 12, 4656. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Liu, J.; Zhang, H. The first complete mitochondrial genome of the Mariana Trench Freyastera benthophila (Asteroidea: Brisingida: Brisingidae) allows insights into the deep-sea adaptive evolution of Brisingida. Ecol. Evol. 2018, 8, 10673–10686. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, M.; Komatsu, M.; Araki, T.; Asakawa, S.; Yokobori, S.-I.; Watanabe, K.; Wada, H. The phylogenetic status of Paxillosida (Asteroidea) based on complete mitochondrial DNA sequences. Mol. Phylogenet. Evol. 2005, 36, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Tan, M.; Meng, G.; Yang, S.; Su, X.; Liu, S.; Song, W.; Li, Y.; Wu, Q.; Zhang, A. Multiplex sequencing of pooled mitochondrial genomes—A crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 2014, 42, e166. [Google Scholar] [CrossRef] [PubMed]
- Han, K.; Zeng, X.; Ni, G. The complete mitochondrial genome of the starfish Coscinasterias acutispina Stimpson, 1862 (Echinodermata, Forcipulatida) from the East China Sea. Mitochondrial DNA Part B 2022, 7, 1843–1844. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Shin, S. Complete mitochondrial genome analysis of Distolasterias nipon (Echinodermata, Asteroidea, Forcipulatida). Mitochondrial DNA Part B 2018, 3, 1290–1291. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Liu, J.; Zhang, H. The complete mitochondrial genome of Styracaster yapensis (Paxillosida: Porcellanasteridae): Characterization and phylogenetic position. Mitochondrial DNA Part B 2019, 4, 81–82. [Google Scholar] [CrossRef]
- Payne, C.Y.; Tilic, E.; Boschen-Rose, R.E.; Gannon, A.; Stiller, J.; Hiley, A.S.; Grupe, B.M.; Mah, C.L.; Rouse, G.W. Xyloplax princealberti (Asteroidea, Echinodermata): A New Species That Is Not Always Associated with Wood Falls. Diversity 2023, 15, 1212. [Google Scholar] [CrossRef]
- Seixas, V.C.; Ventura, C.R.R.; Paiva, P.C. The complete mitochondrial genome of the sea star Echinaster (Othilia) brasiliensis (Asteroidea: Echinasteridae). Conserv. Genet. Resour. 2019, 11, 151–155. [Google Scholar] [CrossRef]
- Lee, T.; Shin, S. Complete mitochondrial genome of the taxonomically notorious sea star, Henricia leviuscula (Asteroidea, Spinulosida, Echinasteridae), from South Korea. Mitochondrial DNA Part B 2019, 4, 2656–2657. [Google Scholar] [CrossRef]
- Lee, T.; Shin, S. Complete mitochondrial genome of Henricia pachyderma (Asteroidea, Spinulosida, Echinasteridae) and phylogenetic analysis. Mitochondrial DNA Part B 2020, 5, 1483–1484. [Google Scholar] [CrossRef]
- Yasuda, N.; Hamaguchi, M.; Sasaki, M.; Nagai, S.; Saba, M.; Nadaoka, K. Complete mitochondrial genome sequences for Crown-of-thorns starfish Acanthaster planci and Acanthaster brevispinus. BMC Genom. 2006, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, H.; Kajitani, R.; Nakamura, Y.; Takahashi, K.; Okuno, M.; Kobayashi, F.; Shinoda, T.; Toyoda, A.; Suzuki, Y.; Thongtham, N. Elucidation of the speciation history of three sister species of crown-of-thorns starfish (Acanthaster spp.) based on genomic analysis. DNA Res. 2021, 28, dsab012. [Google Scholar] [CrossRef] [PubMed]
- Quek, Z.B.R.; Chang, J.J.M.; Ip, Y.C.A.; Huang, D. Complete mitochondrial genome of the sea star Archaster typicus (Asteroidea: Archasteridae). Mitochondrial DNA Part B 2019, 4, 3130–3132. [Google Scholar] [CrossRef] [PubMed]
- Quek, Z.B.R.; Chang, J.J.M.; Ip, Y.C.A.; Chan, Y.K.S.; Huang, D. Mitogenomes reveal alternative initiation codons and lineage-specific gene order conservation in echinoderms. Mol. Biol. Evol. 2021, 38, 981–985. [Google Scholar] [CrossRef]
- Asakawa, S.; Himeno, H.; Miura, K.-I.; Watanabe, K. Nucleotide sequence and gene organization of the starfish Asterina pectinifera mitochondrial genome. Genetics 1995, 140, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hiruta, S.F.; Arai, M.; Shimizu, M.; Mah, C.L.; Fujita, T.; Setiamarga, D.H. The first complete mitochondrial genome of the Northern Pacific deep-sea goniasterid sea star Ceramaster japonicus (Sladen, 1889) determined using NGS-based shotgun sequencing. Mitochondrial DNA Part B 2021, 6, 1406–1408. [Google Scholar] [CrossRef] [PubMed]
- Hiruta, S.F.; Arai, M.; Chavanich, S.; Viyakarn, V.; Fujita, T. Complete mitochondrial genome of a sea star, Linckia laevigata (Echinodermata, Asteroidea, Valvatida, Ophidiasteridae). Mitochondrial DNA Part B 2020, 5, 1342–1343. [Google Scholar] [CrossRef]
- Alboasud, M.; Jeong, H.; Lee, T. The complete mitochondrial genome of Poraniopsis inflata (Asteroidea: Valvatida: Poraniidae) from Dokdo Island, Korea. Mitochondrial DNA Part B 2024, 9, 290–294. [Google Scholar] [CrossRef]
- Guo, J.; Fan, S.; Xu, Q. Study on genetic diversity of Ophiura sarsii population in the Arctic. Prog. Mar. Sci. 2021, 39. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wang, X.-T.; Dong, Y.; Fan, S.-L.; Xu, Q.-Z. The complete mitochondrial genome of Ophiura kinbergi (Ophiuroidea, Ophiurina): Genome structure and phylogenetics. Mitochondrial DNA Part B 2020, 5, 1309–1310. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and high-performance computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Rubinoff, D.; Holland, B.S. Between two extremes: Mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference. Syst. Biol. 2005, 54, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Waikagul, J.; Thaekham, U. Approaches to Research on the Systematics of Fish-Borne Trematodes; Academic Press: Waltham, MA, USA, 2014; Available online: https://books.google.co.kr/books?hl=en&lr=&id=Nsu7AgAAQBAJ&oi=fnd&pg=PP1&dq=28.%09Waikagul,+J.%3B+Thaekham,+U.+Approaches+to+Research+on+the+Systematics+of+Fish-Borne+Trematodes%3B+Academic+Press:+Waltham+Cam-bridge,+MA,+USA++,+2014.&ots=M9pQKTwzm6&sig=HswsYJ_TFPysOjH_z-s1YBYH_1U&redir_esc=y#v=onepage&q&f=false (accessed on 1 February 2014).
- Harrison, R.G. Animal mitochondrial DNA as a genetic marker in population and evolutionary biology. Trends Ecol. Evol. 1989, 4, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, S.; Komuro, T.; Izawa, H.; Tsutsumi, H. Analysis of human mitochondrial DNA polymorphisms in the Japanese population. Biochem. Genet. 2013, 51, 33–70. [Google Scholar] [CrossRef] [PubMed]
- Chichvarkhin, A.; Chichvarkhina, O.; Wakita, D. Sea stars of the genus Henricia Gray, 1840 (Echinodermata, Asteroidea) from Vostok Bay, Sea of Japan. PeerJ 2019, 7, e6585. [Google Scholar] [CrossRef] [PubMed Central]
- Ubagan, M.D.; Alboasud, M.S.A.; Lee, T. Morphological Description and Molecular Analysis of Newly Recorded Asteroid, Henricia djakonovi Chichvarkhin, 2017 (Asteroidea: Spinulosida: Echinasteridae), from Dokdo Island, Korea. Taxonomy 2023, 3, 46–54. [Google Scholar] [CrossRef]
- Chichvarkhin, A. Henricia djakonovi sp. nov.(Echinodermata, Echinasteridae): A new sea star species from the Sea of Japan. PeerJ 2017, 5, e2863. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Madsen, F.J. The Henricia Sanguinolenta Complex (Echinodermata, Asteroidea) of the Norwegian Sea and Adjacent Waters: A Re-Evaluation, with Notes on Related Species; Zoological Museum, University of Copenhagen: Copenhagen, Denmark, 1987. [Google Scholar]
- Ubagan, M.D.; Lee, T.; Kim, P.; Shin, S. A new species of the genus Henricia (Asteroidea, Spinulosida, Echinasteridae) from South Korea. ZooKeys 2020, 997, 1. [Google Scholar] [CrossRef] [PubMed]
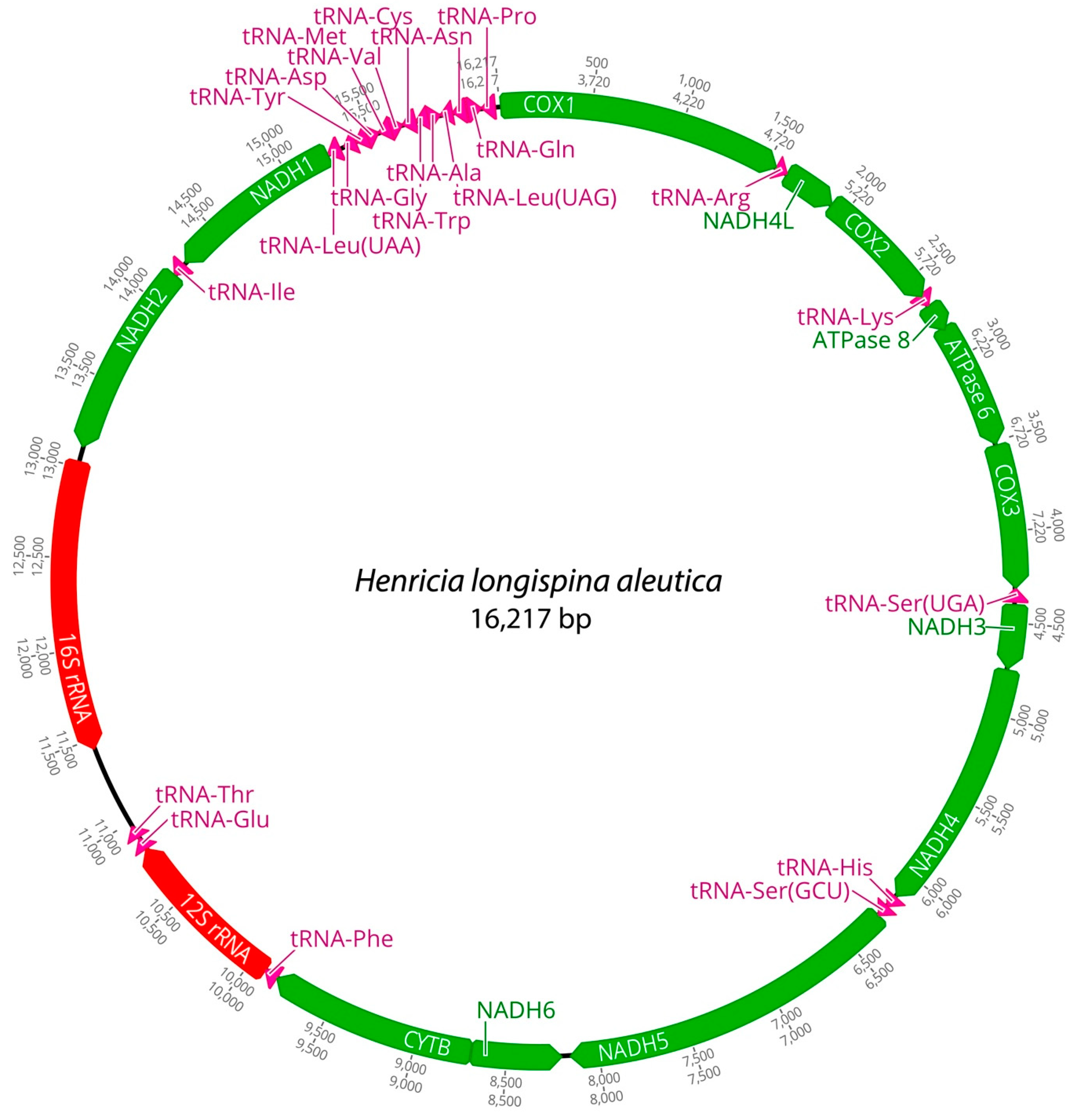

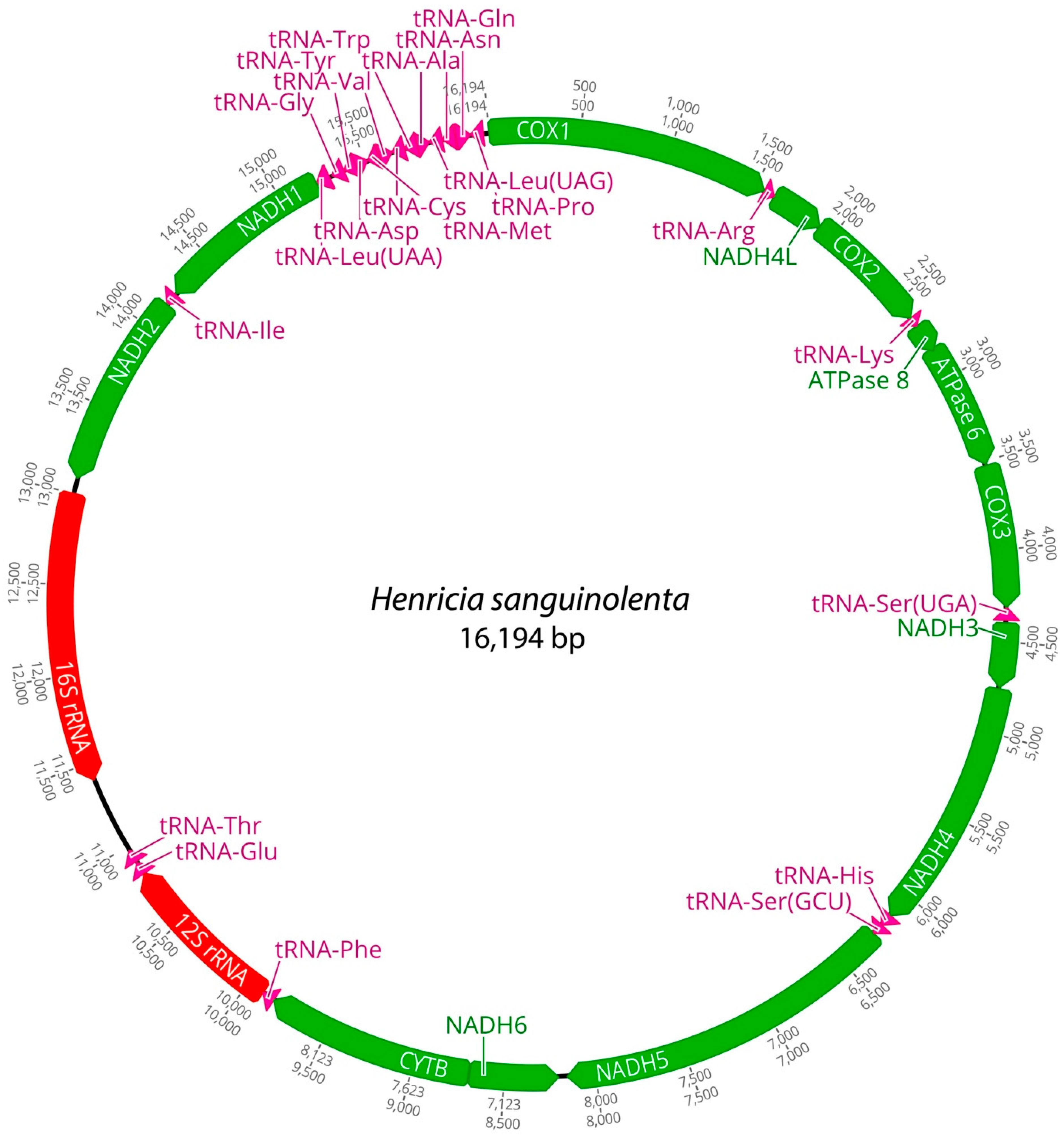
| Species | Region | Size (bp) | A% | G% | C% | T% | AT% | GC% | ATskew | GCskew |
|---|---|---|---|---|---|---|---|---|---|---|
| Henricia longispina aleutica | Mitogenome | 16,217 | 36.2 | 13.5 | 24.1 | 26.2 | 62.4 | 37.5 | 0.15 | −0.28 |
| PCGs | 11,473 | 36.3 | 13.1 | 24.9 | 25.6 | 61.9 | 38.0 | 0.17 | −0.30 | |
| tRNAs | 1537 | 35.7 | 14.1 | 21.1 | 29.1 | 64.8 | 35.1 | 0.10 | −0.19 | |
| rRNAs | 2516 | 37.3 | 13.9 | 23.5 | 25.3 | 62.5 | 37.4 | 0.19 | −0.25 | |
| Henricia reniossa | Mitogenome | 16,223 | 35.4 | 13.9 | 25.0 | 25.7 | 61.1 | 38.8 | 0.15 | −0.28 |
| PCGs | 11,476 | 35.3 | 14.0 | 25.8 | 25.0 | 60.2 | 39.7 | 0.17 | −0.29 | |
| tRNAs | 1541 | 34.9 | 14.7 | 21.6 | 28.8 | 63.7 | 36.2 | 0.09 | −0.19 | |
| rRNAs | 2535 | 37.1 | 13.8 | 24.0 | 25.1 | 51.4 | 37.7 | 0.19 | −0.26 | |
| Henricia sanguinolenta | Mitogenome | 16,194 | 35.6 | 13.9 | 24.6 | 25.8 | 61.4 | 38.5 | 0.15 | −0.27 |
| PCGs | 11,473 | 35.5 | 13.9 | 25.5 | 25.1 | 60.6 | 39.3 | 0.17 | −0.29 | |
| tRNAs | 1531 | 35.5 | 14.2 | 21.3 | 29.0 | 64.4 | 35.5 | 0.10 | −0.19 | |
| rRNAs | 2516 | 37.1 | 14.1 | 24.0 | 24.8 | 61.9 | 38.0 | 0.19 | −0.25 |
| Species | Gene | Type | Strand | Amino Acids | Start | Stop | Length (bp) | Start | Stop | Anti-Codon |
|---|---|---|---|---|---|---|---|---|---|---|
| Henricia longispina aleutica | COX1 | PCG | H | 518 | 1 | 1554 | 1554 | ATG | TGA | |
| tRNA-Arg | tRNA | H | 1555 | 1621 | 67 | CCU | ||||
| NAD4L | PCG | H | 98 | 1622 | 1915 | 294 | ATC | TAA | ||
| COX2 | PCG | H | 234 | 1916 | 2617 | 702 | ATG | TAA | ||
| tRNA-Lys | tRNA | H | 2604 | 2675 | 72 | GAA | ||||
| ATP8 | PCG | H | 55 | 2676 | 2840 | 165 | ATG | TAA | ||
| ATP6 | PCG | H | 231 | 2825 | 3517 | 693 | ATG | TAA | ||
| COX3 | PCG | H | 261 | 3524 | 4306 | 783 | ATG | TAA | ||
| tRNA-Ser (UGA) | tRNA | L | 4312 | 4382 | 71 | GAA | ||||
| NADH3 | PCG | H | 117 | 4398 | 4748 | 351 | ATT | TAA | ||
| NADH4 | PCG | H | 461 | 4753 | 6135 | 1383 | ATG | TGA | ||
| tRNA-His | tRNA | H | 6140 | 6206 | 67 | GUC | ||||
| tRNA-Ser (GCU) | tRNA | H | 6207 | 6275 | 69 | UCG | ||||
| NADH5 | PCG | H | 626 | 6276 | 8153 | 1878 | ATG | TAA | ||
| NADH6 | PCG | L | 163 | 8200 | 8688 | 489 | ATG | TAA | ||
| CYTB | PCG | H | 379 | 8697 | 9834 | 1138 | ATG | TAG | ||
| tRNA-Phe | tRNA | H | 9835 | 9905 | 71 | GCA | ||||
| 12S rRNA | rRNA | H | 9906 | 10,836 | 931 | |||||
| tRNA-Glu | tRNA | H | 10,837 | 10,905 | 69 | AUC | ||||
| tRNA-Thr | tRNA | H | 10,909 | 10,979 | 71 | AUU | ||||
| 16S rRNA | rRNA | L | 11,444 | 13,028 | 1585 | |||||
| NADH2 | PCG | L | 355 | 13,116 | 14,180 | 1065 | ATG | TGA | ||
| tRNA-Ile | tRNA | L | 14,181 | 14,251 | 71 | AAA | ||||
| NADH1 | PCG | L | 326 | 14,265 | 15,242 | 978 | ATG | TAA | ||
| tRNA-Leu (UAA) | tRNA | L | 15,243 | 15,314 | 72 | AAC | ||||
| tRNA-Gly | tRNA | L | 15,336 | 15,403 | 68 | UAU | ||||
| tRNA-Tyr | tRNA | L | 15,404 | 15,472 | 69 | UAA | ||||
| tRNA-Asp | tRNA | H | 15,473 | 15,539 | 67 | UCU | ||||
| tRNA-Met | tRNA | L | 15,540 | 15,608 | 69 | AAA | ||||
| tRNA-Val | tRNA | H | 15,614 | 15,618 | 68 | CGA | ||||
| tRNA-Cys | tRNA | L | 15,680 | 15,751 | 72 | GAA | ||||
| tRNA-Trp | tRNA | L | 15,752 | 15,821 | 70 | CCA | ||||
| tRNA-Ala | tRNA | H | 15,822 | 15,889 | 68 | ACA | ||||
| tRNA-Leu (UAG) | tRNA | L | 15,890 | 15,960 | 71 | AUU | ||||
| tRNA-Asn | tRNA | L | 15,961 | 16,031 | 72 | CUG | ||||
| tRNA-Gln | tRNA | H | 16,043 | 16,113 | 71 | UAA | ||||
| tRNA-Pro | tRNA | L | 16,117 | 16,188 | 72 | UCA | ||||
| D-loop | - | H | 10,982 | 11,442 | 460 | |||||
| Henricia reniossa | COX1 | PCG | H | 518 | 1 | 1554 | 1554 | ATG | TGA | |
| tRNA-Arg | tRNA | H | 1555 | 1621 | 67 | CCU | ||||
| NAD4L | PCG | H | 98 | 1622 | 1915 | 294 | ATC | TAA | ||
| COX2 | PCG | H | 234 | 1916 | 2617 | 702 | ATG | TAA | ||
| tRNA-Lys | tRNA | H | 2604 | 2674 | 71 | GGA | ||||
| ATP8 | PCG | H | 55 | 2676 | 2840 | 165 | ATG | TAA | ||
| ATP6 | PCG | H | 231 | 2825 | 3517 | 693 | ATG | TAA | ||
| COX3 | PCG | H | 261 | 3525 | 4307 | 783 | ATG | TAA | ||
| tRNA-Ser (UGA) | tRNA | L | 4308 | 4377 | 70 | GAG | ||||
| NADH3 | PCG | H | 117 | 4394 | 4744 | 351 | ATT | TAA | ||
| NADH4 | PCG | H | 461 | 4749 | 6131 | 1383 | ATG | TAA | ||
| tRNA-His | tRNA | H | 6136 | 6202 | 67 | GUC | ||||
| tRNA-Ser (GCU) | tRNA | H | 6203 | 6271 | 69 | UCG | ||||
| NADH5 | PCG | H | 627 | 6272 | 8152 | 1881 | ATG | TAA | ||
| NADH6 | PCG | L | 163 | 8204 | 8692 | 489 | ATG | TAA | ||
| CYTB | PCG | H | 379 | 8701 | 9838 | 1138 | ATG | TGA | ||
| tRNA-Phe | tRNA | H | 9839 | 9910 | 72 | GCA | ||||
| 12S rRNA | rRNA | H | 9911 | 10,838 | 928 | |||||
| tRNA-Glu | tRNA | H | 10,839 | 10,910 | 72 | AUU | ||||
| tRNA-Thr | tRNA | H | 10,918 | 10,987 | 70 | CUU | ||||
| 16S rRNA | rRNA | L | 11,420 | 13,026 | 1607 | |||||
| NADH2 | PCG | L | 355 | 13,121 | 14,185 | 1065 | GTG | TAA | ||
| tRNA-Ile | tRNA | L | 14,186 | 14,256 | 71 | AAA | ||||
| NADH1 | PCG | L | 326 | 14,269 | 15,246 | 978 | GTG | TAA | ||
| tRNA-Leu (UAA) | tRNA | L | 15,247 | 15,318 | 72 | AAC | ||||
| tRNA-Gly | tRNA | L | 15,339 | 15,406 | 68 | UAU | ||||
| tRNA-Tyr | tRNA | L | 15,407 | 15,475 | 69 | UAA | ||||
| tRNA-Asp | tRNA | H | 15,476 | 15,544 | 69 | CUA | ||||
| tRNA-Met | tRNA | L | 15,545 | 15,612 | 68 | AAC | ||||
| tRNA-Val | tRNA | H | 15,618 | 15,686 | 69 | CGA | ||||
| tRNA-Cys | tRNA | L | 15,685 | 15,757 | 73 | GAA | ||||
| tRNA-Trp | tRNA | L | 15,758 | 15,827 | 70 | CAG | ||||
| tRNA-Ala | tRNA | H | 15,828 | 15,896 | 69 | ACA | ||||
| tRNA-Leu (UAG) | tRNA | L | 15,897 | 15,967 | 71 | GUU | ||||
| tRNA-Asn | tRNA | L | 15,970 | 16,041 | 72 | CUG | ||||
| tRNA-Gln | tRNA | H | 16,050 | 16,120 | 71 | UAA | ||||
| tRNA-Pro | tRNA | L | 16,123 | 16,193 | 71 | UCA | ||||
| D-loop | - | H | 10,989 | 11,417 | 428 | |||||
| Henricia sanguinolenta | COX1 | PCG | H | 518 | 1 | 1554 | 1554 | ATG | TGA | |
| tRNA-Arg | tRNA | H | 1555 | 1621 | 67 | CCU | ||||
| NAD4L | PCG | H | 98 | 1622 | 1915 | 294 | ATC | TAA | ||
| COX2 | PCG | H | 234 | 1916 | 2617 | 702 | ATG | TAA | ||
| tRNA-Lys | tRNA | H | 2618 | 2674 | 57 | GGA | ||||
| ATP8 | PCG | H | 55 | 2676 | 2840 | 165 | ATG | TAA | ||
| ATP6 | PCG | H | 231 | 2825 | 3517 | 693 | ATG | TAA | ||
| COX3 | PCG | H | 261 | 3524 | 4306 | 783 | ATG | TAA | ||
| tRNA-Ser (UGA) | tRNA | L | 4312 | 4382 | 71 | AAG | ||||
| NADH3 | PCG | H | 117 | 4398 | 4748 | 351 | ATT | TAA | ||
| NADH4 | PCG | H | 461 | 4753 | 6135 | 1383 | ATG | TGA | ||
| tRNA-His | tRNA | H | 6140 | 6206 | 67 | GUC | ||||
| tRNA-Ser (GCU) | tRNA | H | 6207 | 6275 | 69 | UCG | ||||
| NADH5 | PCG | H | 626 | 6276 | 8153 | 1878 | ATG | TAA | ||
| NADH6 | PCG | L | 163 | 8203 | 8691 | 489 | ATG | TAA | ||
| CYTB | PCG | H | 379 | 8700 | 9837 | 1138 | ATG | TAG | ||
| tRNA-Phe | tRNA | H | 9838 | 9909 | 72 | GCA | ||||
| 12S rRNA | rRNA | H | 9910 | 10,831 | 922 | |||||
| tRNA-Glu | tRNA | H | 10,832 | 10,903 | 72 | AUC | ||||
| tRNA-Thr | tRNA | H | 10,908 | 10,977 | 70 | GAU | ||||
| 16S rRNA | rRNA | L | 11,407 | 13,000 | 1594 | |||||
| NADH2 | PCG | L | 355 | 13,087 | 14,151 | 1065 | ATG | TAA | ||
| tRNA-Ile | tRNA | L | 14,152 | 14,224 | 73 | AAA | ||||
| NADH1 | PCG | L | 326 | 14,236 | 15,213 | 978 | ATG | TAA | ||
| tRNA-Leu (UAA) | tRNA | L | 15,214 | 15,285 | 72 | AAC | ||||
| tRNA-Gly | tRNA | L | 15,307 | 15,374 | 68 | UAU | ||||
| tRNA-Tyr | tRNA | L | 15,375 | 15,443 | 69 | UAA | ||||
| tRNA-Asp | tRNA | H | 15,444 | 15,512 | 69 | CUA | ||||
| tRNA-Met | tRNA | L | 15,512 | 15,580 | 69 | AAA | ||||
| tRNA-Val | tRNA | H | 15,586 | 15,654 | 69 | AGA | ||||
| tRNA-Cys | tRNA | L | 15,653 | 15,724 | 72 | GAA | ||||
| tRNA-Trp | tRNA | L | 15,726 | 15,794 | 69 | CAG | ||||
| tRNA-Ala | tRNA | H | 15,795 | 15,864 | 70 | ACA | ||||
| tRNA-Leu (UAG) | tRNA | L | 15,865 | 15,935 | 71 | GUU | ||||
| tRNA-Asn | tRNA | L | 15,938 | 16,009 | 72 | CUG | ||||
| tRNA-Gln | tRNA | H | 16,020 | 16,090 | 71 | UAA | ||||
| tRNA-Pro | tRNA | L | 16,093 | 16,164 | 72 | AUC | ||||
| D-loop | - | H | 10,980 | 11,404 | 424 |
| Henricia longispina aleutica | Henricia reniossa | Henricia sanguinolenta | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | Codon | Count | RSCU | AA | Codon | Count | RSCU | AA | Codon | Count | RSCU | AA | Codon | Count | RSCU | AA | Codon | Count | RSCU | AA | Codon | Count | RSCU |
| Ala | GCA | 60 | 1.36 | Pro | CCA | 105 | 1 | Ala | GCA | 36 | 1.12 | Pro | CCA | 104 | 1.16 | Ala | GCA | 53 | 1.48 | Pro | CCA | 86 | 0.54 |
| GCC | 73 | 1.68 | CCC | 153 | 1.44 | GCC | 55 | 1.72 | CCC | 111 | 1.24 | GCC | 59 | 1.64 | CCC | 102 | 1.32 | ||||||
| GCG | 9 | 0.2 | CCG | 43 | 0.4 | GCG | 6 | 0.16 | CCG | 31 | 0.4 | GCG | 6 | 0.16 | CCG | 21 | 0.24 | ||||||
| GCU | 32 | 0.72 | CCU | 115 | 1.08 | GCU | 29 | 0.92 | CCU | 102 | 1.16 | GCU | 24 | 0.64 | CCU | 99 | 1.28 | ||||||
| Cys | UGC | 48 | 1.14 | Gln | CAA | 182 | 1.44 | Cys | UGC | 22 | 1.06 | Gln | CAA | 122 | 1.54 | Cys | UGC | 32 | 1.16 | Gln | CAA | 108 | 1.38 |
| UGU | 35 | 0.84 | CAG | 68 | 0.54 | UGU | 19 | 0.92 | CAG | 36 | 0.44 | UGU | 23 | 0.82 | CAG | 47 | 0.6 | ||||||
| Asp | GAC | 57 | 0.92 | Arg | CGA | 39 | 0.56 | Asp | GAC | 48 | 1.04 | Arg | CGA | 38 | 1.12 | Asp | GAC | 49 | 1.02 | Arg | CGA | 35 | 0.96 |
| GAU | 67 | 1.08 | CGC | 23 | 0.32 | GAU | 36 | 0.86 | CGC | 32 | 0.6 | GAU | 47 | 0.98 | CGC | 14 | 0.36 | ||||||
| Glu | GAA | 82 | 1.4 | CGG | 26 | 0.36 | Glu | GAA | 54 | 1.24 | CGG | 21 | 0.4 | Glu | GAA | 65 | 1.38 | CGG | 12 | 0.32 | |||
| GAG | 35 | 0.6 | CGU | 23 | 0.32 | GAG | 33 | 0.73 | CGU | 11 | 0.2 | GAG | 28 | 0.6 | CGU | 15 | 0.4 | ||||||
| Phe | UUC | 96 | 0.78 | Ser | UCA | 104 | 1.6 | Phe | UUC | 92 | 1.04 | Ser | UCA | 55 | 1.44 | Phe | UUC | 79 | 0.88 | Ser | UCA | 55 | 1.68 |
| UUU | 150 | 1.22 | UCC | 111 | 1.76 | UUU | 83 | 0.94 | UCC | 64 | 1.68 | UUU | 97 | 1.1 | UCC | 64 | 1.84 | ||||||
| Gly | GGA | 68 | 1.56 | UCG | 24 | 0.32 | Gly | GGA | 59 | 1.76 | UCG | 10 | 0.24 | Gly | GGA | 75 | 2.44 | UCG | 10 | 0.32 | |||
| GGC | 33 | 0.76 | UCU | 100 | 1.52 | GGC | 27 | 0.8 | UCU | 63 | 1.68 | GGC | 19 | 0.6 | UCU | 63 | 1.68 | ||||||
| GGG | 31 | 0.72 | AGC | 84 | 1.28 | GGG | 23 | 0.68 | AGC | 64 | 1.68 | GGG | 10 | 3.2 | AGC | 51 | 1.2 | ||||||
| GGU | 40 | 0.92 | AGU | 82 | 1.28 | GGU | 23 | 0.68 | AGU | 37 | 0.96 | GGU | 18 | 0.54 | AGU | 45 | 1.04 | ||||||
| His | CAC | 93 | 1.06 | AGA | 111 | 3.2 | His | CAC | 66 | 0.86 | AGA | 67 | 2.56 | His | CAC | 59 | 0.96 | AGA | 52 | 2.8 | |||
| CAU | 82 | 0.94 | AGG | 52 | 1.52 | CAU | 86 | 1.12 | AGG | 40 | 1.52 | CAU | 62 | 1.02 | AGG | 18 | 0.96 | ||||||
| Ile | AUA | 160 | 1.32 | Thr | ACA | 139 | 1.32 | Ile | AUA | 93 | 1.11 | Thr | ACA | 93 | 1.4 | Ile | AUA | 128 | 1.35 | Thr | ACA | 123 | 1.48 |
| AUC | 71 | 0.57 | ACC | 125 | 1.2 | AUC | 68 | 0.81 | ACC | 69 | 1.04 | AUC | 58 | 0.6 | ACC | 93 | 1.12 | ||||||
| AUU | 127 | 1.05 | ACG | 37 | 0.36 | AUU | 91 | 1.08 | ACG | 19 | 0.28 | AUU | 98 | 1.02 | ACG | 23 | 0.28 | ||||||
| Lys | AAA | 330 | 1.52 | ACU | 112 | 1.08 | Lys | AAA | 229 | 1.5 | ACU | 82 | 1.24 | Lys | AAA | 260 | 1.62 | ACU | 86 | 1.04 | |||
| AAG | 103 | 0.46 | Val | GUA | 52 | 2 | AAG | 75 | 0.4 | Val | GUA | 49 | 1.6 | AAG | 58 | 0.36 | Val | GUA | 43 | 1.88 | |||
| Leu | CUA | 108 | 1.2 | GUC | 37 | 1.45 | Leu | CUA | 30 | 0.48 | GUC | 21 | 0.68 | Leu | CUA | 94 | 1.5 | GUC | 21 | 0.92 | |||
| CUC | 74 | 0.78 | GUG | 12 | 0.45 | CUC | 82 | 1.26 | GUG | 11 | 0.36 | CUC | 45 | 0.72 | GUG | 8 | 0.36 | ||||||
| CUG | 50 | 0.54 | GUU | 26 | 1 | CUG | 30 | 0.48 | GUU | 40 | 1.32 | CUG | 30 | 0.48 | GUU | 18 | 0.8 | ||||||
| CUU | 115 | 1.26 | Trp | UGG | 46 | 1 | CUU | 82 | 1.26 | Trp | UGG | 31 | 1 | CUU | 71 | 1.14 | Trp | UGG | 38 | 1 | |||
| UUA | 128 | 1.38 | Tyr | UAC | 89 | 0.8 | UUA | 76 | 1.2 | Tyr | UAC | 50 | 0.78 | UUA | 90 | 1.44 | Tyr | UAC | 79 | 0.98 | |||
| UUG | 67 | 0.72 | UAU | 130 | 1.18 | UUG | 27 | 0.42 | UAU | 76 | 1.2 | UUG | 35 | 0.54 | UAU | 82 | 1.02 | ||||||
| Met | AUG | 64 | 1 | Stop | UAA | 196 | 1.77 | Met | AUG | 51 | 1 | Stop | UAA | 125 | 1.68 | Met | AUG | 48 | 1 | Stop | UAA | 113 | 1.5 |
| Asn | AAC | 178 | 1.08 | UAG | 80 | 0.72 | Asn | AAC | 133 | 0.96 | UAG | 51 | 0.69 | Asn | AAC | 116 | 1 | UAG | 60 | 0.78 | |||
| AAU | 149 | 0.9 | UGA | 57 | 0.51 | AAU | 140 | 1.02 | UGA | 46 | 0.6 | AAU | 113 | 0.98 | UGA | 52 | 0.69 | ||||||
| Species | Collection Method | Collection Depth (m) | Collection Site (GPS) | Collection Date | MERBK Voucher Number |
|---|---|---|---|---|---|
| Henricia longispina aleutica | Trimix SCUBA diving | 42 | Ulleung island, Korea (37°14′58.2″ N, 131°52′1.3″ E) | 23 August 2023 | MERBK-A0093 |
| Henricia reniossa | Netting | 51 | Yangyang, Korea (37°58′42.7″ N, 128°48′42.9″ E) | 1 September 2022 | MERBK-A0018 |
| Henricia sanguinolenta | SCUBA Diving | 26 | Ulleung island, Korea (37°32′32.5″ N, 130°55′13.7″ E) | 21 May 2023 | MERBK-A0066 |
| Class | Order | Family | Species | GenBank Accession No. | References | |
|---|---|---|---|---|---|---|
| 1 | Asteroidea | Brisingida | Brisingidae | Brisinga sp. | MZ702705 | [22] |
| 2 | Freyellidae | Freyastera benthophila | NC_039982 | [23] | ||
| 3 | Forcipulatida | Asteriidae | Asterias amurensis | NC_006665 | [24] | |
| 4 | Aphelasterias japonica | NC_025766 | [25] | |||
| 5 | Coscinasterias acutispina | NC_063787 | [26] | |||
| 6 | Distolasterias nipon | MH473231 | [27] | |||
| 7 | Lethasterias fusca | OR466204 | Unpublished | |||
| 8 | Pisaster ochraceus | NC_042741 | Unpublished | |||
| 9 | Paulasteriidae | Paulasterias sp. | MZ702702 | [22] | ||
| 10 | Zoroasteridae | Zoroaster ophiactis | NC_063669 | [22] | ||
| 11 | Paxillosida | Astropectinidae | Astropecten polyacanthus | NC_006666 | [24] | |
| 12 | Leptychaster arcticus | NC_081967 | Unpublished | |||
| 13 | Benthopectinidae | Cheiraster sp. | MZ702701 | [22] | ||
| 14 | Ctenodiscidae | Ctenodiscus crispatus | NC_071876 | Unpublished | ||
| 15 | Luidiidae | Luidia quinaria | NC_006664 | [24] | ||
| 16 | Porcellanasteridae | Styracaster yapensis | NC_041450 | [28] | ||
| 17 | Peripodida | Xyloplacidae | Xyloplax princealberti | OR818549 | [29] | |
| 18 | Spinulosida | Echinasteridae | Echinaster brasiliensis | NC_037943 | [30] | |
| 19 | Henricia leviuscula | MK947912 | [31] | |||
| 20 | Henricia longispina aleutica | PP384217 | This study | |||
| 21 | Henricia pachyderma | MT079801 | [32] | |||
| 22 | Henricia reniossa | PP384218 | This study | |||
| 23 | Henricia sanguinolenta | PP384219 | This study | |||
| 24 | Valvatida | Acanthasteridae | Acanthaster brevispinus | NC_007789 | [33] | |
| 25 | Acanthaster planci | LC566216 | [34] | |||
| 26 | Archasteridae | Archaster typicus | MN052674 | [35] | ||
| 27 | Asterinidae | Aquilonastra batheri | MH507076 | [27] | ||
| 28 | Nepanthia sp. | MT476595 | [36] | |||
| 29 | Patiria pectinifera | NC_001627 | [37] | |||
| 30 | Goniasteridae | Ceramaster japonicus | LC592775 | [38] | ||
| 31 | Iconaster longimanus | NC_054228 | [36] | |||
| 32 | Ophidiasteridae | Linckia laevigata | LC505032 | [39] | ||
| 33 | Ophidiaster granifer | NC_054227 | [36] | |||
| 34 | Oreasteridae | Anthenea aspera | NC_054230 | [36] | ||
| 35 | Culcita novaeguineae | NC_054229 | [36] | |||
| 36 | Pentaceraster mammillatus | NC_054225 | [36] | |||
| 37 | Protoreaster nodosus | NC_054226 | [36] | |||
| 38 | Poraniidae | Poraniopsis inflata | NC_083191 | [40] | ||
| 39 | Solasteridae | Crossaster japonicus | NC_081981 | Unpublished | ||
| 40 | Crossaster papposus | NC_053361 | Unpublished | |||
| 41 | Velatida | Myxasteridae | Asthenactis papyraceus | NC_063668 | [22] | |
| 42 | Pterasteridae | Euretaster insignis | NC_054231 | [36] | ||
| 43 | Ophiuroidea | Ophiurida | Ophiuridae | Ophiura sarsii | MH780492 | [41] |
| 44 | Ophiura kinbergi | MH910618 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alboasud, M.; Jeong, H.; Lee, T. Complete Mitochondrial Genomes and Phylogenetic Analysis of Genus Henricia (Asteroidea: Spinulosida: Echinasteridae). Int. J. Mol. Sci. 2024, 25, 5575. https://doi.org/10.3390/ijms25115575
Alboasud M, Jeong H, Lee T. Complete Mitochondrial Genomes and Phylogenetic Analysis of Genus Henricia (Asteroidea: Spinulosida: Echinasteridae). International Journal of Molecular Sciences. 2024; 25(11):5575. https://doi.org/10.3390/ijms25115575
Chicago/Turabian StyleAlboasud, Maria, Hoon Jeong, and Taekjun Lee. 2024. "Complete Mitochondrial Genomes and Phylogenetic Analysis of Genus Henricia (Asteroidea: Spinulosida: Echinasteridae)" International Journal of Molecular Sciences 25, no. 11: 5575. https://doi.org/10.3390/ijms25115575
APA StyleAlboasud, M., Jeong, H., & Lee, T. (2024). Complete Mitochondrial Genomes and Phylogenetic Analysis of Genus Henricia (Asteroidea: Spinulosida: Echinasteridae). International Journal of Molecular Sciences, 25(11), 5575. https://doi.org/10.3390/ijms25115575





