Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin–Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions
Abstract
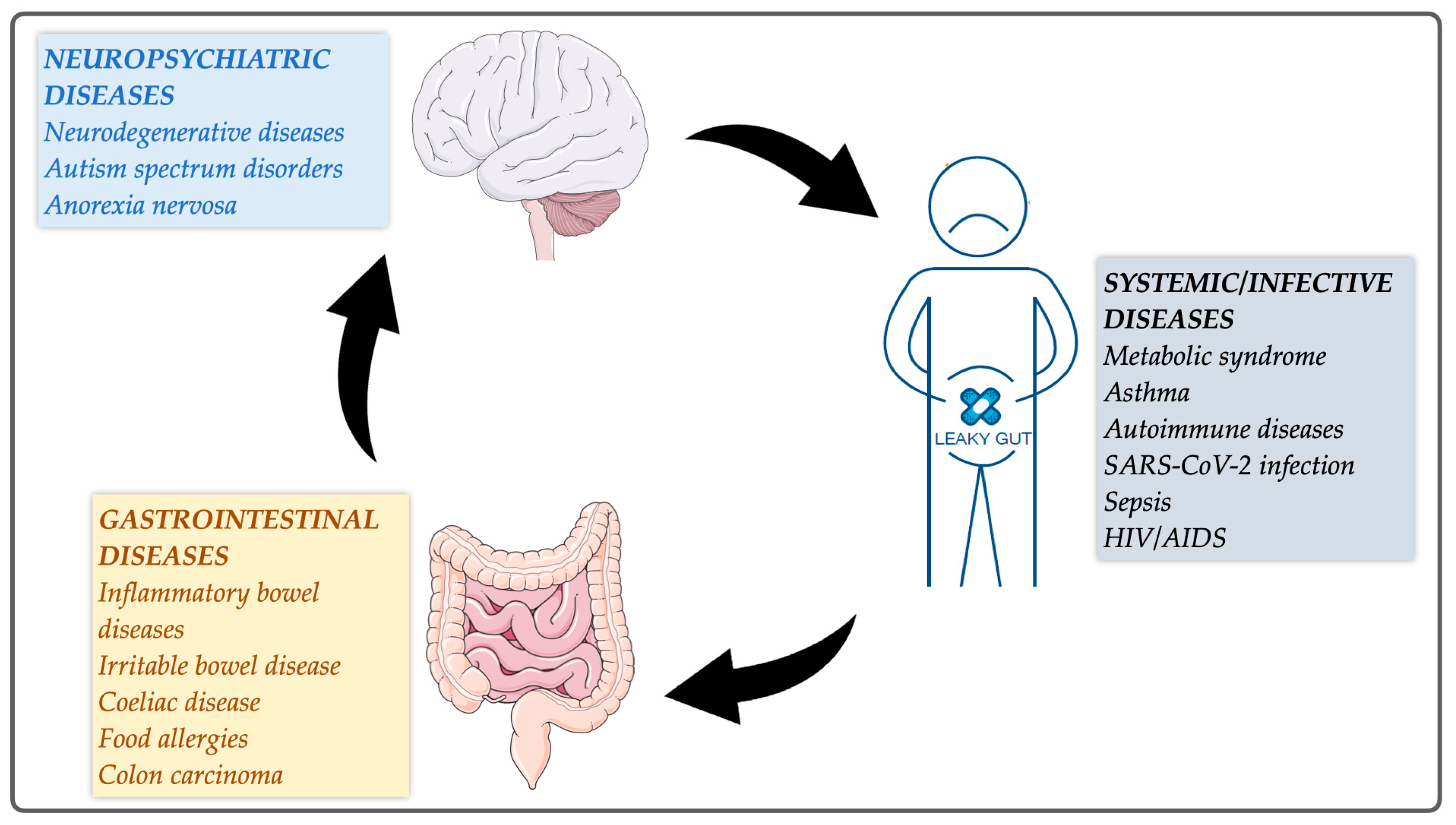
1. Introduction
2. Materials and Methods
3. Results
3.1. Part 1—A Narrative Review
3.1.1. What Are the Morphological and Functional Characteristics of Intestinal Epithelial Cells in the Context of Intestinal Permeability?
3.1.2. How TJs Impact Intestinal Permeability?
3.1.3. Are Intestinal Epithelial Na+/K+ATPase and Tight Junctions Structurally and Functionally Interconnected?
3.1.4. What Are the Key Components of the Renin–Angiotensin System in the Gastrointestinal Tract?
3.1.5. What Are the Key Components of the Dopaminergic System in the Gastrointestinal Tract?
3.2. Part 2—A Systematic Review
3.2.1. Coexpression and Cosynthesis Studies on Epithelial DAergic System and RAS in the Intestines
3.2.2. The Influence of Intestinal DAergic System and RAS on Epithelial Permeability
3.2.3. Intestinal B0AT1 and RAS
3.2.4. Intestinal Na+/K+ ATPase Activity
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular permeability and tight junction regulation in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The intestinal barrier: A fundamental role in health and disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Schulzke, J.D.; Ploeger, S.; Amasheh, M.; Fromm, A.; Zeissig, S.; Troeger, H.; Richter, J.; Bojarski, C.; Schumann, M.; Fromm, M. Epithelial tight junctions in intestinal inflammation. Ann. N. Y. Acad. Sci. 2009, 1165, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Kuo, W.T.; Turner, J.R. Tight Junctions as Targets and Effectors of Mucosal Immune Homeostasis. Cell Mol. Gastroenterol. Hepatol. 2020, 10, 327–340. [Google Scholar] [CrossRef] [PubMed]
- van IJzendoorn, S.C.D.; Derkinderen, P. The Intestinal Barrier in Parkinson’s Disease: Current State of Knowledge. J. Parkinson’s Dis. 2019, 9, S323–S329. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Yazici, D.; Cagan, E.; Tan, G.; Li, M.; Do, E.; Kucukkase, O.C.; Simsek, A.; Kizmaz, M.A.; Bozkurt, T.; Aydin, T.; et al. Disrupted epithelial permeability as a predictor of severe COVID-19 development. Allergy 2023, 78, 2644–2658. [Google Scholar] [CrossRef]
- Gildea, J.J. Dopamine and angiotensin as renal counterregulatory systems controlling sodium balance. Curr. Opin. Nephrol. Hypertens. 2009, 18, 28–32. [Google Scholar] [CrossRef]
- Khan, F.; Spicarová, Z.; Zelenin, S.; Holtbäck, U.; Scott, L.; Aperia, A. Negative reciprocity between angiotensin II type 1 and dopamine D1 receptors in rat renal proximal tubule cells. Am. J. Physiol. Renal Physiol. 2008, 295, F1110–F1116. [Google Scholar] [CrossRef]
- Padia, S.H.; Kemp, B.A.; Howell, N.L.; Keller, S.R.; Gildea, J.J.; Carey, R.M. Mechanisms of dopamine D1 and angiotensin type 2 receptor interaction in natriuresis. Hypertension 2012, 59, 437–445. [Google Scholar] [CrossRef]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain Renin-Angiotensin System and Microglial Polarization: Implications for Aging and Neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef]
- Jackson, L.; Eldahshan, W.; Fagan, S.C.; Ergul, A. Within the Brain: The Renin Angiotensin System. Int. J. Mol. Sci. 2018, 19, 876. [Google Scholar] [CrossRef]
- Campos, J.; Rodrigo, P. Involvement of dopaminergic signaling in the cross talk between the renin-angiotensin system and inflammation. Semin. Immunopathol. 2020, 42, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.R.; Fernandez, A.; Kneer, C.; Cerda, J.J.; Phillips, M.I.; Woodward, E.R. Human intestinal brush border angiotensin-converting enzyme activity and its inhibition by antihypertensive Ramipril. Gastroenterology 1988, 94, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- de Santa Barbara, P.; van den Brink, G.R.; Roberts, D.J. Development and differentiation of the intestinal epithelium. Cell. Mol. Life Sci. CMLS 2003, 60, 1322–1332. [Google Scholar] [CrossRef]
- van der Flier, L.G.; Clevers, H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Rev. Physiol. 2009, 71, 241–260. [Google Scholar] [CrossRef]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Farquhar, M.G.; Palade, G.E. Junctional complexes in various epithelia. J. Cell Biol. 1963, 17, 375–412. [Google Scholar] [CrossRef]
- Staehelin, L.A. Three types of gap junctions interconnecting intestinal epitbelial cells visualized by freeze-etching. Proc. Nat. Acad. Sci. USA 1972, 69, 1318–1321. [Google Scholar] [CrossRef] [PubMed]
- Forster, C. Tight junctions and the modulation of barrier function in disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, N.S.; Antonetti, D.A. Regulation of tight junction and loss of barrier function in pathophysiology. Inter. J. Biochem. Cell Biol. 2004, 36, 1206–1237. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hirase, T.; Itoh, M.; Nagafuchi, A.; Yonemura, S.; Tsukita, S.; Tsukita, S. Occludin: A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993, 123, 1777–1788. [Google Scholar] [CrossRef] [PubMed]
- Gumbiner, B.M. Proteins associated with the cytoplasmic surface of adhesion molecules. Neuron 1993, 11, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Cereijido, M.; Contreras, R.G.; Shoshani, L. Cell adhesion, polarity and epithelia in the dawn of metazoans. Physiol. Rev. 2004, 84, 1229–1262. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Nelson, W.J. Re-solving the cadherin-catenin-actin conundrum. J. Biol. Chem. 2006, 281, 35593–35607. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Kim, C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018, 2018, 2645465. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Turner, J.R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 2008, 181, 683–695. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Tapia, R.; David, C. Crosstalk of tight junction components with signaling pathways. Biochim. Biophys. Acta 2008, 1778, 729–756. [Google Scholar] [CrossRef]
- Cummins, P.M. Occludin: One protein, many forms. Mol. Cell. Biol. 2012, 32, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Weber, C.R.; Raleigh, D.R.; Yu, D.; Turner, J.R. Tight junction pore and leak pathways: A dynamic duo. Annu. Rev. Physiol. 2011, 73, 283–309. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.; Winkler, L.; Mueller, S.L.; Haseloff, R.F.; Piontek, J.; Blasig, I.E. Structure and function of claudins. Biochim. Biophys. Acta 2008, 1778, 631–645. [Google Scholar] [CrossRef]
- Günzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann. N. Y. Acad. Sci. 2000, 915, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and its regulation of intestinal barrier function: The biological door to inflammation, autoimmunity, and cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Citi, S.; Guerrera, D.; Spadaro, D.; Shah, J. Epithelial junctions and Rho family GTPases: The zonular signalosome. Small GTPases 2014, 5, e973760-15. [Google Scholar] [CrossRef] [PubMed]
- Rouaud, F.; Sluysmans, S.; Flinois, A.; Shah, J.; Vasileva, E.; Citi, S. Scaffolding proteins of vertebrate apical junctions: Structure, functions and biophysics. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183399. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, B.R.; Siliciano, J.D.; Mooseker, M.S.; Goodenough, D.A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (Zonula occludens) in a variety of epithelia. J. Cell Biol. 1986, 103, 755–766. [Google Scholar] [CrossRef]
- Bazzoni, G.; Dejana, E. Endothelial cell-to-cell junctions: Molecular organization and role in vascular homeostasis. Physiol. Rev. 2004, 84, 869–901. [Google Scholar] [CrossRef]
- Rao, R. Occludin phosphorylation in regulation of epithelial tight junctions. Ann. N. Y. Acad. Sci. 2009, 1165, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Elias, B.C.; Suzuki, T.; Seth, A.; Giorgianni, F.; Kale, G.; Shen, L.; Turner, J.R.; Naren, A.; Desiderio, D.M.; Rao, R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J. Biol. Chem. 2009, 284, 1559–1569. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Felinski, E.A.; Antonetti, D.A. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability. J. Biol. Chem. 2009, 284, 21036–21046. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Furuse, M.; Sasaki, H.; Schulzke, J.D.; Fromm, M.; Takano, H.; Noda, T.; Tsukita, S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell 2000, 11, 4131–4142. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.C.; Higashi, T.; Fukazawa, Y.; Otani, T.; Tauchi, M.; Higashi, A.Y.; Furuse, M.; Chiba, H. Occludin and tricellulin facilitate formation of anastomosing tight-junction strand network to improve barrier function. Mol. Biol. Cell 2021, 32, 722–738. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J.; Brown, G.T.; Gerner-Smidt, K.; Buret, A.G.; Meddings, J.B.; Quan, C.; Koval, M.; Nusrat, A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am. J. Pathol. 2008, 172, 905–915. [Google Scholar] [CrossRef]
- Amasheh, S.; Meiri, N.; Gitter, A.H.; Schöneberg, T.; Mankertz, J.; Schulzke, J.D.; Fromm, M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002, 115, 4969–4976. [Google Scholar] [CrossRef] [PubMed]
- Van Itallie, C.M.; Fanning, A.S.; Anderson, J.M. Reversal of charge selectivity in cation or anion-selective epithelial lines by expression of different claudins. Am. J. Physiol. Renal Physiol. 2003, 285, F1078–F1084. [Google Scholar] [CrossRef]
- Hou, J.; Paul, D.L.; Goodenough, D.A. Good enough, Paracellin-1 and the modulation of ion selectivity of tight junctions. J. Cell Sci. 2005, 118, 5109–5118. [Google Scholar] [CrossRef]
- Beggs, M.R.; Young, K.; Pan, W.; O’Neill, D.D.; Saurette, M.; Plain, A.; Rievaj, J.; Doschak, M.R.; Cordat, E.; Dimke, H.; et al. Claudin-2 and claudin-12 form independent, complementary pores required to maintain calcium homeostasis. Proc. Natl. Acad. Sci. USA 2021, 118, e2111247118. [Google Scholar] [CrossRef]
- Wada, M.; Tamura, A.; Takahashi, N.; Tsukita, S. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterology 2013, 144, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Klaus, E. Junctional adhesion molecules (JAMs): Cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol. Rev. 2017, 97, 1529–1554. [Google Scholar] [CrossRef] [PubMed]
- Kummer, D.; Ebnet, K. Junctional Adhesion Molecules (JAMs): The JAM-Integrin Connection. Cells 2018, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, T.; Kummer, D.; Ebnet, K. Junctional adhesion molecule-A: Functional diversity through molecular promiscuity. Cell. Mol. Life Sci. 2018, 75, 1393–1409. [Google Scholar] [CrossRef] [PubMed]
- Laukoetter, M.G.; Nava, P.; Lee, W.Y.; Severson, E.A.; Capaldo, C.T.; Babbin, B.A.; Williams, I.R.; Koval, M.; Peatman, E.; Campbell, J.A.; et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J. Exp. Med. 2007, 204, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Smith, M.S.; Keeney, J.; O’Leary, M.N.; Nusrat, A.; Parkos, C.A. JAM-A signals through the Hippo pathway to regulate intestinal epithelial proliferation. iScience 2022, 25, 104316. [Google Scholar] [CrossRef] [PubMed]
- Contreras, R.G.; Shoshani, L.; Flores-Maldonado, C.; Lázaro, A.; Cereijido, M. Relationship between Na+,K+-ATPase and cell attachment. J. Cell Sci. 1999, 112, 4223–4232. [Google Scholar] [CrossRef]
- Nguyen, A.N.; Wallace, D.P.; Blanco, G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J. Am. Soc. Nephrol. 2007, 18, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.I.; Tian, J. Signaling through the Na/K-ATPase: Implications for cardiac fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H29–H30. [Google Scholar] [CrossRef]
- Pavlov, K.V.; Sokolov, V.S. Electrogenic ion transport by Na+,K+-ATPase. Membr. Cell Biol. 2000, 13, 745–788. [Google Scholar]
- Schultz, S.G.; Curran, P.F. The role of sodium in non-electrolyte transport across animal cell membranes. Physiologist 1969, 12, 437–452. [Google Scholar] [PubMed]
- Cereijido, M.; Shoshani, L.; Contreras, R.G. The polarized distribution of Na+, K+-ATPase and active transport across epithelia. J. Membr. Biol. 2001, 184, 299–304. [Google Scholar] [CrossRef]
- Lingrel, J.B.; Orlowski, J.; Shull, M.M.; Price, E.M. Molecular genetics of Na,K-ATPase. Prog. Nucleic Acid. Res. Mol. Biol. 2008, 38, 37–89. [Google Scholar]
- Robinson, J.D.; Flashner, M.S. The (Na+ + K+)-activated ATPase. Enzymatic and transport properties. Biochim. Biophys. Acta 1979, 549, 145–176. [Google Scholar] [CrossRef] [PubMed]
- Jewell, E.A.; Shamraj, O.I.; Lingrel, J.B. Isoforms of the alpha subunit of Na,K-ATPase and their significance. Acta Physiol. Scand. Suppl. 1992, 607, 161–169. [Google Scholar]
- Pressley, T.A.; Allen, J.C.; Clarke, C.H.; Odebunmi, T.; Higham, S.C. Amino-terminal processing of the catalytic subunit from Na+-K+-ATPase. Am. J. Physiol. 1996, 271, C825–C832. [Google Scholar] [CrossRef]
- Ackermann, U.; Geering, K. Mutual dependence of Na,K-ATPase alpha- and beta-subunits for correct posttranslational processing and intracellular transport. FEBS Lett. 1990, 269, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. The functional role of the beta-subunit in the maturation and intracellular transport of Na,K-ATPase. FEBS Lett. 1991, 285, 189–193. [Google Scholar] [CrossRef]
- Aperia, A.C. Regulation of sodium transport. Curr. Opin. Nephrol. Hypertens. 1995, 4, 416–420. [Google Scholar] [CrossRef]
- Bertorello, A.M.; Katz, A.I. Short-term regulation of renal Na-K-ATPase activity: Physiological relevance and cellular mechanisms. Am. J. Physiol. 1993, 265, F743–F755. [Google Scholar] [CrossRef]
- Sundaram, U.; Wisel, S.; Rajendren, V.M.; West, A.B. Mechanism of inhibition of Na+-glucose cotransport in the chronically inflamed rabbit ileum. Am. J. Physiol. 1997, 273, G913–G919. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, U.; Wisel, S.; Fromkes, J.J. Unique mechanism of inhibition of Na+-amino acid cotransport during chronic ileal inflammation. Am. J. Physiol. 1998, 275, G483–G489. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Manoharan, P.; Arthur, S.; Sundaram, S.; Kekuda, R.; Sundaram, U. Molecular mechanism of regulation of villus cell Na-K-ATPase in the chronically inflamed mammalian small intestine. Biochim. Biophys. Acta 2015, 1848, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.A.; Palmer, L.G.; Moon, S.Y.; Peralta Soler, A.; Apodaca, G.L.; Harper, J.F.; Zheng, Y.; Rajasekaran, A.K. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol. Biol. Cell 2001, 12, 3717–3732. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Han, W.; Pagel, P.; Nairn, A.C.; Caplan, M.J. Protein phosphatase 2A interacts with the Na,K-ATPase and modulates its trafficking by inhibition of its association with arrestin. PLoS ONE 2011, 6, e29269. [Google Scholar] [CrossRef] [PubMed]
- Seth, A.; Sheth, P.; Elias, B.C.; Rao, R. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 2007, 282, 11487–11498. [Google Scholar] [CrossRef] [PubMed]
- Nunbhakdi-Craig, V.; Machleidt, T.; Ogris, E.; Bellotto, D.; White, C.L.; Sontag, E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J. Cell Biol. 2002, 158, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.A.; Barwe, S.P.; Gopal, J.; Ryazantsev, S.; Schneeberger, E.E.; Rajasekaran, A.K. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G124–G133. [Google Scholar] [CrossRef] [PubMed]
- Basso, N.; Terragno, N.A. History about the discovery of the renin-angiotensin system. Hypertension 2001, 38, 1246–1249. [Google Scholar] [CrossRef]
- Peach, M.J. Renin-angiotensin system: Biochemistry and mechanisms of action. Physiol. Rev. 1977, 57, 313–370. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Derkx, F.; Schalekamp, M. Human prorenin: Pathophysiological and clinical implications. Clin. Exp. Hypertens. 1988, 10, 1213–1225. [Google Scholar]
- Stubbs, A.J.; Skinner, S.L. Lectin chromatography of extrarenal renin protein in human plasma and tissues: Potential endocrine function via the renin receptor. J. Renin. Angiotensin. Aldosterone. Syst. 2004, 5, 189–196. [Google Scholar] [CrossRef]
- Fleming, I. Signaling by the angiotensin-converting enzyme. Circ. Res. 2006, 98, 887–896. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 532, 107–110. [Google Scholar] [CrossRef]
- Tipnis, S.R.; Hooper, N.M.; Hyde, R.; Karran, E.; Christie, G.; Turner, A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000, 275, 33238–33243. [Google Scholar] [CrossRef]
- McCarthy, C.A.; Widdop, R.E.; Denton, K.M.; Jones, E.S. Update on the angiotensin AT2 receptor. Curr. Hypertens. Rep. 2013, 15, 25–30. [Google Scholar] [CrossRef]
- Santos, R.A.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef]
- Turker, R.K.; Kayaalp, S.O. Inhibitory effect of angiotensin on the intestinal motility of the cat and its relation to sympa-thetic nervous system. Arch. Int. Physiol. Biochim. 1967, 75, 735–744. [Google Scholar]
- Beleslin, D.B. The effect of angiotensin on the peristaltic reflex of the isolated guinea-pig ileum. Br. J. Pharmacol. 1968, 32, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Koper, M.; Ufnal, M. Gut microbiota and renin-angiotensin system: A complex interplay at local and systemic levels. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G355–G366. [Google Scholar] [CrossRef] [PubMed]
- Fändriks, L. The angiotensin II type 2 receptor and the gastrointestinal tract. J. Renin Angiotensin Aldosterone Syst. 2010, 11, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Fändriks, L. The renin-angiotensin system and the gastrointestinal mucosa. Acta Physiol. 2011, 201, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Angus, P.W.; Burrell, L.M.; Herath, C.; Gibson, P.R.; Lubel, J.S. Review article: The pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment. Pharmacol. Ther. 2012, 35, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J.; Habener, J.F. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J. Clin. Investig. 1986, 78, 31–39. [Google Scholar] [CrossRef]
- Hirasawa, K.; Sato, Y.; Hosoda, Y.; Yamamoto, T.; Hanai, H. Immunohistochemical localization of angiotensin II receptor and local renin-angiotensin system in human colonic mucosa. J. Histochem. Cytochem. 2002, 50, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.P.; Debnam, E.S.; Leung, P.S. Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J. Physiol. 2007, 584, 613–623. [Google Scholar] [CrossRef]
- Spak, E.; Casselbrant, A.; Olbers, T.; Lönroth, H.; Fändriks, L. Angiotensin II-induced contractions in human jejunal wall musculature in vitro. Acta Physiol. 2008, 193, 181–190. [Google Scholar] [CrossRef]
- Wong, T.P.; Debnam, E.S.; Leung, P.S. Diabetes mellitus and expression of the enterocyte renin-angiotensin system: Implications for control of glucose transport across the brush border membrane. Am. J. Physiol. Cell Physiol. 2009, 297, C601–C610. [Google Scholar] [CrossRef]
- Hallersund, P.; Elfvin, A.; Helander, H.F.; Fändriks, L. The expression of renin-angiotensin system components in the human gastric mucosa. J. Renin Angiotensin Aldosterone Syst. 2011, 12, 54–64. [Google Scholar] [CrossRef]
- Hume, G.E.; Doecke, J.D.; Huang, N.; Fowler, E.V.; Brown, I.S.; Simms, L.A.; Radford-Smith, G.L. Altered Expression of Angiotensinogen and Mediators of Angiogenesis in Ileal Crohn’s Disease. J. Gastrointest. Liver Dis. 2016, 25, 39–48. [Google Scholar] [CrossRef]
- Pasanen, L.; Launonen, H.; Siltari, A.; Korpela, R.; Vapaatalo, H.; Salmenkari, H.; Forsgard, R.A. Age-related changes in the local intestinal renin-angiotensin system in normotensive and spontaneously hypertensive rats. J. Physiol. Pharmacol. 2019, 70. [Google Scholar] [CrossRef]
- Jaszewski, R.; Tolia, V.; Ehrinpreis, M.N.; Bodzin, J.H.; Peleman, R.R.; Korlipara, R.; Weinstock, J.V. Increased colonic mucosal angiotensin I and II concentrations in Crohn’s colitis. Gastroenterology 1990, 98, 1543–1548. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Jardé, T.; McCarthy, A.; Ashworth, A.; de Leng, W.W.; Offerhaus, G.J.; Resta, N.; Dale, T.; Clarke, A.R. Intestinal renin-angiotensin system is stimulated after deletion of Lkb1. Gut 2012, 61, 202–213. [Google Scholar] [CrossRef]
- Wong, T.P.; Ho, K.Y.; Ng, E.K.; Debnam, E.S.; Leung, P.S. Upregulation of ACE2-ANG-(1–7)-Mas axis in jejunal enterocytes of type 1 diabetic rats: Implications for glucose transport. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E669–E681. [Google Scholar] [CrossRef]
- Duggan, K.A.; Mendelsohn, F.A.; Levens, N.R. Angiotensin receptors and angiotensin I-converting enzyme in rat intestine. Am. J. Physiol. 1989, 257, G504–G510. [Google Scholar] [CrossRef]
- Schinke, M.; Doods, H.N.; Ganten, D.; Wienen, W.; Entzeroth, M. Characterization of the angiotensin II receptor subtype in rat ileum. Eur. J. Pharmacol. 1991, 204, 165–170. [Google Scholar] [CrossRef]
- Sechi, L.A.; Valentin, J.P.; Griffin, C.A.; Schambelan, M. Autoradiographic characterization of angiotensin II receptor subtypes in rat intestine. Am. J. Physiol. 1993, 265, G21–G27. [Google Scholar] [CrossRef]
- Ewert, S.; Spak, E.; Olbers, T.; Johnsson, E.; Edebo, A.; Fändriks, L. Angiotensin II induced contraction of rat and human small intestinal wall musculature in vitro. Acta Physiol. 2006, 188, 33–40. [Google Scholar] [CrossRef]
- Casselbrant, A.; Edebo, A.; Hallersund, P.; Spak, E.; Helander, H.F.; Jönson, C.; Fändriks, L. Angiotensin II receptors are expressed and functional in human esophageal mucosa. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1019–G1027. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Zennaro, C.; Palmisano, S.; Velkoska, E.; Sabato, N.; Toffoli, B.; Giacomel, G.; Buri, L.; Zanconati, F.; Bellini, G.; et al. Characterization and significance of ACE2 and Mas receptor in human colon adenocarcinoma. J. Renin Angiotensin Aldosterone Syst. 2012, 13, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Mastropaolo, M.; Zizzo, M.G.; Mulè, F.; Serio, R. Angiotensin II contractile effects in mouse colon: Role for pre- and post-junctional AT1A receptors. Acta Physiol. 2013, 207, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Ha, G.W.; Kim, J.H.; Kim, S.H. Effects of angiotensin peptides on colonic motility in rats. Ann. Coloproctol. 2023, 39, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Avula, L.R.; Buckinx, R.; Favoreel, H.; Cox, E.; Adriaensen, D.; Van Nassauw, L.; Timmermans, J.P. Expression and distribution patterns of Mas-related gene receptor subtypes A-H in the mouse intestine: Inflammation-induced changes. Histochem. Cell Biol. 2013, 139, 639–658. [Google Scholar] [CrossRef] [PubMed]
- Casselbrant, A.; Kostic, S.; Lönroth, H. The muscular expression of RAS in patients with achalasia. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Fukamizu, A.; Saito, T.; Murakami, K. Identification of a previously unrecognized production site of human renin. Biochim. Biophys. Acta 1991, 1129, 87–89. [Google Scholar] [CrossRef] [PubMed]
- Bruneval, P.; Hinglais, N.; Alhenc-Gelas, F.; Tricottet, V.; Corvol, P.; Menard, J.; Camilleri, J.P.; Bariety, J. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry 1986, 85, 73–80. [Google Scholar] [CrossRef]
- Carl-McGrath, S.; Gräntzdörffer, I.; Lendeckel, U.; Ebert, M.P.; Röcken, C. Angiotensin II-generating enzymes, angiotensin-converting enzyme (ACE) and mast cell chymase (CMA1), in gastric inflammation may be regulated by H. pylori and associated cytokines. Pathology 2009, 41, 419–427. [Google Scholar] [CrossRef]
- Vuille-dit-Bille, R.N.; Camargo, S.M.; Emmenegger, L.; Sasse, T.; Kummer, E.; Jando, J.; Hamie, Q.M.; Meier, C.F.; Hunziker, S.; Forras-Kaufmann, Z.; et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 2015, 47, 693–705. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A. Thirty years of dopamine research. Adv. Neurol. 1993, 60, 1–10. [Google Scholar] [PubMed]
- Eisenhofer, G.; Aneman, A.; Friberg, P.; Hooper, D.; Fåndriks, L.; Lonroth, H.; Hunyady, B.; Mezey, E. Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 1997, 82, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Musacchio, J.M. Chapter 1: Enzymes involved in the biosynthesis and degradation of catecholamines. In Bio-Chemistry of Biogenic Amines; Iverson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–35. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; pp. 589–768. [Google Scholar]
- Elango, R.; Ball, R.O.; Pencharz, P.B. Recent advances in determining protein and amino acid requirements in humans. Br. J. Nutr. 2012, 108 (Suppl. S2), S22–S30. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol. Rev. 2008, 88, 249–286. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Pham, T.D.; Tamir, H.; Chen, J.J.; Gershon, M.D. Enteric dopaminergic neurons: Definition, developmental lineage, and effects of extrinsic denervation. J. Neurosci. 2004, 24, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Anlauf, M.; Schäfer, M.K.-H.; Eiden, L.; Weihe, E. Chemical coding of the human gastrointestinal nervous system: Cholinergic, VIPergic, and catecholaminergic phenotypes. J. Comp. Neurol. 2003, 459, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Zhang, X.-L.; Feng, X.-Y.; Liu, C.-Z.; Zhang, X.-N.; Quan, Z.-S.; Yan, J.-T.; Zhu, J.-X. Dopamine promotes colonic mucus secretion through dopamine D5 receptor in rats. Am. J. Physiol. Cell Physiol. 2019, 316, C393–C403. [Google Scholar] [CrossRef]
- Li, Z.S.; Schmauss, C.; Cuenca, A.; Ratcliffe, E.; Gershon, M.D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: Analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 2006, 26, 2798–2807. [Google Scholar] [CrossRef]
- Vaughan, C.J.; Aherne, A.M.; Lane, E.; Power, O.; Carey, R.M.; O’Connell, D.P. Identification and regional distribution of the dopamine D(1A) receptor in the gastrointestinal tract. Am. J. Physiol. Integr. Comp. Physiol. 2000, 279, R599–R609. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Liu, J.F. Expression of dopamine receptors in human lower esophageal sphincter. J. Gastroenterol. Hepatol. 2012, 27, 945–950. [Google Scholar] [CrossRef]
- Feng, X.Y.; Li, Y.; Li, L.S.; Li, X.F.; Zheng, L.F.; Zhang, X.L.; Fan, R.F.; Song, J.; Hong, F.; Zhang, Y.; et al. Dopamine D1 receptors mediate dopamine-induced duodenal epithelial ion transport in rats. Transl. Res. 2013, 161, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Marmon, L.M.; Albrecht, F.; Canessa, L.M.; Hoy, G.R.; Jose, P.A. Identification of dopamine1A receptors in the rat small intestine. J. Surg. Res. 1993, 54, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.G.; Bellanca, A.; Amato, A.; Serio, R. Opposite effects of dopamine on the mechanical activity of circular and longitudinal muscle of human colon. Neurogastroenterol. Motil. 2020, 32, e13811. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef]
- Jose, P.A.; Yang, Z.; Zeng, C.; Felder, R.A. The importance of the gastrorenal axis in the control of body sodium homeostasis. Exp. Physiol. 2016, 101, 465–470. [Google Scholar] [CrossRef]
- Serio, R.; Zizzo, M.G. The multiple roles of dopamine receptor activation in the modulation of gastrointestinal motility and mucosal function. Auton. Neurosci. 2023, 244, 103041. [Google Scholar] [CrossRef]
- Kurnik-Łucka, M.; Pasieka, P.; Łączak, P.; Wojnarski, M.; Jurczyk, M.; Gil, K. Gastrointestinal Dopamine in Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 12932. [Google Scholar] [CrossRef]
- Hamamah, S.; Aghazarian, A.; Nazaryan, A.; Hajnal, A.; Covasa, M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines 2022, 10, 436. [Google Scholar] [CrossRef]
- Nataf, S.; Pays, L. Molecular Insights into SARS-CoV2-Induced Alterations of the Gut/Brain Axis. Int. J. Mol. Sci. 2021, 22, 10440. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Gil, P.; Dominguez-Meijide, A.; Moratalla, R.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-related dysregulation in enteric dopamine and angiotensin system interactions: Implications for gastrointestinal dysfunction in the elderly. Oncotarget 2018, 9, 10834–10846. [Google Scholar] [CrossRef] [PubMed]
- Chittimalli, K.; Jahan, J.; Sakamuri, A.; McAdams, Z.L.; Ericsson, A.C.; Jarajapu, Y.P.R. Restoration of the gut barrier integrity and restructuring of the gut microbiome in aging by angiotensin-(1–7). Clin. Sci. 2023, 137, 913–930. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Zhang, D.N.; Wang, Y.A.; Fan, R.F.; Hong, F.; Zhang, Y.; Li, Y.; Zhu, J.X. Dopamine enhances duodenal epithelial permeability via the dopamine D 5 receptor in rodent. Acta Physiol. 2017, 220, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Singer, D.; Camargo, S.M.; Ramadan, T.; Schäfer, M.; Mariotta, L.; Herzog, B.; Huggel, K.; Wolfer, D.; Werner, S.; Penninger, J.M.; et al. Defective intestinal amino acid absorption in Ace2 null mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G686–G695. [Google Scholar] [CrossRef] [PubMed]
- Camargo, S.M.; Singer, D.; Makrides, V.; Huggel, K.; Pos, K.M.; Wagner, C.A.; Kuba, K.; Danilczyk, U.; Skovby, F.; Kleta, R.; et al. Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 2009, 136, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Kowalczuk, S.; Bröer, A.; Tietze, N.; Vanslambrouck, J.M.; Rasko, J.E.; Bröer, S. A protein complex in the brush-border membrane explains a Hartnup disorder allele. FASEB J. 2008, 22, 2880–2887. [Google Scholar] [CrossRef]
- Kowalczuk, S.; Bröer, A.; Tietze, N.; Vanslambrouck, J.M.; Rasko, J.E.; Bröer, S. Circulating Ouabain Modulates Expression of Claudins in Rat Intestine and Cerebral Blood Vessels. Int. J. Mol. Sci. 2020, 21, 5067. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Serrão, P.; Hussain, T.; Lokhandwala, M.F.; Soares-da-Silva, P. Salt intake and intestinal dopaminergic activity in adult and old Fischer 344 rats. Life Sci. 2001, 69, 1957–1968. [Google Scholar] [CrossRef]
- Vieira-Coelho, M.A.; Soares-da-Silva, P. Comparative study on sodium transport and Na+,K+-ATPase activity in Caco-2 and rat jejunal epithelial cells: Effects of dopamine. Life Sci. 2001, 69, 1969–1981. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Teixeira, V.A.; Vieira-Coelho, M.A.; Serrão, P.; Pestana, M.; Soares-da-Silva, P. Salt intake and sensitivity of intestinal and renal Na+-K+ atpase to inhibition by dopamine in spontaneous hypertensive and Wistar-Kyoto rats. Clin. Exp. Hypertens. 2000, 22, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Teixeira, V.; Serrão, M.P.; Soares-Da-Silva, P. Effect of salt intake on jejunal dopamine, Na+,K+-ATPase activity and electrolyte transport. Acta Physiol. Scand. 2000, 168, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Coelho, M.A.; Soares-da-Silva, P. Ontogenic aspects of D1 receptor coupling to G proteins and regulation of rat jejunal Na+, K+ ATPase activity and electrolyte transport. Br. J. Pharmacol. 2000, 129, 573–581. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vieira-Coelho, M.A.; Teixeira, V.A.; Finkel, Y.; Soares-Da-Silva, P.; Bertorello, A.M. Dopamine-dependent inhibition of jejunal Na+-K+-ATPase during high-salt diet in young but not in adult rats. Am. J. Physiol. 1998, 275, G1317–G1323. [Google Scholar] [CrossRef] [PubMed]
- Finkel, Y.; Eklöf, A.C.; Granquist, L.; Soares-da-Silva, P.; Bertorello, A.M. Endogenous dopamine modulates jejunal sodium absorption during high-salt diet in young but not in adult rats. Gastroenterology 1994, 107, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Gutman, Y.; Shamir, Y.; Glushevitzky, D.; Hochman, S. Angiotensin increases microsomal (Na+-K+)-ATPase activity in several tissues. Biochim. Biophys. Acta 1972, 273, 401–405. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, B.; Jiang, Y.; Lin, L.; Li, X.; Yang, S. DA-DRD5 signaling controls colitis by regulating colonic M1/M2 macrophage polarization. Cell Death Dis. 2021, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef]
- Stevens, B.R.; Ross, H.J.; Wright, E.M. Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J. Membr. Biol. 1982, 66, 213–225. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Y.H.; Zhang, W. Regulation of Intestinal Epithelial Cells Properties and Functions by Amino Acids. BioMed Res. Int. 2018, 2018, 2819154. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Wu, C.; Li, P.; Li, N.; Zhang, D.; Zhu, Q.; Ren, W.; Peng, Y. Functions and Signaling Pathways of Amino Acids in Intestinal Inflammation. BioMed Res. Int. 2018, 2018, 9171905. [Google Scholar] [CrossRef]
- Nagarjun, S.; Dhadde, S.B.; Veerapur, V.P.; Thippeswamy, B.S.; Chandakavathe, B.N. Ameliorative effect of chromium-d-phenylalanine complex on indomethacin-induced inflammatory bowel disease in rats. Biomed. Pharmacother. 2017, 89, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Tan, B.; Huang, B.; Li, J.; Wang, J.; Liao, P.; Guan, G.; Ji, P.; Yin, Y. Involvement of calcium-sensing receptor activation in the alleviation of intestinal inflammation in a piglet model by dietary aromatic amino acid supplementation. Br. J. Nutr. 2018, 120, 1321–1331. [Google Scholar] [CrossRef]
- Yin, L.; Gupta, R.; Vaught, L.; Grosche, A.; Okunieff, P.; Vidyasagar, S. An amino acid-based oral rehydration solution (AA-ORS) enhanced intestinal epithelial proliferation in mice exposed to radiation. Sci. Rep. 2016, 6, 37220. [Google Scholar] [CrossRef] [PubMed]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int. J. Tryptophan Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Klaessens, S.; Stroobant, V.; De Plaen, E.; Van den Eynde, B.J. Systemic tryptophan homeostasis. Front. Mol. Biosci. 2022, 9, 897929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yoshihara, K.; Miyata, N.; Hata, T.; Altaisaikhan, A.; Takakura, S.; Asano, Y.; Izuno, S.; Sudo, N. Dietary tryptophan, tyrosine, and phenylalanine depletion induce reduced food intake and behavioral alterations in mice. Physiol. Behav. 2022, 244, 113653. [Google Scholar] [CrossRef] [PubMed]
- Maini Rekdal, V.; Bess, E.N.; Bisanz, J.E.; Turnbaugh, P.J.; Balskus, E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science 2019, 364, eaau6323. [Google Scholar] [CrossRef]
- González-Arancibia, C.; Urrutia-Piñones, J.; Illanes-González, J.; Martinez-Pinto, J.; Sotomayor-Zárate, R.; Julio-Pieper, M.; Bravo, J.A. Do your gut microbes affect your brain dopamine? Psychopharmacology 2019, 236, 1611–1622. [Google Scholar] [CrossRef]
- Stevens, B.R.; Ellory, J.C.; Preston, R.L. B0AT1 Amino Acid Transporter Complexed With SARS-CoV-2 Receptor ACE2 Forms a Heterodimer Functional Unit: In Situ Conformation Using Radiation Inactivation Analysis. Function 2021, 2, zqab027. [Google Scholar] [CrossRef]
- Khajah, M.A.; Fateel, M.M.; Ananthalakshmi, K.V.; Luqmani, Y.A. Anti-inflammatory action of angiotensin 1–7 in experimental colitis. PLoS ONE 2016, 11, e0150861. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Fateel, M.M.; Ananthalakshmi, K.V.; Luqmani, Y.A. Anti-inflammatory action of angiotensin 1–7 in experimental colitis may be mediated through modulation of serum cytokines/chemokines and immune cell functions. Dev. Comp. Immunol. 2017, 74, 200–208. [Google Scholar] [CrossRef]
- Salmenkari, H.; Korpela, R.; Vapaatalo, H. Renin-angiotensin system in intestinal inflammation-Angiotensin inhibitors to treat inflammatory bowel diseases? Basic Clin. Pharmacol. Toxicol. 2021, 129, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Hakam, A.C.; Hussain, T. Angiotensin II type 2 receptor agonist directly inhibits proximal tubule sodium pump activity in obese but not in lean Zucker rats. Hypertension 2006, 47, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Hakam, A.C.; Hussain, T. Angiotensin II AT2 receptors inhibit proximal tubular Na+-K+-ATPase activity via a NO/cGMP-dependent pathway. Am. J. Physiol. Renal Physiol. 2006, 290, F1430–F1436. [Google Scholar] [CrossRef]
- Shakibfar, S.; Allin, K.H.; Jess, T.; Barbieri, M.A.; Battini, V.; Simoncic, E.; Kirchgesner, J.; Ulven, T.; Sessa, M. Drug Repurposing in Crohn’s Disease Using Danish Real-World Data. Pragmat. Obs. Res. 2024, 15, 17–29. [Google Scholar] [CrossRef]
- Salazar, A.M.; Aparicio, R.; Clark, R.I.; Rera, M.; Walker, D.W. Intestinal barrier dysfunction: An evolutionarily conserved hallmark of aging. Dis. Model Mech. 2023, 16, dmm049969. [Google Scholar] [CrossRef]

| Class of TJ Protein | Type of Protein | Molecular Mass | Major Function | References |
|---|---|---|---|---|
| Integral (intrinsic) transmembrane | Occludin (OCLN, BLCPMG, PPP1R115, PTORCH1) | ~65 kDa | barrier properties, expression of glucose transporters, activation of transcription factors | [31,32] |
| Claudin (CLDN) 1–27 | 21–34 kDa | selective permeability—charge and size selectivity | [2,32,33,34] | |
| Junctional adhesion molecules (JAMs) | ~40 kDa | barrier and cell motility, polarity, and proliferation | [32] | |
| Tricellulin (MARVELD2, MARVEL domain containing 2) | ~66–72 kDa | formation of the epithelial barrier | ||
| Peripheral (extrinsic plaque) membrane protein | Zonulin 1, 2 and 3 (ZO-1, 2, and 3 OR Tight junction protein-1, 2, and 3) | ~47 kDa | a primary barrier—diffusion of solutes and a fence, maintenance of polarity | [35,36] |
| Cingulin (CGN) | 140–160 kDa | adaptor protein | [37,38] | |
| Paracingulin (Cingulin-like 1 protein, CGNL1, or JACOP) | ~160 kDa |
| RAS Components | GI Distribution | References | |
|---|---|---|---|
| Quantification of Transcript Levels | Quantitative and Qualitative Protein Abundance | ||
| Angiotensinogen | stomach, colon, and mesentery (r); enterocytes of jejunum and ileum (r); jejunal muscle wall (h); ileum (h); jejunum and colon (r); sigmoid and ascending colon (h) | antral and corporal gastric mucosa (WB, h); resident mesenchymal cells in the lamina propria, and vascular endothelial cells in antral and corporal gastric mucosa (IHC, h); small intestinal brush border membrane and lamina propria, muscularis mucosa, the muscle layer, and submucosal blood vessels (IHC, r); enterocytes of ileum and jejunum (WB, r), | [96,97,98,99,100,101,102,103] |
| Angiotensin I | N/A | colonic mucosa (RIA; h) | [104] |
| Angiotensin II | crypt and crypt–villus junction small intestinal epithelial cells (m) | [104,105] | |
| Angiotensin (1–7) | jejunal enterocytes (r) | jejunal enterocytes (WB, r) | [106] |
| AT1R | esophageal mucosa (h); small intestinal epithelial brush border (m), enterocytes of jejunum and ileum (r); jejunal muscle wall (h); jejunum and colon (r); colon mucosa (h); sigmoid colon and ascending colon (h); proximal and distal colon (m); colon (r) | esophageal mucosa (WB, h); esophageal mucosa epithelium and stratum superficiale and spinosum, blood vessel walls supplying the epithelium and the lamina propria (IHC, h); antral and corporal gastric mucosa (WB, h); antral and corporal gastric mucosa—basal parts of most epithelial cells, endothelial cells of vessels, some resident mesenchymal cells in the lamina propria, and only antral mucosal endocrine cells (IHC, h); enterocytes of jejunum and ileum (WB, r); jejunum and entire ileum villus length at both brush border and basolateral membranes, lamina propria, muscularis mucosa, muscle layers, and submucosal blood vessels (IHC, r); jejunal myenteric plexus and musculature (IHC, h); muscularis of duodenum, jejunum, ileum, and colon (autoradiography, r); duodenum and ileum (especially longitudinal smooth muscle; autoradiography, r); mucosa, and the muscularis of jejunum, ileum, and colon (autoradiography, r); sigmoid colon and ascending colon vessel walls, surface epithelium, crypt bases, mesenchymal cells in the lamina propria (especially macrophages), and myofibroblasts (IHC, h) | [97,98,99,100,101,103,105,106,107,108,109,110,111,112,113,114] |
| AT2R | esophageal mucosa (h); enterocytes of jejunum and ileum (r); jejunal muscle wall (h); sigmoid colon and ascending colon (h); distal colon (m); colon (r) | esophageal mucosa (WB, h); esophageal mucosa epithelium and in superficial stratum and spinosum, as well as in blood vessel walls supplying the epithelium in the lamina propria (IHC, h); antral and corporal gastric mucosa (WB, h); antral and corporal gastric muscosa—basal parts of most epithelial cells, endothelial cells of vessels, and some resident mesenchymal cells in the lamina propria (IHC, h); jejunal myenteric plexus (IHC, h); enterocytes of jejunum and ileum (WB, r); entire jejunum villus length at the brush border membrane (IHC, r); mucosa and muscularis of jejunum, ileum, and colon (autoradiography, r); sigmoid colon and ascending colon—mesenchymal cells and parts of surface epithelium, as well as a small number of crypt cells (IHC, h); | [97,98,99,100,101,103,104,109,111] |
| MasR | muscular layer of distal esophagus (h); jejunal enterocytes (r); ileum (m); jejunum and colon (r); colon mucosa (h); colon (r) | jejunal enterocytes (WB, IHC, r); ileum—with a neuron-specific pattern (IHC, m); colon mucosa (IHC, h) | [103,104,105,106,112,114,115,116] |
| Renin | muscular layer of distal esophagus (h); small intestine (m, r, h); sigmoid colon and ascending colon (h) | antral and corporal gastric mucosa (WB, h); resident mesenchymal cells in the lamina propria and vascular endothelial cells in antral and corporal gastric mucosa (IHC, h); sigmoid colon and ascending colon—surface epithelium, vessel walls, muscularis mucosae, mesenchymal cells in the lamina propria (IHC, h) | [97,101,116,117] |
| ACE | esophageal mucosa (h); duodenum, jejunum, ileum (h); jejunal muscle wall (h); duodenal and ileal mucosa (h); enterocytes of jejunum and ileum (r); jejunum and colon (r); sigmoid colon and ascending colon (h); colon mucosa (h); colon (r) | esophageal mucosa (WB, h)—the capillary walls located at the tip of the papillae and in the blood vessel walls in the lamina propria (IHC, h); stomach fundic chief cells, and the mucin-secreting cells of the antral and pyloric region (IHC, h); antral and corporal gastric mucosa (WB, h); vascular endothelial cells in both the antral and corporal gastric mucosa (IHC, h); entire villus length at the brush border membrane of the jejunum (IHC, r); enterocytes of jejunum and ileum (WB, r); small intestinal epithelial cells, microvilli, brush borders, and microvascular endothelium (IHC, h); mucosa and muscularis of duodenum, jejunum, ileum, and colon (autoradiography, r); jejunum and colon (WB, r); vessel walls and mesenchymal cells in lamina propria and submucosa and weakly in parts of the surface epithelium in ascending colon (IHC, h) | [86,97,98,99,100,101,103,107,111,112,114,118,119,120] |
| ACE2 | jejunal enterocytes (r); duodenum, jejunum, ileum, caecum, and colon (h); duodenal and ileal mucosa (h); jejunum and colon (r); colonic mucosa (h); colon (r) | jejunal enterocytes (WB, r); small intestinal enterocytes (brush border), smooth muscle cells and endothelium of vessels from the stomach, small intestine and colon, smooth muscle cells of the muscularis mucosae and the muscularis propria (IHC, h); along the villi and in the crypts of duodenum and terminal ileum, as well as in crypts in ascending colon (IHC, h); jejunum and colon (WB, r) | [96,103,106,112,114,120,121] |
| References | Animal Model(s) | Ex Vivo Study (ies) | Human Sample (s) | Major Conclusions |
|---|---|---|---|---|
| Co-expression and co-synthesis studies on epithelial DAergic system and RAS in the intestines | ||||
| Nataf and Pays 2021 [143] | X | X | Enterocytes of the small intestine | ACE2 coregulated not only with DDC but also with other genes involved in DA/trace amines metabolic pathway and the absorption of microbiota-derived L-DOPA, as well as in neutral amino acids serving as precursors to neurotransmitters. |
| Garrido-Gil et al., 2018 [144] | Male (2- to 3-month-old and 18- to 20-month-old) Sprague Dawley rats and C57/BL6 mice—D1 or D2 receptor knockout and AT1 or AT2 receptor knockout | X | X | Aged rats showed a decrease in colonic dopamine D2 receptor, an increase in AT1 receptor, a decrease in AT2 receptor expression and synthesis, and exhibited increased levels of colonic DA and NA. |
| The influence of intestinal DAergic system and RAS on epithelial permeability | ||||
| Chittimalli et al., 2023 [145] | C57BL/6 male (3–7-month-old) mice | Ogranoids from mouse colons | X | Ang (1–7) restored gut barrier integrity and increased levels of claudin 1 and occludin in the aging colon. |
| Feng et al., 2017 [146] | Male Sprague Dawley rats (210–240 g), the rat model of hyperendogenous enteric DA, C57BL/6J mice (20–25 g), transgenic mice with differentially mutated D5R | X | Duodenal mucosa | DA altered duodenal permeability via D5R. |
| Hashimoto et al., 2012 [147] | Wild-type and ACE2 knockout mice treated with DDS | X | X | Deficiency in murine ACE2 resulted in highly increased susceptibility to intestinal inflammation induced by epithelial damage. |
| Intestinal B0AT1 and RAS | ||||
| Vuille-dit-bille et al., 2015 [112] | X | X | Mucosal biopsies from four different parts of the gastrointestinal tract from patients treated with either ACE inhibitors, AT1-receptor blockers, or controls. | Increased intestinal mRNA levels of ACE2 and B0AT1 were noted in samples from patients treated with ACE inhibitors. |
| Singer et al., 2012 [148] | Wild-type and ACE2 knockout mice on a standard diet or a low-protein/low-niacin diet for up to 85 days | Proximal small intestine rings | X | All neutral amino acids required functional B0AT1 to be efficiently absorbed along the small intestine. |
| Camargo et al., 2009 [149] | Wild-type and ACE2 knockout male mice | Brush border membrane vesicles from wild-type and ACE2 knockout murine small intestine | X | B0AT1 in small intestine was dependent on ACE2. |
| Kowalczuk et al., 2008 [150] | X | X. laevis oocytes | Jejunum, ileum | B0AT1 and ACE2 coexpressed in the apical membrane of human jejunal and ileal enterocytes. |
| Intestinal Na+, K+-ATPase activity | ||||
| Markov et al., 2020 [151] | Male Wistar (180–230 g) rats | X | X | Chronic ouabain treatment differently affected claudin synthesis in jejunal and colonic enterocytes. |
| Vieira-Coelho et al., 2001 [152] | Adult (6 months) and old (24 months) Fischer 344 rats on normal and high-salt intake (24 h). | Rat jejunal epithelial cells | X | Intestinal dopaminergic tonus in old rats was higher than in adult rats and was accompanied by a lower basal intestinal Na+/K+-ATPase activity. High-sodium diet failed to alter intestinal dopaminergic tonus or Na+/K+ ATPase activity in old rats, whereas in adult rats, an increase in AADC activity was accompanied by decreased Na+/K+ ATPase activity. |
| Vieira-Coelho et al., 2001 [153] | Male Wistar rats (18- to 20-day-old) | Rat jejunal epithelial cells | X | DA significantly decreased Na+/K+ ATPase activity in rat jejunal cells, and the effect was antagonized by D1 receptor antagonist SKF 83566 but not affected by D2-like receptor antagonist S-sulpiride and was mimicked by D1-like receptor agonist SKF 38393, but not by D2-like receptor agonist quinerolane. |
| Lucas-Teixeira et al., 2000 [154] | Male 12-week-old spontaneous hypertensive rats (SHR) and Wistar Kyoto rats (WKR) on normal, low-, and high-sodium intake | X | X | Inhibition of jejunal Na+/K+ ATPase activity by D1 dopamine receptor activation was dependent on salt intake in WKY rats, and SHR animals failed to respond to DA, irrespective of their salt intake. |
| Lucas-Teixeira et al., 2000 [155] | Male (60-day-old) Wistar rats on normal, low-, and high-sodium diet | X | X | Prolonged low salt intake decreased activity of jejunal Na+/K+ ATPase and increased rate of DA synthesis (with significantly increased levels of DA and decreased of L-DOPA). |
| Vieira-Coelho et al., 2000 [156] | Adult (60-day-old) and young (18–20-day-old) male Wistar rats | Membrane preparations from rat intestinal mucosa; rat jejunal epithelial cells | X | Na+/K+ ATPase activity in isolated jejunal epithelial cells from adult rats was 2.4 fold that in young rats. Levels of DA in the jejunal mucosa were more than twice in young rats compared to the adult animals, and DA/NA tissue ratios in young animals were 7.5 fold those in adult animals. |
| Vieira-Coelho et al., 1998 [157] | Sprague Dawley rats aged 20 (weaning) and 40 (adult) days | Rat jejunal epithelial cells | X | In 20-day-old but not in 40-day-old rats Na+/K+ ATPase activity was significantly reduced during high-salt diet, and the inhibition was abolished by a blocker of DA synthesis. Decreased Na+/K+ ATPase activity was associated with a decrease in α1 subunit at the plasma membrane. |
| Finkel et al., 1994 [158] | Sprague Dawley rats, aged 20 (weanling), 40 (adolescent), and 80 (adult) days | X | X | Weanling animals had a greater jejunal sodium absorption than older animals. High-salt diet resulted in a decreased intestinal sodium absorption in weanling rats but not in adult rats, and an endogenous DA should play a role in this regulation. |
| Gutman et al., 1972 [159] | X | Mucosal scrapings from rat colon | X | Ang II increased Na+/K+ ATPase activity in colonic microsomes. |
| Indications | Generic Name | Target Site |
|---|---|---|
| Hypertension, Heart failure, Coronary artery disease, Myocardial infarction | Candesartan | Angiotensin II receptor blockers |
| Valsartan | ||
| Losartan | ||
| Irbesartan | ||
| Eprosartan | ||
| Olmesartan | ||
| Left ventricle hypertrophy, Chronic kidney disease, Hypertension, Diabetic nephropathy | Benzapril | Angiotensin converting enzyme inhibitors |
| Captopril | ||
| Enalapril | ||
| Quinapril | ||
| Ramipril | ||
| Lisinopril | ||
| Perindopril | ||
| Fosinopril |
| Indications | Generic Name | Receptors |
|---|---|---|
| Agonists | ||
| Parkinson’s disease, Restless leg syndrome, Hyperprolactinemia | Apomorphine | D1, D2 |
| Bromocriptine | D1, D2 | |
| Dihydroergocryptine | D1–D3 | |
| Fenoldopam | D1 | |
| Rotigotine | D1–D5 | |
| Cabergoline | D2 | |
| Antagonists | ||
| Schizophrenia, Bipolar disorders, Mania, Alcohol withdrawls, Depression | Chlorpromazine | D1–D5 |
| Haloparidol | D2–D4 | |
| Amisulpride | D2, D3 | |
| Aripiprazole | D2 | |
| Olanzapine | D1–D5 | |
| Ziprasidone | D1–D4 | |
| Loxapine | D2–D4 | |
| Risperidone | D2–D4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Kurnik-Łucka, M.; Latacz, G.; Gil, K. Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin–Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions. Int. J. Mol. Sci. 2024, 25, 5566. https://doi.org/10.3390/ijms25105566
Khan N, Kurnik-Łucka M, Latacz G, Gil K. Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin–Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions. International Journal of Molecular Sciences. 2024; 25(10):5566. https://doi.org/10.3390/ijms25105566
Chicago/Turabian StyleKhan, Nadia, Magdalena Kurnik-Łucka, Gniewomir Latacz, and Krzysztof Gil. 2024. "Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin–Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions" International Journal of Molecular Sciences 25, no. 10: 5566. https://doi.org/10.3390/ijms25105566
APA StyleKhan, N., Kurnik-Łucka, M., Latacz, G., & Gil, K. (2024). Systematic-Narrative Hybrid Literature Review: Crosstalk between Gastrointestinal Renin–Angiotensin and Dopaminergic Systems in the Regulation of Intestinal Permeability by Tight Junctions. International Journal of Molecular Sciences, 25(10), 5566. https://doi.org/10.3390/ijms25105566






