The Presence of TGFβ3 in Human Ovarian Intrafollicular Fluid and Its Involvement in Thromboxane Generation in Follicular Granulosa Cells through a Canonical TGFβRI, Smad2/3 Signaling Pathway and COX-2 Induction
Abstract
1. Introduction
2. Results
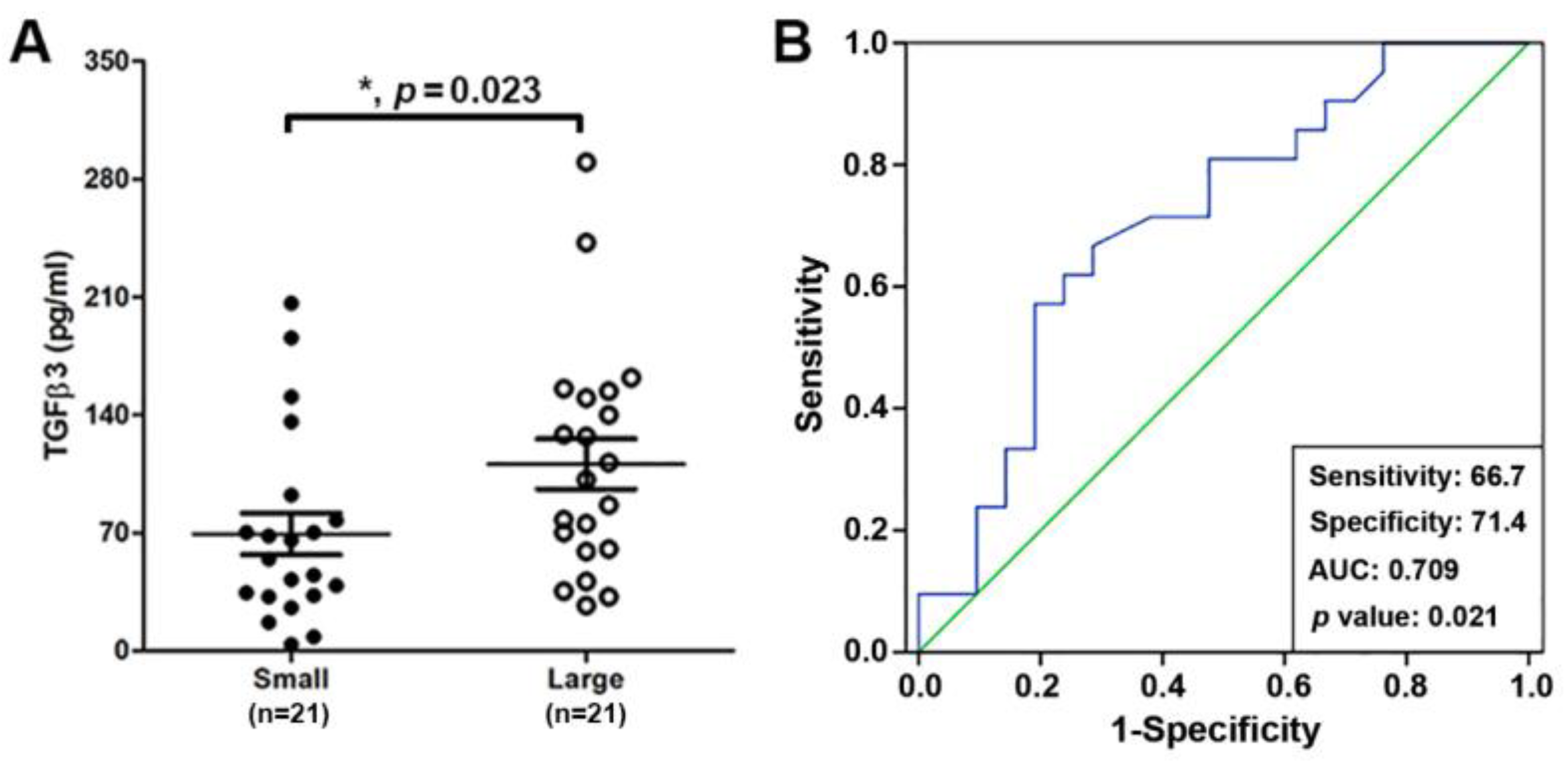
2.1. The TGFβ3 Is Present in the Ovarian FF of IVF Patients and Its Levels Are Positively Correlated with Oocyte Maturity
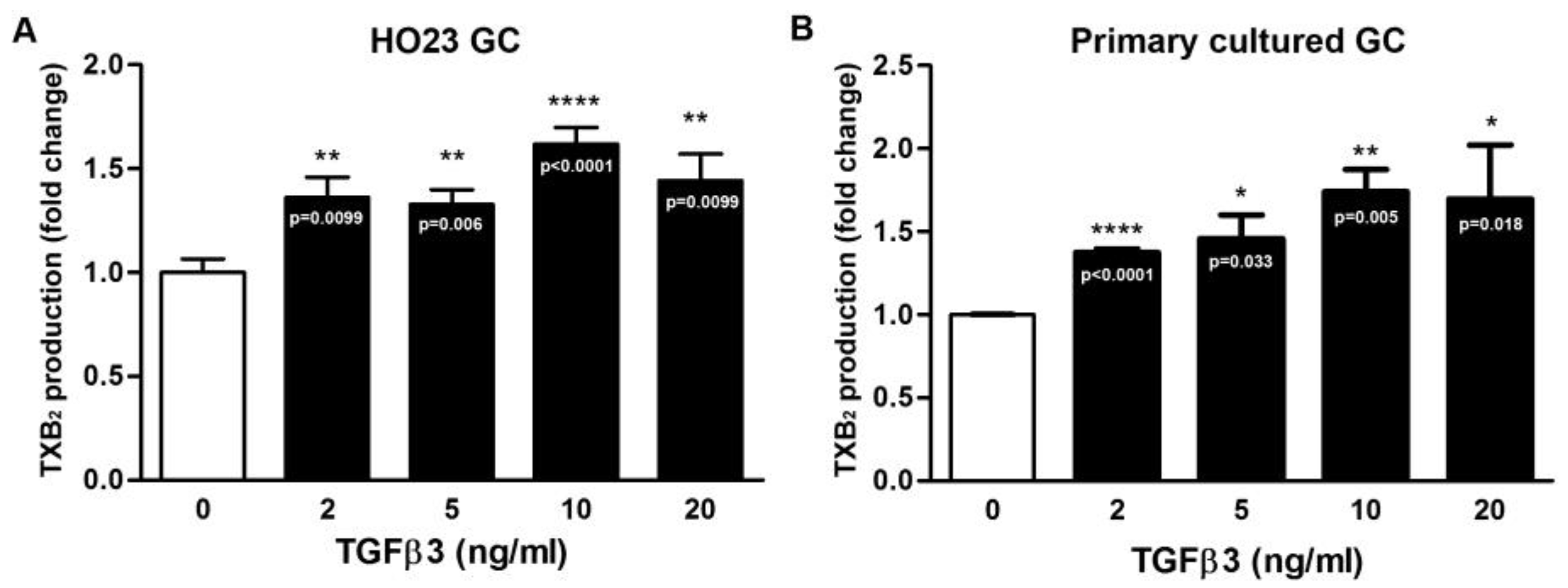
2.2. TGFβ3 Promotes COX-2 but Not COX-1 Expression in Human HO23 GC Cell Line and Primary Cultured Ovarian Follicular GCs
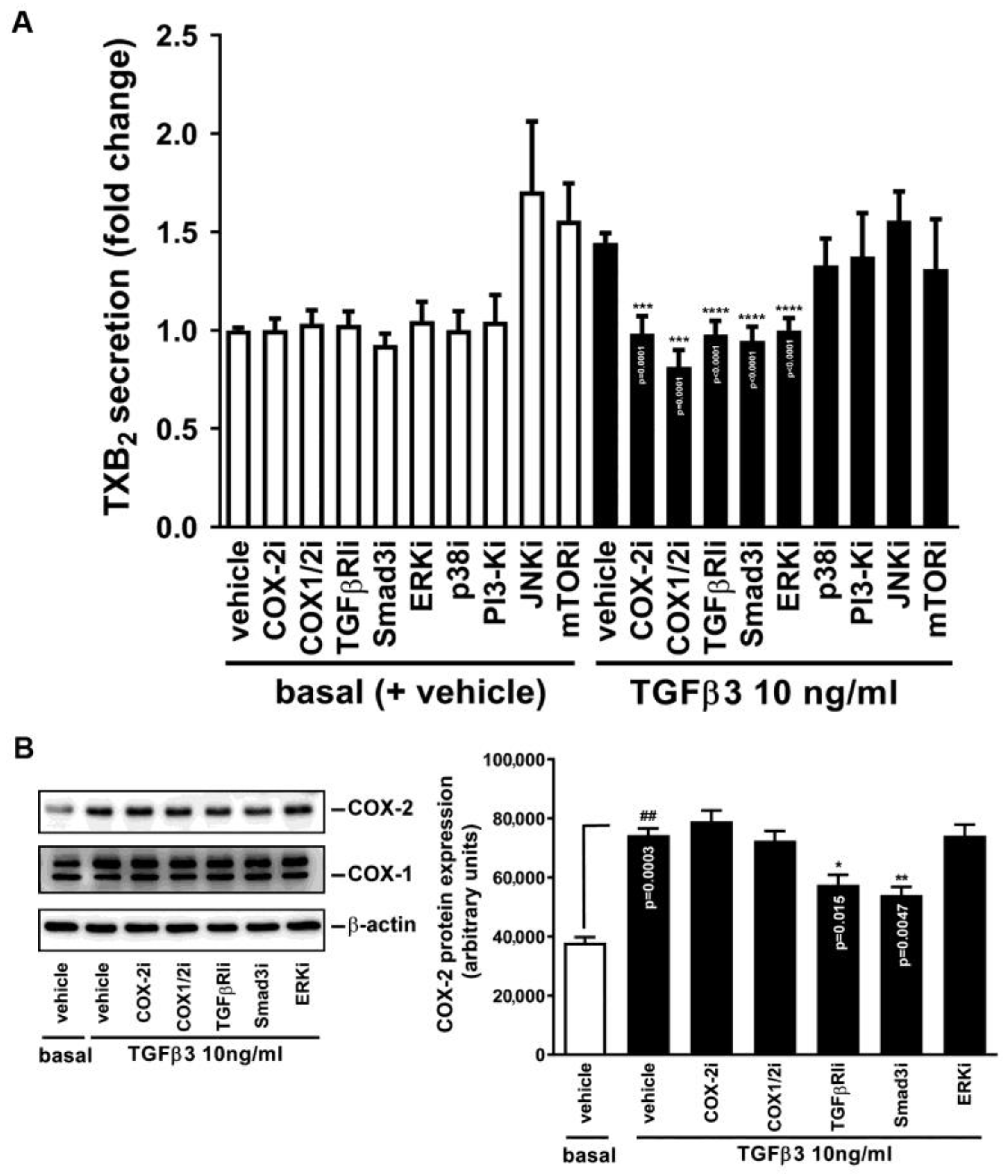
2.3. Participation of TGFβRI, Smad3, COX-2, and MAPK in TGFβ3-Induced TXB2 Production
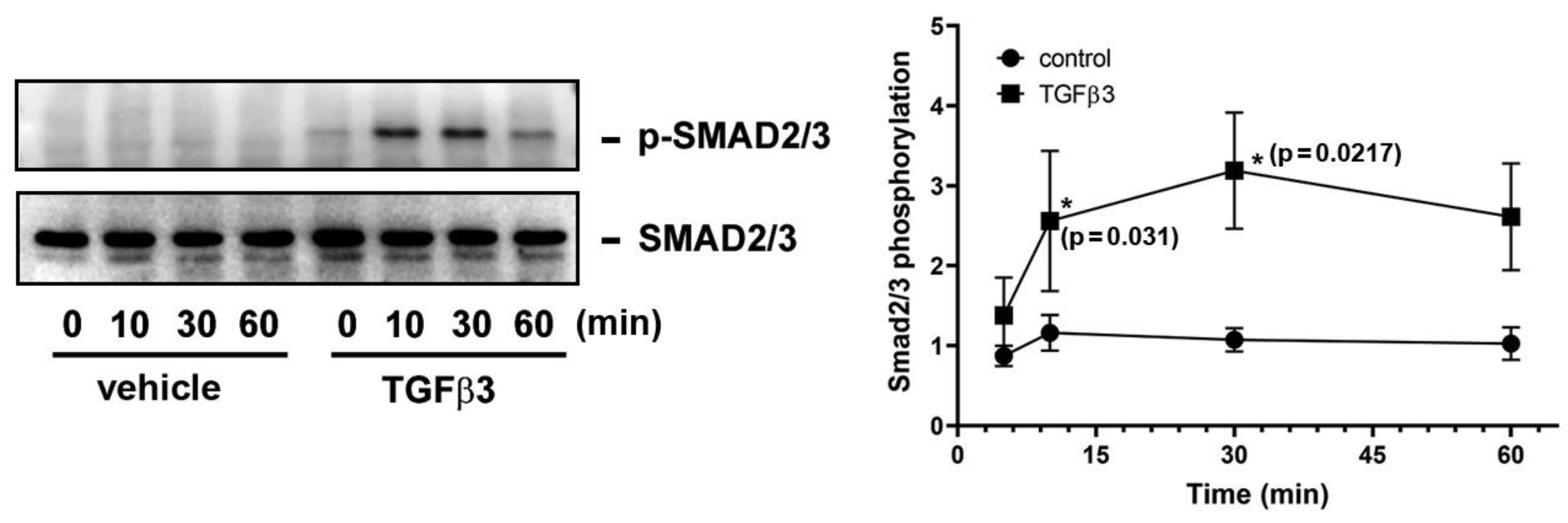
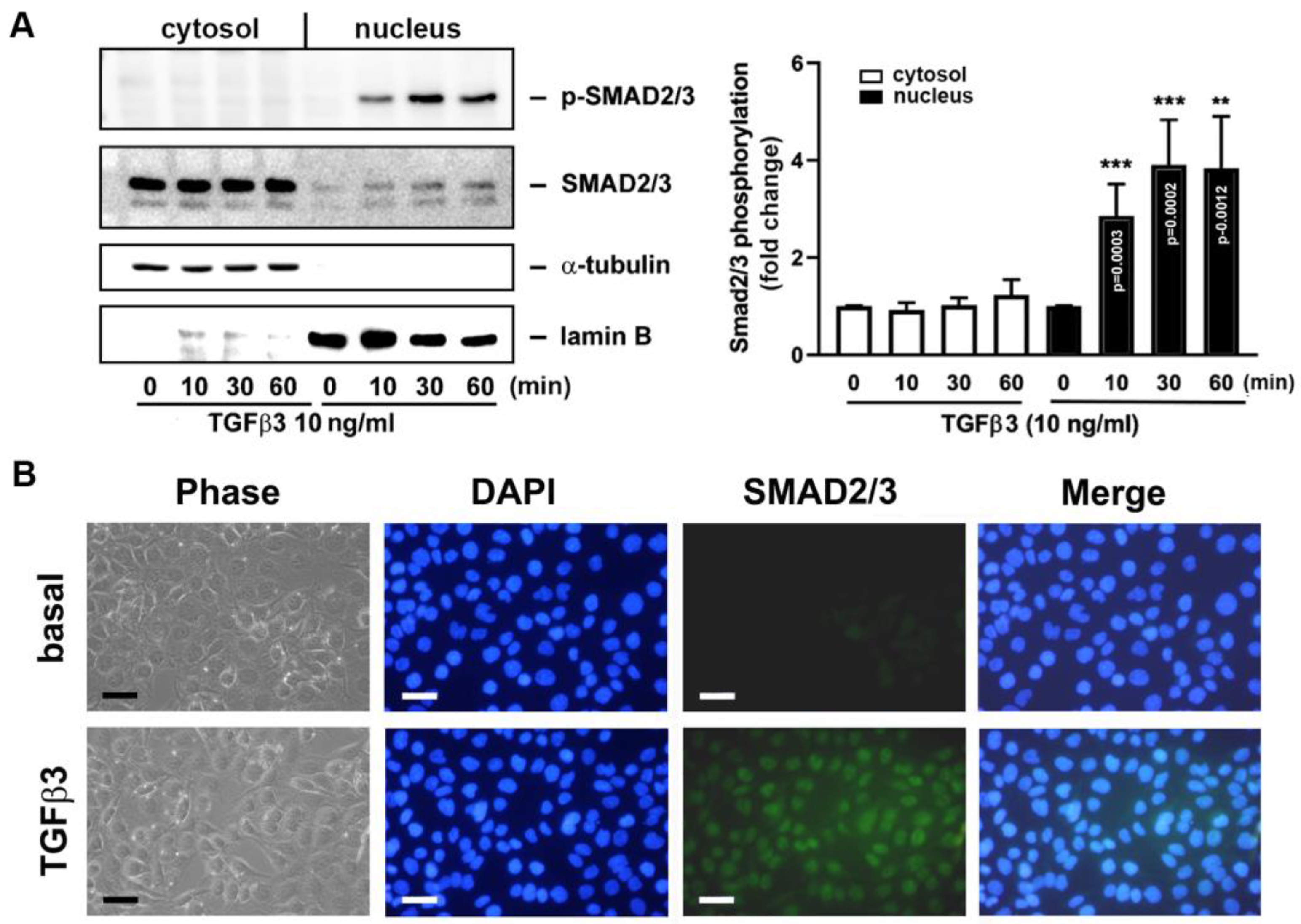
2.4. Direct Application of TGFβ3 to GCs Leads to Smad2/3 Phosphorylation and Translocation from Cytosol to Nucleus
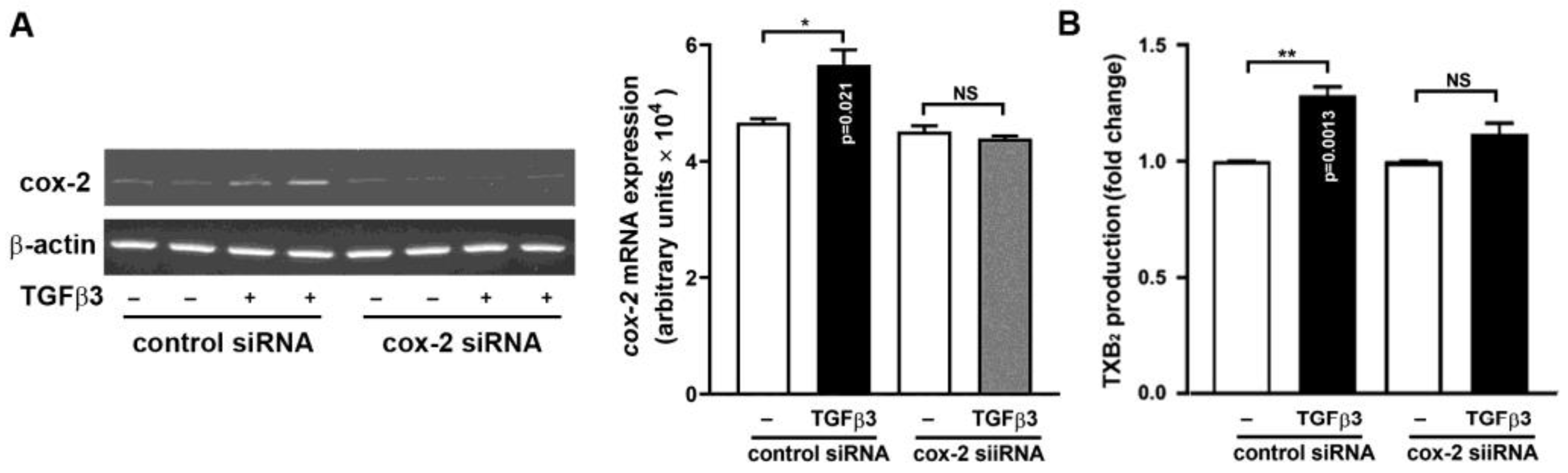
2.5. Requirement of COX-2 Induction for TGFβ3-Mediated TXB2 Production
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Patient Recruitment and Ovarian FF Collection
4.3. Preparation and Culture of Ovarian Follicular GCs
4.4. ELISA
4.5. Western Blot Analysis
4.6. RT-PCR
4.7. Pharmacological Interventions
4.8. Cell Fractionation and Smad2/3 Translocation
4.9. Immunofluorescence Microscopy
4.10. SiRNA Interference
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shrestha, D.; La, X.; Feng, H.L. Comparison of different stimulation protocols used in in vitro fertilization: A review. Ann. Transl. Med. 2015, 3, 137. [Google Scholar] [PubMed]
- Edwards, R.G. Follicular fluid. J. Reprod. Fertil. 1974, 37, 189–219. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Meldrum, D.R.; Katz-Jaffe, M.G.; Krisher, R.L.; Schoolcraft, W.B. Oocyte environment: Follicular fluid and cumulus cells are critical for oocyte health. Fertil. Steril. 2015, 103, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Bayasula; Iwase, A.; Kobayashi, H.; Goto, M.; Nakahara, T.; Nakamura, T.; Kondo, M.; Nagatomo, Y.; Kotani, T.; Kikkawa, F. A proteomic analysis of human follicular fluid: Comparison between fertilized oocytes and non-fertilized oocytes in the same patient. J. Assist. Reprod. Genet. 2013, 30, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Piane, L.D.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, T.; Matsumoto, K.; Hosoi, Y.; Morimoto, Y. Evaluation of antioxidant status and oxidative stress markers in follicular fluid for human in vitro fertilization outcome. Reprod. Med. Biol. 2018, 17, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Inazumi, T.; Tsuchiya, S. Roles of prostaglandin receptors in female reproduction. J. Biochem. 2015, 157, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Salih, S.M.; Bormann, C.L.; Wiltbank, M.C. Induction of chemokines and prostaglandin synthesis pathways in luteinized human granulosa cells: Potential role of luteotropin withdrawal and prostaglandin f2α in regression of the human corpus luteum. Reprod. Biol. 2015, 15, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Pier, B.; Edmonds, J.W.; Wilson, L.; Arabshahi, A.; Moore, R.; Bates, G.W.; Prasain, J.K.; Miller, M.A. Comprehensive profiling of prostaglandins in human ovarian follicular fluid using mass spectrometry. Prostaglandins Other Lipid Mediat. 2018, 134, 7–15. [Google Scholar] [CrossRef]
- Sugimoto, Y.; Narumiya, S.; Ichikawa, A. Distribution and function of prostanoid receptors: Studies from knockout mice. Prog. Lipid Res. 2000, 39, 289–314. [Google Scholar] [CrossRef]
- Szczuko, M.; Kikut, J.; Komorniak, N.; Bilicki, J.; Celewicz, Z.; Ziętek, M. The role of arachidonic and linoleic acid derivatives in pathological pregnancies and the human reproduction process. Int. J. Mol. Sci. 2020, 21, 9628. [Google Scholar] [CrossRef] [PubMed]
- Sales, K.J.; Jabbour, H.N. Cyclooxygenase enzymes and prostaglandins in pathology of the endometrium. Reproduction 2003, 126, 559–567. [Google Scholar] [CrossRef]
- Brown, C.G.; Poyser, N.L. Studies on ovarian prostaglandin production in relation to ovulation in the rat. J. Reprod. Fertil. 1984, 72, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Dey, S.K. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef]
- Hizaki, H.; Segi, E.; Sugimoto, Y.; Hirose, M.; Saji, T.; Ushikubi, F.; Matsuoka, T.; Noda, Y.; Tanaka, T.; Yoshida, N.; et al. Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin e receptor subtype ep(2). Proc. Natl. Acad. Sci. USA 1999, 96, 10501–10506. [Google Scholar] [CrossRef]
- Chen, H.T.; Wu, W.B.; Lin, J.J.; Lai, T.H. Identification of potential angiogenic biomarkers in human follicular fluid for predicting oocyte maturity. Front. Endocrinol. 2023, 14, 1173079. [Google Scholar] [CrossRef]
- Chen, H.-T.; Lai, T.-H.; Lin, J.-J.; Wu, W.-B. An inverse correlation between intrafollicular thromboxane level and oocyte maturation: Novel role of thrombin and thromboxane in folliculogenesis. Biochem. Pharmacol. 2024. in submission. [Google Scholar]
- David, C.J.; Massagué, J. Contextual determinants of tgfβ action in development, immunity and cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-β in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J. How cells read tgf-β signals. Nat. Rev. Mol. Cell Biol. 2000, 1, 169. [Google Scholar] [CrossRef]
- Peng, D.; Fu, M.; Wang, M.; Wei, Y.; Wei, X. Targeting tgf-β signal transduction for fibrosis and cancer therapy. Mol. Cancer 2022, 21, 104. [Google Scholar] [CrossRef]
- Field, S.L.; Dasgupta, T.; Cummings, M.; Orsi, N.M. Cytokines in ovarian folliculogenesis, oocyte maturation and luteinisation. Mol. Reprod. Dev. 2014, 81, 284–314. [Google Scholar] [CrossRef] [PubMed]
- Barberi, M.; Ermini, B.; Morelli, M.B.; Ermini, M.; Cecconi, S.; Canipari, R. Follicular fluid hormonal profile and cumulus cell gene expression in controlled ovarian hyperstimulation with recombinant fsh: Effects of recombinant lh administration. J. Assist. Reprod. Genet. 2012, 29, 1381–1391. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katzung, B.G.; Katzung, B.G. Basic & Clinical Pharmacology/by Betram g. Katzung, 14th ed.; McGraw-Hill: New York, NY, USA, 2018. [Google Scholar]
- Camacho, M.; Rodriguez, C.; Salazar, J.; Martinez-Gonzalez, J.; Ribalta, J.; Escudero, J.R.; Masana, L.; Vila, L. Retinoic acid induces pgi synthase expression in human endothelial cells. J. Lipid Res. 2008, 49, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Rucker, D.; Dhamoon, A.S. Physiology, thromboxane a2. In Statpearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Priddy, A.R.; Killick, S.R.; Elstein, M.; Morris, J.; Sullivan, M.; Patel, L.; Elder, M.G. Ovarian follicular fluid eicosanoid concentrations during the pre-ovulatory period in humans. Prostaglandins 1989, 38, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ylikorkala, O.; Tenhunen, A. Follicular fluid prostaglandins in endometriosis and ovarian hyperstimulation. Fertil. Steril. 1984, 41, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.D.; Sertich, P.L. Concentrations of arachidonate metabolites, steroids and histamine in preovulatory horse follicles after administration of human chorionic gonadotrophin and the effect of intrafollicular injection of indomethacin. J. Endocrinol. 1991, 129, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Juengel, J.L.; Bibby, A.H.; Reader, K.L.; Lun, S.; Quirke, L.D.; Haydon, L.J.; McNatty, K.P. The role of transforming growth factor-beta (tgf-beta) during ovarian follicular development in sheep. Reprod. Biol. Endocrinol. 2004, 2, 78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, N.; Xu, Y.; Yin, Y.; Yao, G.; Tian, H.; Wang, G.; Lian, J.; Wang, Y.; Sun, F. Steroidogenic factor-1 is required for tgf-beta3-mediated 17beta-estradiol synthesis in mouse ovarian granulosa cells. Endocrinology 2011, 152, 3213–3225. [Google Scholar] [CrossRef]
- Steffl, M.; Schweiger, M.; Amselgruber, W.M. Expression of transforming growth factor-beta3 (tgf-beta3) in the porcine ovary during the oestrus cycle. Histol. Histopathol. 2008, 23, 665–671. [Google Scholar]
- Jackowska, M.; Kempisty, B.; Woźna, M.; Piotrowska, H.; Antosik, P.; Zawierucha, P.; Bukowska, D.; Nowicki, M.; Jaśkowski, J.M.; Brüssow, K.P. Differential expression of gdf9, tgfb1, tgfb2 and tgfb3 in porcine oocytes isolated from follicles of different size before and after culture in vitro. Acta Vet. Hung. 2013, 61, 99–115. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Doraiswamy, V.; Skinner, M.K. Transforming growth factor-beta isoform expression during bovine ovarian antral follicle development. Mol. Reprod. Dev. 2003, 66, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jia, Q.; Hong, I.S.; Dang, X.; Wu, Z.; Wang, H.; Cheng, J.C.; Fang, L. Tgf-β1 and tgf-β3, but not tgf-β2, are upregulated in the ovaries of ovarian hyperstimulation syndrome. Biol. Reprod. 2024, 110, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.H.; Chen, H.T.; Wu, W.B. Tgfβ1 induces in-vitro and ex-vivo angiogenesis through vegf production in human ovarian follicular fluid-derived granulosa cells during in-vitro fertilization cycle. J. Reprod. Immunol. 2021, 145, 103311. [Google Scholar] [CrossRef] [PubMed]
- Poormoosavi, S.M.; Behmanesh, M.A.; Janati, S.; Najafzadehvarzi, H. Level of bisphenol a in follicular fluid and serum and oocyte morphology in patients undergoing ivf treatment. J. Fam. Reprod. Health 2019, 13, 154–159. [Google Scholar] [CrossRef]
- Cavallo, I.K.; Dela Cruz, C.; Oliveira, M.L.; Del Puerto, H.L.; Dias, J.A.; Lobach, V.N.; Casalechi, M.; Camargos, M.G.; Reis, A.M.; Santos, R.A.; et al. Angiotensin-(1-7) in human follicular fluid correlates with oocyte maturation. Hum. Reprod. 2017, 32, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Cook-Andersen, H.; Curnow, K.J.; Su, H.I.; Chang, R.J.; Shimasaki, S. Growth and differentiation factor 9 promotes oocyte growth at the primary but not the early secondary stage in three-dimensional follicle culture. J. Assist. Reprod. Genet. 2016, 33, 1067–1077. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, C. Lysophosphatidic acid is associated with oocyte maturation by enhancing autophagy via pi3k-akt-mtor signaling pathway in granulosa cells. J. Ovarian Res. 2023, 16, 137. [Google Scholar] [CrossRef]
- Hood, R.B.; Liang, D.; Tan, Y.; Ford, J.B.; Souter, I.; Chavarro, J.E.; Jones, D.P.; Hauser, R.; Gaskins, A.J. Serum and follicular fluid metabolome and markers of ovarian stimulation. Hum. Reprod. 2023, 38, 2196–2207. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Liu, C.M.; Jia, X.Z.; Zhao, S.B.; Nie, Z.Y.; Lv, C.T.; Jiang, Q.; Hao, Y.L. Expression of mitochondrial transcription factor a in granulosa cells: Implications for oocyte maturation and in vitro fertilization outcomes. J. Assist. Reprod. Genet. 2024, 41, 363–370. [Google Scholar] [CrossRef]
- Huang, T.; David, L.; Mendoza, V.; Yang, Y.; Villarreal, M.; De, K.; Sun, L.; Fang, X.; López-Casillas, F.; Wrana, J.L.; et al. Tgf-β signalling is mediated by two autonomously functioning tβri:Tβrii pairs. EMBO J. 2011, 30, 1263–1276. [Google Scholar] [CrossRef]
- Du, X.; Cai, L.; Xie, J.; Zhou, X. The role of tgf-beta3 in cartilage development and osteoarthritis. Bone Res. 2023, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Liu, J.; Derynck, R. Post-translational regulation of tgf-β receptor and smad signaling. FEBS Lett. 2012, 586, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Clayton, S.W.; Ban, G.I.; Liu, C.; Serra, R. Canonical and noncanonical tgf-β signaling regulate fibrous tissue differentiation in the axial skeleton. Sci. Rep. 2020, 10, 21364. [Google Scholar] [CrossRef] [PubMed]
- Koinuma, D.; Tsutsumi, S.; Kamimura, N.; Taniguchi, H.; Miyazawa, K.; Sunamura, M.; Imamura, T.; Miyazono, K.; Aburatani, H. Chromatin immunoprecipitation on microarray analysis of smad2/3 binding sites reveals roles of ets1 and tfap2a in transforming growth factor beta signaling. Mol. Cell. Biol. 2009, 29, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.S.; Kujubu, D.A.; Perrin, D.M.; Herschman, H.R. Structure of the mitogen-inducible tis10 gene and demonstration that the tis10-encoded protein is a functional prostaglandin g/h synthase. J. Biol. Chem. 1992, 267, 4338–4344. [Google Scholar] [CrossRef]
- Matsumura, T.; Suzuki, T.; Aizawa, K.; Sawaki, D.; Munemasa, Y.; Ishida, J.; Nagai, R. Regulation of transforming growth factor-beta-dependent cyclooxygenase-2 expression in fibroblasts. J. Biol. Chem. 2009, 284, 35861–35871. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Chaudhry, P.; Parent, S.; Asselin, E. Ubiquitin-proteasomal degradation of cox-2 in tgf-β stimulated human endometrial cells is mediated through endoplasmic reticulum mannosidase i. Endocrinology 2012, 153, 426–437. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ekart, J.; McNatty, K.; Hutton, J.; Pitman, J. Ranking and selection of mii oocytes in human icsi cycles using gene expression levels from associated cumulus cells. Hum. Reprod. 2013, 28, 2930–2942. [Google Scholar] [CrossRef]
- Tsai, Y.-J.; Hao, S.-P.; Chen, C.-L.; Wu, W.-B. Thromboxane A2 regulates CXCL1 and CXCL8 chemokine expression in the nasal mucosa–derived fibroblasts of chronic rhinosinusitis patients. PLoS ONE 2016, 11, e0158438. [Google Scholar] [CrossRef]

 indicates a pharmacological and/or siRNA intervention used in this study.
indicates a pharmacological and/or siRNA intervention used in this study.
 indicates a pharmacological and/or siRNA intervention used in this study.
indicates a pharmacological and/or siRNA intervention used in this study.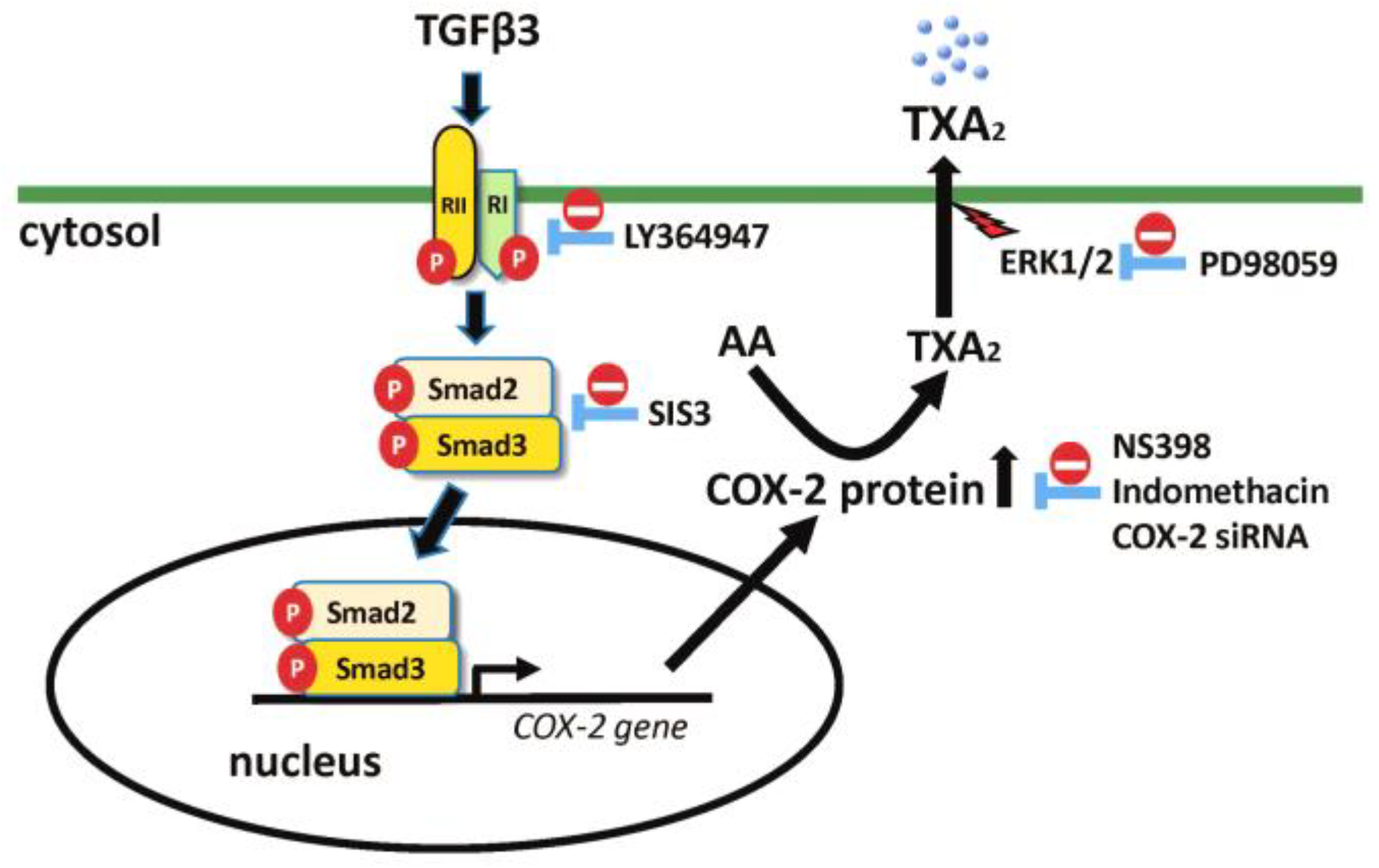
| Variable | Odds Ratio | 95% C.I. | p-Value |
|---|---|---|---|
| TGFβ3 | 1.012 | 1.001/1.024 | 0.039 * (<0.05) |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size (bp) |
|---|---|---|---|
| COX-1 | ACATTCAGTTCCCACCATCT | TCACTGCTGTTGGGTCTCTG | 601 |
| COX-2 | CAGCAAATCCTTGCTGTTCC | TGGGCAAAGAATGCAAACATC | 517 |
| β-actin | ATCATGTTTGAGACCTTCAA | CATCTCTTGCTCGAAGTCCA | 314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, T.-H.; Chen, H.-T.; Wu, P.-H.; Wu, W.-B. The Presence of TGFβ3 in Human Ovarian Intrafollicular Fluid and Its Involvement in Thromboxane Generation in Follicular Granulosa Cells through a Canonical TGFβRI, Smad2/3 Signaling Pathway and COX-2 Induction. Int. J. Mol. Sci. 2024, 25, 5558. https://doi.org/10.3390/ijms25105558
Lai T-H, Chen H-T, Wu P-H, Wu W-B. The Presence of TGFβ3 in Human Ovarian Intrafollicular Fluid and Its Involvement in Thromboxane Generation in Follicular Granulosa Cells through a Canonical TGFβRI, Smad2/3 Signaling Pathway and COX-2 Induction. International Journal of Molecular Sciences. 2024; 25(10):5558. https://doi.org/10.3390/ijms25105558
Chicago/Turabian StyleLai, Tsung-Hsuan, Hsuan-Ting Chen, Pi-Hui Wu, and Wen-Bin Wu. 2024. "The Presence of TGFβ3 in Human Ovarian Intrafollicular Fluid and Its Involvement in Thromboxane Generation in Follicular Granulosa Cells through a Canonical TGFβRI, Smad2/3 Signaling Pathway and COX-2 Induction" International Journal of Molecular Sciences 25, no. 10: 5558. https://doi.org/10.3390/ijms25105558
APA StyleLai, T.-H., Chen, H.-T., Wu, P.-H., & Wu, W.-B. (2024). The Presence of TGFβ3 in Human Ovarian Intrafollicular Fluid and Its Involvement in Thromboxane Generation in Follicular Granulosa Cells through a Canonical TGFβRI, Smad2/3 Signaling Pathway and COX-2 Induction. International Journal of Molecular Sciences, 25(10), 5558. https://doi.org/10.3390/ijms25105558







