Abstract
Age-related hearing loss (HL), or presbycusis, is a complex and heterogeneous condition, affecting a significant portion of older adults and involving various interacting mechanisms. Metabolic presbycusis, a type of age-related HL, is characterized by the dysfunction of the stria vascularis, which is crucial for maintaining the endocochlear potential necessary for hearing. Although attention on metabolic presbycusis has waned in recent years, research continues to identify strial pathology as a key factor in age-related HL. This narrative review integrates past and recent research, bridging findings from animal models and human studies, to examine the contributions of the stria vascularis to age-related HL. It provides a brief overview of the structure and function of the stria vascularis and then examines mechanisms contributing to age-related strial dysfunction, including altered ion transport, changes in pigmentation, inflammatory responses, and vascular atrophy. Importantly, this review outlines the contribution of metabolic mechanisms to age-related HL, highlighting areas for future research. It emphasizes the complex interdependence of metabolic and sensorineural mechanisms in the pathology of age-related HL and highlights the importance of animal models in understanding the underlying mechanisms. The comprehensive and mechanistic investigation of all factors contributing to age-related HL, including cochlear metabolic dysfunction, remains crucial to identifying the underlying mechanisms and developing personalized, protective, and restorative treatments.
1. Introduction
Globally, more than 1.5 billion people currently suffer from some form of hearing loss (HL). The prevalence of HL of this severity increases exponentially with age, from 15.4% in people in their sixties to 58.2% in people above the age of ninety [1]. Given the increasing lifespan, the number individuals with HL is projected to increase to 2.5 billion (or 1 in 4) by 2050. Of those, nearly 700 million are predicted to have moderate to higher levels of HL [1]. Age-related HL, or presbycusis, has been classically subdivided by Schuknecht into four types based on human histopathological findings [2,3]. One type, termed metabolic presbycusis, relates to the dysfunction and atrophy of the stria vascularis (SV) [4,5]. The SV is a complex epithelial structure lining the lateral wall of the cochlea and plays an essential role in maintaining ion and fluid homeostasis in the cochlea [6]. Strial atrophy is usually detected as a loss in the cross-sectional area of the stria vascularis [7,8]. In humans, especially over the age of 60 years, the atrophy of the SV is observed frequently and is most severe in the apical and extreme basal regions of the cochlea [9]. By transporting potassium ions (K+) into the endolymph, the SV is essential for generating and maintaining the endocochlear potential (EP) of about +80 mV [10]. The EP provides the electrochemical gradient necessary for the influx of K+ and calcium (Ca2+) ions from the endolymph into the sensory hair cells via mechanoelectrical transduction channels located at the top of the hair cell stereocilia. The EP, and thus SV, is therefore necessary for mechanotransduction [11].
In recent years, research investigating (age-related) HL has increasingly concentrated on the role of the sensory hair cells and the ribbon synapses that form connections between the sensory hair cells and the spiral ganglion neurons, the primary auditory neurons [12]. Consequently, the interest in metabolic HL has declined. However, as evidenced by Schuknecht’s earlier research and supported by recent studies [13,14], age-related HL is highly heterogenous and involves various potentially interacting mechanisms. Recently a genome-wide association meta-analysis highlighted the role of the SV in human HL [15]. The SV has also been linked to Ménière’s disease, and relatively rare hereditary diseases such as Alport Syndrome, Waardenburg syndrome and Norrie disease, as well as various neglected otologic diseases (reviewed by [16,17,18]). Ménière’s disease also involves vestibular pathology, however, it is unlikely that the SV is involved directly, since the endovestibular potential is maintained independently of the cochlea [19]. Given the complexity of age-related HL, the comprehensive and mechanistic investigation of all contributing factors, including cochlear metabolic dysfunction, remains crucial to identifying the underlying mechanisms and developing personalized, protective, and restorative treatments [20,21].
In turn, comprehensive and mechanistic investigation requires research using animal models due to several limitations in investigating presbycusis directly in humans. Firstly, age-related HL is compounded by environmental factors, especially noise exposure. In humans, accurately determining lifetime noise exposure is challenging, making it difficult to disentangle noise-induced from ageing-specific pathology. Secondly, in vivo measurements beyond standard audiological assessments are difficult due to the location of the cochlea within the dense temporal bone and, therefore, tissue samples are necessary for more invasive investigation of the cochlea. The quality and quantity of donated cochlear tissue is, however, extremely limited and often insufficient for mechanistic investigation. Finally, it is very often not ethically permissible to implement invasive experimental manipulations. These various limitations can be largely overcome when using animal models, which can be studied in a controlled environment and experimentally manipulated and, therefore, provide more accurate data on the direct causes of age-related HL.
Key animal models in presbycusis research are the Mongolian gerbil (Meriones unguiculatus) as well as several different mouse strains, such as BALB/cJ, C57BL/6J, CBA/CaJ and CBA/J [22]. In gerbils, in contrast to mice, genetic manipulation is not readily available. However, in contrast to mice, gerbils have sensitive hearing that extends to lower frequency ranges comparable to humans [23,24]. Studying animal models, particularly the gerbil, has proven valuable in distinguishing between metabolic and sensory HL. When gerbils age while exposed to continuous noise, they exhibit variable hair cell loss, specifically affecting the outer hair cells [25]. In contrast, quiet-aged gerbils, with minimal noise exposure throughout their lifespan, consistently show only minimal inner and outer hair cell loss [25,26], in accordance with predominantly metabolic presbycusis. Synaptopathy, or the loss of auditory nerve fibers on the inner hair cells, does occur in quiet-aged gerbils [27,28,29], However, not to such an extent that it necessarily affects hearing thresholds [30,31,32]. Despite reduced acoustic exposure in quiet-aged gerbils, significant HL can still occur, providing an excellent chance to investigate the effects of strial dysfunction [33]. Furthermore, it is possible to pharmacologically reduce the EP in young gerbils using furosemide, allowing for the investigation of EP loss in the absence of other age-related effects [34,35,36].
This narrative review specifically emphasizes the critical role of the SV in age-related hearing impairment. It comprehensively integrates classical and recent studies, while also aiming to link findings from animal models with those from humans. The review begins by introducing the structure and function of the various layers of cells in the SV and the normal capillary network. Subsequently, it delves into the mechanisms contributing to the age-related loss of strial function, exploring the decline in ion transport in SV cells, alterations in pigmentation, the involvement of tissue-resident macrophages and inflammatory responses, and the role of vascular atrophy. Furthermore, this review sheds light on strial degeneration as a significant contributor to presbycusis, highlighting the value of animal models for investigating the underlying mechanisms. By focusing on the cellular and molecular processes underlying strial dysfunction, it provides insight into the causes of dysfunction and targets for potential interventions.
2. Structure and Function of the Stria Vascularis
The SV is a highly vascularized tissue located in the lateral wall of the cochlea. It comprises three main cell types: marginal, intermediate, and basal cells (Figure 1). In addition, an extensive network of capillaries is present within the SV, from which the name “stria vascularis” is derived. Marginal cells directly face the endolymph, intermediate cells form the middle layer, and basal cells, which are closely associated with fibrocytes in the spiral ligament, are located most laterally [37]. Together, the different cell types of the SV are responsible for K+ transport into the scala media and the generation of the endocochlear potential (EP). Inner hair cells, the main sensory receptors of the cochlea, as well as outer hair cells, which act as a cochlear gain control, depend on the EP for their proper functioning. The K+ flowing through the hair cells via their transduction and basolateral channels is then circulated back to the SV and released into the endolymph again [38]. The disturbance of this cochlear homeostasis is the cause underlying many types of HL [39]. The electrochemical details of EP generation are beyond the scope of this text and are explored in more detail elsewhere [10]. In the following sections, the functional role of each of the cell types within the SV as well as the capillary network that forms the blood labyrinth barrier are discussed.
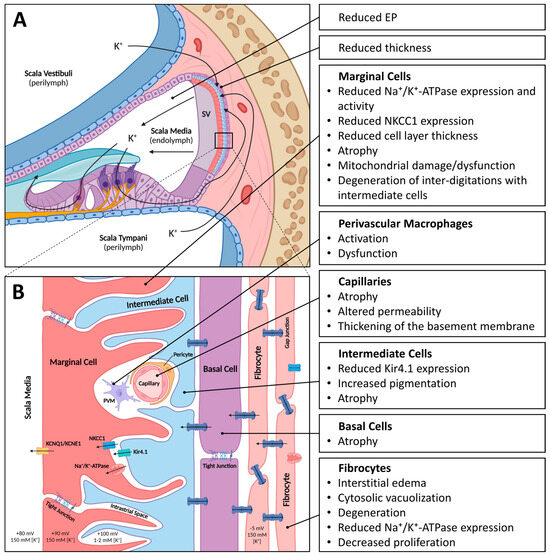
Figure 1.
Schematic of the stria vascularis (SV) with age-related changes indicated. (A). Schematic cross-section of the cochlea with SV located in the lateral wall. The SV with its three main cell layers, consisting of marginal cells, intermediate cells, and basal cells, is responsible for generating and maintaining the high potassium ion (K+) concentration in the endolymph and resulting endocochlear potential (EP). (B). Magnified schematic of the cell layers of the SV. Marginal cells, connected via tight junctions, directly face the endolymph and express several ion channels and transporters (KCNQ1/KNCE1, NKCC1 and Na+/K+-ATPase). Marginal cells form extensive inter-digitations with intermediate cells, which express the ion channel Kir4.1. The intrastrial space, which has a relatively low K+ concentration and high positive potential, is located between the marginal and intermediate cell layers. Capillaries run through the SV and their endothelial cells, and pericytes and perivascular macrophages form the blood–labyrinth barrier (BLB). Basal cells, connected via tight junctions, form gap junctions with intermediate cells and fibrocytes to form a syncytium. Image created with BioRender.com.
2.1. Marginal Cells
Marginal cells form a monolayer epithelium that directly faces the endolymph (Figure 1). They are derived from the epithelial cells of the cochlear duct [40], and connected via tight junctions. Reflecting the importance of K+ transport by the SV, two key K+ transporters are enriched in the marginal cells of the SV: the Na+/K+-ATPase pump and the Na+-K+-2Cl− cotransporter 1 [38].
The Na+/K+-ATPase pump is abundantly expressed in the basolateral membrane of marginal cells [38,41] (Figure 1B). This pump is a ubiquitously expressed transmembrane protein composed of a catalytic α subunit, a β subunit that can modulate the kinetic characteristics and acts as a chaperone during structural and functional maturation, and tissue-specific FXYD proteins that modulate activity. There are four α, four β, and seven FXYD mammalian subunit isoforms (reviewed by [42,43,44]). Na+/K+-ATPase is expressed in tissues throughout the body in various isoform combinations. The isoform combinations in a given type of tissue can also vary between species. This heterogeneity suggests that Na+/K+-ATPase adapts its activity to the tissue- and species-specific needs. The α1 isoform is the most ubiquitous isoform, while, for example, the α3 isoform is typically found in neuronal tissue. In addition to being abundantly expressed in the marginal cells of the gerbil SV (α1, β2), the Na+/K+-ATPase is also found in spiral ganglion neurons (α3, β1), including their central and peripheral processes, in subpopulations of fibrocytes (α1, α2, β1), and various other cell types [41,45]. In mice, the Na+/K+-ATPase is found in marginal cells of the SV (α1, β1, β2), subpopulations of fibrocytes (α1, α2, β1), a number of spiral ganglion neurons (α2, β1), as well as other cell types [46]. In CBA/CaJ mice, the Na+/K+-ATP α1 isoform was found to be the most abundant protein in the strial capillaries, which plays an important role in the blood–labyrinth barrier integrity [47]. In rats, the Na+/K+-ATPase α3 is expressed in spiral ganglion somata, afferent and efferent terminals, and supporting cells neighboring the inner hair cells [48]. In the human cochlea, Na+/K+-ATPase is expressed in marginal cells of the SV (α1, β1), subpopulations of fibrocytes (α1, β1), spiral ganglion neurons (α1, α3, β1), and various other cell types [49,50].
The Na+-K+-2Cl− cotransporter 1, commonly known as NKCC1, is also located in the basolateral membrane of marginal cells [51]. Like the Na+/K+-ATPase pump, NKCC1 is a ubiquitously expressed transmembrane protein [52]. Unlike the Na+/K+-ATPase pump, NKCC1 is not powered by ATP but rather harnesses the Na+ gradient created by the Na+/K+-ATPase pump to allow the movement of two molecules of Cl− and one molecule of K+ together with one molecule of Na+ into the cell [52]. In the gerbil, in addition to the marginal cells, NKCC1 is also found in subpopulations of spiral ligament fibrocytes [51]. In C57BL/6J mice, NKCC1 is present in the stria vascularis as well as in fibrocytes in the spiral ligament [53]. In humans, NKCC1 mutations are associated with HL and deafness [54].
2.2. Intermediate Cells
Intermediate cells are located between the marginal and basal cell layer (Figure 1). Intermediate cells show diversity in their types within and across species but commonly contribute to melanin production, protection against oxidative damage, and K+ transport within the SV. Intermediate cells of both melanoblast and Schwann-cell precursor origin have been found [55]. During development, they invade the lateral wall and penetrate the basement membrane beneath the marginal cells following a basal-to-apical spatiotemporal gradient [55,56,57]. The intermediate cells are also melanocytes and, like other melanocytes, produce the pigment melanin. Mammalian species have varying levels of SV pigmentation, which consists largely of melanin. No melanin is present in the albino inner ear [58]. Melanocytes are best known for their protection against UV-induced DNA damage in the skin. They produce the melanin pigment in specialized organelles called melanosomes, which are then transferred to keratinocytes [59]. Since melanin is involved in free radical scavenging [60], it likely also serves a protective role in intermediate cells. The various properties of melanin that are involved in this protective function are discussed in detail in Section 3.2.
In several species, two populations of melanin-containing cells can be identified. The two populations observed in different species are, however, not clearly equivalent and/or may represent different stages of the same cell type. In the chinchilla (Chinchilla lanigera), one population of melanin-containing cells are called melanocyte-like cells, which are heavily pigmented with dense pigment granules and are closely associated with blood vessels. The other population consists of intermediate cells, which contain small, dense pigment granules [61]. Similarly, in the cat, one population of melanin-containing cells are called melanocytes, which are closely associated with capillaries and contain varying numbers of fine-grained pigment granules. Intermediate cells, by contrast, are not obviously associated with capillaries and less frequently contain pigmented organelles [58]. In mice, one population of melanin containing cells are called light intermediate cells, and they are present from birth and contain pigment granules in different stages of development. Light intermediate cells appear less frequently in old animals. The other population, called dark intermediate cells, which are only present in adult mice, contain pigment granules in lysosomal bodies. There are few other organelles, and their nuclei are often pyknotic, a state characterized by the irreversible condensation of chromatin. Neither light nor dark intermediate cells are obviously associated with capillaries. These two apparently different populations may represent different stages in the life cycle of a single cell type [62].
In newborn gerbils, melanosomes in the SV are found in large, dendritic cells, called intermediate cells, while the adult gerbil SV contains a population of melanin containing cells called star-shaped, triangular, or fusiform pigment cells (STFPCs). These STFPCs correspond morphologically to the melanocyte-like cells in the chinchilla and cat, which contain most of the melanin in the adult SV. It is possible that melanosomes are transferred from intermediate cells to these STFPCs, similar to how in the dermal melanin system melanosomes are transferred to keratinocytes [63]. To a lesser extent, basal cells can also contain melanin granules, which are likely transferred from nearby melanin-producing cells [61,62]. More recently, in the gerbil, ultrastructural studies distinguished a basal subtype of intermediate cell (BIC) from an upper subtype of intermediate cell (UIC), referring to their relative positions within the strial layers. Marginal cells projected distinct processes to each of these subtypes suggesting a functional difference [64].
In addition to their role in melanin production, intermediate cells play an important role in K+ transport via inwardly rectifying Kir4.1 channels [65]. In the healthy adult SV, marginal cells and intermediate cells form extensive inter-digitations to maximize the surface area for ion exchange (Figure 1B) through the extension of primary and secondary processes, as was shown by ultrastructural analysis in the gerbil [66]. In the viable dominant spotting mouse mutant, which lacks intermediate cells in the SV, the development of inter-digitations between the remaining cell types is reduced and the EP remains at 0 mV, failing to show the normal gradual developmental increases to about +100 mV in adulthood. Intermediate cells are, therefore, regarded as essential for the normal development and functioning of the SV [67].
2.3. Basal Cells and Fibrocytes
Basal cells form the third and final layer of the SV (Figure 1). They are derived from the otic mesenchyme [68]. The basal cell layer is located next to the spiral ligament, which contains five different types of fibrocytes (I–V) bathed in perilymph. Gap junctions composed of connexins connect the basal cells to the fibrocytes and intermediate cells in the SV, forming a functional syncytium called the “connective tissue gap junction network” [38,69]. To form a barrier between the intrastrial space and the perilymph in the spiral ligament, basal cells are connected to each other via tight junctions containing the adhesion molecule claudin-11 [70].
Fibrocytes play an active role in regulating ion concentrations. Specifically, Na+/K+-ATPase and NKCC1 are abundantly expressed in type II and IV fibrocytes [41,46,51,71]. Via the gap junction network with the basal cells and intermediate cells, the K+ taken up by the fibrocytes is transported to the SV [69]. Together, these various cell types play essential roles in maintaining the appropriate ionic environment and fluid balance in the cochlea.
2.4. Capillary Network and the Blood–Labyrinth Barrier
The SV contains an extensive capillary system (Figure 1B) and, similar to the brain, which is protected by the blood–brain barrier (BBB), the cochlea is protected by the blood–labyrinth barrier (BLB). Like the BBB, the BLB restricts the entry of most bloodborne compounds. Both in the BBB and the BLB, the endothelial cells of the capillaries are connected via tight junctions, and the vessels are populated with pericytes [72,73]. The permeability and active transport mechanisms of the BLB, however, differ from those in the BBB and are not yet well characterized. The measurements of permeability using a radioactive tracer showed that the BLB is less permeable than the BBB at the choroid plexus [74]. Interestingly, the permeability appears to be molecule specific: for example, gentamycin can cross the BLB but not the BBB [75]. The restrictive entry of bloodborne compounds into the cochlea due to the BLB and the incomplete characterization of its permeability also complicates the development of drug delivery via the strial vasculature [73]. The permeability of the blood vessels is further regulated by perivascular macrophages, a subset of tissue resident macrophages [76]. Tissue resident macrophages within the cochlea can have different origins. In mice, during development, resident macrophages originating from the yolk sac as well as resident macrophages originating from the fetal liver are found [77]. Resident macrophages in the SV only appear after birth [77]. In the adult mouse cochlea, bone marrow-derived resident macrophages are found in the spiral ligament, the auditory nerve, and the SV [78,79,80]. Their function and especially role in inflammatory responses is discussed in Section 3.3.
3. Mechanisms Contributing to Age-Related Loss of Strial Function
To assess the impact of SV dysfunction on the EP and hearing, researchers have employed various functional and structural measures. In humans, distinguishing between the metabolic component of HL, resulting from SV dysfunction, and the sensory component, resulting from hair cell dysfunction, remains challenging because there is currently no method for assessing these tissues directly in a noninvasive and independent manner. Based on the shape of the audiogram, presbycusis has been categorized as metabolic, sensory, or both [81,82]. These categories, however, did not indicate the specific extent to which either component contributed to HL. More recently, a promising quantitative approach has been developed to estimate indirectly the extent of metabolic and sensory HL through analyzing the shape of the audiogram [14]. While it is not possible to completely define or differentiate the underlying pathologies solely based on the audiogram, the findings indicate that the audiogram provides valuable insights into both types of HL. This method was recently validated using datasets from gerbils and humans, including histopathologic assessments. The model could potentially be improved in the future by including the interactions between the two pathologies [14]. A study using human samples confirmed that a flatter audiometric shape is associated with greater strial atrophy, however, it was argued that not the flatness but the degree of low-frequency threshold shift is predictive of strial degeneration [83].
In animal models, the EP and, thus, SV function can be measured directly but invasively using microelectrodes placed in the cochlea [84,85]. These measurements detect the loss of the EP in response to various perturbations of the SV, including the absence of melanocytes or melanin [67,86], potassium ion channel knockout [87], and furosemide application [34,88]. Structural measures of the SV, used to estimate the extent of strial atrophy, commonly include measures of the SV thickness, cross-sectional area, and/or cell density [7,8,89]. These changes can be assessed postmortem in animal models as well as humans, with the caveat that histological changes in humans are often examined with a limited knowledge of the individual’s hearing history and well after the onset of HL, complicating the interpretation of the causative pathology [7,8,83]. In humans, structural measurements reveal correlations between the extent of strial atrophy and HL, supporting the basis of Schuknecht’s original classification of metabolic presbycusis [2,3,7,83]. Various correlations have also been observed in animal models. A decrease in marginal cell density, for example, was shown to be a good predictor of EP across the lifespan in BALB/cJ mice. In addition, spiral ligament thickness was also found to be a good predictor of EP across the lifespan of BALB/cJ mice [89], supporting the involvement of spiral ligament fibrocytes in age-dependent lateral wall atrophy [90]. Interestingly, the examination of CD/1 mice, which show accelerated presbycusis, suggests that the fibrocyte pathology of the SV preceded the age-related pathology of the sensorineural structures in the cochlea [91].
Nevertheless, there is considerable discrepancy between functional and structural measures of the SV, with functional deficits sometimes observed in the absence of structural pathology and vice versa. For example, in BALB/cJ mice, strial thickness was found to be only a weak predictor of the age-related loss of the EP, while in C57BL/6J mice no significant correlation was found. Moreover, other morphological changes that showed highly significant correlations with age-related changes in the EP in BALB/cJ mice, including marginal cell density and spiral ligament thickness, failed to show significant correlations in C57BL/6J mice [89]. In guinea pigs with sensorineural HL induced by kanamycin and xylazine, the EP returned to normal after 56 days despite the severe atrophy of the SV [92]. In CBA/CaJ mice showing permanent, noise-induced threshold shifts, the EP recovered despite strial edema and the degeneration of type II fibrocytes in the spiral ligament [93].
Such discrepancies may be explained by additionally considering the role of the sensory hair cells. Hair-cell loss per se does not affect the EP, as demonstrated in CBA/J mice, where the EP was maintained even after severe hair cell loss induced by kanamycin and furosemide [94]. A reduction in hair cells would nevertheless decrease the metabolic load on the SV by reducing the continuous K+ current flow through active hair cells [92]. Depending on the extent of hair cell loss, a normal EP could still be observed despite SV dysfunction. By analogy, the SV is often likened to a battery that powers the hair cells. Outer hair cells, and to a lesser extent inner hair cells, draw current from this battery, and a reduced load resulting from hair cell loss would allow the EP to remain relatively normal even if the SV generating the EP was substantially weakened [13]. In support of this phenomenon, C57BL/6 mice with significant outer hair cell loss showed preserved EPs, despite a decreased expression of the K+ channels Kir4.1 and KCNQ1 and a decreased expression of the transcripts encoding Na+/K+-ATPase α1 and α2 and NKCC1 in the SV [95]. Moreover, studies in gerbils have shown that outer hair cells can adapt their transducer operation point in response to an acutely lowered EP, recovering nearly full cochlear amplification [96]. It is not known if this adaptive mechanism also operates in the long term when the EP is chronically reduced due to aging [96]. These findings highlight the complicated interaction between the SV and the hair cells and, thus, metabolic and sensory HL.
Perhaps not surprisingly then, there is considerable controversy over the relative contributions of strial atrophy and sensorineural loss to age-related HL in humans. A recent analysis of hair cells, auditory nerve fibers, and strial tissue in human post-mortem tissue showed that most of the variance in audiometric thresholds could be explained by hair cell loss and not strial damage [8]. More recently, a related study with additional samples did find a significant predictive value of strial degeneration for threshold shift in the low frequency region, although the contribution of outer hair cell damage was still greater [83]. However, as further discussed below, strial degeneration, assessed by the common structural measures outlined above, is not necessarily a reliable indicator of the functional status of the SV. Thus, the contribution of strial function, beyond just strial degeneration, in age-related HL in humans warrants increased investigation. Therefore, the following sections will explore the various factors implicated in SV function and their potential for assessing strial dysfunction during aging. These factors include disrupted ion transport, altered pigmentation, inflammatory responses, and vascular atrophy.
3.1. Disrupted Ion Transport
This section summarizes how aging and experimental reduction in the expression and function of crucial K+ channels and transporters, specifically Na+/K+-ATPase, NKCC1, and Kir4.1 in the SV, impact the generation of the EP, strial function, and hearing thresholds.
In the quiet-aged gerbil, the expression level of the Na+/K+-ATPase, based on immunoreactivity observed in the marginal cells of the SV, has been shown to decline with age [97]. Similarly, in aged CBA/CaJ mice, Na+/K+-ATPase expression in the SV decreases by approximately 80% despite a reduction in strial thickness by only 20% [98]. This observation indicates that strial atrophy is a poor predictor of ion transport function in the SV. In the quiet aged gerbil, in 21–22-month-old animals, only a limited, patchy loss in immunoreactivity was observed in apical cochlear turns. In contrast, in 29–31-month-old gerbils, the loss of Na+/K+-ATPase immunoreactivity expanded to most of the apical turn and parts of the basal turn [97]. In the oldest group of gerbils, aged 35–38 months, the loss of immunoreactivity extended throughout the apical turn and into basal turns and showed considerable variation, involving the entire basal turn in the most severe cases. In regions of advanced strial atrophy, fibrocytes in the lateral wall also showed a decrease in Na+/K+-ATPase content [97]. In quiet-aged gerbils, aged 33–36 months, fibrocytes in the spiral ligament showed cytosolic vacuolization and degenerated cells, as well as interstitial edema. These conditions may be the result of impaired K+ diffusion through marginal cells [90]. Since Na+/K+-ATPase immunoreactivity is only an indirect indicator for its enzymatic activity, the enzymatic activity in the lateral wall has also been assessed directly. In some 36–39 months old gerbils, a marked decrease in Na+/K+-ATPase activity was observed compared to other age groups ranging from 1 to 19 months old [99]. Importantly, in the human cochlea, a decrease in Na+/K+-ATPase α1 in the SV has been related to presbycusis [100].
In gerbils, the proportion of the SV showing immunostaining for Na+/K+-ATPase was found to correlate with the magnitude of the EP. For evaluation purposes, the intensity of the staining was not taken into account, primarily because regions exhibiting reduced immunoreactivity, rather than complete loss of immunoreactivity, were uncommon [97]. In aged gerbils (30 months old), the decline in EP compared to young gerbils (under 8 months old) was most pronounced near both the apex and base of the cochlea [101]. Specifically, there was a decrease of 18 mV at 0.5 kHz, 3 mV at 2 kHz, 7 mV at 16 kHz, and 16 mV at 40 kHz equivalent frequency locations. In contrast, even older gerbils (36 months old) showed a more uniform decline in EP across the cochlea, with decreases of 27 mV at 0.5 kHz, 19 mV at 2 kHz, 23 mV at 16 kHz, and 31 mV at 40 kHz [101]. Furthermore, in aged gerbils (32–39 months old), there was a strong correlation between reduced Na+/K+-ATPase activity and reduced EP, suggesting that this reduced activity can explain most of the decline in EP [102]. No significant difference in endolymphatic K+ concentration was found in quiet-aged versus young gerbils, although the intersubject variability was much greater in aged compared to young gerbils. There was a weak but significant correlation between the K+ concentration in endolymph and the EP in aged gerbils (R2 = 0.23, p < 0.01). K+ concentrations below 150 mM were associated with EPs below 60 mV [103].
The loss of NKCC1, a Na+-K+-2Cl− cotransporter located in the marginal cells of the SV and subpopulations of fibrocytes of the spiral ligament, can, just like the loss of Na+/K+-ATPase, contribute to hearing problems. In the gerbil, NKCC1 shows both a developmental increase and age-related loss of expression similar to that of Na+/K+-ATPase [71]. With age, diminished NKCC1 immunoreactivity in the SV and, eventually, along with the advanced atrophy of the SV, a complete loss of immunoreactivity was reported [71]. In the final stages of atrophy, the SV was replaced by a thin squamous cell layer [71]. In aging C57BL/6 mice, the level of NKCC1 mRNA as well as NKCC1 protein expression in the lateral wall was reduced [53]. It has been shown that mice lacking NKCC1 are deaf and the membranous labyrinth in the inner ear is collapsed [104,105]. NKCC1 has also been implicated in human HL. In three cases, deafness in children was associated with inherited mutations that led to a total absence of NKCC1. Additionally, other cases have been reported where single allele mutations in the SLC12A2 gene, which encodes NKCC1, were linked to HL. These findings linking the loss of NKCC1 function and HL in humans underscore the validity of animal models in studying the contribution of NKCC1 to metabolic HL [54].
In the gerbil, as mentioned above, NKCC1 immunostaining shows a similar developmental and age-related expression pattern to Na+/K+-ATPase, consistent with a high level of functional cooperation [71]. In Black Swiss-129/SvJ mice, heterozygote deletion of either Na+/K+-ATPase α1, Na+/K+-ATPase α2, or NKCC1 lead to progressive, age-related HL. Interestingly, when both Na+/K+-ATPase α2 (located in fibrocytes but not marginal cells) and NKCC1 (found in marginal cells and certain fibrocyte subpopulations) were simultaneously deleted, hearing remained largely preserved in Black Swiss-129/SvJ mice. This observation suggests that the dual deletion may have a protective effect on hearing. Double deletion presumably leads to downregulated but balanced K+ flux, potentially offering insights for future drug treatment strategies. If the downregulation or inhibition of a certain transporter due to ototoxic drugs or a genetic predisposition leads to HL, then deliberately inhibiting the counterbalancing transporter may mitigate the potential damage [106].
NKCC1 decreases with age but can also be pharmacologically manipulated. Furosemide is a loop diuretic that disturbs the function of the SV by blocking NKCC1. By applying this drug, the effects of a dysfunctional SV can be simulated in young animals, thereby allowing the evaluation of EP decline while excluding potentially confounding effects of aging. Chronic furosemide application (up to 28 days) was shown to lower the EP and induce HL, quantified using compound action potential (CAP) measurements [34]. Increased CAP thresholds were the most prominent at high frequencies, likely reflecting the greater gain of the cochlear amplifier (mediated by the outer-hair-cell function) in the high compared to low frequency regions of the cochlea [34]. Furthermore, the loss of EP and the audiogram profile resulting from furosemide treatment in young gerbils closely matched profiles of old gerbils. This observation suggests that the reduction in EP in quiet-aged gerbils is the main cause of their age-related HL [34]. A separate study revealed that when young gerbils were treated with furosemide for seven days, there was a reduction in EP and an increase in CAP thresholds [35]. Notably, two weeks after furosemide treatment was stopped, both the EP and the CAP thresholds began to recover. This recovery coincided with an observed increase in the cell division of fibrocytes within the spiral ligament. In contrast, older gerbils exhibited a decrease in fibrocyte proliferation compared to the younger controls [35]. These observations suggest that decreased fibrocyte proliferation plays a role in the pathology of the lateral wall of the cochlea, resulting in the loss of EP.
Another factor that can result in disturbed ion transport through the SV is cellular damage due to metabolic dysfunction. Since the SV has a high metabolic rate [107], it is likely highly susceptible to such a dysfunction. In 30–36-month-old gerbils, the first sign of strial atrophy was the degeneration of secondary marginal cell processes, apparently caused by oxidative self-damage to mitochondria in the primary processes, resulting in insufficient ATP for the Na+/K+-ATPase of the secondary processes. Eventually, the primary processes themselves were also affected [66]. Aging, as well as noise overexposure and ototoxic drugs, can trigger mitochondrial dysfunction resulting in oxidative stress by the excess production of reactive oxygen species (reviewed by [108,109]). Cisplatin treatment, for example, leads to an increase in free radicals within the SV, resulting in mitochondrial membrane permeabilization and the apoptosis of marginal cells [110].
The dysfunction of the K+ channel Kir4.1, located in the intermediate cells of the SV, contributes to HL as well. In knockout mice lacking Kir4.1, the EP is abolished [87]. The loss of Kir 4.1 protein expression in the intermediate cells of the SV is likely the direct cause of abolished EP and deafness in a mouse model of Pendred syndrome, caused by the homozygous deletion of Slc26a4 [111]. This Pendred syndrome mouse model also showed the hyperpigmentation of the SV as well as increased macrophage proliferation and activation, potentially as a result of excess free radicals [111,112]. The hyperpigmentation of the SV and tissue-resident macrophages are discussed further in Section 3.2 and Section 3.3, respectively. In aging CBA/CaJ mice, a decline in Kir4.1 immunoreactivity has been shown in intermediate cells as well as outer sulcus cells and satellite cells in the spiral ganglion [113]. The animal models of the dysfunction of Kir4.1 are highly relevant for the understanding of human age-related HL, since the expression of Kir4.1 was found to be altered in these same cell types in the aging human cochlea [113].
Collectively, these studies linking reduced EP with HL predict that artificially increasing the EP in animals suffering from age-related HL would restore hearing to some extent. This has been tested by injecting a current in the scala media of old gerbils to increase the EP, resulting in a 20 dB reduction in the CAP threshold [101]. Although this manipulation was invasive and acute, it nevertheless provides a proof of principle that restoring the EP artificially, potentially using pharmacological approaches and/or implanted biological devices, can improve hearing. Furthermore, the studies discussed above show that different kinds of dysfunction produce the same HL because the elements discussed in this section are interdependent; if any element is taken out, the whole ion transport loop suffers. This underscores the importance of differential diagnosis in this context, as the identification of the specific dysfunction or combination of dysfunctions at play allows for personalized treatment strategies targeting the root cause of the condition.
3.2. Variation in Pigmentation in the Stria Vascularis
This section focuses on the role that pigment plays in the SV in the context of age-related HL. As briefly mentioned in the introduction, melanin has a number of different properties that can be of importance in the SV. Certain drugs are believed to accumulate in the inner ear due to their affinity to melanin [114]. Additionally, melanin is capable of scavenging paramagnetic metal [115], consistent with its potential cytoprotective function as a scavenger of toxic substances [116]. Melanin also plays a role in free radical homeostasis and is able to scavenge and bind free radicals, which is important for combatting oxidative stress [60].
While the precise role of melanin in age-related HL is not fully understood, studies involving albino animals offer some, albeit conflicting, insights. Most notably, an investigation comparing C57BL/6J mice, which have normal melanin production, and C57BL/6J-Tyr(c-2J) mice, which carry a tyrosinase mutation resulting in the absence of melanin, found that both pigmented and albino mice exhibited similar rates of HL and sensory cell loss but that the EP in the basal cochlea was reduced in albino compared to pigmented mice. A reduced EP in aged albino mice was correlated with reduced strial thickness and a greater loss of marginal cells [86]. BALB mice, which are albino, demonstrate a significant reduction in endocochlear potential (EP) with age. Initially, albino C57BL/6J mice exhibit an aging pattern similar to their pigmented counterparts, suggesting that melanin alone may not fully account for EP loss in aging BALB/cJ mice [89]. These findings are consistent with reports of accelerated age-related EP decline in the albino strains of rats (Fischer 344/NHsd) [117], further albino mouse strains (NOD.NON-H2nb1) [118] and albino guinea pigs [119]; however, these models lack appropriate pigmented controls. Importantly, the lack of melanin does not appear to affect the development of the inner ear in any of these models. In addition to albinism, NOD.NON-H2nb1 mice show a broader pathology including hair cell and neuronal loss, reduced strial thickness, as well as vascular atrophy in the SV [118], which is further discussed below. Interestingly, there are also indications that melanin is not essential for maintaining normal auditory function. Transgenic YRT2 mice, carrying a functional copy of the Tyr locus and indistinguishable from wild-type pigmented animals, as well as transgenic TyrTH mice, which can only generate the melanin precursor L-DOPA, but not melanin, maintained normal auditory thresholds as they aged, while their non-transgenic albino NMRI littermates showed premature HL with age, suggesting that the melanin precursor L-DOPA is sufficient to provide a protective effect against premature deafness [120]. Finally, no correlation between the presence or absence of melanin and the rate of strial degeneration with age was found when comparing the pathology between two albino rat strains (Fischer 344 and Lewis) and two pigmented rat strains (Lewis–Brown Norway F1 and Brown Norway) [121], arguing against a protective role of melanin in age-related HL.
The evidence in favor of melanin’s involvement in the susceptibility to strial pathology comes from experiments examining the susceptibility of albino animals to noise-induced HL. The albino compared to pigmented guinea pigs showed an increased susceptibility to HL due to noise overexposure [122]. Moreover, in normally pigmented chinchillas, the hyperpigmentation of the SV, due to melanin granules in the intermediate cells and, less frequently, the marginal and basal cells, has been observed after noise overexposure [123].
In humans, ethnicity is associated with the development of HL, with adult African American individuals showing a lower prevalence of HL than adult Caucasian individuals [124,125]. A correlation also exists between the ethnicity and pigmentation in the SV. African American SV samples contain significantly more pigmentation than Caucasian specimens, both as children and as adults [126,127]. Consistent with this, several indicators of melanin content, such as eye color and skin sun-sensitivity, are correlated with susceptibility to temporary auditory threshold shift after noise overexposure [128,129,130]. In aging humans, as well as in C57BL/6 and CBA/CaJ mice, strial melanin content significantly increases with age. It is currently unclear whether this increase with age is related to the potential protective role of melanin or whether it is simply a metabolic by-product [126,127,131].
3.3. Tissue-Resident Macrophages and Inflammatory Responses
In this section, the role of inflammation and tissue-resident macrophages in the SV are discussed. Tissue-resident macrophages are sentinel immune cells that are involved in initiating inflammatory responses, clearing debris and aggregated pigment granules, maintaining a homeostatic tissue environment, and supporting angiogenesis [132,133]. Tissue-resident macrophages are part of the blood–labyrinth barrier discussed in Section 3.3. Perivascular macrophages (PVMs) are a unique subset of tissue-resident macrophages associated with blood vessels [134]. A number of studies have started to use the term “perivascular macrophage-like melanocytes” (PVM/Ms) for a hybrid cell type containing both macrophage as well as melanocyte markers [73,135,136,137]. Other studies, however, argue against the existence of this hybrid cell type [133,138].
PVMs are integrated into the cochlear vessels and maintain the integrity of the barrier between the blood and the intrastrial space by affecting the expression of tight- and adherens-junction proteins [135]. Their exact role in the regulation of barrier integrity is not yet clear. One study demonstrated that the absence of PVMs resulted in the increased capillary permeability of cultured endothelial cell monolayers to fluorescein isothiocyanate (FITC)-dextran, the increased capillary leakage of various tracers, and a reduction in the EP [135]. In contrast, however, it has also been reported that PVM depletion does not alter the baseline permeability of capillaries for fluorescein, and, in fact, activated PVMs may play a role in the breakdown of the BLB after systemic exposure to lipopolysaccharide [138]. Since different tracers were used to assess the leakage of intact capillaries, selective permeability for different tracers could explain the discrepancy in permeability between the two studies. More recently, an increase in cochlear macrophages was found during inflammation induced by lipopolysaccharide [139]. The excess proliferation and activation of PVMs could result in dysfunctional BLB maintenance. Investigating the numbers and activation states of PVMs under different circumstances could provide insight into these potentially pathological mechanisms.
Recently, a study by Lang et al. [140] revealed that in mice, early pathological changes in the SV are associated with increased macrophage activation and “inflammaging”, which is characterized by chronic low-grade inflammation with a dysregulation of immune cell activity. The age dependent increase in macrophage activation was correlated with an increase in cochlear auditory brainstem response (ABR) thresholds. Strial thickness was, however, not correlated with age or thresholds, highlighting once more that strial thickness is not a reliable indicator of strial function [140]. These results indicate that the dysregulation of the immune system and macrophage dysfunction could be early indicators of age-related strial pathology. Macrophage activation has been demonstrated before in the human cochlea [141,142]. As in mice, in human strial samples the number of activated macrophages also increased significantly with age while strial thickness did not differ significantly [140].
3.4. Vascular Atrophy
This section synthesizes numerous findings from animal models linking the atrophy of the strial vasculature to the age-related decline in cochlear function. In C57BL/6 mice aged 6–12 months, corrosion casts, which create detailed three-dimensional replicas of blood vessels, revealed the degeneration of the vasculature in the SV, especially in basal turns, where the capillary diameter was significantly reduced [143]. In C57BL/6 and BALB/cJ mice, the age-related thickening of the basement membrane and reduced capillary diameter were observed, both of which may contribute to chronic ischemia within the cochlea [89]. Surprisingly, however, there was no correlation between the EP and either the basement membrane thickness or capillary diameter in either strain [89]. In fact, in old BALB/cJ mice, low EPs tended to be associated with larger capillaries. Thus, the dependency of the EP on physical features of the basement membrane and the strial vasculature is not straightforward.
Gerbils also show age-related changes in strial vasculature. As in mice, the basement membrane thickness of SV capillaries was increased in gerbils aged 33 months and older [144]. With advancing age, the loss of capillaries in regions of the SV progressed from the extreme apical and basal ends and into the middle regions of the cochlea [145]. Small areas of the SV with few or no capillaries were, in fact, already observed at the extreme apical and basal ends of the SV by 5–9 months of age. By 33 months of age, the regions of the SV with normal vasculature were found only in the middle and upper basal turns, with the rest of the SV showing a loss of capillaries and, in some cases, the atrophy of the marginal cells as well. These findings suggest that changes in the strial microvasculature begin before the onset of HL and may initiate the degeneration of other parts of the SV, including the marginal cells [145]. In a subsequent study, the decline in the strial capillary area in quiet-aged gerbils was quantified, showing a significant reduction in the individual capillary area of 8–29% at 2, 20, and 40 kHz in gerbils aged 33–40 months [146]. The functional consequences of the loss of strial capillaries are, nevertheless, not clear. Although blood flow within the cochlea, and especially SV, is reduced in 2–3-year-old gerbils, reduced blood flow is not clearly related to the loss of strial capillaries but possibly results from decreased perfusion pressure or increased vascular resistance instead [147].
As in mice, in gerbils the link between age-related changes in strial vasculature and the EP is not straightforward and may be difficult to identify in cross-sections given the patchy nature of strial degeneration [146]. Vascular abnormalities assessed in whole-mount preparations were associated with a decrease in EP in gerbils aged in quiet for 36–39 months [148]. Areas with atrophied capillaries were mainly found in the apex, lower base and hook region of the cochlea. The total area in which the capillaries remained normal ranged from 19 to 87% [148]. The EP also varied greatly, ranging from 23 to 83 mV. A significant correlation was found between the area with normal vasculature and the mean EP (measured at several locations) [148].
Recent work has also uncovered a link between HL caused by cytomegalovirus (CMV) infection and a decreased EP in BALB/c and C57BL/6 mice [149]. Congenital CMV infection is the most common congenital viral infection and often results in sensorineural HL in infants [150]. In BALB/c mice, CMV infection is associated with capillary network damage in the SV and elevated ABR thresholds [151]. CMV pathology is, however, not limited to the SV, and even within the SV it remains unclear whether capillary damage is the initial step in strial degeneration. Although capillary damage and insufficient oxygen supply could lead to disrupted ion transport and decreased EP, ion transport may be disrupted before vascular damage occurs [149]. It has also been suggested that damage to the SV could be caused by an inflammatory response in the inner ear [152]. The role of CMV infection in HL has been reviewed more extensively elsewhere [16,17,18].
4. Conclusions
This review summarizes a large body of research identifying correlations between age-related changes in the structure and function of the SV with disruptions in ion transport, altered pigmentation, inflammatory responses, and vascular abnormalities. These insights underscore the crucial role of the SV in cochlear function and its contribution to age-related HL and, moreover, motivate continued research to identify the mechanisms linking age-related changes in the SV and age-related HL. Identifying these mechanisms is crucial for developing an array of individualized strategies to treat age-related HL and requires increased attention as part of ongoing research endeavors, which are increasingly focused on the contribution of the sensorineural structures within the cochlea.
Future research should explore the complex interplay between the SV, EP generation, and its impact over time on age-related HL. To this end, experiments should recognize that, as highlighted in this review, the SV comprises distinct cell types that operate in an interdependent and synergistic manner and, thus, requires a diverse toolkit that permits investigation from cells to tissue. Single-cell approaches include electrophysiological approaches capable of probing ion transport mechanisms in living cells to transcriptomic and proteomic approaches capable of investigating, in high throughput, age-related changes in gene and protein expression within single, identified cell types. At the tissue level, advanced imaging techniques, such as high-resolution microscopy and imaging modalities that capture dynamic blood flow, will be necessary to visualize and analyze gradual age-related changes in the size and blood flow within the SV. These methods should be applied to existing animal models and, in addition, further explore strain differences in mice, better utilize existing transgenic models, and potentially employ CRISPR-Cas technology to generate new transgenic animal, and especially gerbil, models.
Ultimately, these approaches and others, in combination with clinical research, will delineate the various mechanisms including genetic and epigenetic factors and environmental stressors that determine and influence the susceptibility of the SV to age-related changes and identify strategies for intervention and prevention. Achieving this ambitious goal necessitates ongoing interdisciplinary collaboration, a longstanding strength of auditory research.
Author Contributions
Conceptualization, S.B., G.M.K., C.K. and S.J.P.; validation, G.M.K., C.K. and S.J.P.; formal analysis, S.B.; writing—original draft preparation, S.B.; writing—review and editing, G.M.K., C.K. and S.J.P.; visualization, S.B.; supervision, G.M.K., C.K. and S.J.P.; project administration, G.M.K., C.K. and S.J.P.; funding acquisition, G.M.K., C.K. and S.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy–EXC 2177/1-Project ID 390895286 to C.K. and G.M.K. and the Heinsius Houbolt Foundation to S.J.P.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization. World Report on Hearing; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002048-1.
- Schuknecht, H.F. Presbycusis. Laryngoscope 1955, 65, 402–419. [Google Scholar] [CrossRef]
- Schuknecht, H.F. Further Observations on the Pathology of Presbycusis. Arch. Otolaryngol. Head Neck Surg. 1964, 80, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H.F.; Watanuki, K.; Takahashi, T.; Aziz Belal, A.; Kimura, R.S.; Jones, D.D.; Ota, C.Y. Atrophy of the Stria Vascularis, a Common Cause for Hearing Loss. Laryngoscope 1974, 84, 1777–1821. [Google Scholar] [CrossRef] [PubMed]
- Schuknecht, H.F.; Gacek, M.R. Cochlear Pathology in Presbycusis. Ann. Otol. Rhinol. Laryngol. 1993, 102, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P.; Marcus, D.C. Ion and Fluid Homeostasis in the Cochlea. In Understanding the Cochlea; Manley, G.A., Gummer, A.W., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research; Springer International Publishing: Cham, Switzerland, 2017; Volume 62, pp. 253–286. ISBN 978-3-319-52071-1. [Google Scholar]
- Pauler, M.; Schuknecht, H.F.; White, J.A. Atrophy of the Stria Vascularis as a Cause of Sensorineural Hearing Loss. Laryngoscope 1988, 98, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; O’Malley, J.T.; de Gruttola, V.; Liberman, M.C. Age-Related Hearing Loss Is Dominated by Damage to Inner Ear Sensory Cells, Not the Cellular Battery That Powers Them. J. Neurosci. 2020, 40, 6357–6366. [Google Scholar] [CrossRef]
- Takahashi, T. The Ultrastructure of the Pathologic Stria Vascularis and Spiral Prominence in Man. Ann. Otol. Rhinol. Laryngol. 1971, 80, 721–735. [Google Scholar] [CrossRef]
- Hibino, H.; Nin, F.; Tsuzuki, C.; Kurachi, Y. How Is the Highly Positive Endocochlear Potential Formed? The Specific Architecture of the Stria Vascularis and the Roles of the Ion-Transport Apparatus. Pflug. Arch. Eur. J. Physiol. 2010, 459, 521–533. [Google Scholar] [CrossRef]
- Chan, D.K.; Hudspeth, A.J. Ca2+ Current–Driven Nonlinear Amplification by the Mammalian Cochlea in Vitro. Nat. Neurosci. 2005, 8, 149–155. [Google Scholar] [CrossRef]
- Liberman, M.C.; Kujawa, S.G. Cochlear Synaptopathy in Acquired Sensorineural Hearing Loss: Manifestations and Mechanisms. Hear. Res. 2017, 349, 138–147. [Google Scholar] [CrossRef]
- Eckert, M.A.; Harris, K.C.; Lang, H.; Lewis, M.A.; Schmiedt, R.A.; Schulte, B.A.; Steel, K.P.; Vaden, K.I.; Dubno, J.R. Translational and Interdisciplinary Insights into Presbyacusis: A Multidimensional Disease. Hear. Res. 2021, 402, 108109. [Google Scholar] [CrossRef] [PubMed]
- Vaden, K.I.; Eckert, M.A.; Matthews, L.J.; Schmiedt, R.A.; Dubno, J.R. Metabolic and Sensory Components of Age-Related Hearing Loss. J. Assoc. Res. Otolaryngol. 2022, 23, 253–272. [Google Scholar] [CrossRef]
- Trpchevska, N.; Freidin, M.B.; Broer, L.; Oosterloo, B.C.; Yao, S.; Zhou, Y.; Vona, B.; Bishop, C.; Bizaki-Vallaskangas, A.; Canlon, B.; et al. Genome-Wide Association Meta-Analysis Identifies 48 Risk Variants and Highlights the Role of the Stria Vascularis in Hearing Loss. Am. J. Hum. Genet. 2022, 109, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zong, S.; Du, P.; Zhou, P.; Li, H.; Wang, E.; Xiao, H. Role of the Stria Vascularis in the Pathogenesis of Sensorineural Hearing Loss: A Narrative Review. Front. Neurosci. 2021, 15, 774585. [Google Scholar] [CrossRef]
- Thulasiram, M.R.; Ogier, J.M.; Dabdoub, A. Hearing Function, Degeneration, and Disease: Spotlight on the Stria Vascularis. Front. Cell Dev. Biol. 2022, 10, 841708. [Google Scholar] [CrossRef]
- Johns, J.D.; Adadey, S.M.; Hoa, M. The Role of the Stria Vascularis in Neglected Otologic Disease. Hear. Res. 2023, 428, 108682. [Google Scholar] [CrossRef]
- Schmidt, R.S. Independence of the Endovestibular Potential in Homeotherms. J. Gen. Physiol. 1963, 47, 371–378. [Google Scholar] [CrossRef]
- Bielefeld, E.C.; Tanaka, C.; Chen, G.; Henderson, D. Age-Related Hearing Loss: Is It a Preventable Condition? Hear. Res. 2010, 264, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Translating Animal Models to Human Therapeutics in Noise-Induced and Age-Related Hearing Loss. Hear. Res. 2019, 377, 44–52. [Google Scholar] [CrossRef]
- Ohlemiller, K.K. Mechanisms and Genes in Human Strial Presbycusis from Animal Models. Brain Res. 2009, 1277, 70–83. [Google Scholar] [CrossRef]
- Ryan, A. Hearing Sensitivity of the Mongolian Gerbil, Meriones unguiculatis. J. Acoust. Soc. Am. 1976, 59, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Gleich, O.; Strutz, J. The Mongolian Gerbil as a Model for the Analysis of Peripheral and Central Age-Dependent Hearing Loss. In Hearing Loss; Naz, S., Ed.; InTechOpen: London, UK, 2012; ISBN 978-953-51-0366-0. [Google Scholar]
- Schmiedt, R.A.; Mills, J.H.; Adams, J.C. Tuning and Suppression in Auditory Nerve Fibers of Aged Gerbils Raised in Quiet or Noise. Hear. Res. 1990, 45, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, B.I.; Schmiedt, R.A.; Hellstrom, L.I.; Lee, F.S.; Adams, J.C. Age-Related Changes in Cochleas of Mongolian Gerbils. Hear. Res. 1991, 54, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Gleich, O.; Semmler, P.; Strutz, J. Behavioral Auditory Thresholds and Loss of Ribbon Synapses at Inner Hair Cells in Aged Gerbils. Exp. Gerontol. 2016, 84, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Steenken, F.; Heeringa, A.N.; Beutelmann, R.; Zhang, L.; Bovee, S.; Klump, G.M.; Köppl, C. Age-Related Decline in Cochlear Ribbon Synapses and Its Relation to Different Metrics of Auditory-Nerve Activity. Neurobiol. Aging 2021, 108, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Bovee, S.; Klump, G.M.; Pyott, S.J.; Sielaff, C.; Köppl, C. Cochlear Ribbon Synapses in Aged Gerbils. Int. J. Mol. Sci. 2024, 25, 2738. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Bourien, J.; Tang, Y.; Batrel, C.; Huet, A.; Lenoir, M.; Ladrech, S.; Desmadryl, G.; Nouvian, R.; Puel, J.-L.; Wang, J. Contribution of Auditory Nerve Fibers to Compound Action Potential of the Auditory Nerve. J. Neurophysiol. 2014, 112, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Heeringa, A.N.; Köppl, C. The Aging Cochlea: Towards Unraveling the Functional Contributions of Strial Dysfunction and Synaptopathy. Hear. Res. 2019, 376, 111–124. [Google Scholar] [CrossRef]
- Mills, J.; Schmiedt, R.; Schulte, B.; Dubno, J. Age-Related Hearing Loss: A Loss of Voltage, Not Hair Cells. Semin. Hear. 2006, 27, 228–236. [Google Scholar] [CrossRef]
- Schmiedt, R.A.; Lang, H.; Okamura, H.; Schulte, B.A. Effects of Furosemide Applied Chronically to the Round Window: A Model of Metabolic Presbyacusis. J. Neurosci. 2002, 22, 9643–9650. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Schulte, B.A.; Schmiedt, R.A. Effects of Chronic Furosemide Treatment and Age on Cell Division in the Adult Gerbil Inner Ear. J. Assoc. Res. Otolaryngol. 2003, 4, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; Jyothi, V.; Smythe, N.M.; Dubno, J.R.; Schulte, B.A.; Schmiedt, R.A. Chronic Reduction of Endocochlear Potential Reduces Auditory Nerve Activity: Further Confirmation of an Animal Model of Metabolic Presbyacusis. J. Assoc. Res. Otolaryngol. 2010, 11, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. K+ Cycling and the Endocochlear Potential. Hear. Res. 2002, 165, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hibino, H.; Kurachi, Y. Molecular and Physiological Bases of the K+ Circulation in the Mammalian Inner Ear. Physiology 2006, 21, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Wangemann, P. Supporting Sensory Transduction: Cochlear Fluid Homeostasis and the Endocochlear Potential: Cochlear Homeostasis. J. Physiol. 2006, 576, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Sagara, T.; Furukawa, H.; Makishima, K.; Fujimoto, S. Differentiation of the Rat Stria Vascularis. Hear. Res. 1995, 83, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.A.; Adams, J.C. Distribution of Immunoreactive Na+,K+-ATPase in Gerbil Cochlea. J. Histochem. Cytochem. 1989, 37, 127–134. [Google Scholar] [CrossRef]
- Sweadner, K.J.; Rael, E. The FXYD Gene Family of Small Ion Transport Regulators or Channels: cDNA Sequence, Protein Signature Sequence, and Expression. Genomics 2000, 68, 41–56. [Google Scholar] [CrossRef]
- Blanco, G. Na,K-ATPase Subunit Heterogeneity as a Mechanism for Tissue-Specific Ion Regulation. Semin. Nephrol. 2005, 25, 292–303. [Google Scholar] [CrossRef]
- Geering, K. Functional Roles of Na,K-ATPase Subunits. Curr. Opin. Nephrol. Hypertens. 2008, 17, 526–532. [Google Scholar] [CrossRef]
- McGuirt, J.P.; Schulte, B.A. Distribution of Immunoreactive Alpha- and Beta-Subunit Isoforms of Na,K-ATPase in the Gerbil Inner Ear. J. Histochem. Cytochem. 1994, 42, 843–853. [Google Scholar] [CrossRef]
- Schulte, B.A.; Steel, K.P. Expression of α and β Subunit Isoforms of Na,K-ATPase in the Mouse Inner Ear and Changes with Mutations at the Wv or Sld Loci. Hear. Res. 1994, 78, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dai, M.; Wilson, T.M.; Omelchenko, I.; Klimek, J.E.; Wilmarth, P.A.; David, L.L.; Nuttall, A.L.; Gillespie, P.G.; Shi, X. Na+/K+-ATPase A1 Identified as an Abundant Protein in the Blood-Labyrinth Barrier That Plays an Essential Role in the Barrier Integrity. PLoS ONE 2011, 6, e16547. [Google Scholar] [CrossRef] [PubMed]
- McLean, W.J.; Smith, K.A.; Glowatzki, E.; Pyott, S.J. Distribution of the Na,K-ATPase α Subunit in the Rat Spiral Ganglion and Organ of Corti. J. Assoc. Res. Otolaryngol. 2009, 10, 37–49. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Luque, M.; Glueckert, R.; Danckwardt-Lillieström, N.; Nordström, C.K.; Schrott-Fischer, A.; Rask-Andersen, H. Expression of Na/K-ATPase Subunits in the Human Cochlea: A Confocal and Super-Resolution Microscopy Study with Special Reference to Auditory Nerve Excitation and Cochlear Implantation. Upsala J. Med. Sci. 2019, 124, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Rask-Andersen, H. Na/K-ATPase Gene Expression in the Human Cochlea: A Study Using mRNA in Situ Hybridization and Super-Resolution Structured Illumination Microscopy. Front. Mol. Neurosci. 2022, 15, 857216. [Google Scholar] [CrossRef]
- Crouch, J.J.; Sakaguchi, N.; Lytle, C.; Schulte, B.A. Immunohistochemical Localization of the Na-K-Cl Co-Transporter (NKCC1) in the Gerbil Inner Ear. J. Histochem. Cytochem. 1997, 45, 773–778. [Google Scholar] [CrossRef]
- Russell, J.M. Sodium-Potassium-Chloride Cotransport. Physiol. Rev. 2000, 80, 211–276. [Google Scholar] [CrossRef]
- Liu, Y.; Chu, H.; Chen, J.; Zhou, L.; Chen, Q.; Yu, Y.; Wu, Z.; Wang, S.; Lai, Y.; Pan, C.; et al. Age-Related Change in the Expression of NKCC1 in the Cochlear Lateral Wall of C57BL/6J Mice. Acta Oto. Laryngol. 2014, 134, 1047–1051. [Google Scholar] [CrossRef]
- Koumangoye, R.; Bastarache, L.; Delpire, E. NKCC1: Newly Found as a Human Disease-Causing Ion Transporter. Function 2020, 2, zqaa028. [Google Scholar] [CrossRef] [PubMed]
- Renauld, J.M.; Khan, V.; Basch, M.L. Intermediate Cells of Dual Embryonic Origin Follow a Basal to Apical Gradient of Ingression into the Lateral Wall of the Cochlea. Front. Cell Dev. Biol. 2022, 10, 867153. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Hilding, D.A. The Development of the Stria Vascularis in the Mouse. Acta Oto. Laryngol. 1966, 62, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Locher, H.; de Groot, J.C.M.J.; van Iperen, L.; Huisman, M.A.; Frijns, J.H.M.; Chuva de Sousa Lopes, S.M. Development of the Stria Vascularis and Potassium Regulation in the Human Fetal Cochlea: Insights into Hereditary Sensorineural Hearing Loss. Dev. Neurobiol. 2015, 75, 1219–1240. [Google Scholar] [CrossRef]
- Conlee, J.W.; Parks, T.N.; Schwartz, I.R.; Creel, D.J. Comparative Anatomy of Melanin Pigment in the Stria Vascularis: Evidence for a Distinction between Melanocytes and Intermediate Cells. Cat. Acta Oto. Laryngol. 1989, 107, 48–58. [Google Scholar] [CrossRef]
- Park, H.-Y.; Yaar, M. Chapter 72. Biology of Melanocytes. In Fitzpatrick’s Dermatology in General Medicine, 8th ed.; Goldsmith, L.A., Katz, S.I., Gilchrest, B.A., Paller, A.S., Leffell, D.J., Wolff, K., Eds.; The McGraw-Hill Companies: New York, NY, USA, 2012. [Google Scholar]
- Różanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free Radical Scavenging Properties of Melanin. Free. Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.G.; Lee, D.H. Pigmented Cells of the Stria Vascularis and Spiral Ligament of the Chinchilla. Acta Oto. Laryngol. 1989, 108, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Cable, J.; Steel, K.P. Identification of Two Types of Melanocyte Within the Stria Vascularis of the Mouse Inner Ear. Pigment. Cell Res. 1991, 4, 87–101. [Google Scholar] [CrossRef]
- Barrenäs, M.-L.; Axelsson, A. The Development of Melanin in the Stria Vascularis of the Gerbil. Acta Oto. Laryngol. 1992, 112, 50–58. [Google Scholar] [CrossRef]
- Spicer, S.S.; Schulte, B.A. Novel Structures in Marginal and Intermediate Cells Presumably Relate to Functions of Apical versus Basal Strial Strata. Hear. Res. 2005, 200, 87–101. [Google Scholar] [CrossRef]
- Ando, M.; Takeuchi, S. Immunological Identification of an Inward Rectifier K+ Channel (Kir4.1) in the Intermediate Cell (Melanocyte) of the Cochlear Stria Vascularis of Gerbils and Rats. Cell Tissue Res. 1999, 298, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Spicer, S.S.; Schulte, B.A. Pathologic Changes of Presbycusis Begin in Secondary Processes and Spread to Primary Processes of Strial Marginal Cells. Hear. Res. 2005, 205, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Steel, K.P.; Barkway, C. Another Role for Melanocytes: Their Importance for Normal Stria Vascularis Development in the Mammalian Inner Ear. Development 1989, 107, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Trowe, M.-O.; Maier, H.; Petry, M.; Schweizer, M.; Schuster-Gossler, K.; Kispert, A. Impaired Stria Vascularis Integrity upon Loss of E-Cadherin in Basal Cells. Dev. Biol. 2011, 359, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Adams, J.C.; Miyabe, Y.; So, E.; Kobayashi, T. Potassium Ion Recycling Pathway via Gap Junction Systems in the Mammalian Cochlea and Its Interruption in Hereditary Nonsyndromic Deafness. Med. Electron Microsc. 2000, 33, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kitajiri, S.; Furuse, M.; Morita, K.; Saishin-Kiuchi, Y.; Kido, H.; Ito, J.; Tsukita, S. Expression Patterns of Claudins, Tight Junction Adhesion Molecules, in the Inner Ear. Hear. Res. 2004, 187, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, N.; Crouch, J.J.; Lytle, C.; Schulte, B.A. Na-K-Cl Cotransporter Expression in the Developing and Senescent Gerbil Cochlea. Hear. Res. 1998, 118, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Han, W.; Yamamoto, H.; Tang, W.; Lin, X.; Xiu, R.; Trune, D.R.; Nuttall, A.L. The Cochlear Pericytes. Microcirculation 2008, 15, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.; Abbott, N.J.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of Therapeutics to the Inner Ear: The Challenge of the Blood-Labyrinth Barrier. Sci. Transl. Med. 2019, 11, eaao0935. [Google Scholar] [CrossRef]
- Inamura, N.; Salt, A.N. Permeability Changes of the Blood-Labyrinth Barrier Measured in Vivo during Experimental Treatments. Hear. Res. 1992, 61, 12–18. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Baker, D.E.; Pagel, M.A.; Blank, N.K. Cerebrovascular Permeability and Delivery of Gentamicin to Normal Brain and Experimental Brain Abscess in Rats. J. Neurosurg. 1984, 61, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kurata, N.; Fukunaga, Y. Tissue-Resident Macrophages in the Stria Vascularis. Front. Neurol. 2022, 13, 818395. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, I.; Okano, T.; Nishimura, K.; Motohashi, T.; Omori, K. Early Development of Resident Macrophages in the Mouse Cochlea Depends on Yolk Sac Hematopoiesis. Front. Neurol. 2019, 10, 1115. [Google Scholar] [CrossRef]
- Sato, E.; Shick, H.E.; Ransohoff, R.M.; Hirose, K. Repopulation of Cochlear Macrophages in Murine Hematopoietic Progenitor Cell Chimeras: The Role of CX3CR1. J. Comp. Neurol. 2008, 506, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Okano, T.; Nakagawa, T.; Kita, T.; Kada, S.; Yoshimoto, M.; Nakahata, T.; Ito, J. Bone Marrow-Derived Cells Expressing Iba1 Are Constitutively Present as Resident Tissue Macrophages in the Mouse Cochlea. J. Neurosci. Res. 2008, 86, 1758–1767. [Google Scholar] [CrossRef]
- Shi, X. Resident Macrophages in the Cochlear Blood-Labyrinth Barrier and Their Renewal via Migration of Bone-Marrow-Derived Cells. Cell Tissue Res. 2010, 342, 21–30. [Google Scholar] [CrossRef]
- Schmiedt, R.A. The Physiology of Cochlear Presbycusis. In The Aging Auditory System; Gordon-Salant, S., Frisina, R.D., Popper, A.N., Fay, R.R., Eds.; Springer Handbook of Auditory Research; Springer: New York, NY, USA, 2010; Volume 34, pp. 9–38. ISBN 978-1-4419-0992-3. [Google Scholar]
- Dubno, J.R.; Eckert, M.A.; Lee, F.-S.; Matthews, L.J.; Schmiedt, R.A. Classifying Human Audiometric Phenotypes of Age-Related Hearing Loss from Animal Models. J. Assoc. Res. Otolaryngol. 2013, 14, 687–701. [Google Scholar] [CrossRef]
- Kaur, C.; Wu, P.-Z.; O’Malley, J.T.; Liberman, M.C. Predicting Atrophy of the Cochlear Stria Vascularis from the Shape of the Threshold Audiogram. J. Neurosci. 2023, 43, 8801–8811. [Google Scholar] [CrossRef]
- Békésy, V.G. DC Resting Potentials Inside the Cochlear Partition. J. Acoust. Soc. Am. 1952, 24, 72–76. [Google Scholar] [CrossRef]
- Tasaki, I.; Spyropoulos, C.S. Stria Vascularis as Source of Endocochlear Potential. J. Neurophysiol. 1959, 22, 149–155. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Rybak Rice, M.E.; Lett, J.M.; Gagnon, P.M. Absence of Strial Melanin Coincides with Age-Associated Marginal Cell Loss and Endocochlear Potential Decline. Hear. Res. 2009, 249, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.C.; Wu, T.; Wangemann, P.; Kofuji, P. KCNJ10 (Kir4.1) Potassium Channel Knockout Abolishes Endocochlear Potential. Am. J. Physiol. Cell Physiol. 2002, 282, C403–C407. [Google Scholar] [CrossRef] [PubMed]
- Sewell, W.F. The Effects of Furosemide on the Endocochlear Potential and Auditory-Nerve Fiber Tuning Curves in Cats. Hear. Res. 1984, 14, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; Lett, J.M.; Gagnon, P.M. Cellular Correlates of Age-Related Endocochlear Potential Reduction in a Mouse Model. Hear. Res. 2006, 220, 10–26. [Google Scholar] [CrossRef]
- Spicer, S.S.; Schulte, B.A. Spiral Ligament Pathology in Quiet-Aged Gerbils. Hear. Res. 2002, 172, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Mahendrasingam, S.; MacDonald, J.A.; Furness, D.N. Relative Time Course of Degeneration of Different Cochlear Structures in the CD/1 Mouse Model of Accelerated Aging. J. Assoc. Res. Otolaryngol. 2011, 12, 437–453. [Google Scholar] [CrossRef]
- Hellier, W.P.L.; Wagstaff, S.A.; O’Leary, S.J.; Shepherd, R.K. Functional and Morphological Response of the Stria Vascularis Following a Sensorineural Hearing Loss. Hear. Res. 2002, 172, 127–136. [Google Scholar] [CrossRef]
- Hirose, K.; Liberman, M.C. Lateral Wall Histopathology and Endocochlear Potential in the Noise-Damaged Mouse Cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 339–352. [Google Scholar] [CrossRef]
- Xiong, H.; Chu, H.; Zhou, X.; Huang, X.; Cui, Y.; Zhou, L.; Chen, J.; Li, J.; Wang, Y.; Chen, Q.; et al. Conservation of Endocochlear Potential in Mice with Profound Hearing Loss Induced by Co-Administration of Kanamycin and Furosemide. Lab. Anim. 2011, 45, 95–102. [Google Scholar] [CrossRef]
- Yang, H.; Xiong, H.; Huang, Q.; Pang, J.; Zheng, X.; Chen, L.; Yu, R.; Zheng, Y. Compromised Potassium Recycling in the Cochlea Contributes to Conservation of Endocochlear Potential in a Mouse Model of Age-Related Hearing Loss. Neurosci. Lett. 2013, 555, 97–101. [Google Scholar] [CrossRef]
- Wang, Y.; Fallah, E.; Olson, E.S. Adaptation of Cochlear Amplification to Low Endocochlear Potential. Biophys. J. 2019, 116, 1769–1786. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.A.; Schmiedt, R.A. Lateral Wall Na, K-ATPase and Endocochlear Potentials Decline with Age in Quiet-Reared Gerbils. Hear. Res. 1992, 61, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Walton, J.P.; Zhu, X.; Frisina, R.D. Age-Related Changes in Na, K-ATPase Expression, Subunit Isoform Selection and Assembly in the Stria Vascularis Lateral Wall of Mouse Cochlea. Hear. Res. 2018, 367, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Gratton, M.A.; Smyth, B.J.; Schulte, B.A.; Vincent, D.A. Na,K-ATPase Activity Decreases in the Cochlear Lateral Wall of Quiet-Aged Gerbils. Hear. Res. 1995, 83, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.; Mangasarian, A.; Ishiyama, G.; Hosokawa, K.; Hosokawa, S.; Ishiyama, A.; Lopez, I.A. Immunohistochemical Location of Na+, K+-ATPase A1 Subunit in the Human Inner Ear. Hear. Res. 2021, 400, 108113. [Google Scholar] [CrossRef] [PubMed]
- Schmiedt, R.A. Cochlear Potentials in Quiet-Aged Gerbils: Does the Aging Cochlea Need a Jump Start? In Sensory Research: Multimodal Perspectives; Verrillo, R.T., Zwislocki, J.J., Eds.; L. Erlbaum Associates: Hillsdale, NJ, USA, 1993; pp. 91–103. ISBN 978-0-8058-1342-5. [Google Scholar]
- Gratton, M.A.; Smyth, B.J.; Lam, C.F.; Boettcher, F.A.; Schmiedt, R.A. Decline in the Endocochlear Potential Corresponds to Decreased Na,K-ATPase Activity in the Lateral Wall of Quiet-Aged Gerbils. Hear. Res. 1997, 108, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Schmiedt, R.A. Effects of Aging on Potassium Homeostasis and the Endocochlear Potential in the Gerbil Cochlea. Hear. Res. 1996, 102, 125–132. [Google Scholar] [CrossRef]
- Delpire, E.; Lu, J.; England, R.; Dull, C.; Thorne, T. Deafness and Imbalance Associated with Inactivation of the Secretory Na-K-2Cl Co-Transporter. Nat. Genet. 1999, 22, 192–195. [Google Scholar] [CrossRef] [PubMed]
- Flagella, M.; Clarke, L.L.; Miller, M.L.; Erway, L.C.; Giannella, R.A.; Andringa, A.; Gawenis, L.R.; Kramer, J.; Duffy, J.J.; Doetschman, T.; et al. Mice Lacking the Basolateral Na-K-2Cl Cotransporter Have Impaired Epithelial Chloride Secretion and Are Profoundly Deaf. J. Biol. Chem. 1999, 274, 26946–26955. [Google Scholar] [CrossRef]
- Diaz, R.C.; Vazquez, A.E.; Dou, H.; Wei, D.; Cardell, E.L.; Lingrel, J.; Shull, G.E.; Doyle, K.J.; Yamoah, E.N. Conservation of Hearing by Simultaneous Mutation of Na,K-ATPase and NKCC1. J. Assoc. Res. Otolaryngol. 2007, 8, 422–434. [Google Scholar] [CrossRef]
- Marcus, D.C.; Thalmann, R.; Marcus, N.Y. Respiratory Rate and Atp Content of Stria Vascularis of Guinea Pig In Vitro. Laryngoscope 1978, 88, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Böttger, E.C.; Schacht, J. The Mitochondrion: A Perpetrator of Acquired Hearing Loss. Hear. Res. 2013, 303, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kamogashira, T.; Fujimoto, C.; Yamasoba, T. Reactive Oxygen Species, Apoptosis, and Mitochondrial Dysfunction in Hearing Loss. BioMed Res. Int. 2015, 2015, 617207. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Nakagawa, T.; Kita, T.; Kim, T.S.; Iguchi, F.; Endo, T.; Shiga, A.; Lee, S.H.; Ito, J. Mechanisms of Apoptosis Induced by Cisplatin in Marginal Cells in Mouse Stria Vascularis. ORL 2004, 66, 111–118. [Google Scholar] [CrossRef]
- Wangemann, P.; Itza, E.M.; Albrecht, B.; Wu, T.; Jabba, S.V.; Maganti, R.J.; Ho Lee, J.; Everett, L.A.; Wall, S.M.; Royaux, I.E.; et al. Loss of KCNJ10 Protein Expression Abolishes Endocochlear Potential and Causes Deafness in Pendred Syndrome Mouse Model. BMC Med. 2004, 2, 30. [Google Scholar] [CrossRef] [PubMed]
- Jabba, S.V.; Oelke, A.; Singh, R.; Maganti, R.J.; Fleming, S.; Wall, S.M.; Everett, L.A.; Green, E.D.; Wangemann, P. Macrophage Invasion Contributes to Degeneration of Stria Vascularis in Pendred Syndrome Mouse Model. BMC Med. 2006, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, G.; Noble, K.V.; Li, Y.; Barth, J.L.; Schulte, B.A.; Lang, H. Age-Dependent Alterations of Kir4.1 Expression in Neural Crest–Derived Cells of the Mouse and Human Cochlea. Neurobiol. Aging 2019, 80, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Gottesberge, A.M.Z. Physiology and Pathophysiology of Inner Ear Melanin. Pigment. Cell Res. 1988, 1, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Enochs, W.S.; Petherick, P.; Bogdanova, A.; Mohr, U.; Weissleder, R. Paramagnetic Metal Scavenging by Melanin: MR Imaging. Radiology 1997, 204, 417–423. [Google Scholar] [CrossRef]
- Larsson, B.S. Interaction Between Chemicals and Melanin. Pigment. Cell Res. 1993, 6, 127–133. [Google Scholar] [CrossRef]
- Bielefeld, E.C.; Coling, D.; Chen, G.-D.; Li, M.; Tanaka, C.; Hu, B.-H.; Henderson, D. Age-Related Hearing Loss in the Fischer 344/NHsd Rat Substrain. Hear. Res. 2008, 241, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ohlemiller, K.K.; Rice, M.E.R.; Gagnon, P.M. Strial Microvascular Pathology and Age-Associated Endocochlear Potential Decline in NOD Congenic Mice. Hear. Res. 2008, 244, 85–97. [Google Scholar] [CrossRef]
- Dum, N.; Schmidt, U.; Wedel, H. Age-Dependence of the Neural Auditory Thresholds of Albino and Pigmented Guinea Pigs. Arch. Otorhinolaryngol. 1980, 229, 191–199. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Contreras, J.; Zurita, E.; Cediel, R.; Cantero, M.; Varela-Nieto, I.; Montoliu, L. Melanin Precursors Prevent Premature Age-Related and Noise-Induced Hearing Loss in Albino Mice: Albino Mice Become Deaf Prematurely. Pigment. Cell Melanoma Res. 2010, 23, 72–83. [Google Scholar] [CrossRef]
- Keithley, E.M.; Ryan, A.F.; Feldman, M.L. Cochlear Degeneration in Aged Rats of Four Strains. Hear. Res. 1992, 59, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Conlee, J.W.; Abdul-Baqi, K.J.; McCandless, G.A.; Creel, D.J. Differential Susceptibility to Noise-Induced Permanent Threshold Shift between Albino and Pigmented Guinea Pigs. Hear. Res. 1986, 23, 81–91. [Google Scholar] [CrossRef]
- Gratton, M.A.; Wright, C.G. Hyperpigmentation of Chinchilla Stria Vascularis Following Acoustic Trauma. Pigment. Cell Res. 1992, 5, 30–37. [Google Scholar] [CrossRef]
- Agrawal, Y. Prevalence of Hearing Loss and Differences by Demographic Characteristics Among US Adults: Data from the National Health and Nutrition Examination Survey, 1999–2004. Arch. Intern. Med. 2008, 168, 1522. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Maas, P.; Chien, W.; Carey, J.P.; Ferrucci, L.; Thorpe, R. Association of Skin Color, Race/Ethnicity, and Hearing Loss Among Adults in the USA. J. Assoc. Res. Otolaryngol. 2012, 13, 109–117. [Google Scholar] [CrossRef]
- Sun, D.Q.; Zhou, X.; Lin, F.R.; Francis, H.W.; Carey, J.P.; Chien, W.W. Racial Difference in Cochlear Pigmentation Is Associated with Hearing Loss Risk. Otol. Neurotol. 2014, 35, 1509–1514. [Google Scholar] [CrossRef]
- Andresen, N.S.; Coreas, S.; Villavisanis, D.F.; Lauer, A.M. Comparison of Age-Related Pigmentary Changes in the Auditory and Vestibular Systems Within Mouse and Human Temporal Bones. Front. Neurosci. 2021, 15, 680994. [Google Scholar] [CrossRef]
- Hood, J.D.; Poole, J.P.; Freedman, L. The Influence of Eye Colour upon Temporary Threshold Shift. Int. J. Audiol. 1976, 15, 449–464. [Google Scholar] [CrossRef]
- Barrenäs, M.-L.; Lindgren, F. The Influence of Inner Ear Melanin on Susceptibility to TTS in Humans. Scand. Audiol. 1990, 19, 97–102. [Google Scholar] [CrossRef]
- Barrenäs, M.-L.; Lindgren, F. The Influence of Eye Colour on Susceptibility to TTS in Humans. Br. J. Audiol. 1991, 25, 303–307. [Google Scholar] [CrossRef]
- Hayashi, H.; Sone, M.; Schachern, P.A.; Wakamatsu, K.; Paparella, M.M.; Nakashima, T. Comparison of the Quantity of Cochlear Melanin in Young and Old C57BL/6 Mice. Arch. Otolaryngol. Head. Neck. Surg. 2007, 133, 151. [Google Scholar] [CrossRef][Green Version]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-Resident Macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Ito, T.; Li, X.; Kurima, K.; Choi, B.Y.; Wangemann, P.; Griffith, A.J. Slc26a4-Insufficiency Causes Fluctuating Hearing Loss and Stria Vascularis Dysfunction. Neurobiol. Dis. 2014, 66, 53–65. [Google Scholar] [CrossRef]
- Koizumi, T.; Kerkhofs, D.; Mizuno, T.; Steinbusch, H.W.M.; Foulquier, S. Vessel-Associated Immune Cells in Cerebrovascular Diseases: From Perivascular Macrophages to Vessel-Associated Microglia. Front. Neurosci. 2019, 13, 1291. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, M.; Fridberger, A.; Hassan, A.; DeGagne, J.; Neng, L.; Zhang, F.; He, W.; Ren, T.; Trune, D.; et al. Perivascular-Resident Macrophage-like Melanocytes in the Inner Ear Are Essential for the Integrity of the Intrastrial Fluid–Blood Barrier. Proc. Natl. Acad. Sci. USA 2012, 109, 10388–10393. [Google Scholar] [CrossRef]
- Neng, L.; Zhang, F.; Kachelmeier, A.; Shi, X. Endothelial Cell, Pericyte, and Perivascular Resident Macrophage-Type Melanocyte Interactions Regulate Cochlear Intrastrial Fluid–Blood Barrier Permeability. J. Assoc. Res. Otolaryngol. 2013, 14, 175–185. [Google Scholar] [CrossRef]
- Shi, X. Pathophysiology of the Cochlear Intrastrial Fluid-Blood Barrier (Review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef]
- Hirose, K.; Li, S.-Z. The Role of Monocytes and Macrophages in the Dynamic Permeability of the Blood-Perilymph Barrier. Hear. Res. 2019, 374, 49–57. [Google Scholar] [CrossRef]
- Bae, S.H.; Kwak, S.H.; Yoo, J.E.; Kim, K.M.; Hyun, Y.M.; Choi, J.Y.; Jung, J. Three-Dimensional Distribution of Cochlear Macrophages in the Lateral Wall of Cleared Cochlea. Clin. Exp. Otorhinolaryngol. 2021, 14, 179–184. [Google Scholar] [CrossRef]
- Lang, H.; Noble, K.V.; Barth, J.L.; Rumschlag, J.A.; Jenkins, T.R.; Storm, S.L.; Eckert, M.A.; Dubno, J.R.; Schulte, B.A. The Stria Vascularis in Mice and Humans Is an Early Site of Age-Related Cochlear Degeneration, Macrophage Dysfunction, and Inflammation. J. Neurosci. 2023, 43, 5057–5075. [Google Scholar] [CrossRef]
- Noble, K.V.; Liu, T.; Matthews, L.J.; Schulte, B.A.; Lang, H. Age-Related Changes in Immune Cells of the Human Cochlea. Front. Neurol. 2019, 10, 895. [Google Scholar] [CrossRef]
- Liu, W.; Danckwardt-Lillieström, N.; Schrott-Fischer, A.; Glueckert, R.; Rask-Andersen, H. Distribution of Immune Cells Including Macrophages in the Human Cochlea. Front. Neurol. 2021, 12, 781702. [Google Scholar] [CrossRef]
- Carraro, M.; Harrison, R.V. Degeneration of Stria Vascularis in Age-Related Hearing Loss; a Corrosion Cast Study in a Mouse Model. Acta Oto. Laryngol. 2016, 136, 385–390. [Google Scholar] [CrossRef]
- Thomopoulos, G.N.; Spicer, S.S.; Gratton, M.A.; Schulte, B.A. Age-Related Thickening of Basement Membrane in Stria Vascularis Capillaries. Hear. Res. 1997, 111, 31–41. [Google Scholar] [CrossRef]
- Gratton, M.A.; Schulte, B.A. Alterations in Microvasculature Are Associated with Atrophy of the Stria Vascularis in Quiet-Aged Gerbils. Hear. Res. 1995, 82, 44–52. [Google Scholar] [CrossRef]
- Gratton, M.A.; Schulte, B.A.; Smythe, N.M. Quantification of the Stria Vascularis and Strial Capillary Areas in Quiet-Reared Young and Aged Gerbils. Hear. Res. 1997, 114, 1–9. [Google Scholar] [CrossRef]
- Prazma, J.; Carrasco, V.N.; Butler, B.; Waters, G.; Anderson, T.; Pillsbury, H.C. Cochlear Microcirculation in Young and Old Gerbils. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Gratton, M.A.; Schmiedt, R.A.; Schulte, B.A. Age-Related Decreases in Endocochlear Potential Are Associated with Vascular Abnormalities in the Stria Vascularis. Hear. Res. 1996, 102, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shi, K.; Nielson, C.; Graham, E.M.; Price, M.S.; Haller, T.J.; Carraro, M.; Firpo, M.A.; Park, A.H.; Harrison, R.V. Hearing Loss Caused by CMV Infection Is Correlated with Reduced Endocochlear Potentials Caused by Strial Damage in Murine Models. Hear. Res. 2022, 417, 108454. [Google Scholar] [CrossRef]
- Goderis, J.; De Leenheer, E.; Smets, K.; Van Hoecke, H.; Keymeulen, A.; Dhooge, I. Hearing Loss and Congenital CMV Infection: A Systematic Review. Pediatrics 2014, 134, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Almishaal, A.; Hillas, E.; Firpo, M.; Park, A.; Harrison, R.V. Cytomegalovirus (CMV) Infection Causes Degeneration of Cochlear Vasculature and Hearing Loss in a Mouse Model. J. Assoc. Res. Otolaryngol. 2017, 18, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Bradford, R.D.; Yoo, Y.-G.; Golemac, M.; Pugel, E.P.; Jonjic, S.; Britt, W.J. Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons. PLoS Pathog. 2015, 11, e1004774. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).