Is Cardiac Transplantation Still a Contraindication in Patients with Muscular Dystrophy-Related End-Stage Dilated Cardiomyopathy? A Systematic Review
Abstract
1. Introduction
1.1. Cardiac Involvement in Muscular Dystrophies
1.1.1. Dystrophinopathies
1.1.2. Emery–Dreifuss Muscular Dystrophies
1.1.3. Limb-Girdle Muscular Dystrophies
1.1.4. Myotonic Dystrophy Type 1
1.1.5. Myofibrillar Myopathies
1.1.6. Gender Differences
1.2. Association of Cardiac Disease to Molecular Defects
1.2.1. Dystrophinopathies
1.2.2. Emery–Dreifuss Muscular Dystrophies
1.2.3. Limb-Girdle Muscular Dystrophies
1.2.4. Myotonic Dystrophy Type 1
1.2.5. Desminopathies
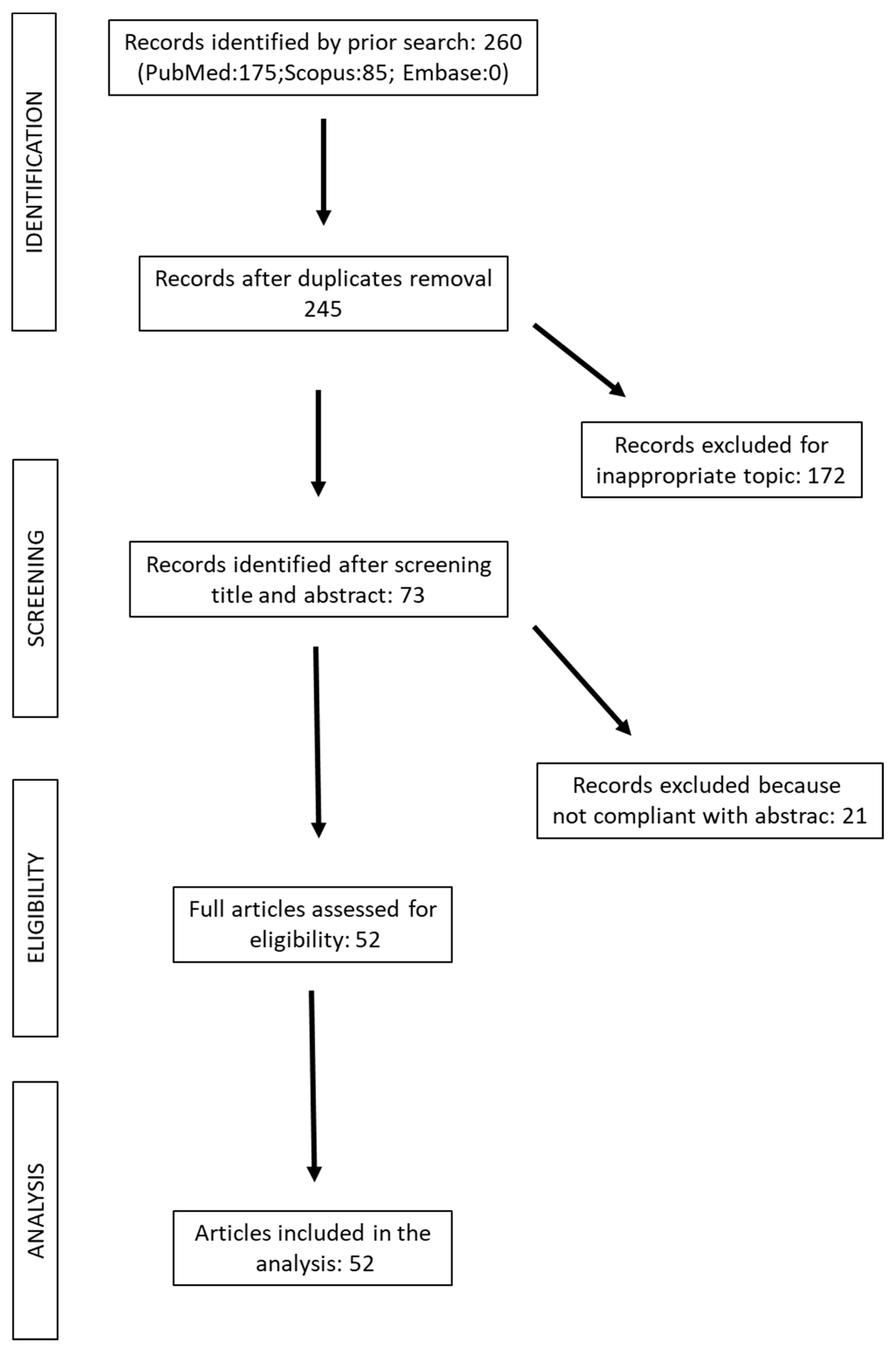
2. Methods
Search and Selection
- −
- inclusion of patients with muscular dystrophies in the cohort;
- −
- systematic reviews, prospective and retrospective cohort studies including case series to capture all published material;
- −
- defined outcomes, such as dilated heart transplantation and survival.
3. Results and Discussion
3.1. Dystrophinopathies
3.1.1. Duchenne Muscular Dystrophy
3.1.2. Becker Muscular Dystrophy and XL-DCM
3.1.3. DMD/BMD Carriers
3.2. Emery–Dreifuss Muscular Dystrophies
3.3. Limb-Girdle Muscular Dystrophies
3.4. Myotonic Dystrophy Type 1
3.5. Myofibrillar Myopathies
3.6. Discussion
4. Conclusions
Future Directions
- −
- HTx remains the treatment of choice for patients with mild-to-moderate skeletal muscle impairment and advanced dilated cardiomyopathy.
- −
- The residual reluctance to accept patients with muscular dystrophy-related cardiomyopathy for heart transplantation due to presumed shortened life expectancy must be overcome.
- −
- Long-term prognosis in these patients is closely linked to the possibility of being transplanted.
- −
- The development of neuromuscular cardiology teams (NMCT) that include specialists in cardiology, neuromuscular diseases, pulmonology, orthopedics, endocrinology, nutrition, palliative care, and physical/occupational therapies, such as that developed by Wells et al. [169] in the USA, has proven to be quite effective in the evaluation and treatment of these patients. This, therefore, should be a good model to follow in clinical practice.
- −
- The use of correct regimens of immunosuppression therapy provides good long-term results.
- −
- −
- Guidelines, preferentially developed by the NMCT for the pre-transplant evaluation of patients, should take into account the type of myopathy, the prognosis based on the muscle disease, the cardiac and respiratory conditions of the patient, and his/her life expectancy. Furthermore, the choice of the most appropriate criteria for their selection is also advisable to optimize outcomes.
- −
- Future studies should focus on detailed outcomes to assess quality of life in these patients and better guide the NMCT in transplant decisions.
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| MDs | Muscular dystrophies |
| DMD | Duchenne muscular dystrophy |
| DM1 | Distrofia myotonica type 1 |
| EDMD | Emery–Dreifuss muscular dystrophy |
| EDMD1 | X-linked Emery–Dreifuss muscular dystrophy |
| EDMD2 | Autosomal Emery–Dreifuss muscular dystrophy |
| BMD | Becker muscular dystrophy |
| MFMs | Myofibrillar myopathies |
| DCM | Dilated cardiomyopathy |
| XL-DCM | X-linked dilated cardiomyopathy |
| LGMD | Limb-girdle muscular dystrophy |
| HTx | Heart transplantation |
| HF | Heart failure |
| CK | Creatin kinase |
| GCS | Glucocorticoid steroids |
| ACEi | Angiotensin-converting enzyme inhibitors |
| ARBs | Angiotensin receptor blockers |
| AD | Autosomal dominant |
| AR | Autosomal recessive |
| ICD | Implantable cardioverter-defibrillator |
| FSHD | Facioscapulohumeral dystrophy |
| NGS | Next generation sequencing |
| LVSD | Left ventricle systolic dysfunction |
| LVEF | Left ventricle ejection fraction |
| VSD | Ventricle systolic dysfunction |
| ACM | Arrhythmogenic cardiomyopathy |
| LoF | Loss of function |
| ARVC | Arrhythmogenic right ventricle cardiomyopathy |
| A-V Block | Atrio-ventricular block |
| FU | Follow-up |
References
- Emery, A.E. The muscular dystrophies. Lancet 2002, 359, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Nigro, G.; Comi, L.I.; Politano, L.; Nigro, G. Cardiomyopathies associated with Muscular Dystrophies. In Engel & Franzini-Armstrong: Myology; McGRAW-HILL: New York, NY, USA, 2004; Chapter 45; pp. 1239–1256. [Google Scholar]
- Silvestri, N.J.; Ismail, H.; Zimetbaum, P.; Raynor, E.M. Cardiac involvement in the muscular dystrophies. Muscle Nerve 2018, 57, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Palladino, A.; D’Ambrosio, P.; Papa, A.A.; Petillo, R.; Orsini, C.; Scutifero, M.; Nigro, G.; Politano, L. Management of cardiac involvement in muscular dystrophies: Pediatric versus adult forms. Acta Myol. 2016, 35, 128–134. [Google Scholar] [PubMed]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Cecio, M.R.; Torre, V.; De Luca, F.; Picillo, E.; et al. Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myol. 2012, 31, 121–125. [Google Scholar] [PubMed]
- Broomfield, J.; Hill, M.; Guglieri, M.; Crowther, M.; Abrams, K. Life Expectancy in Duchenne Muscular Dystrophy: Reproduced Individual Patient Data Meta-analysis. Neurology 2021, 97, e2304–e2314. [Google Scholar] [CrossRef] [PubMed]
- San Martín, P.P.; Solis, F.F.; Cavada, C.G. Survival of patients with Duchenne muscular dystrophy. Rev. Chil. Pediatr. 2018, 89, 477–483, (In English and Spanish). [Google Scholar] [CrossRef]
- Seijger, C.; Raaphorst, J.; Vonk, J.; van Engelen, B.; Heijerman, H.; Stigter, N.; Wijkstra, P. New Insights in Adherence and Survival in Myotonic Dystrophy Patients Using Home Mechanical Ventilation. Respiration 2021, 100, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Lechner, A.; Herzig, J.J.; Kientsch, J.G.; Kohler, M.; Bloch, K.E.; Ulrich, S.; Schwarz, E.I. Cardiomyopathy as cause of death in Duchenne muscular dystrophy: A longitudinal observational study. ERJ Open Res. 2023, 9, 00176–2023. [Google Scholar] [CrossRef]
- Nikhanj, A.; Yogasundaram, H.; Nichols, B.M.; Richman-Eisenstat, J.; Phan, C.; Bakal, J.A.; Siddiqi, Z.A.; Oudit, G.Y. Cardiac intervention improves heart disease and clinical outcomes in patients with muscular dystrophy in a multidisciplinary care setting. J. Am. Heart Assoc. 2020, 9, e014004. [Google Scholar] [CrossRef]
- El-Aloul, B.; Altamirano-Diaz, L.; Zapata-Aldana, E.; Rodrigues, R.; Malvankar-Mehta, M.S.; Nguyen, C.-T.; Campbell, C. Pharmacological therapy for the prevention and management of cardiomyopathy in Duchenne muscular dystrophy: A systematic review. Neuromuscul. Disord. 2017, 27, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, Y.; Ross, H.J. Keeping an Open Mind: Heart Transplant in Patients with Muscular Dystrophy. Circ. Heart Fail. 2020, 13, e005872. [Google Scholar] [CrossRef]
- Hoffman, E.P.; Brown, R.H., Jr.; Kunkel, L.M. Dystrophin: The protein product of the Duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, M.A.; Flanigan, K.M. Update in Duchenne and Becker muscular dystrophy. Curr. Opin. Neurol. 2019, 32, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P. Causes of clinical variability in Duchenne and Becker muscular dystrophies and implications for exon skipping therapies. Acta Myol. 2020, 39, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; Hejtmancik, J.F.; Brink, P.; Gelb, B.; Zhu, X.M.; Chamberlain, J.S.; McCabe, E.R.; Swift, M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation 1993, 87, 1854–1865. [Google Scholar] [CrossRef]
- Prior, T.W. Genetic analysis of the Duchenne muscular dystrophy gene. Arch. Pathol. Lab. Med. 1991, 115, 984–990. [Google Scholar] [PubMed]
- Viggiano, E.; Picillo, E.; Passamano, L.; Onore, M.E.; Piluso, G.; Scutifero, M.; Torella, A.; Nigro, V.; Politano, L. Spectrum of Genetic Variants in the Dystrophin Gene: A Single Centre Retrospective Analysis of 750 Duchenne and Becker Patients from Southern Italy. Genes 2023, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Comi, L.I.; Politano, L.; Limongelli, F.M.; Nigro, V.; De Rimini, M.L.; Giugliano, M.A.M.; Petretta, V.R.; Passamano, L.; Restucci, B.; et al. Evaluation of the cardiomyopathy in Becker muscular dystrophy. Muscle Nerve 1995, 18, 283–291. [Google Scholar] [CrossRef]
- Hermans, M.C.E.; Pinto, Y.M.; Merkies, I.S.J.; de Die-Smulders, C.E.M.; Crijns, H.J.G.M.; Faber, C.G. Hereditary muscular dystrophies and the heart. Neuromuscul. Disord. 2010, 20, 479–492. [Google Scholar] [CrossRef]
- Kamdar, F.; Garry, D.J. Dystrophin-Deficient Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Arbustini, E.; Di Toro, A.; Giuliani, L.; Favalli, V.; Narula, N.; Grasso, M. Cardiac Phenotypes in Hereditary Muscle Disorders: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2485–2506. [Google Scholar] [CrossRef]
- Politano, L.; Nigro, V.; Nigro, G.; Petretta, V.R.; Passamano, L.; Papparella, S.; Di Somma, S.; Comi, L.I. Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA 1996, 275, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Sheri, N.; Nguyen, Q.; Yokota, T. Cardiac Involvement in Dystrophin-Deficient Females: Current Understanding and Implications for the Treatment of Dystrophinopathies. Genes 2020, 11, 765. [Google Scholar] [CrossRef]
- Sarkozy, A.; Quinlivan, R.; Bourke, J.P.; Ferlini, A.; ENMC 263rd Workshop Study Group. 263rd ENMC International Workshop: Focus on female carriers of dystrophinopathy: Refining recommendations for prevention, diagnosis, surveillance, and treatment. Hoofddorp, The Netherlands, 13–15 May 2022. Neuromuscul. Disord. 2023, 33, 274–284. [Google Scholar] [CrossRef]
- Nigro, G.; Comi, L.; Politano, L.; Bain, R. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int. J. Cardiol. 1990, 26, 271–277. [Google Scholar] [CrossRef]
- Fayssoil, A.; Nardi, O.; Orlikowski, D.; Annane, D. Cardiomyopathy in Duchenne muscular dystrophy: Pathogenesis and therapeutics. Heart Fail. Rev. 2010, 15, 103–107. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 2016, CD003725. [Google Scholar] [CrossRef]
- Biggar, W.; Harris, V.; Eliasoph, L.; Alman, B. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul. Disord. 2006, 16, 249–255. [Google Scholar] [CrossRef]
- Silversides, C.K.; Webb, G.D.; Harris, V.A.; Biggar, D.W. Effects of deflazacort on left ventricular function in patients with Duchenne muscular dystrophy. Am. J. Cardiol. 2003, 91, 769–772. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Politano, L.; Nigro, G. Treatment of dystrophinopathic cardiomyopathy: Review of the literature and personal results. Acta Myol. 2012, 31, 24–30. [Google Scholar]
- Politano, L.; Nigro, G. Managing dystrophinopathic cardiomyopathy. Expert Opin. Orphan Drugs 2016, 4, 1159–1178. [Google Scholar] [CrossRef]
- Feingold, B.; Mahle, W.T.; Auerbach, S.; Clemens, P.; Domenighetti, A.A.; Jefferies, J.L.; Judge, D.P.; Lal, A.K.; Markham, L.W.; Parks, W.J.; et al. Management of Cardiac Involvement Associated with Neuromuscular Diseases: A Scientific Statement From the American Heart Association. Circulation 2017, 136, e200–e231. [Google Scholar] [CrossRef]
- Buddhe, S.; Cripe, L.; Friedland-Little, J.; Kertesz, N.; Eghtesady, P.; Finder, J.; Hor, K.; Judge, D.P.; Kinnett, K.; McNally, E.M.; et al. Cardiac Management of the Patient with Duchenne Muscular Dystrophy. Pediatrics 2018, 142 (Suppl. 2), S72–S81. [Google Scholar] [CrossRef]
- Meyers, T.A.; Townsend, D. Cardiac Pathophysiology and the Future of Cardiac Therapies in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2019, 20, 4098. [Google Scholar] [CrossRef]
- Nguyen, Q.; Yokota, T. Antisense oligonucleotides for the treatment of cardiomyopathy in Duchenne muscular dystrophy. Am. J. Transl. Res. 2019, 11, 1202–1218. [Google Scholar] [PubMed]
- Mbakam, C.H.; Tremblay, J.P. Gene therapy for Duchenne muscular dystrophy: An update on the latest clinical developments. Expert Rev. Neurother. 2023, 23, 905–920. [Google Scholar] [CrossRef]
- Emery, A.E.; Dreifuss, F.E. Unusual type of benign x-linked muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 1966, 29, 338–342. [Google Scholar] [CrossRef]
- Bione, S.; Maestrini, E.; Rivella, S.; Mancini, M.; Regis, S.; Romeo, G.; Toniolo, D. Identification of a novel X-linked gene responsible for Emery-Dreifuss muscular dystrophy. Nat. Genet. 1994, 8, 323–327. [Google Scholar] [CrossRef]
- Nigro, V.; Bruni, P.; Ciccodicola, A.; Politano, L.; Nigro, G.; Piluso, G.; Cappa, V.; Covone, A.E.; Romeo, G.; D’Urso, M. SSCP detection of novel mutations in patients with Emery-Dreifuss muscular dystrophy: Definition of a small C-terminal region required for emerin function. Hum. Mol. Genet. 1995, 4, 2003–2004. [Google Scholar] [CrossRef] [PubMed]
- Bonne, G.; Di Barletta, M.R.; Varnous, S.; Bécane, H.-M.; Hammouda, E.-H.; Merlini, L.; Muntoni, F.; Greenberg, C.R.; Gary, F.; Urtizberea, J.-A.; et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat. Genet. 1999, 21, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, M.; Tsuchiya, Y.; Arahata, K. Emerin and cardiomyopathy in Emery–Dreifuss muscular dystrophy. Neuromuscul. Disord. 1999, 9, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Fatkin, D.; Macrae, C.; Sasaki, T.; Wolff, M.R.; Porcu, M.; Frenneaux, M.; Atherton, J.; Vidaillet, H.J., Jr.; Spudich, S.; De Girolami, U.; et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999, 341, 1715–1724. [Google Scholar] [CrossRef]
- Raharjo, W.H.; Enarson, P.; Sullivan, T.; Stewart, C.L.; Burke, B. Nuclear envelope defects associated with LMNA mutations cause dilated cardiomyopathy and Emery-Dreifuss muscular dystrophy. J. Cell Sci. 2001, 114 Pt 24, 4447–4457. [Google Scholar] [CrossRef] [PubMed]
- Captur, G.; Arbustini, E.; Bonne, G.; Syrris, P.; Mills, K.; Wahbi, K.; Mohiddin, S.A.; McKenna, W.J.; Pettit, S.; Ho, C.Y.; et al. Lamin and the heart. Heart 2017, 104, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Hasselberg, N.E.; Haland, T.F.; Saberniak, J.; Brekke, P.H.; Berge, K.E.; Leren, T.P.; Edvardsen, T.; Haugaa, K.H. Lamin A/C cardiomyopathy: Young onset, high penetrance, and frequent need for heart transplantation. Eur. Heart J. 2017, 39, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Madej-Pilarczyk, A. Clinical aspects of Emery-Dreifuss muscular dystrophy. Nucleus 2018, 9, 314–320. [Google Scholar] [CrossRef]
- Ditaranto, R.; Boriani, G.; Biffi, M.; Lorenzini, M.; Graziosi, M.; Ziacchi, M.; Pasquale, F.; Vitale, G.; Berardini, A.; Rinaldi, R.; et al. Differences in cardiac phenotype and natural history of laminopathies with and without neuromuscular onset. Orphanet J. Rare Dis. 2019, 14, 263. [Google Scholar] [CrossRef]
- D’Ambrosio, P.; Petillo, R.; Torella, A.; Papa, A.A.; Palladino, A.; Orsini, C.; Ergoli, M.; Passamano, L.; Novelli, A.; Nigro, V.; et al. Cardiac diseases as a predictor warning of hereditary muscle diseases. The case of laminopathies. Acta Myol. 2019, 38, 33–36. [Google Scholar] [PubMed]
- Wang, S.; Peng, D. Cardiac Involvement in Emery-Dreifuss Muscular Dystrophy and Related Management Strategies. Int. Heart J. 2019, 60, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kułach, A.; Majewski, M.; Gąsior, Z.; Gardas, R.; Gościńska-Bis, K.; Gołba, K.S. Dilated cardiomyopathy with severe arrhythmias in Emery-Dreifuss muscular dystrophy. Cardiol. J. 2020, 27, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Marchel, M.; Madej-Pilarczyk, A.; Tymińska, A.; Steckiewicz, R.; Ostrowska, E.; Wysińska, J.; Russo, V.; Grabowski, M.; Opolski, G. Cardiac Arrhythmias in Muscular Dystrophies Associated with Emerinopathy and Laminopathy: A Cohort Study. J. Clin. Med. 2021, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Golzio, P.G.; Chiribiri, A.; Gaita, F. ‘Unexpected’ sudden death avoided by implantable cardioverter defibrillator in Emery Dreifuss patient. Europace 2007, 9, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Nigro, G.; Russo, V.; Ventriglia, V.M.; Della Cioppa, N.; Palladino, A.; Nigro, V.; Calabrò, R.; Nigro, G.; Politano, L. Early onset of cardiomyopathy and primary prevention of sudden death in X-linked Emery–Dreifuss muscular dystrophy. Neuromuscul. Disord. 2010, 20, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stollberger, C.; Maeztu, C. Sudden cardiac death in neuromuscular disorders. Int. J. Cardiol. 2016, 203, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Peretto, G.; Di Resta, C.; Perversi, J.; Forleo, C.; Maggi, L.; Politano, L.; Barison, A.; Previtali, S.C.; Carboni, N.; Brun, F.; et al. Cardiac and Neuromuscular Features of Patients with LMNA-Related Cardiomyopathy. Ann. Intern. Med. 2019, 171, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Barriales-Villa, R.; Ochoa, J.P.; Larrañaga-Moreira, J.M.; Salazar-Mendiguchía, J.; Díez-López, C.; Restrepo-Córdoba, M.A.; Álvarez-Rubio, J.; Robles-Mezcua, A.; Olmo-Conesa, M.C.; Nicolás-Rocamora, E.; et al. Risk predictors in a Spanish cohort with cardiac laminopathies. The REDLAMINA registry. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 216–224, (In English and Spanish). [Google Scholar] [CrossRef]
- Angelini, C. LGMD. Identification, description, and classification. Acta Myol. 2020, 39, 207–217. [Google Scholar] [CrossRef]
- Taghizadeh, E.; Rezaee, M.; Barreto, G.E.; Sahebkar, A. Prevalence, pathological mechanisms, and genetic basis of limb-girdle muscular dystrophies: A review. J. Cell. Physiol. 2019, 234, 7874–7884. [Google Scholar] [CrossRef]
- Walton, J.N.; Nattrass, F.J. On the classification, natural history and treatment of the myopathies. Brain 1954, 77, 169–231. [Google Scholar] [CrossRef]
- Rideau, Y. An unknown type of muscular dystrophy. I. Analysis of two cases reports. Sem. Hop. Paris 1989, 65, 1835–1846. (In French) [Google Scholar]
- Rideau, Y. An unknown type of muscular dystrophy. II. Autosomal recessive inheritance. Sem. Hop. Paris 1989, 31, 1901–1910. (In French) [Google Scholar]
- Rideau, Y.; Delaubier, A.; Foucault, P.; Goulet, G.; Guillou, C.; Renardel, A.; Bonneau, D.; Colbert, A.; Bianchi, C.; Politano, L.; et al. Une forme méconnue de myopathie. III. Definition et caractères cliniques. Sem. Hop. Paris 1991, 67, 1343–1349. (In French) [Google Scholar]
- Beckmann, J.; Brown, R.; Muntoni, F.; Urtizberea, A.; Bonnemann, C.; Bushby, K. 66th/67th ENMC sponsored international workshop: The limb-girdle muscular dystrophies, 26-28 March 1999, Naarden, The Netherlands. Neuromuscul. Disord. 1999, 9, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Nigro, V.; Savarese, M. Genetic basis of limb-girdle muscular dystrophies: The 2014 update. Acta Myol. 2014, 33, 1–12. [Google Scholar] [PubMed]
- Savarese, M.; Di Fruscio, G.; Torella, A.; Fiorillo, C.; Magri, F.; Fanin, M.; Ruggiero, L.; Ricci, G.; Astrea, G.; Passamano, L.; et al. The genetic basis of undiagnosed muscular dystrophies and myopathies: Results from 504 patients. Neurology 2016, 87, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Straub, V.; Murphy, A.; Udd, B.; LGMD Workshop Study Group. 229th ENMC international workshop: Limb girdle muscular dystrophies—Nomenclature and reformed classification. Neuromuscul. Disord. 2018, 28, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Bourke, J.; Eagle, M.; Frosk, P.; Wrogemann, K.; Greenberg, C.; Muntoni, F.; Voit, T.; Straub, V.; Hilton-Jones, D.; et al. Cardiac and respiratory failure in limb-girdle muscular dystrophy 2I. Ann. Neurol. 2004, 56, 738–741. [Google Scholar] [CrossRef]
- Müller, T.; Krasnianski, M.; Witthaut, R.; Deschauer, M.; Zierz, S. Dilated cardiomyopathy may be an early sign of the C826A Fukutin-related protein mutation. Neuromuscul. Disord. 2005, 15, 372–376. [Google Scholar] [CrossRef]
- Gaul, C.; Deschauer, M.; Tempelmann, C.; Vielhaber, S.; Klein, H.; Heinze, H.; Zierz, S.; Grothues, F. Cardiac involvement in limb-girdle muscular dystrophy 2I: Conventional cardiac diagnostic and cardiovascular magnetic resonance. J. Neurol. 2006, 253, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Kefi, M.; Amouri, R.; Chabrak, S.; Mechmeche, R.; Hentati, F. Variable cardiac involvement in Tunisian siblings harboring FKRP gene mutations. Neuropediatrics 2008, 39, 113–115. [Google Scholar] [CrossRef]
- Wahbi, K.; Meune, C.; Hamouda, E.H.; Stojkovic, T.; Laforêt, P.; Bécane, H.M.; Eymard, B.; Duboc, D. Cardiac assessment of limb–girdle muscular dystrophy 2I patients: An echography, Holter ECG and magnetic resonance imaging study. Neuromuscul. Disord. 2008, 18, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Bourteel, H.; Vermersch, P.; Cuisset, J.-M.; Maurage, C.-A.; Laforet, P.; Richard, P.; Stojkovic, T. Clinical and mutational spectrum of limb-girdle muscular dystrophy type 2I in 11 French patients. J. Neurol. Neurosurg. Psychiatry 2009, 80, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Libell, E.M.; Richardson, J.A.; Lutz, K.L.; Ng, B.Y.; Mockler, S.R.H.; Laubscher, K.M.; Stephan, C.M.; Zimmerman, B.M.; Edens, E.R.; Reinking, B.E.; et al. Cardiomyopathy in limb girdle muscular dystrophy R9, FKRP related. Muscle Nerve 2020, 62, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Barresi, R.; Di Blasi, C.; Negri, T.; Brugnoni, R.; Vitali, A.; Felisari, G.; Salandi, A.; Daniel, S.; Cornelio, F.; Morandi, L.; et al. Disruption of heart sarcoglycan complex and severe cardiomyopathy caused by beta sarcoglycan mutations. J. Med. Genet. 2000, 37, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Politano, L.; Nigro, V.; Passamano, L.; Petretta, V.; Comi, L.; Papparella, S.; Nigro, G.; Rambaldi, P.; Raia, P.; Pini, A.; et al. Evaluation of cardiac and respiratory involvement in sarcoglycanopathies. Neuromuscul. Disord. 2001, 11, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Fanin, M.; Melacini, P.; Boito, C.; Pegoraro, E.; Angelini, C. LGMD2E patients risk developing dilated cardiomyopathy. Neuromuscul. Disord. 2003, 13, 303–309. [Google Scholar] [CrossRef]
- Fayssoil, A. Cardiac diseases in sarcoglycanopathies. Int. J. Cardiol. 2010, 144, 67–68. [Google Scholar] [CrossRef]
- Fayssoil, A.; Nardi, O.; Annane, D.; Orlikowski, D. Left ventricular function in alpha-sarcoglycanopathy and gamma-sarcoglycanopathy. Acta Neurol. Belg. 2014, 114, 257–259. [Google Scholar] [CrossRef]
- van Westrum, S.M.S.; Dekker, L.R.; de Voogt, W.G.; Wilde, A.A.; Ginjaar, I.B.; de Visser, M.; van der Kooi, A.J. Cardiac involvement in Dutch patients with sarcoglycanopathy: A cross-sectional cohort and follow-up study. Muscle Nerve 2014, 50, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Fayssoil, A.; Ogna, A.; Chaffaut, C.; Chevret, S.; Guimarães-Costa, R.; Leturcq, F.; Wahbi, K.; Prigent, H.; Lofaso, F.; Nardi, O.; et al. Natural History of Cardiac and Respiratory Involvement, Prognosis and Predictive Factors for Long-Term Survival in Adult Patients with Limb Girdle Muscular Dystrophies Type 2C and 2D. PLoS ONE 2016, 11, e0153095. [Google Scholar] [CrossRef] [PubMed]
- Magri, F.; Nigro, V.; Angelini, C.; Mongini, T.; Mora, M.; Moroni, I.; Toscano, A.; D’Angelo, M.G.; Tomelleri, G.; Siciliano, G.; et al. The Italian limb girdle muscular dystrophy registry: Relative frequency, clinical features, and differential diagnosis. Muscle Nerve 2017, 55, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Pérez, J.; González-Quereda, L.; Bello, L.; Guglieri, M.; Straub, V.; Gallano, P.; Semplicini, C.; Pegoraro, E.; Zangaro, V.; Nascimento, A.; et al. New genotype-phenotype correlations in a large European cohort of patients with sarcoglycanopathy. Brain 2020, 143, 2696–2708. [Google Scholar] [CrossRef] [PubMed]
- Guimarães-Costa, R.; Fernández-Eulate, G.; Wahbi, K.; Leturcq, F.; Malfatti, E.; Behin, A.; Leonard-Louis, S.; Desguerre, I.; Barnerias, C.; Nougues, M.C.; et al. Clinical correlations and long-term follow-up in 100 patients with sarcoglycanopathies. Eur. J. Neurol. 2021, 28, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Carson, L.; Merrick, D. Genotype–phenotype correlations in alpha-sarcoglycanopathy: A systematic review. Ir. J. Med. Sci. 2022, 191, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Hayashi, Y.K.; Noguchi, S.; Ogawa, M.; Nonaka, I.; Tanabe, Y.; Ogino, M.; Takada, F.; Eriguchi, M.; Kotooka, N.; et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann. Neurol. 2006, 60, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Molina, M.T.; Rosas-Madrigal, S.; López-Mora, E.; Avila, A.L.C.; Rodríguez-Zanella, H.; Romero-Hidalgo, S.; Rosendo-Gutierrez, R.; Carnevale, A. Homozygous Fukutin Missense Mutation in Two Mexican Siblings with Dilated Cardiomyopathy. Rev. Investig. Clin. 2020, 73, 132–137. [Google Scholar] [CrossRef]
- Gaertner, A.; Burr, L.; Klauke, B.; Brodehl, A.; Laser, K.T.; Klingel, K.; Tiesmeier, J.; Schulz, U.; zu Knyphausen, E.; Gummert, J.; et al. Compound Heterozygous FKTN Variants in a Patient with Dilated Cardiomyopathy Led to an Aberrant α-Dystroglycan Pattern. Int. J. Mol. Sci. 2022, 23, 6685. [Google Scholar] [CrossRef]
- Bos, J.M.; Poley, R.N.; Ny, M.; Tester, D.J.; Xu, X.; Vatta, M.; Towbin, J.A.; Gersh, B.J.; Ommen, S.R.; Ackerman, M.J. Genotype–phenotype relationships involving hypertrophic cardiomyopathy-associated mutations in titin, muscle LIM protein, and telethonin. Mol. Genet. Metab. 2006, 88, 78–85. [Google Scholar] [CrossRef]
- Zambon, A.A.; Ridout, D.; Main, M.; Mein, R.; Phadke, R.; Muntoni, F.; Sarkozy, A. LAMA2-related muscular dystrophy: Natural history of a large pediatric cohort. Ann. Clin. Transl. Neurol. 2020, 7, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Steinert, H. Über das klinische und anatomische Bild des Muskelschwunds der Myotoniker. Nervenheilkd 1909, 37, 58–104. (In German) [Google Scholar] [CrossRef]
- Thornton, C.A. Myotonic dystrophy. Neurol. Clin. 2014, 32, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.E. Myotonic Muscular Dystrophies. Continuum (Minneap Minn) 2019, 25, 1682–1695. [Google Scholar] [CrossRef]
- Harper, P.S. Trinucleotide repeat disorders. J. Inherit. Metab. Dis. 1997, 20, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.-P.; Frishman, W.H. Myotonic dystrophies and the heart. Cardiol. Rev. 2012, 20, 001–003. [Google Scholar] [CrossRef]
- Nigro, G.; Papa, A.A.; Politano, L. The heart and cardiac pacing in Steinert disease. Acta Myol. 2012, 31, 110–116. [Google Scholar] [PubMed]
- Lau, J.; Sy, R.; Corbett, A.; Kritharides, L. Myotonic dystrophy and the heart: A systematic review of evaluation and management. Int. J. Cardiol. 2015, 184, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Wahbi, K.; Algalarrondo, V.; Bécane, H.M.; Fressart, V.; Beldjord, C.; Azibi, K.; Lazarus, A.; Berber, N.; Radvanyi-Hoffman, H.; Stojkovic, T.; et al. Brugada syndrome and abnormal splicing of SCN5A in myotonic dystrophy type 1. Arch Cardiovasc Dis. 2013, 106, 635–643. [Google Scholar] [CrossRef]
- McNally, E.M.; Mann, D.L.; Pinto, Y.; Bhakta, D.; Tomaselli, G.; Nazarian, S.; Groh, W.J.; Tamura, T.; Duboc, D.; Itoh, H.; et al. Clinical Care Recommendations for Cardiologists Treating Adults with Myotonic Dystrophy. J. Am. Heart Assoc. 2020, 9, e014006. [Google Scholar] [CrossRef]
- Russo, V.; Sperlongano, S.; Gallinoro, E.; Rago, A.; Papa, A.A.; Golino, P.; Politano, L.; Nazarian, S.; Nigro, G. Prevalence of Left Ventricular Systolic Dysfunction in Myotonic Dystrophy Type 1: A Systematic Review. J. Card. Fail. 2020, 26, 849–856. [Google Scholar] [CrossRef]
- Selcen, D.; Engel, A.G. Myofibrillar myopathies. Handb. Clin. Neurol. 2011, 101, 143–154. [Google Scholar] [CrossRef]
- Claeys, K.G.; Fardeau, M. Myofibrillar myopathies. Handb. Clin. Neurol. 2013, 113, 1337–1342. [Google Scholar] [CrossRef]
- Maggi, L.; Mavroidis, M.; Psarras, S.; Capetanaki, Y.; Lattanzi, G. Skeletal and Cardiac Muscle Disorders Caused by Mutations in Genes Encoding Intermediate Filament Proteins. Int. J. Mol. Sci. 2021, 22, 4256. [Google Scholar] [CrossRef]
- Fichna, J.P.; Maruszak, A.; Żekanowski, C. Myofibrillar myopathy in the genomic context. J. Appl. Genet. 2018, 59, 431–439. [Google Scholar] [CrossRef]
- Brodehl, A.; Gaertner-Rommel, A.; Milting, H. Molecular insights into cardiomyopathies associated with desmin (DES) mutations. Biophys. Rev. 2018, 10, 983–1006. [Google Scholar] [CrossRef]
- Goldfarb, L.G.; Vicart, P.; Goebel, H.H.; Dalakas, M.C. Desmin myopathy. Brain 2004, 127 Pt 4, 723–734. [Google Scholar] [CrossRef]
- Clemen, C.S.; Herrmann, H.; Strelkov, S.V.; Schröder, R. Desminopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 47–75. [Google Scholar] [CrossRef]
- Tsikitis, M.; Galata, Z.; Mavroidis, M.; Psarras, S.; Capetanaki, Y. Intermediate filaments in cardiomyopathy. Biophys. Rev. 2018, 10, 1007–1031. [Google Scholar] [CrossRef]
- Dalakas, M.C.; Park, K.-Y.; Semino-Mora, C.; Lee, H.S.; Sivakumar, K.; Goldfarb, L.G. desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N. Engl. J. Med. 2000, 342, 770–780. [Google Scholar] [CrossRef]
- McLendon, P.M.; Robbins, J.; Guichard, J.L.; Rogowski, M.; Agnetti, G.; Fu, L.; Powell, P.; Wei, C.-C.; Collawn, J.; Yancey, D.M.; et al. Desmin-related cardiomyopathy: An unfolding story. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1220–H1228. [Google Scholar] [CrossRef]
- Kuhl, A.; Melberg, A.; Meinl, E.; Nürnberg, G.; Nürnberg, P.; Kehrer-Sawatzki, H.; Jenne, D.E. Myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy 7: Corroboration and narrowing of the critical region on 10q22.3. Eur. J. Hum. Genet. 2008, 16, 367–373. [Google Scholar] [CrossRef][Green Version]
- Lorenzon, A.; Beffagna, G.; Bauce, B.; De Bortoli, M.; Mura, I.E.L.; Calore, M.; Dazzo, E.; Basso, C.; Nava, A.; Thiene, G.; et al. Desmin mutations and arrhythmogenic right ventricular cardiomyopathy. Am. J. Cardiol. 2013, 111, 400–405. [Google Scholar] [CrossRef]
- Onore, M.E.; Savarese, M.; Picillo, E.; Passamano, L.; Nigro, V.; Politano, L. Bi-Allelic DES Gene Variants Causing Autosomal Recessive Myofibrillar Myopathies Affecting Both Skeletal Muscles and Cardiac Function. Int. J. Mol. Sci. 2022, 23, 15906. [Google Scholar] [CrossRef]
- Richard, I.; Hogrel, J.; Stockholm, D.; Payan, C.A.M.; Fougerousse, F.; Calpainopathy Study Group; Eymard, B.; Mignard, C.; de Munain, A.L.; Fardeau, M.; et al. Natural history of LGMD2A for delineating outcome measures in clinical trials. Ann. Clin. Transl. Neurol. 2016, 3, 248–265. [Google Scholar] [CrossRef]
- Hicks, D.; Sarkozy, A.; Muelas, N.; Köehler, K.; Huebner, A.; Hudson, G.; Chinnery, P.F.; Barresi, R.; Eagle, M.; Polvikoski, T.; et al. A founder mutation in Anoctamin 5 is a major cause of limb girdle muscular dystrophy. Brain 2011, 134, 171–182. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Coimbra-Neto, A.R.; Souza, P.V.S.; Winckler, P.B.; Gonçalves, M.V.M.; Cavalcanti, E.B.U.; Carvalho, A.A.D.S.; Sobreira, C.F.D.R.; Camelo, C.G.; Mendonça, R.D.H.; et al. Clinical and molecular findings in a cohort of ANO5-related myopathy. Ann. Clin. Transl. Neurol. 2019, 6, 1225–1238. [Google Scholar] [CrossRef]
- Zatz, M.; de Paula, F.; Starling, A.; Vainzof, M. The 10 autosomal recessive limb-girdle muscular dystrophies. Neuromuscul. Disord. 2003, 13, 532–544. [Google Scholar] [CrossRef]
- Diaz-Manera, J.; Fernandez-Torron, R.; LLauger, J.; James, M.K.; Mayhew, A.; Smith, F.E.; Moore, U.R.; Blamire, A.M.; Carlier, P.G.; Rufibach, L.; et al. Muscle MRI in patients with dysferli-nopathy: Pattern recognition and implications for clinical trials. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1071–1081. [Google Scholar] [CrossRef]
- Murphy, L.B.; Schreiber-Katz, O.; Rafferty, K.; Robertson, A.; Topf, A.; Willis, T.A.; Heidemann, M.; Thiele, S.; Bindoff, L.; Laurent, J.; et al. Global FKRP Registry: Observations in more than 300 patients with Limb Girdle Muscular Dystrophy R9. Ann. Clin. Transl. Neurol. 2020, 7, 757–766. [Google Scholar] [CrossRef]
- Jensen, S.M.; Müller, K.I.; Mellgren, S.I.; Bindoff, L.A.; Rasmussen, M.; Ørstavik, K.; Jonsrud, C.; Tveten, K.; Nilssen, Ø.; Van Ghelue, M.; et al. Epidemiology and natural history in 101 subjects with FKRP-related limb-girdle muscular dystrophy R9. The Norwegian LGMDR9 cohort study (2020). Neuromuscul. Disord. 2023, 33, 119–132. [Google Scholar] [CrossRef]
- Monaco, A.P.; Neve, R.L.; Colletti-Feener, C.; Bertelson, C.J.; Kurnit, D.M.; Kunkel, L.M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature 1986, 323, 646–650. [Google Scholar] [CrossRef]
- Leturcq, F.; Kaplan, J.-C. Bases moléculaires des dystrophinopathies [Molecular bases of dystrophinopathies]. J. Soc. Biol. 2005, 199, 5–11. [Google Scholar] [CrossRef]
- Politano, L.; Colonna-Romano, S.; Esposito, M.G.; Nigro, V.; Comi, L.I.; Nigro, G. Genotype-phenotype correlation in patients with deletions of Duchenne/Becker gene. Acta Cardiomyol. 1991, 2, 239–244. [Google Scholar]
- Nigro, G.; Politano, L.; Nigro, V.; Petretta, V.R.; Comi, L.I. Mutation of dystrophin gene and cardiomyopathy. Neuromuscul. Disord. 1994, 4, 371–379. [Google Scholar] [CrossRef]
- Melacini, P.; Fanin, M.; Danieli, G.A.; Fasoli, G.; Villanova, C.; Angelini, C.; Vitiello, L.; Miorelli, M.; Buja, G.F.; Mostacciuolo, M.L.; et al. Cardiac involvement in Becker muscular dystrophy. J. Am. Coll. Cardiol. 1993, 22, 1927–1934. [Google Scholar] [CrossRef]
- Kaspar, R.W.; Allen, H.D.; Ray, W.C.; Alvarez, C.E.; Kissel, J.T.; Pestronk, A.; Weiss, R.B.; Flanigan, K.M.; Mendell, J.R.; Montanaro, F.; et al. Analysis of dystrophin deletion mutations predicts age of cardiomyopathy onset in becker muscular dystrophy. Circ. Cardiovasc. Genet. 2009, 2, 544–551. [Google Scholar] [CrossRef]
- Bostick, B.; Yue, Y.; Long, C.; Marschalk, N.; Fine, D.M.; Chen, J.; Duan, D. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged mdx mice. Mol. Ther. 2009, 17, 253–261. [Google Scholar] [CrossRef]
- Restrepo-Cordoba, M.A.; Wahbi, K.; Florian, A.R.; Jiménez-Jáimez, J.; Politano, L.; Arad, M.; Paya, V.C.; Garcia-Alvarez, A.; Hansen, R.B.; Larrañaga-Moreira, J.M.; et al. Prevalence and clinical outcomes of dystrophin-associated dilated cardiomyopathy without severe skeletal myopathy. Eur. J. Heart Fail. 2021, 23, 1276–1286. [Google Scholar] [CrossRef]
- Holaska, J.M. Emerin and the nuclear lamina in muscle and cardiac disease. Circ. Res. 2008, 103, 16–23. [Google Scholar] [CrossRef]
- Parks, S.B.; Kushner, J.D.; Nauman, D.; Burgess, D.; Ludwigsen, S.; Peterson, A.; Li, D.; Jakobs, P.; Litt, M.; Porter, C.B.; et al. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am. Heart J. 2008, 156, 161–169. [Google Scholar] [CrossRef]
- Perrot, A.; Hussein, S.; Ruppert, V.; Schmidt, H.H.J.; Wehnert, M.S.; Duong, N.T.; Posch, M.G.; Panek, A.; Dietz, R.; Kindermann, I.; et al. Identification of mutational hot spots in LMNA encoding lamin A/C in patients with familial dilated cardiomyopathy. Basic Res. Cardiol. 2009, 104, 90–99. [Google Scholar] [CrossRef]
- Kumar, S.; Baldinger, S.H.; Gandjbakhch, E.; Maury, P.; Sellal, J.M.; Androulakis, A.F.; Waintraub, X.; Charron, P.; Rollin, A.; Richard, P.; et al. Long-term arrhythmic and non-arrhythmic outcomes of lamin A/C mutation carriers. J. Am. Coll. Cardiol. 2016, 68, 2299–2307. [Google Scholar] [CrossRef]
- Brodt, C.; Siegfried, J.D.; Hofmeyer, M.; Martel, J.; Rampersaud, E.; Li, D.; Morales, A.; Hershberger, R.E. Temporal relationship of conduction system disease and ventricular dysfunction in LMNA cardiomyopathy. J. Card. Fail. 2013, 19, 233–239. [Google Scholar] [CrossRef]
- Ollila, L.; Nikus, K.; Holmström, M.; Jalanko, M.; Jurkko, R.; Kaartinen, M.; Koskenvuo, J.; Kuusisto, J.; Kärkkäinen, S.; Palojoki, E.; et al. Clinical disease presentation and ECG characteristics of LMNA mutation carriers. Open Heart 2017, 4, e000474. [Google Scholar] [CrossRef]
- Tesson, F.; Saj, M.; Uvaize, M.M.; Nicolas, H.; Płoski, R.; Bilińska, Z. Lamin A/C mutations in dilated cardiomyopathy. Cardiol. J. 2014, 21, 331–342. [Google Scholar] [CrossRef]
- Bertrand, A.T.; Chikhaoui, K.; Ben Yaou, R.; Bonne, G. Clinical and genetic heterogeneity in laminopathies. Biochem. Soc. Trans. 2011, 39, 1687–1692. [Google Scholar] [CrossRef]
- Benedetti, S.; Menditto, I.; Degano, M.; Rodolico, C.; Merlini, L.; D’Amico, A.; Palmucci, L.; Berardinelli, A.; Pegoraro, E.; Trevisan, C.P.; et al. Phenotypic clustering of lamin A/C mutations in neuromuscular patients. Neurology 2007, 69, 1285–1292. [Google Scholar] [CrossRef]
- Maggi, L.; D’Amico, A.; Pini, A.; Sivo, S.; Pane, M.; Ricci, G.; Vercelli, L.; D’Ambrosio, P.; Travaglini, L.; Sala, S.; et al. LMNA -associated myopathies: The Italian experience in a large cohort of patients. Neurology 2014, 83, 1634–1644. [Google Scholar] [CrossRef]
- Szeverenyi, I.; Cassidy, A.J.; Chung, C.W.; Lee, B.T.K.; Common, J.E.A.; Ogg, S.C.; Chen, H.; Sim, S.Y.; Goh, W.L.P.; Ng, K.W.; et al. The Human Intermediate Filament Database: Comprehensive information on a gene family involved in many human diseases. Hum. Mutat. 2008, 29, 351–360. [Google Scholar] [CrossRef]
- van Berlo, J.H.; de Voogt, W.G.; van der Kooi, A.J.; van Tintelen, J.P.; Bonne, G.; Ben Yaou, R.; Duboc, D.; Rossenbacker, T.; Heidbüchel, H.; de Visser, M.; et al. Meta-analysis of clinical characteristics of 299 carriers of LMNA gene mutations: Do lamin A/C mutations portend a high risk of sudden death? J. Mol. Med. 2005, 83, 79–83. [Google Scholar] [CrossRef]
- Holt, I.; Ostlund, C.; Stewart, C.L.; Man, N.T.; Worman, H.J.; Morris, G.E. Effect of pathogenic mis-sense mutations in lamin A on its interaction with emerin in vivo. J. Cell Sci. 2003, 116 Pt 14, 3027–3035. [Google Scholar] [CrossRef]
- Wolf, C.M.; Wang, L.; Alcalai, R.; Pizard, A.; Burgon, P.G.; Ahmad, F.; Sherwood, M.; Branco, D.M.; Wakimoto, H.; Fishman, G.I.; et al. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J. Mol. Cell. Cardiol. 2008, 44, 293–303. [Google Scholar] [CrossRef]
- Angelini, C.; Fanin, M.; Freda, M.P.; Duggan, D.J.; Siciliano, G.; Hoffman, E.P. The clinical spectrum of sarcoglycanopathies. Neurology 1999, 52, 176–179. [Google Scholar] [CrossRef]
- Alonso-Pérez, J.; González-Quereda, L.; Bruno, C.; Panicucci, C.; Alavi, A.; Nafissi, S.; Nilipour, Y.; Zanoteli, E.; Isihi, L.M.d.A.; Melegh, B.; et al. Clinical and genetic spectrum of a large cohort of patients with δ-sarcoglycan muscular dystrophy. Brain 2022, 145, 596–606. [Google Scholar] [CrossRef]
- Kang, P.B.; Feener, C.A.; Estrella, E.; Thorne, M.; White, A.J.; Darras, B.T.; Amato, A.A.; Kunkel, L.M. LGMD2I in a North American population. BMC Musculoskelet. Disord. 2007, 8, 115. [Google Scholar] [CrossRef]
- Sveen, M.; Schwartz, M.; Vissing, J. High prevalence and phenotype–genotype correlations of limb girdle muscular dystrophy type 2I in Denmark. Ann. Neurol. 2006, 59, 808–815. [Google Scholar] [CrossRef]
- Redman, J.B.; Fenwick, R.G.; Fu, Y.-H.; Pizzuti, A.; Caskey, C.T. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA 1993, 269, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Villanova, C.; Menegazzo, E.; Novelli, G.; Danieli, G.; Rizzoli, G.; Fasoli, G.; Angelini, C.; Buja, G.; Miorelli, M.; et al. Correlation between cardiac involvement and CTG trinucleotide repeat length in myotonic dystrophy. J. Am. Coll. Cardiol. 1995, 25, 239–245. [Google Scholar] [CrossRef]
- Finsterer, J.; Gharehbaghi-Schnell, E.; Stöllberger, C.; Fheodoroff, K.; Seiser, A. Relation of cardiac abnormalities and CTG-repeat size in myotonic dystrophy. Clin. Genet. 2001, 59, 350–355. [Google Scholar] [CrossRef]
- Chong-Nguyen, C.; Wahbi, K.; Algalarrondo, V.; Bécane, H.M.; Radvanyi-Hoffman, H.; Arnaud, P.; Furling, D.; Lazarus, A.; Bassez, G.; Béhin, A.; et al. Association Between Mutation Size and Cardiac Involvement in Myotonic Dystrophy Type 1: An Analysis of the DM1-Heart Registry. Circ. Cardiovasc. Genet. 2017, 10, e001526. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, N.; Park, D. Diastolic heart dysfunction is correlated with CTG repeat length in myotonic dystrophy type 1. Neurol. Sci. 2018, 39, 1935–1943. [Google Scholar] [CrossRef]
- Goldfarb, L.G.; Dalakas, M.C. Tragedy in a heartbeat: Malfunctioning desmin causes skeletal and cardiac muscle disease. J. Clin. Investig. 2009, 119, 1806–1813. [Google Scholar] [CrossRef]
- Hedberg, C.; Melberg, A.; Kuhl, A.; Jenne, D.; Oldfors, A. Autosomal dominant myofibrillar myopathy with arrhythmogenic right ventricular cardiomyopathy 7 is caused by a DES mutation. Eur. J. Hum. Genet. 2012, 20, 984–985. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.; van Hessem, L.; Jongbloed, J.; de Walle, H.; Capetanaki, Y.; van der Kooi, A.; van Langen, I.; Berg, M.v.D.; van Tintelen, J. Desmin-related myopathy. Clin. Genet. 2011, 80, 354–366. [Google Scholar] [CrossRef]
- Fan, P.; Lu, C.-X.; Dong, X.-Q.; Zhu, D.; Yang, K.-Q.; Liu, K.-Q.; Zhang, D.; Zhang, Y.; Meng, X.; Tan, H.-Q.; et al. A novel phenotype with splicing mutation identified in a Chinese family with desminopathy. Chin. Med. J. (Engl.) 2019, 132, 127–134. [Google Scholar] [CrossRef]
- McLaughlin, H.M.; Kelly, M.A.; Hawley, P.P.; Darras, B.T.; Funke, B.; Picker, J. Compound heterozygosity of predicted loss-of-function DES variants in a family with recessive des-minopathy. BMC Med. Genet. 2013, 14, 68. [Google Scholar] [CrossRef]
- van Tintelen, J.P.; Van Gelder, I.C.; Asimaki, A.; Suurmeijer, A.J.; Wiesfeld, A.C.; Jongbloed, J.D.; Wijngaard, A.v.D.; Kuks, J.B.; van Spaendonck-Zwarts, K.Y.; Notermans, N.; et al. Severe cardiac phenotype with right ventricular predominance in a large cohort of patients with a single missense mutation in the DES gene. Heart Rhythm. 2009, 6, 1574–1583. [Google Scholar] [CrossRef]
- van Spaendonck-Zwarts, K.Y.; van der Kooi, A.J.; van den Berg, M.P.; Ippel, E.F.; Boven, L.G.; Yee, W.-C.; van den Wijngaard, A.; Brusse, E.; Hoogendijk, J.E.; Doevendans, P.A.; et al. Recurrent and founder mutations in the Netherlands: The cardiac phenotype of DES founder mutations p.S13F and p.N342D. Neth. Heart J. 2012, 20, 219–228. [Google Scholar] [CrossRef]
- Klauke, B.; Kossmann, S.; Gaertner, A.; Brand, K.; Stork, I.; Brodehl, A.; Dieding, M.; Walhorn, V.; Anselmetti, D.; Gerdes, D.; et al. De novo desmin-mutation N116S is associated with arrhythmogenic right ventricular cardiomyopathy. Hum. Mol. Genet. 2010, 19, 4595–4607. [Google Scholar] [CrossRef]
- Bermúdez-Jiménez, F.J.; Carriel, V.; Brodehl, A.; Alaminos, M.; Campos, A.; Schirmer, I.; Milting, H.; Abril, B.; Álvarez, M.; López-Fernández, S.; et al. Novel Desmin Mutation p.Glu401Asp Impairs Filament Formation, Disrupts Cell Membrane Integrity, and Causes Severe Arrhythmogenic Left Ventricular Cardiomyopathy/Dysplasia. Circulation 2018, 137, 1595–1610. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Dittmann, S.; Brodehl, A.; Unger, A.; Stallmeyer, B.; Paul, M.; Seebohm, G.; Kayser, A.; Peischard, S.; Linke, W.A.; et al. Functional characterization of novel alpha-helical rod domain desmin (DES) pathogenic variants associated with dilated cardiomyopathy, atrioventricular block and a risk for sudden cardiac death. Int. J. Cardiol. 2021, 329, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Spezzacatene, A.; Sinagra, G.; Merlo, M.; Barbati, G.; Graw, S.L.; Brun, F.; Slavov, D.; Di Lenarda, A.; Salcedo, E.E.; Towbin, J.A.; et al. Arrhythmogenic phenotype in dilated cardiomyopathy: Natural history and predictors of life-threatening arrhythmias. J. Am. Heart Assoc. 2015, 4, e002149. [Google Scholar] [CrossRef] [PubMed]
- Mestroni, L.; Sbaizero, O. Arrhythmogenic Cardiomyopathy: Mechanotransduction Going Wrong. Circulation 2018, 137, 1611–1613. [Google Scholar] [CrossRef] [PubMed]
- Protonotarios, A.; Brodehl, A.; Asimaki, A.; Jager, J.; Quinn, E.; Stanasiuk, C.; Ratnavadivel, S.; Futema, M.; Akhtar, M.M.; Gossios, T.D.; et al. The Novel Desmin Variant p.Leu115Ile Is Associated with a Unique Form of Biventricular Arrhythmogenic Cardiomyopathy. Can. J. Cardiol. 2021, 37, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Rees, W.; Schüler, S.; Hummel, M.; Hetzer, R. Heart transplantation in patients with muscular dystrophy associated with end-stage cardiomyopathy. J. Heart Lung Transplant. 1993, 12, 804–807. [Google Scholar] [PubMed]
- Wu, R.S.; Gupta, S.; Brown, R.N.; Yancy, C.W.; Wald, J.W.; Kaiser, P.; Kirklin, N.M.; Patel, P.C.; Markham, D.W.; Drazner, M.H.; et al. Clinical outcomes after cardiac transplantation in muscular dystrophy patients. J. Heart Lung Transplant. 2010, 29, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.; Rizwan, R.; Jefferies, J.L.; Bryant, R., 3rd; Ryan, T.D.; Lorts, A.; Chin, C.; Zafar, F.; Morales, D.L. Heart transplantation in muscular dystrophy patients. Is it a viable option? Circ. Heart Fail. 2020, 13, e005447. [Google Scholar] [CrossRef] [PubMed]
- Cripe, L.; Kinnett, K.; Uzark, K.; Eghtesady, P.; Wong, B.; Spicer, R. P1.14 Cardiac transplantation in Duchenne muscular dystrophy: A case report. Neuromuscul. Disord. 2011, 21, 645. [Google Scholar] [CrossRef]
- Piperata, A.; Bottio, T.; Toscano, G.; Avesani, M.; Vianello, A.; Gerosa, G. Is heart transplantation a real option in patients with Duchenne syndrome? Inferences from a case report. ESC Heart Fail. 2020, 7, 3198–3202. [Google Scholar] [CrossRef]
- Casazza, F.; Brambilla, G.; Salvato, A.; Morandi, L.; Gronda, E.; Bonacina, E. Dilated cardiomyopathy and successful cardiac transplantation in Becker’s muscular distrophy. Follow-up after two years. G. Ital. Cardiol. 1988, 18, 753–757. [Google Scholar] [PubMed]
- Donofrio, P.D.; Challa, V.R.; Hackshaw, B.T.; Mills, S.A.; Cordell, A.R. Cardiac transplantation in a patient with muscular dystrophy and cardiomyopathy. Arch. Neurol. 1989, 46, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Sakata, C.; Yamada, H.; Sunohara, N.; Arahata, K.; Nonaka, I. [Cardiomyopathy in Becker muscular dystrophy]. Rinsho Shinkeigaku 1990, 30, 952–955. (In Japanese) [Google Scholar] [PubMed]
- Anthuber, M.; Kemkes, B.M.; Schuetz, A.; Kugler, C.; Sudhoff, F.; Spes, C.; Angermann, C. Heart transplantation in patients with “so-called” contraindications. Transplant. Proc. 1990, 22, 1451–1453. [Google Scholar] [PubMed]
- Piccolo, G.; Azan, G.; Tonin, P.; Arbustini, E.; Gavazzi, A.; Banfi, P.; Mora, M.; Morandi, L.; Tedeschi, S. Dilated cardiomyopathy requiring cardiac transplantation as initial manifestation of Xp21 Becker type muscular dystrophy. Neuromuscul. Disord. 1994, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, R.; Vernocchi, A.; Gariboldi, F.; Senni, M.; Mamprin, F.; Gamba, A. Troponin I as a specific marker for heart damage after heart transplantation in a patient with Becker type muscular dystrophy. J. Heart Lung Transplant. 1997, 16, 969–973. [Google Scholar] [PubMed]
- Finsterer, J.; Bittner, R.; Grimm, M. Cardiac involvement in Becker’s muscular dystrophy, necessitating heart transplantation, 6 years before apparent skeletal muscle involvement. Neuromuscul. Disord. 1999, 9, 598–600. [Google Scholar] [CrossRef] [PubMed]
- Melacini, P.; Gambino, A.; Caforio, A.; Barchitta, A.; Valente, M.; Angelini, A.; Fanin, M.; Thiene, G.; Angelini, C.; Casarotto, D.; et al. Heart transplantation in patients with inherited myopathies associated with end-stage cardiomyopathy: Molecular and biochemical defects on cardiac and skeletal muscle. Transplant. Proc. 2001, 33, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Leprince, P.; Heloire, F.; Eymard, B.; Léger, P.; Duboc, D.; Pavie, A. Successful bridge to transplantation in a patient with Becker muscular dystrophy–associated cardiomyopathy. J. Heart Lung Transplant. 2002, 21, 822–824. [Google Scholar] [CrossRef]
- Ruiz-Cano, M.; Delgado, J.; Jiménez, C.; Jiménez, S.; Cea-Calvo, L.; Sánchez, V.; Escribano, P.; Gómez, M.; Gil-Fraguas, L.; de la Calzada, C.S. Successful heart transplantation in patients with inherited myopathies associated with end-stage cardiomyopathy. Transplant. Proc. 2003, 35, 1513–1515. [Google Scholar] [CrossRef]
- Srinivasan, R.; Hornyak, J.E.; Badenhop, D.T.; Koch, L.G. Cardiac rehabilitation after heart transplantation in a patient with becker’s muscular dystrophy: A case report. Arch. Phys. Med. Rehabil. 2005, 86, 2059–2061. [Google Scholar] [CrossRef] [PubMed]
- Komanapalli, C.B.; Sera, V.; Slater, M.S.; Burdette, M.; Tripathy, U.; Brady, G.; Hegnell, L.; Ravichandran, P.S.; Hershberger, R.E.; Song, H.K. Becker’s muscular dystrophy and orthotopic heart transplantation: Perioperative considerations. Heart Surg. Forum. 2006, 9, E604-6. [Google Scholar] [PubMed]
- Patanè, F.; Zingarelli, E.; Attisani, M.; Sansone, F. Successful heart transplantation in Becker’s muscular dystrophy. Eur. J. Cardio-Thorac. Surg. 2006, 29, 250. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katzberg, H.; Karamchandani, J.; So, Y.T.; Vogel, H.; Wang, C.H. End-stage cardiac disease as an initial presentation of systemic myopathies: Case series and literature review. J. Child Neurol. 2010, 25, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.; Lairez, O.; Marachet, M.-A.; Roncalli, J. Unusual right heart failure in a patient with heart transplant. Eur. Heart J. 2010, 31, 2431. [Google Scholar] [CrossRef] [PubMed]
- Papa, A.A.; D’Ambrosio, P.; Petillo, R.; Palladino, A.; Politano, L. Heart transplantation in patients with dystrophinopathic cardiomyopathy: Review of the literature and personal series. Intractable Rare Dis. Res. 2017, 6, 95–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Steger, C.M.; Höfer, D.; Antretter, H. Cardiac manifestation in muscular dystrophies leading to heart transplantation. Eur. Surg. 2013, 45, 245–250. [Google Scholar] [CrossRef]
- Ketelsen, U.P.; Trenk, D.; Eschenbruch, E.M.; Tollenaere, P.J. Myopathy/rhabdomyolisis in patients after heart transplantation by presurgical treatment with lipid-lowering drugs? Interaction of cyclosporine and HMG-CoA reductase inhibitor therapy? Neuromuscul. Disord. 1997, 7, 446. [Google Scholar] [CrossRef]
- Bajoras, V.; Zuoziene, G.; Maneikiene, V.; Janusauskas, V.; Zorinas, A.; Rucinskas, K. Rapidly manifested heart failure treated with left ventricle assist device and heart transplantation in patient with Becker muscular dystrophy. In Abstracts of the Heart Failure 2017 and the 4th World Congress on Acute Heart Failure, Paris, France, 29 April–2 May 2017. Eur. J. Heart Fail 2017, 19 (Suppl. S1), 534. [Google Scholar]
- Ascencio-Lemus, M.G.; Barge-Caballero, E.; Paniagua-Martín, M.J.; Barge-Caballero, G.; Couto-Mallón, D.; Crespo-Leiro, M.G. Is Becker Dystrophinopathy a Contraindication to Heart Transplant? Experience in a Single Institution. Rev. Esp. Cardiol. (Engl. Ed.) 2019, 72, 584–585, (In English and Spanish). [Google Scholar] [CrossRef]
- Visrodia, P.; Patel, N.J.; Burford, M.; Hamilton, M.A.; Patel, J.K.; Kobashigawa, J.A.; Kittleson, M.M. Heart transplantation in muscular dystrophy: Single-center analysis. Clin. Transplant. 2022, 36, e14645. [Google Scholar] [CrossRef]
- Melacini, P.; Fanin, M.; Angelini, A.; Pegoraro, E.; Livi, U.; Danieli, G.; Hoffman, E.; Thiene, G.; Volta, S.D.; Angelini, C. Cardiac transplantation in a Duchenne muscular dystrophy carrier. Neuromuscul. Disord. 1998, 8, 585–590. [Google Scholar] [CrossRef]
- Davies, J.E.; Winokur, T.S.; Aaron, M.F.; Benza, R.L.; Foley, B.A.; Holman, W.L. Cardiomyopathy in a carrier of Duchenne’s muscular dystrophy. J. Heart Lung Transplant. 2001, 20, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Cullom, C.; Vo, V.; McCabe, M.D. Orthotopic Heart Transplantation in Manifesting Carrier of Duchenne Muscular Dystrophy. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Merchut, M.P.; Zdonczyk, D.; Gujrati, M. Cardiac transplantation in female Emery-Dreifuss muscular dystrophy. J. Neurol. 1990, 237, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Kichuk Crisant, M.R.; Drummond-Webb, J.; Hallowell, S.; Friedman, N.R. Cardiac transplantation in twins with autosomal dominant Emery-Dreifuss muscular dystrophy. J. Heart Lung Transplant. 2004, 23, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Ben Yaou, R.; Bécane, H.-M.; Demay, L.; Laforet, P.; Hannequin, D.; Bohu, P.-A.; Drouin-Garraud, V.; Ferrer, X.; Mussini, J.-M.; Ollagnon, E.; et al. Autosomal dominant limb-girdle muscular dystrophy associated with conduction defects (LGMD1B): A description of 8 new families with the LMNA gene mutations]. Rev. Neurol. 2005, 161, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, S.; Reissell, E.; Heliö, T.; Kaartinen, M.; Tuomainen, P.; Toivonen, L.; Kuusisto, J.; Kupari, M.; Nieminen, M.S.; Laakso, M.; et al. Novel mutations in the lamin A/C gene in heart transplant recipients with end stage dilated cardiomyopathy. Heart 2006, 92, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Holdt, L.M.; Klingel, K.; Kandolf, R.; Tebbe, U. Kardiologische Befunde bei Hauptmann-Thannhauser-Muskeldystrophie (autosomal dominante Emery-Dreifuss-Muskeldystrophie) [Cardiologic findings in Hauptmann-Thannhauser muscular dystrophy (autosomal dominant Emery-Dreifuss muscular dystrophy)]. DMW—Dtsch. Med. Wochenschr. 2007, 132, 2006–2009. (In German) [Google Scholar] [CrossRef]
- Dell’Amore, A.; Botta, L.; Suarez, S.M.; Forte, A.L.; Mikus, E.; Camurri, N.; Ortelli, L.; Arpesella, G. Heart transplantation in patients with emery-dreifuss muscular dystrophy: Case reports. Transplant. Proc. 2007, 39, 3538–3540. [Google Scholar] [CrossRef]
- Ambrosi, P.; Mouly-Bandini, A.; Attarian, S.; Habib, G. Heart transplantation in 7 patients from a single family with limb-girdle muscular dystrophy caused by lamin A/C mutation. Int. J. Cardiol. 2009, 137, e75–e76. [Google Scholar] [CrossRef]
- Volpi, L.; Ricci, G.; Passino, C.; Di Pierri, E.; Alì, G.; Maccherini, M.; Benedetti, S.; Lattanzi, G.; Columbaro, M.; Ferrari, M.; et al. Prevalent cardiac phenotype resulting in heart transplantation in a novel LMNA gene duplication. Neuromuscul. Disord. 2010, 20, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Blagova, O.; Nedostup, A.; Shumakov, D.; Poptsov, V.; Shestak, A.; Zaklyasminskaya, E. Dilated cardiomyopathy with severe arrhythmias in Emery-Dreifuss muscular dystrophy: From ablation to heart transplantation. J. Atr. Fibrillation 2016, 9, 1468. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Cuervo, M.; Vilhena, I. Heart transplant anesthetic approach in a patient with Emery Dreyfuss muscular dystrophy: A case report. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.) 2023, 70, 540–544. [Google Scholar] [CrossRef]
- Loureiro, M.; Branco, C.; Duarte, J.; Coutinho, G.; Martins, M.M.; Novo, A. Cardiac Rehabilitation in a Transplanted Person with Emery-Dreifuss Muscular Dystrophy. Arq. Bras. Cardiol. 2023, 120, e20220560. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.I.; Bonser, R.S.; Griffith, M.J. Radiofrequency catheter ablation for atrial flutter following orthotopic heart transplantation. Heart 1998, 79, 412–413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- D’Amico, A.; Petrini, S.; Parisi, F.; Tessa, A.; Francalanci, P.; Grutter, G.; Santorelli, F.M.; Bertini, E. Heart transplantation in a child with LGMD2I presenting as isolated dilated cardiomyopathy. Neuromuscul. Disord. 2008, 18, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.; Connolly, A.M.; Winder, T.L.; Pestronk, A.; Moore, S.A. Cardiac pathology exceeds skeletal muscle pathology in two cases of limb-girdle muscular dystrophy type 2I. Muscle Nerve 2009, 40, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, M.K.; Shin, M.-J.; Shin, Y.B.; Lee, H.W.; Yun, R.Y.; Lee, B.-J. Early cardiac rehabilitation after heart transplantation in a patient with limb-girdle muscular dystrophy: A case report. Medicine 2022, 101, e29180. [Google Scholar] [CrossRef] [PubMed]
- Goenen, M.J.; Jacquet, L.; De Kock, M.; Van Dyck, M.; Schoevardts, J.C.; Chalant, C.H. Aortic valve replacement thirty-one months after orthotopic heart transplantation. J. Heart Lung Transplant. 1991, 10, 604–607. [Google Scholar] [PubMed]
- Conraads, V.M.; Beckers, P.J.; Vorlat, A.; Vrints, C.J. Importance of physical rehabilitation before and after cardiac transplantation in a patient with myotonic dystrophy: A case report. Arch. Phys. Med. Rehabil. 2002, 83, 724–726. [Google Scholar] [CrossRef]
- Papa, A.A.; Verrillo, F.; Scutifero, M.; Rago, A.; Morra, S.; Cassese, A.; Cioppa, N.D.; Magliocca, M.C.G.; Galante, D.; Palladino, A.; et al. Heart transplantation in a patient with Myotonic Dystrophy type 1 and end-stage dilated cardiomyopathy: A short term follow-up. Acta Myol. 2018, 37, 267–271. [Google Scholar] [PubMed]
- El-Menyar, A.A.; Al-Suwaidi, J.; Gehani, A.A.; Bener, A. Clinical and histologic studies of a Qatari family with myofibrillar myopathy. Saudi Med. J. 2004, 25, 1723–1726. [Google Scholar]
- Shelly, S.; Talha, N.; Pereira, N.L.; Engel, A.G.; Johnson, J.N.; Selcen, D. Expanding Spectrum of Desmin-Related Myopathy, Long-term Follow-up, and Cardiac Transplantation. Neurology 2021, 97, E1150–E1158. [Google Scholar] [CrossRef] [PubMed]
- Bittner, R.E.; Shorny, S.; Streubel, B.; Hübner, C.; Voit, T.; Kress, W. Serum antibodies to the deleted dystrophin sequence after cardiac transplantation in a patient with Becker’s muscular dystrophy. N. Engl. J. Med. 1995, 333, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, J.; Schessl, J.; Schara, U.; Reitter, B.; Stettner, G.M.; Hobbiebrunken, E.; Wilichowski, E.; Bernert, G.; Weiss, S.; Stehling, F.; et al. Treatment of Duchenne muscular dystrophy with ciclosporin A: A randomised, double-blind, placebo-controlled multicentre trial. Lancet Neurol. 2010, 9, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Politano, L. Females with dystrophinopathy: A neglected patient population. Dev. Med. Child Neurol. 2023, 65, 1001–1002. [Google Scholar] [CrossRef] [PubMed]
- Seguchi, O.; Kuroda, K.; Fujita, T.; Kumai, Y.; Nakajima, S.; Watanabe, T.; Yanase, M.; Matsumoto, Y.; Fukushima, S.; Kimura, K.; et al. Heart Transplantation Ameliorates Ambulation Capacity in Patients with Muscular Dystrophy—An Analysis of 9 Cases. Circ. J. 2019, 83, 684–686. [Google Scholar] [CrossRef]
- Rosenbaum, A.N.; Kremers, W.K.; Schirger, J.A.; Thomas, R.J.; Squires, R.W.; Allison, T.G.; Daly, R.C.; Kushwaha, S.S.; Edwards, B.S. Association between early cardiac rehabilitation and long-term survival in cardiac transplant recipients. Mayo Clin. Proc. 2016, 91, 149–156. [Google Scholar] [CrossRef]
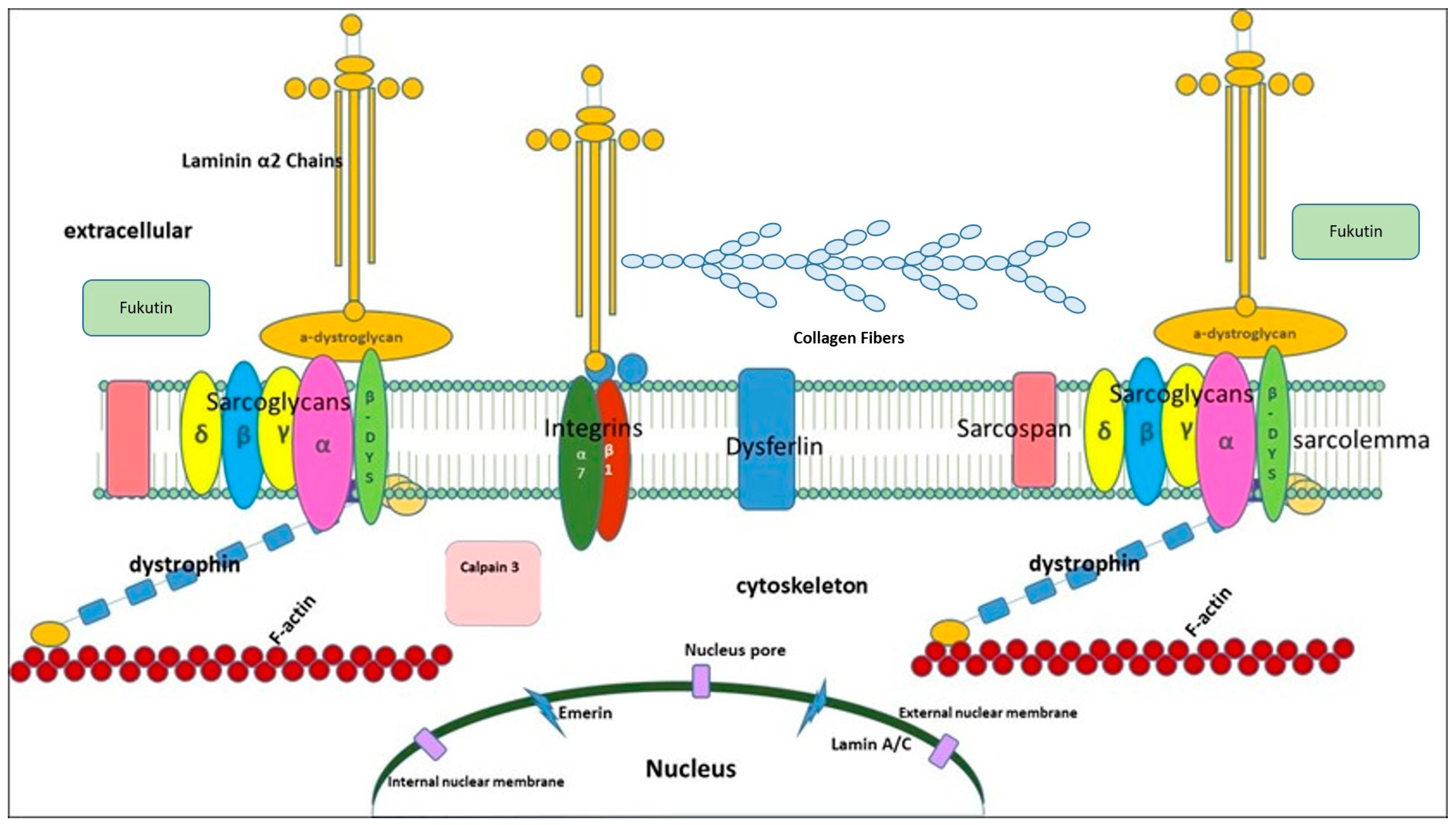
| Disease | Disease MIM Number | Locus | Gene Symbol | Protein |
|---|---|---|---|---|
| Duchenne | 310200 | Xp21 | DMD | Dystrophin |
| Becker | 300376 | Xp21 | DMD | Dystrophin |
| XL-DCM | 302045 | Xp21 | DMD | Dystrophin |
| Emery-Dreifuss | 310300 | Xq28 | EMD | Emerin |
| Myotonic Distrophy Type 1 | 160900 | 19q13 | DMPK | Myotonin |
| Myofibrillar Myopathies | 601419 | 2q35 | Several | Several |
| LGMD Type Nomenclature | MIM Disease Number | Locus | Gene Symbol | Protein | |
|---|---|---|---|---|---|
| New | Old | ||||
| 1A | 159000 | 5q31.2 | MYOT | Myotilin | |
| 1B | 159001 | 1q22 | LMNA | Lamin A/C | |
| 1C | 607801 | 3p25.3 | CAV3 | Caveolin-3 | |
| D1 | 1D | 603511 | 7q36.3 | DNAJB6 | DNAJ/HSP40 homologue |
| 1E | 615325 | 2q35 | DES | Desmin | |
| D2 | 1F | 608423 | 7q32.1 | TPNO3 | Transportin-3 |
| D3 | 1G | 609115 | 4p21 | HNRNPDL | Heterogeneous molecular ribonucleic D-like protein |
| 1H | ? | ? | ? | Not confirmed | |
| D4 | 1I | 613350 | 15q15.1 | CAPN3 | Calpain-3 |
| D5 | COL6A1, COL6A2, COL6A3 | Collagen 6α1, α2, α3 | |||
| R1 | 2A | 253600 | 15q15.1 | CAPN3 | Calpain-3 |
| R2 | 2B | 253601 | 2p13.2 | DYSF | Dysferlin |
| R3 | 2D | 608099 | 17q21.33 | SGCA | α-sarcoglycan |
| R4 | 2E | 604286 | 4q12 | SGCB | β-sarcoglycan |
| R5 | 2C | 253700 | 13q12.12 | SGCG | γ-sarcoglycan |
| R6 | 2F | 601287 | 5q33.3 | SGCD | δ-sarcoglycan |
| R7 | 2G | 601954 | 17q12 | TCAP | Telethonin |
| R8 | 2H | 254110 | 9q33.1 | TRIM32 | Tripartite motif containing protein-32 |
| R9 | 2I | 607155 | 19q13.32 | FKRP | Fukutin-related protein |
| R10 | 2J | 608807 | 2q32.2 | TTN | Titin |
| R11 | 2K | 609308 | 9q34.13 | POMT1 | Protein O-mannosyl transferase-1 |
| R12 | 2L | 611307 | 11p14.3 | ANO5 | Anoctamin-5 |
| R13 | 2M | 611588 | 9q31.2 | FKTN | Fukutin |
| R14 | 2N | 613158 | 14q24.3 | POMT2 | Protein O-mannosyl transferase-2 |
| R15 | 2O | 613157 | 1p34.1 | POMGnT1 | Protein O-mannose N-acetyl-glucosaminyl transferase-1 |
| R16 | 2P | 613818 | 3p21 | DAG1 | Dystroglycan |
| R17 | 2Q | 613723 | 8q24.3 | PLEC1 | Plectin |
| R18 | 2R | 615325 | 2q35 | DES | Desmin |
| R19 | 2S | 615356 | 4q35.1 | TRAPPC11 | Transpo-protein-particle-complex-11 |
| R20 | 2T | 615352 | 3p21.31 | GMPPB | GDP-mannose-pyrophosphorylase B |
| R21 | 615618 | 3q13.33 | POGLUT1 | Protein O-glucosyltranferase-1 | |
| R22 | COL6A1, COL6A2, COL6A3 | Collagen 6α1, α2, α3 | |||
| R23 | 156225 | 6q22.33 | LAMA2 | Laminin α2 | |
| R24 | 614828 | 3p22.1 | POMGNT2 | Protein O-linked Mannose N-acetylglucosaminyl transferase-2 | |
| R25 | BVES | Blood vessel epicardial substance | |||
| Authors [Year of Publication] | DMD | BMD/ XL-DCM | DMD Carriers | EDMD | LGMDs | DM1 | MFMs | Unspecified Type | Age or Mean Age at Transplant in Years (Range) | FU or Mean FU in Years (Range) | Ref. Number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Casazza et al. (1988) | 1 | 23 | 2.0 | [172] | |||||||
| D’Onofrio et al. (1989) | 1 | 17 | 2.0 | [173] | |||||||
| Sakata et al. (1990) | 3 | n.r. | n.r. | [174] | |||||||
| Merchut et al. (1990) | 1 | n.r. | n.r. | [196] | |||||||
| Anthuber et al. (1990) | 1 | 23 | 2.7 | [175] | |||||||
| Anthuber et al. (1990) | 1 | 47 | 3.3 | [175] | |||||||
| Goenen et al. (1991) | 1 | 25 | 2.8 | [211] | |||||||
| Rees et al. (1993) | 3 | 22.3 (12–31) | 2.8 (0.10–5.4) | [167] | |||||||
| Rees et al. (1993) | 1 | 45 | 1.5 | [167] | |||||||
| Rees et al. (1993) | 1 | 33 | 2.4 | [167] | |||||||
| Rees et al. (1993) | 1 | 9 | 7.0 | [167] | |||||||
| Piccolo et al. (1994) | 1 | 32 | n.r. | [176] | |||||||
| Fiocchi et al. (1997) | 1 | 15 | 0.6 | [177] | |||||||
| Pitt et al. (1998) | 1 | 26 | 0.2 | [207] | |||||||
| Melacini et al. (1998) | 1 | 41 | 3.6 | [193] | |||||||
| Finsterer et al. (1999) | 1 | 27 | 6.0 | [178] | |||||||
| Melacini et al. (2001) | 1 | 24 | 0.4 | [179] | |||||||
| Davis et al. (2001) | 1 | 25 | n.r. | [194] | |||||||
| Conraads et al. (2002) | 1 | 40 | 5.0 | [212] | |||||||
| Leprince et al. (2002) | 1 | 28 | 1.6 | [180] | |||||||
| Ruiz-Cano et al. (2003) | 3 | 39.5 (24–55) | 4.8 (1.1–10.8) | [181] | |||||||
| Ruiz-Cano et al. (2003) | 1 | 42 | 6.0 | [181] | |||||||
| Ruiz-Cano et al. (2003) | 1 | 35 | 5.8 | [181] | |||||||
| Kickuk-Chrisant et al. (2004) | 2 | n.r. | n.r. | [197] | |||||||
| El-Menyar et al. (2004) | 1 | n.r. | n.r. | [214] | |||||||
| Srinivasan et al. (2005) | 1 | 38 | n.r. | [182] | |||||||
| Ben Yahou et al. (2005) | 2 | 38 (14–62) | n.r. | [198] | |||||||
| Komanapalli et al. (2006) | 1 | n.r. | n.r. | [183] | |||||||
| Patané et al. (2006) | 1 | 27 | 1.0 | [184] | |||||||
| Kärkkäinen et al. (2006) | 6 | 42 (32–48) | n.r. | [199] | |||||||
| Cuneo et al. (2007) | 1 | 21 | n.r. | [200] | |||||||
| Dell’Amore et al. (2007) | 2 | 44 (43–45) | 4.5 (3.4 -5.6) | [201] | |||||||
| D’Amico et al. (2008) | 1 | 8 | 12.0 | [208] | |||||||
| Margeta et al. (2009) | 2 | 0.8–18 | 9-n.r. | [209] | |||||||
| Ambrosi et al. (2009) | 7 | 46 (21–62) | 8.3 (1–17) | [202] | |||||||
| Wu et al. (2010) | 3 | 15 | 1 | 3 | 4 | 3 | 37 (n.r.) | 5.4 (n.r.—>10) | [168] | ||
| Volpi et al. (2010) | 1 | 58 | n.r. | [203] | |||||||
| Katzberg et al. (2010) | 1 | 14 | n.r. | [185] | |||||||
| Katzberg et al. (2010) | 1 | 12 | n.r. | [185] | |||||||
| Fournier et al. (2010) | 1 | 53 | 0.3 | [186] | |||||||
| Cripe et al. (2011) | 1 | 14 | 4 | [170] | |||||||
| Steger et al. (2013) | 7 | 38.5 (16–56) | 5.7 (1.4–11.6) | [188] | |||||||
| Wahbi et al. (2013) | 1 | 45 | n.r. | [100] | |||||||
| Blagova et al. (2016) | 1 | 38 | 3.4 | [204] | |||||||
| Papa et al. (2017) | 4 | 30.2 (27–34) | 12 (10–14.5) | [187] | |||||||
| Bajoras et al. (2017) | 1 | 25 | n.r. | [190] | |||||||
| Papa et al. (2018) | 1 | 44 | 0.9 | [213] | |||||||
| Hasselberg et al. (2019) | 15 | n.r. | 7.8 ± 6.3 | [48] | |||||||
| Ditaranto et al. (2019) | 26 | 43 (34–48) | 2.4 (0.8–8.5) | [50] | |||||||
| Peretto et al. (2019) | 14 | 48 (35–53) | 10 (7–15) | [58] | |||||||
| Ascencio-Lemus et al. (2019) | 6 | 39.8 (24–48) | 8.4 (0.10–19.1) | [191] | |||||||
| Wells et al. (2020) | 3 | 42 | 11 | 4 | 2 | 18 | n.r. | n.r. | [169] | ||
| Piperata et al. (2020) | 1 | 18 | 0.3 | [215] | |||||||
| Shelly et al. (2021) | 3 | 31.3 (20–39) | n.r. | [166] | |||||||
| Protonotarios et al. (2021) | 1 | n.r. | n.r. | [130] | |||||||
| Kim et al. (2022) | 1 | 38 | n.r. | [210] | |||||||
| Cullom et al. (2022) | 1 | 60 | 2 | [195] | |||||||
| Visrodia et al. (2022) | 6 | 4 | 4 | 2 | 4 | 45 (29–57.5) | 5 | [192] | |||
| Martins et al. (2023) | 1 | 33 | n.r. | [205] | |||||||
| Loureiro et al. (2023) | 1 | 32 | 0.6 | [206] | |||||||
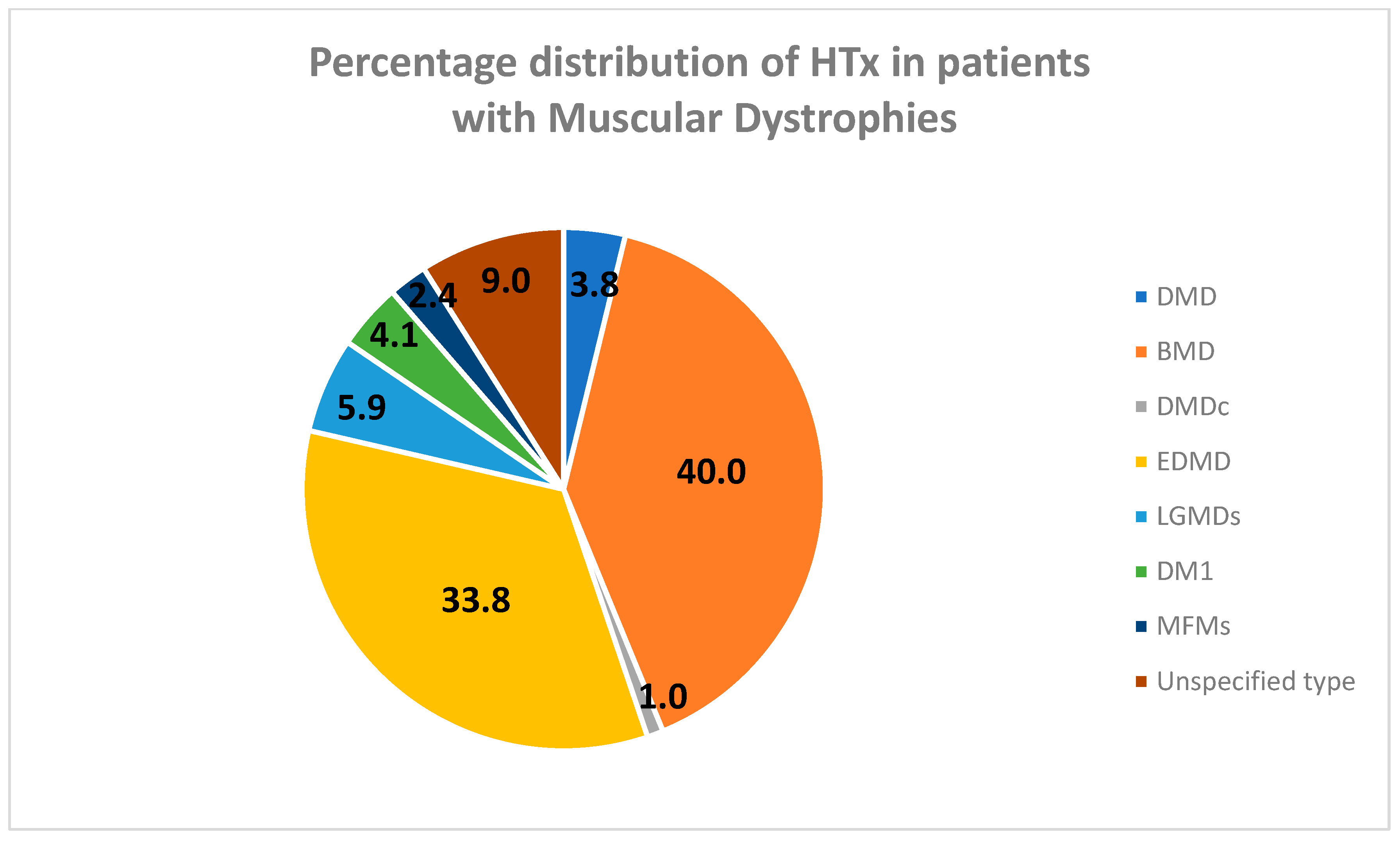
| Total number | 11 | 101 | 3 | 98 | 17 | 12 | 7 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politano, L. Is Cardiac Transplantation Still a Contraindication in Patients with Muscular Dystrophy-Related End-Stage Dilated Cardiomyopathy? A Systematic Review. Int. J. Mol. Sci. 2024, 25, 5289. https://doi.org/10.3390/ijms25105289
Politano L. Is Cardiac Transplantation Still a Contraindication in Patients with Muscular Dystrophy-Related End-Stage Dilated Cardiomyopathy? A Systematic Review. International Journal of Molecular Sciences. 2024; 25(10):5289. https://doi.org/10.3390/ijms25105289
Chicago/Turabian StylePolitano, Luisa. 2024. "Is Cardiac Transplantation Still a Contraindication in Patients with Muscular Dystrophy-Related End-Stage Dilated Cardiomyopathy? A Systematic Review" International Journal of Molecular Sciences 25, no. 10: 5289. https://doi.org/10.3390/ijms25105289
APA StylePolitano, L. (2024). Is Cardiac Transplantation Still a Contraindication in Patients with Muscular Dystrophy-Related End-Stage Dilated Cardiomyopathy? A Systematic Review. International Journal of Molecular Sciences, 25(10), 5289. https://doi.org/10.3390/ijms25105289






