Abstract
Chronic environmental exposure to toxic heavy metals, which often occurs as a mixture through occupational and industrial sources, has been implicated in various neurological disorders, including Parkinsonism. Vanadium pentoxide (V2O5) typically presents along with manganese (Mn), especially in welding rods and high-capacity batteries, including electric vehicle batteries; however, the neurotoxic effects of vanadium (V) and Mn co-exposure are largely unknown. In this study, we investigated the neurotoxic impact of MnCl2, V2O5, and MnCl2-V2O5 co-exposure in an animal model. C57BL/6 mice were intranasally administered either de-ionized water (vehicle), MnCl2 (252 µg) alone, V2O5 (182 µg) alone, or a mixture of MnCl2 (252 µg) and V2O5 (182 µg) three times a week for up to one month. Following exposure, we performed behavioral, neurochemical, and histological studies. Our results revealed dramatic decreases in olfactory bulb (OB) weight and levels of tyrosine hydroxylase, dopamine, and 3,4-dihydroxyphenylacetic acid in the treatment groups compared to the control group, with the Mn/V co-treatment group producing the most significant changes. Interestingly, increased levels of α-synuclein expression were observed in the substantia nigra (SN) of treated animals. Additionally, treatment groups exhibited locomotor deficits and olfactory dysfunction, with the co-treatment group producing the most severe deficits. The treatment groups exhibited increased levels of the oxidative stress marker 4-hydroxynonenal in the striatum and SN, as well as the upregulation of the pro-apoptotic protein PKCδ and accumulation of glomerular astroglia in the OB. The co-exposure of animals to Mn/V resulted in higher levels of these metals compared to other treatment groups. Taken together, our results suggest that co-exposure to Mn/V can adversely affect the olfactory and nigral systems. These results highlight the possible role of environmental metal mixtures in the etiology of Parkinsonism.
1. Introduction
Among potentially toxic elements, heavy metals, including copper (Cu), lead (Pb), manganese (Mn), vanadium (V), and cadmium (Cd), are ubiquitous in nature, and some are essential for a wide range of biological processes, such as maintaining cell structure and regulating metabolic function, gene expression, neurotransmission, and antioxidant responses [1]. However, the excessive accumulation of metals in the nervous system can have harmful effects, resulting in deficits in neurodevelopment, neurobehavior, and cognition, and may cause neurodegeneration depending on the valence states, metal exposure route and duration, bioavailability, chemical forms, and the subject’s life stage [2]. Various metals, including Cd, chromium, nickel, Pb, and Mn, have been shown to alter the ability to smell following chronic exposure [3]. Other studies have reported olfactory dysfunction in cohorts of professional welders [4,5]. The epidemiological, experimental, and clinical evidence indicates a strong correlation between exposure to toxic metals and various neurological diseases, including Alzheimer’s disease, Parkinson’s disease (PD), and amyotrophic lateral sclerosis [2,6]. Currently, no clinically efficacious treatments exist for individuals suffering from chronic neurotoxicity induced by recurrent exposure to toxic metals. Although the exact molecular events remain unclear, the cellular mechanisms underlying toxic heavy metal-induced neurotoxicity likely involve oxidative stress, lipid peroxidation, mitochondrial dysfunction, protein misfolding, apoptosis, DNA damage, the disruption of ATP synthesis, and epigenetic and genetic alterations [7,8].
Among the toxic heavy metals associated with neurological diseases, Mn receives much interest, given its ability to induce manganism, which is characterized by a severe neurological deficit that often resembles the involuntary extrapyramidal symptoms associated with PD [9,10,11,12,13]. Chronic Mn neurotoxicity occurs often from inhalation in occupational settings, including welding, mining and mineral processing, metal manufacturing emissions, fossil fuel combustion, and dry-cell manufacturing [11,14,15,16,17]. The current inhalation reference concentration (RfC) for Mn is 0.05 µg/m3 (USEPA, 1993). Mn toxicity is most commonly associated with occupational exposure to aerosols or dust containing extremely high levels of Mn (>1–5 mg/m3) [18,19,20,21,22,23,24,25]. Mn levels in aerosols, dust, and fumes can be 200–6000 times higher than the RfC. The basal ganglia, which includes the striatum (STR), is a critical target during Mn exposure [26,27,28,29,30]. One of the features distinguishing manganism from PD is the globus pallidus being more severely affected than all the other regions of the brain during the disease pathogenesis of manganism [31]. Mn-induced striatal dopamine (DA) depletion, which is one of the pathological hallmarks of PD, has also been demonstrated in both in vivo [32] and in vitro [33] studies. Dorman et al. [34] showed the accumulation of MnSO4 in the STR and olfactory bulb (OB) of rats following inhalation exposure. V is typically found in the environment as vanadium pentoxide (V2O5) and is often associated with Mn, particularly in welding rods. It is a significant trace element in fossil fuels, the burning of which results in considerable environmental exposure to V. V compounds are widely utilized in numerous industrial applications, including car and aircraft manufacturing, temperature-resistant alloy production, sulfuric acid, and phthalic anhydride manufacturing, pesticide production, and pigment and paint manufacturing, among others [5,8,9]. The increasing use of V in high-capacity battery manufacturing for energy storage and Li-ion batteries in electric cars has also accelerated its widespread demand. The processing of V slag (about 120 g V2O5 per kg) is characterized by the formation of dust, with V concentrations ranging from 5 to 120 mg/m3 [35]. Crude oil from Venezuela is believed to have the highest V concentrations of 1400 mg/kg. Elevated levels of airborne V (4.7 mg/m3) have been found in the breathing zone of steel industry workers [36]. V has been reported in the blood, feces, and urine of workers exposed to airborne V2O5 dust (USEPA, 1987). ICP-MS analyses of roadside dust samples revealed aluminum (55,090 μg/g), V (70 μg/g), Mn (511 μg/g), and iron (Fe) (21,600 μg/g). The ratio of V to Mn in inhaled dust during occupational exposures can vary from 1:1 to 1:8. Despite V’s extensive industrial use, little is known regarding its adverse neurological effects. Previously, our laboratory reported that low-dose V exposure induces neurotoxic effects in dopaminergic neurons in both cell culture and animal models [37]. Notably, intranasal exposure to low-dose V induces olfactory deficits and damages dopaminergic neurons in the glomerular layer of the OBs [38]. Animal studies have also demonstrated that inhaled V2O5 damages the nigrostriatal system and other parts of the central nervous system (CNS) [39,40,41]. Inhalation is the primary route of environmental exposure to V, making it a more suitable experimental model for mimicking its exposure [42]. Given that occupationally related V exposure commonly occurs with co-exposure to Mn [43,44,45,46], Mn/V interactive neurotoxicity has become a true concern for industrial workers exposed to metal-containing substances as well as residents and non-workers in the surrounding environments.
We have demonstrated that like many other neurotoxicants associated with Parkinsonism pathogenesis, V and Mn are neurotoxic to dopaminergic neurons through oxidative stress and the PKCδ pro-apoptotic kinase-mediated pathway in in vitro and ex vivo systems [37,47]. Despite what is known about each metal’s neurotoxicity, the neurotoxic response to an Mn/V mixture has not been investigated. In the current study, we investigated the neurotoxic properties of an Mn/V mixture along with that of each metal alone when exposure occurs intranasally in a mouse model. We determined the neurotoxicological responses in the olfactory and nigrostriatal dopaminergic systems following exposure to different treatment groups.
2. Results
2.1. AP Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Induces Motor Deficits in Mice
Male C57BL/6 mice were intranasally administered appropriate doses of the metals (Mn and V), both alone and mixed, three times a week for up to one month, as described in Section 4.3. At the end of the one-month treatment period, we evaluated the treatment effects on locomotor activity using a VersaMax automated activity monitor. Figure 1A shows a representative locomotor activity map of the control and treatment groups. Quantitative analysis revealed that the treated mice exhibited significant decreases in various motor parameters compared to the control group (Figure 1B–H). Specifically, V2O5- and Mn/V2O5-treated mice exhibited significant decreases in total horizontal movement (Figure 1B); total distance traveled (Figure 1C); total movement time (Figure 1D); rearing activity (Figure 1E); the number of rearing movements (Figure 1F); vertical activity (Figure 1G); and the number of stereotypy counts (Figure 1H) relative to the control group. Notably, the Mn-V co-exposure group showed the most severe locomotor deficits compared to Mn- and V-alone groups. The severity of motor deficits is given in the following order: Mn/V2O5 > V2O5 > Mn. Collectively, these results suggest that chronic intranasal exposure to mixed Mn/V induces severe motor dysfunction in mice.
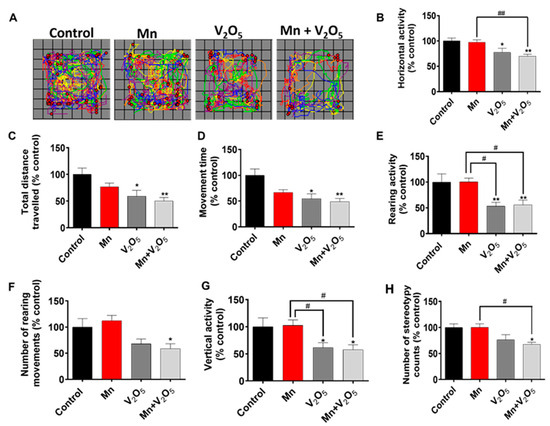
Figure 1.
The effects of intranasally administered Mn, V2O5, and Mn-V2O5 on locomotor activity. (A) Moving track of mice. (B), Horizontal activity. (C), Total distance traveled. (D), Movement time. (E), Rearing activity. (F), Number of rearing movements. (G), Vertical activity. (H), Number of stereotypy counts. The vehicle-treated group served as the control, and it was set at 100%. The data represent the mean ± S.E.M. for at least nine animals per group. Asterisks (*, p ≤ 0.05; **, p < 0.01) indicate significant differences compared with the control unless otherwise indicated, while hashtags (#, p ≤ 0.05; ##, p < 0.01) indicate significant differences between the indicated treatment groups.
2.2. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Induces Olfactory Dysfunction in Male Mice
After the motor deficit measurements, we assessed the effect of Mn, V2O5, and Mn/V2O5 on olfaction by measuring the social discrimination ability of male mice to detect female pheromones. Specifically, we evaluated their ability to find and sniff bedding from female cages, as described in the Methods section. Our results demonstrated a significant decrease in the time spent sniffing the female bedding during a 3-min testing session following the introduction of the bedding into the male mouse cages for the Mn, V2O5, and Mn/V2O5 treatment groups compared to the control group (Figure 2A). We observed a 36%, 66%, and 73% decrease in the time spent sniffing for the Mn, V2O5, and Mn/V2O5 treatment groups, respectively, relative to the controls. Furthermore, we observed a significant loss in the sense of smell in the Mn/V2O5 co-treatment group compared to the Mn-treated group. These findings indicate that prolonged intranasal exposure to these metals causes male mice to experience impaired olfactory function, with the degree of deficits in olfaction following this order: Mn/V2O5 > V2O5 > Mn.
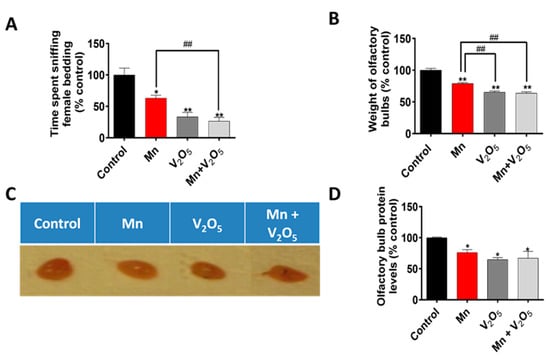
Figure 2.
Effects of intranasally administered Mn, V2O5, and Mn/V2O5 on pheromonal olfaction (sniffing ability) and the size of mouse OBs. (A), Animals were exposed to bedding from female cages at the end of the study, and the amount of time out of three minutes that the animals spent sniffing the bedding was recorded. (B), Animals were sacrificed one week following the treatment, and the OBs were dissected in the same manner every time and weighed. (C), Snapshot showing the different sizes of the OBs following treatments. (D), Graphical representation of the total protein of the homogenized OBs following treatment, as measured with the Bradford assay. The data represent the mean ± S.E.M. from at least six animals per group. Asterisks (*, p ≤ 0.05; **, p < 0.01) indicate significant differences between the treatment and controls unless otherwise indicated, while hashtags (##, p < 0.01) indicate significant differences between the indicated treatment groups.
2.3. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Reduces the Weight of Olfactory Bulbs
To determine the effect of intranasally administered Mn, V2O5, and Mn/V2O5 on OB weight, we dissected and weighed the OBs, as described in the Methods section. Our results show a significant reduction in OB weight in the Mn, V2O5, and Mn/V2O5 combined treatment groups, with reductions of 21%, 34%, and 36%, respectively, compared to the control group (Figure 2B). Morphological changes in the OB (Figure 2C) confirmed the weight loss observed in Figure 2B. The total amount of protein (Figure 2D), as measured by the Bradford assay, also supports the observations in Figure 2B,C. These results indicate that chronic intranasal exposure to neurotoxic metals reduced OB weight and volume as well as olfactory function in male mice, with the severity in the following order: Mn/V2O5 ≥ V2O5 > Mn.
2.4. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Causes the Loss of Tyrosine Hydroxylase (TH) and Dopaminergic Neurons in the Olfactory Bulb
It has been demonstrated that, as the initial synaptic integration site of the whole OB system, the glomerular layer of the OB is rich in dopaminergic neurons [48,49,50]. These neurons are linked with modulating olfactory signal processing since DA is thought to play an important role in proper olfactory function [51,52,53]. TH, which is the rate-limiting enzyme during DA synthesis, is often used as a marker for the presence of healthy dopaminergic neurons. We, therefore, measured the metal-induced changes in TH protein levels and immunoreactivity in the dissected OBs using Western blot (Figure 3) and immunohistochemistry (IHC) (Figure 4). TH protein levels in the OBs of metal-treated groups were significantly reduced relative to the control group (Figure 3A). The densitometric analysis of Western blot revealed a 56%, 74%, and 78% decrease in TH levels in the OBs of the Mn, V2O5, and Mn/V2O5 treatment groups, respectively, compared to the controls (Figure 3B). Furthermore, both V2O5-alone and Mn/V2O5 co-treatment groups showed significantly higher decreases in TH levels than Mn-alone. To provide further anatomical visualization of dopaminergic neurons in the glomerular layer of the OB, we performed a TH IHC analysis. Both V2O5 and Mn/V2O5 groups showed a greater loss of TH-positive neurons (Figure 4A–C), further supporting the Western blot data presented in Figure 3. Taken together, our findings suggest that chronic intranasal exposure to these metals, either alone or mixed, can cause significant neurotoxic effects in dopaminergic OB neurons.
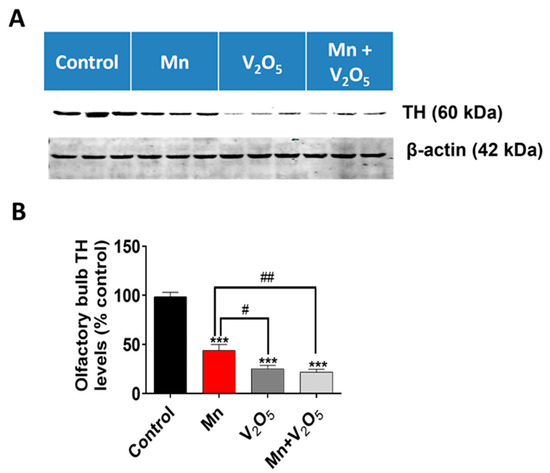
Figure 3.
Effect of intranasally administered Mn, V2O5, and Mn/V2O5 on TH expression level in the OB. (A), Tissues of the OB were homogenized and used for Western blotting, as described in the Methods section. TH expression was detected using a monoclonal mouse antibody raised against TH. The membrane was re-probed with the β-actin antibody to confirm equal protein loading in each lane. (B), Densitometric analysis of the Western blot. The data represent the mean ± S.E.M. from at least seven animals per group. Asterisks (***, p < 0.001) indicate a significant difference between the treatment and control unless otherwise indicated, while hashtags (#, p ≤ 0.05; ##, p < 0.01) indicate significant differences between the indicated treatment groups.
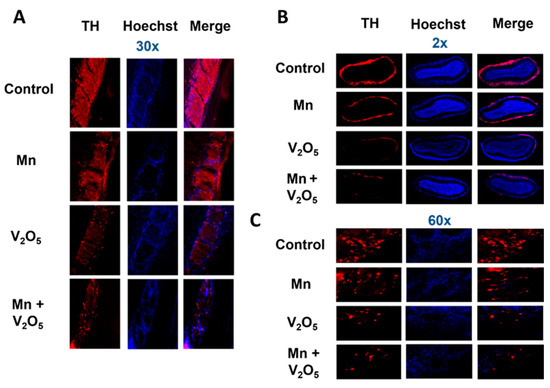
Figure 4.
Loss of TH neurons in the OB following intranasally administered Mn, V2O5 alone, or Mn/V2O5 mixed. (A), Representative 30× pictures of TH neurons in the OB of the control and treated mice. (B), Representative 2× pictures of TH neurons in the OB of control and treated mice. (C), Representative 60× pictures of TH neurons in the OB of control and treated mice. Data were derived from 3 mice per treatment group.
2.5. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Causes the Loss of the Neurotransmitter Dopamine and Its Metabolite 3,4-Dihydroxyphenylacetic Acid (DOPAC) in the Olfactory Bulb
Following the assessment of neurobehavioral and neuropathological changes associated with metal-induced impairment in the olfactory system, we further measured the neurochemical changes in the OBs to determine behavioral and neurochemical correlations. Our HPLC-ECD neurochemical analysis revealed a significant reduction in DA and DOPAC levels in the OBs of the Mn, V2O5, and Mn/V2O5 treatment groups, with a corresponding 57%, 72%, and 79% reduction in DA levels, and a 70%, 80%, and 80% decrease in DOPAC levels, relative to the control group (Figure 5A,B). The Mn/V2O5 treatment group showed significantly lower DA levels than the Mn group (Figure 5A,B). Collectively, these results show that exposure to Mn and V2O5, both alone and mixed, induces a profound dopaminergic neurochemical loss in the OB.
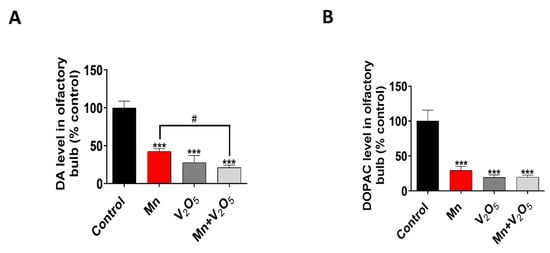
Figure 5.
Effects of intranasally administered Mn, V2O5, and Mn/V2O5 on neurochemical changes in the OB. (A), DA levels and (B), DOPAC levels using HPLC. The data represent the mean ± S.E.M. from at least six animals per group. Asterisks (***, p < 0.001) indicate a significant difference between the treatment and controls unless otherwise indicated, while the hashtag (#, p < 0.05) indicates a significant difference between the indicated treatment groups.
2.6. Exposure to Mn and V2O5 Mixture Results in a Higher Uptake of Both Metals in the Striatum
Next, to ensure that intranasal administration resulted in the uptake of metals by the brain, we measured Mn and V metal levels in the STR using inductively coupled plasma mass spectrometry (ICP-MS). We observed a 4-fold increase in V levels in the STR of the Mn/V2O5 group compared to a 2-fold increase in the V2O5-alone group, both relative to controls (Figure 6A). We also observed a 5-fold increase in Mn levels in the STR of the Mn/V2O5 group compared to a 1.7-fold increase in the Mn-alone group, both relative to the controls (Figure 6B). These findings suggest that an Mn/V2O5 mixture leads to the greater uptake of both metals in the STR compared to their individual exposures.
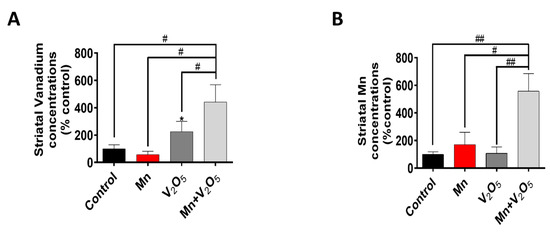
Figure 6.
Striatal levels of Mn and V following intranasal administration of Mn, V2O5, or Mn/V2O5. (A), Mn levels and (B), V levels using ICP-MS. The levels were normalized to striatal tissue weight. The data represent the mean ± S.E.M. from at least four animals per group. The asterisk (*, p ≤ 0.05) indicates a significant difference between the treatment and controls, while hashtags (#, p ≤ 0.05; ##, p < 0.01) indicate significant differences between the indicated treatment groups.
2.7. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Induces Oxidative Stress in the Striatum and Sustantia Nigra
Since oxidative stress has been shown to be an early event during Mn-induced neurotoxicity, we measured the accumulation of the oxidative stress marker 4-hydroxynonenal (4HNE) in the STR and SN using Western blot analysis. We observed a 1.5-, 1.5-, and 1.4-fold increase in 4HNE levels in the STR of animals treated with either Mn, V2O5, or Mn/V2O5, respectively, compared to the controls (Figure 7A,B). Similarly, we observed a 2.4-, 2.3-, and 1.5-fold increase, although not statistically significant, in 4HNE levels in the SN of animals treated with either Mn, V2O5, or Mn/V2O5, respectively, compared to the control group (Figure 7C,D). TH levels in both the SN and the STR did not change, indicating that dopaminergic neurons or their processes in the SN and STR had not yet suffered severe damage.
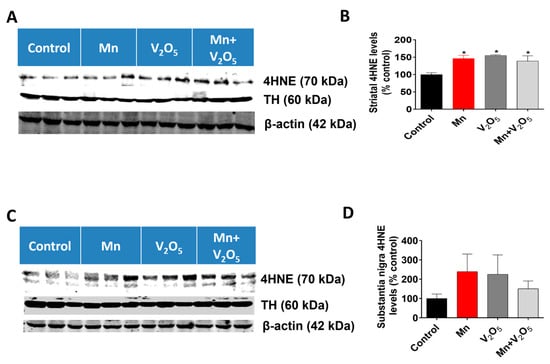
Figure 7.
Effects of intranasally administered Mn, V2O5, and Mn/V2O5 on 4HNE expression levels in the STR and SN. (A), Striatal tissue was homogenized and used for Western blotting, as described in the Methods section. 4HNE and TH were detected using antibodies raised against 4HNE and TH, respectively. The membranes were re-probed with the β-actin antibody to confirm equal protein loading in each lane. (B), 4HNE densitometric analysis of the Western blot. (C), SN tissue was homogenized and used for Western blotting, as described in the Methods section. 4HNE and TH expressions were detected using an antibody raised against 4HNE and TH, respectively. The membranes were re-probed with the β-actin antibody to confirm equal protein loading in each lane. (D), 4HNE densitometric analysis of the Western blot. The data represent the mean ± S.E.M. from at least five animals per group. Asterisks (*, p ≤ 0.05) indicate significant differences between the treatment and controls unless otherwise indicated.
2.8. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Induces the Accumulation of α-Synuclein in the Substantia Nigra
The accumulation of α-synuclein in the SN has been shown to be an early event during dopaminergic neurodegeneration. Hence, we proceeded to measure the accumulation of α-synuclein using Western blots. Remarkably, our results showed a significant increase in α-synuclein accumulation in the SN of animals treated with Mn, V2O5, or Mn/V2O5, with a 2.2-, 4.2-, and 2.6-fold increase, respectively, compared to the control group, as measured by the densitometric analysis of Western blots (Figure 8A,B). These findings suggest that chronic intranasal exposure to these neurotoxic metals, either alone or mixed, triggers α-synuclein accumulation in the SN, which could contribute to the pathogenesis of metal-induced chronic Parkinsonism.
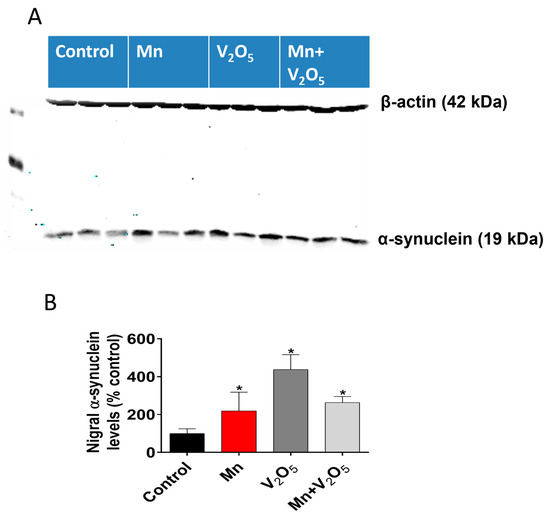
Figure 8.
Effects of intranasally administered Mn, V2O5, and Mn/V2O5 on α-synuclein expression levels in the SN. (A), SN tissue was homogenized and used for Western blotting, as described in the Methods section. Alpha-synuclein expression was detected using a monoclonal antibody raised against α-synuclein. The membrane was re-probed with the β-actin antibody to confirm equal protein loading in each lane. (B), Densitometric analysis of the Western blot. The data represent the mean ± S.E.M. from at least five animals per group. Asterisks (*, p ≤ 0.05) indicate significant differences between the treatment and controls unless otherwise indicated.
2.9. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Induced Upregulation of the Pro-Apoptotic Kinase PKCδ in the Olfactory Bulb
We demonstrated in our laboratory that the pro-apoptotic kinase PKCδ, a member of the novel subfamily of PKC kinases, is critical during metal-induced dopaminergic neurodegeneration [37,47,54]. We, therefore, measured the levels of this protein in the OB with SN as an indicator of ongoing apoptotic events using Western blots. We observed a significant 1.4-, 1.6-, and 1.4-fold increase in native PKCδ levels in the OB lysates following treatment with Mn or V2O5 alone, or with Mn/V2O5, respectively, compared to the controls, as measured by the densitometric analysis of Western blots (Figure 9A,B). Additionally, Western blot analysis showed a 1.4-fold increase in the levels of active PKCδ with phosphorylation at the threonine 505 site in the OB lysate following Mn/V2O5 co-exposure compared to the control group, while the treatment groups with Mn and V2O5 alone showed no significant differences (Figure 9C,D). In the SN, we also observed a modest, statistically insignificant increase in native PKCδ levels following treatment with Mn or V2O5 alone, or with Mn/V2O5, respectively, compared to the control group (Figure 9E,F). These results suggest that the upregulation of pro-apoptotic PKCδ might be involved in mediating the neurotoxic effects induced by these neurotoxic metals in the OB.
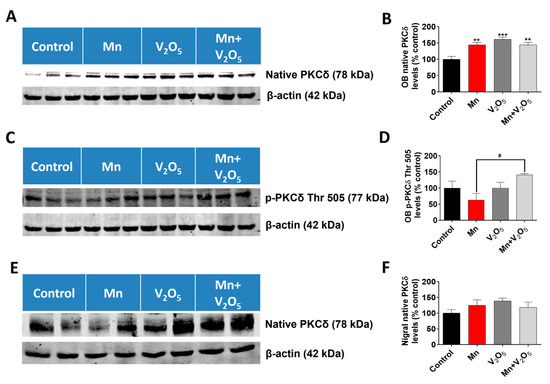
Figure 9.
Effects of intranasally administered Mn, V2O5, and Mn/V2O5 on native PKCδ and PKCδ Thr 505 expression levels in the OB and SN. (A), OB tissue was homogenized and used for Western blotting, as described in the Methods section. PKCδ expression was detected using a monoclonal antibody raised against PKCδ. The membrane was re-probed with the β-actin antibody to confirm equal protein loading in each lane. (B), Densitometric analysis of the Western blot. (C), OB tissue was homogenized and used for Western blotting, as described in the Methods section. PKCδ Thr 505 expression was detected using a monoclonal antibody raised against PKCδ Thr 505. The membrane was re-probed with the β-actin antibody to confirm equal protein loading in each lane. (D), Densitometric analysis of the Western blot. (E), SN tissue was homogenized and used for Western blotting, as described in the text. PKCδ expression was detected using a monoclonal antibody raised against PKCδ. The membrane was re-probed with the β-actin antibody to confirm equal protein loading in each lane. (F), Densitometric analysis of the Western blot. The data represent mean ± S.E.M. from at least five animals per group. Asterisks (**, p < 0.01; ***, p < 0.001) indicate significant differences between the treatment and control unless otherwise indicated, while the hashtag (#, p ≤ 0.05) indicates a significant difference between the indicated treatment groups.
2.10. Chronic Intranasal Exposure to Mn, V2O5, or Their Mixture (Mn/V2O5) Causes Astrocytes to Accumulate in the Glomerular Layer of the Olfactory Bulb
We have shown that dopaminergic neurons in the glomerular layer of the OB degenerate following chronic exposure to neurotoxic metals (Figure 4A–C). Therefore, we examined astrocyte activation as a marker of the neuroinflammatory response in the glomerular layer of the OB using glial fibrillary acidic protein (GFAP) immunostaining. Our results show no GFAP immunoreactivity in the control group; however, a dramatic accumulation of astrocytes occurred in the glomerular layer of the Mn, V2O5, and Mn/V2O5 exposure groups (Figure 10). These findings suggest that metal-induced olfactory damage is associated with astroglial hyperactivation.
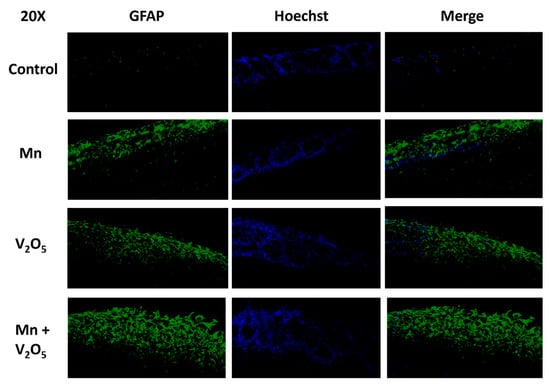
Figure 10.
Astrocyte accumulation in the glomerular layer of the OB following the intranasal administration of Mn or V2O5 alone or Mn/V2O5 mixed. The astrocytes in the OB were identified by staining with GFAP using IHC and were found to accumulate in the glomerular layer following metal exposure. This figure shows representative 20× pictures of GFAP neurons in the OB of control and treated mice. Data were derived from 3 mice per treatment group.
3. Discussion
In this study, we provide evidence for the first time that chronic exposure to low doses of Mn alone, V alone, or a Mn/V mixture via the intranasal route can induce olfactory dysfunction and adversely affect the nigrostriatal system. Observed metal exposure-induced olfactory neurotoxicity was accompanied by reduced OB weights, dramatically decreased DA and DOPAC levels, decreased levels of TH, the accumulation of astroglia in the glomerular layer, and loss of dopaminergic neurotransmission to the OB. Additionally, we observed that chronic exposure to Mn, V, or their mixture via the intranasal route induced locomotor deficits. Our results tend to show a greater severity in the Mn/V mixture group, with Mn alone inducing less severe adverse effects than V alone. Oxidative stress, as measured by the increased levels of 4HNE, increased in the STR. Interestingly, the increased expression of α-synuclein also occurred in the SN following the treatments, suggesting that chronic intranasal exposure to neurotoxic metals can upregulate the key pathological protein involved in PD. Increased Mn and V levels accumulated in the STR during Mn/V2O5 co-exposure compared to exposure to either metal alone. These exposures led to the upregulation of the pro-apoptotic kinase PKCδ in the OB. Our results show evidence of dopaminergic neuronal loss in the OB and not in the nigrostriatal system, suggesting that the olfactory system is adversely affected prior to the effects on the nigrostriatal systems, as has been suggested by Parkinsonism pathology in humans. Environmental factors are believed to contribute to the multifactorial etiology of Parkinsonism pathogenesis [55,56,57,58]. Occupational exposure to environmental neurotoxicants often occurs through inhalation and exposure to metals, which typically occurs in metal mixtures, which is a significant environmental risk factor for various neurodegenerative diseases, including PD [11,59,60,61,62]. Mn and V are both components of welding fumes generated during the welding process [43] and are present in various industries related to steel production, such as construction, car and aircraft manufacturing, and the production of temperature-resistant alloys and batteries, among others [43,63,64,65,66,67]. As the demand for high-capacity batteries grows in the electric vehicle industry, the use of V in developing such batteries grows due to the unique redox transfer property of V oxides [68,69,70]. Recent studies show that highly favorable V chemistry can be adapted to develop high-power and long-life energy storage devices [71].
Chronic exposure studies have ascertained the possible role of Mn in the etiopathogenesis of PD [72,73,74,75,76]. Similarly, Avila-Costa’s group and our lab have shown that V can damage the nigrostriatal dopaminergic systems and different components of the CNS in rodents [37,39,40,41], suggesting that V might also play a role in PD pathology and etiology. The concentrations we used in the current study are comparable to those used in previous studies [39,41,77]. Although strong evidence links welding fumes containing heavy metals, including Mn and V, to an increased risk for the development of neurological diseases, including PD [59,60,78,79,80], epidemiological studies linking metal mixtures to PD have been less certain in their conclusions. Some of these studies, for example, have reported a synergistic effect between Mn and Fe co-exposure and PD [81], even though no studies have shown an association with PD following exposures to Fe alone [82]. Correlations between metal mixture exposure and PD have also been reported for different combinations of Fe and Pb [82,83,84] and Fe and Cu [82,83,84]. Although increased levels of zinc (Zn) have been reported in the brains of PD patients [85,86], Gorell et al. [85] did not show any association between PD and Zn exposure. Co-exposure to oral doses of Mn and Pb can lead to synergistic neurological effects, as shown in rats by Chandra et al. [87], who reported significantly increased motor activity and neurotransmitter levels relative to rats exposed to only one metal. Mn/Pb co-exposed rats showed a greater deficit in learning conditioned avoidance responses compared to those exposed to either metal alone. Additionally, gestational co-exposure to Mn/Pb reduced brain weight more than either metal alone [88]. Exposure to mixtures of Pb, Mn, and arsenic (As) led to even greater changes in neurotransmitter levels in rat brains [89]. It has also been demonstrated that co-exposure to Mn and Pb increases the levels of Pb in the brain approximately three-fold compared to Pb-alone exposure, possibly due to co-exposure-related changes in the affinity of Pb-binding proteins in the brain [90,91]. In the current study, we observed increased striatal levels of Mn and V concentrations during Mn/V co-exposures relative to animals with exposures to either metal alone or in the control group. This finding supports previous research indicating that mid-brain structures critical for motor control, such as the STR and globus pallidus, are targets of Mn neurotoxicity and accumulated Mn [19]. In further support of our observation, Mn and As have also been shown to accumulate more in rat brains with Mn/As co-exposure treatment when compared to single metal exposure controls [92].
Oxidative stress has been shown to be a hallmark of apoptosis in metal neurotoxicity, particularly in relation to Parkinsonism [54,93,94,95]. Even though oxidative stress is known to be a critical factor in PD pathogenesis, with increased oxidative burden expressed in the form of increased levels of the lipid peroxidation product 4HNE in the brains of PD patients when compared to controls [96], the exact role of oxidative stress in metal-induced neurotoxicity is unknown [97]. Our study demonstrates increased levels of 4HNE in both the STR and SN of treated animals relative to controls through Western blots. Our research group and others have also shown that PKCδ is a pro-apoptotic kinase in dopaminergic neurons and a proinflammatory kinase in microglia [98,99,100,101,102]. PKCδ is oxidative stress-dependent in the CNS [98,103,104]. Our laboratory demonstrated in vitro that both V [37] and Mn [47] are neurotoxic to dopaminergic neurons via a caspase-mediated pro-apoptotic PKCδ mechanism. In this study, we observed the upregulation of PKCδ in the OB following exposure to Mn and V alone and during their co-exposure, suggesting ongoing proapoptotic and pro-inflammatory processes in OB.
Olfactory function has been shown to be compromised by metal-induced neurotoxicity in the OB. Various parameters, such as odor recognition memory experiments, odor identification, threshold detection, and odor discrimination, have been used to investigate odor deficits in metal-induced toxicity [105]. Interestingly, PD patients have been reported to exhibit deficits in odor differentiation, detection, and identification [106,107,108]. OB volumes and weight tend to decline with an associated decrease in the sense of smell [109,110,111,112,113]. It has been suggested that the early onset of olfactory dysfunction in PD patients might primarily be due to a damaged OB [112]. Pathological changes have been reported in both the OB [114] and brain regions associated with proper olfaction [115,116,117,118] during postmortem studies in PD patients. A study carried out by Silveira-Moriyama et al. [118] supports the potential of olfactory deficits as a biomarker for progressive cortical atrophy in the first stages of PD. Their study also suggests that symptoms of olfactory dysfunction and a compromised nigrostriatal dopaminergic pathway present themselves concomitantly during the pathological process of PD, as seen in our study. Furthermore, olfaction dysfunction is associated with increased risks of developing motor deficits associated with PD [119]. Therefore, understanding the changes in the OB and recognizing its related behavioral impairments are important.
Our results show that the dopaminergic neurotransmitter system in the OB is adversely affected by exposure to either Mn, V, or a Mn/V mixture. These findings are consistent with those of other studies, which have shown the depletion of DA in the OB after exposure to the classic catecholaminergic neurotoxicants methamphetamine and amphetamine [120,121]. Additionally, another study demonstrated that intranasal irrigation in mice with either ZnSO4 and surgical deafferentation or axotomy in rats decreased levels of DA, DOPAC, TH enzyme activity, and OB weights [122]. They also showed clear correlations between reductions in TH, DA and DOPAC levels and OB weights [122]. In this study, we also observed that a reduction in OB TH levels correlated with the loss of OB DA and DOPAC levels as well as OB weights for the treatment groups, with the greatest severity in the Mn/V co-exposure treatment group. These findings provide further evidence for the detrimental effects of Mn and V exposure on the dopaminergic neurotransmitter system in the OB and suggest that the OB may be particularly vulnerable to these metals, highlighting the importance of further investigating the mechanisms underlying the observed effects.
The OB is made up of five layers, which comprise the subependymal, combined mitral and granule cells, external plexiform, and glomerular layers [123,124]. The glomerular layer has an abundant expression of dopaminergic neurons [48,49]. The results from our study indicate that intranasal exposure to either Mn, V, or their mixture can decrease both TH and DA levels in the OB. DA is believed to play an important role in olfaction [51,125,126,127,128], and changes in DA levels affect olfaction. It has been observed that DA mediates the entry of olfactory information into the brain by inhibiting the transmission between the olfactory epithelium of the OB and the OB glomeruli [51]. In this regard, our results indicate that DA depletion is accompanied by a significantly decreased amount of time spent sniffing female bedding as a measure of hyposmia in all the treatment groups relative to the control. OB DA depletion following intranasal exposure to Mn, V, or their mixture may, therefore, impair pheromonal olfaction since, as previously stated, the disinhibition of olfactory glomeruli can be caused by low DA levels, leading to improperly organized olfactory processing. This supports clinical observations that PD patients experience hyposmia, typically preceding motor deficits [129].
Increased locomotor activity was reported in rats exposed to the Mn phosphate/Mn sulfate mixture [130] and Mn sulfate [131], though similar effects were not observed following exposure to Mn phosphate alone [132,133]. Another study reported decreased locomotor activity and impaired performance in a maze test of spatial memory in rats exposed to gavage doses of 6.5 or 25.9 mg Mn/kg/day for 10 weeks when compared with the controls [134]. Conversely, no changes in locomotor activity were observed in rats exposed to 11 or 22 mg Mn/kg/day for 21 days [135,136]. V exposure is known to cause severe motor deficits in humans [137,138]. Similarly, in this study, we did not observe any locomotor changes in the Mn treatment groups relative to the control. However, we observed significant locomotor deficits in the V-alone and Mn/V co-treatment groups. The olfactory and locomotor deficits tended to be most serious in the co-exposure group, while V alone induced more severe deficits than Mn alone.
The accumulation of abnormal and misfolded α-synuclein within neuronal cell bodies, axons, and synapses in the brains of sporadic PD patients and animal models of Parkinsonism is a pathological hallmark of the diseased state [139,140,141,142,143]. The potential role of α-synuclein in disease progression and development has been strongly supported by genetic evidence that α-synuclein mutations or multiplication of the α-synuclein gene can lead to familial PD [144,145]. Pathological changes attributed to α-synuclein usually occur during the early stages of the disease [115,146]. In this study, we observed an increase in protein levels of α-synuclein in the SN of the brain of metal-treated mice.
While we observed the highest effects in most measurements from the co-exposure group compared to the individual metal exposure groups, our data did not reveal strict additive or synergistic effects for co-exposure to Mn and V. This observation may be attributed to several factors, including differences in the mechanisms of action and the toxicokinetics of Mn and V. These metals may interact differently at the cellular and molecular levels, resulting in diverse responses in various endpoints. Additionally, the non-linear nature of metal toxicology could contribute to the variability of our results. Our goal was to investigate the combined effects comprehensively, and the results presented here reflect the actual outcomes observed under our experimental conditions.
Overall, our results show that V is a more potent neurotoxicant than Mn in inducing damage to OB and nigral dopaminergic systems. Mn/V mixtures induce more pronounced neurotoxic responses compared to individual metal exposure. Notably, intranasal exposure to these metals not only affects the olfactory system but also induces pathological changes in the SN, such as α-synuclein upregulation. Metal exposure also increases oxidative stress in the nigrostriatal system. The growing co-usage of Mn and V in different applications only increases the risk of being environmentally co-exposed to both metals. It is, therefore, invaluable to probe their possible neurotoxic interactive properties. Since it has previously been shown in vitro, ex vivo, and in vivo that V [37,39,40,41] and Mn [47,73] are dopaminergic neurotoxicants, the findings in this study suggest a greater neurotoxic outcome following exposures to Mn/V mixtures when compared to either metal alone. This study expands our understanding of possible metal mixture toxicology relative to individual metals in the brain. Future studies should explore the mechanistic pathways underlying the observed neurotoxic effects, particularly examining the role of other potential modulatory neurotoxic metals and their synergistic interactions. Additionally, longitudinal studies could elucidate the long-term impacts of Mn and V exposure on olfactory function and its correlation with neurodegenerative diseases, enhancing our understanding of disease progression and prevention strategies.
4. Materials and Methods
4.1. Materials
We purchased manganese chloride (MnCl2, Cat. No. 203734), vanadium pentoxide (V2O5, Cat. No. 204854), protease cocktail, and phosphatase inhibitors (Cat. No. 1861281) from Sigma (St. Louis, MO, USA). The Bradford protein assay kit was obtained from Bio-Rad Laboratories (Hercules, CA, USA). Perchloric acid (Cat. No. 244252), EDTA (Cat. No. 79884), DA (Cat. No. PHR1090), and DOPAC (Cat. No. 11569) were purchased from Millipore Sigma (Burlington, MA, USA). NaS2O5 (Cat. No. S244) was purchased from Fisher (Hampton, NH, USA). The primary antibodies used in this study were as follows: anti-mouse tyrosine hydroxylase (TH) obtained from Millipore (Billerica, MA, USA, Cat. No. MAB318); the anti-mouse glial fibrillary acidic protein (GFAP) purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA); anti-mouse 4HNE acquired from R&D Systems, Inc. (Minneapolis, MN, USA, Cat. No. MAB3249); anti-mouse α-synuclein (Cat. No. 610787) and anti-mouse native PKCδ (Cat. No. 610397) obtained from BD Biosciences (Palo Alto, CA, USA); anti-rabbit phospho-PKCδ purchased from Invitrogen (Waltham, MA, USA, Cat. No. PA5–17903); and anti-β-actin purchased from Sigma (Cat. No. A5441). The Alexa 680-conjugated anti-mouse and IRDye800-conjugated anti-rabbit secondary antibodies were purchased from Invitrogen and Rockland Inc., respectively.
4.2. Locomotor Activity
We performed open-field locomotor behavioral experiments both pre- and post-treatment using VersaMax animal activity monitors (model RXYZCM-16; AccuScan Instruments Inc., Columbus, OH, USA), as described in our previous studies [38,147,148,149,150]. The mice were placed in the middle of a transparent Plexiglas chamber for a 12-min test session covered with a ventilated Plexiglas lid. The first two minutes were for acclimatization to reduce the novelty effect, and the last 10 min were analyzed. The data are expressed as a percentage of the de-ionized water-treated control group.
4.3. Animals
A total of 48 (12 mice/group) male C57BL/6 mice aged six to eight weeks were housed in standard conditions using procedures approved and supervised by the Institutional Animal Care and Use Committee (IACUC protocols 12-5-6042, 18-309, 18-321) at Iowa State University (Ames, IA, USA). Based on the amounts used in a published study [39] and our pilot experiments, we intranasally administered MnCl2 alone (252 µg), V2O5 alone (197.91 µg), or a mixture of MnCl2 (252 µg) and V2O5 (197.91 µg) to mice three times a week for up to 4 weeks. The metals were administered intranasally in a 50 µL volume to mice using micropipettes after briefly anesthetizing the mice with isoflurane to prevent a gag reflex. Vehicle control groups received an equal volume of de-ionized water. Intranasal delivery was preferred because it took advantage of the olfactory epithelium’s incomplete blood-brain barrier (BBB) [151]. The olfactory nerves bypass the BBB, enabling chemicals to be taken up by these neurons and transported directly into the brain [151]. This route of administration is similar to the inhalation of airborne contaminants that occur during human occupational exposures. Following the treatments, mice were dissected (8 mice/group) after euthanasia via CO2 or transcardially perfused (4 mice/group) with a 4% solution of paraformaldehyde after being deeply anesthetized with a cocktail of ketamine (200 mg/kg) and xylazine (20 mg/kg) to carry out neurochemical and histological studies. Freshly dissected tissues were stored at −80 °C prior to analyses. Perfused brains were immediately post-fixed with PFA and 30% sucrose, then cryosectioned into 30-μm coronal sections using a cryostat and kept at −20 °C in a 30% sucrose–ethylene glycol solution.
4.4. Weighing Olfactory Bulbs
As previously described [122], we dissected the OB at the junction between the OB and the rest of the brain during the dissection of the mouse brains for various parts of interest. After dissection, the OB was weighed using an electronic balance according to the manufacturer’s guide and good laboratory practice prior to analysis.
4.5. Olfaction Test
The function of intact olfaction was measured by assessing the mouse’s ability to detect and sniff pheromones from female bedding. We combined the principles of the wooden block test, which leverages the ability of mice to discriminate between self- and non-self-odors [152,153] and the fact that the body odor and urine of female rodents contain pheromones that attract males [154,155]. Specifically, a small quantity (approximately the same for every animal) of bedding from a mouse cage housing pregnant females was introduced into the cages of the male mice under study during the week following the last day of treatments. The amount and location of bedding remained constant every time. During a three-minute testing session, the total amount of time that each mouse spent sniffing the foreign bedding was measured with the aid of a stopwatch. This test is an easy and effective way of assessing the olfactory capacity of the mouse to detect a foreign odor, in this instance, female pheromones.
4.6. HPLC Detection of Dopamine
To determine the levels of DA and its metabolite, DOPAC, in OB tissues, we employed a high-performance liquid chromatography method with electrochemical detection (HPLC-ECD). Using OB tissue dissected from the subgroup of mice identified in Section 4.3, the samples were prepared for analyses, as described in our previous publications [148,156]. Using an antioxidant extraction solution composed of 0.1 M of perchloric acid containing 0.05% Na2EDTA and 0.1% Na2S2O5, the neurotransmitters were extracted from the dissected OB on the day of analysis. The extracts were filtered in 0.22-μm spin tubes and diluted 1:2 in a commercial mobile phase (MDTM, Thermo-Scientific, Waltham, MA, USA). The DA and DOPAC from the extracts were separated isocratically using a reversed-phase HPLC C-18 column (ZORBAX Eclipse Plus 4.6 × 100 mm, Agilent, Santa Clara, CA, USA) with a flow rate of 0.7 mL/min. The HPLC-ECD system (ESA Inc., Bedford, MA, USA) was equipped with an automatic sampler with refrigerated temperature control (model 542; ESA Inc.). The ECD system comprises a Coulochem model 5100A with a microanalysis cell (model 5014A) and a guard cell (model 5020) (ESA Inc.). In total, 1 mg/mL standard stock solutions of catecholamines were prepared in the antioxidant extraction solution and diluted to a final working concentration of 50 pg/μL prior to injection. The data were acquired using EZStart HPLC Software version 7.2 (ESA Inc.) and analyzed in Excel 2013 (Microsoft, Redmond, WA, USA) and Prism 4.0 (GraphPad, San Diego, CA, USA), as described previously [147].
4.7. Western Blot
Lysates were prepared by homogenizing dissected SN, STR, and OB tissues from the control and treatment groups with a pestle using the modified RIPA buffer with phosphatase and protease inhibitors. The lysates from the different treatment groups were normalized to contain equal concentrations of the protein. Equal amounts of protein were loaded into each lane and separated on a 10 to 15% SDS-polyacrylamide electrophoresis gel, as described in previous studies [148,157,158]. Following gel electrophoresis, the proteins were transferred to a nitrocellulose membrane and blocked for 1 h with an Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE, USA) to prevent non-specific binding. The nitrocellulose membranes containing the transferred proteins were then incubated with the appropriate primary antibodies (1:1000), followed by incubation with either Alexa Fluor 680-conjugated anti-mouse IgG (1:10,000) or the IR800-conjugate anti-rabbit secondary antibody (1:10,000) for 1 h at room temperature (RT). The membranes were re-probed with a β-actin antibody (1:5000 dilution) to ensure the loading of equal protein in each lane. Western blot images were captured and analyzed using the Odyssey IR Imaging system (LI-COR Biosciences, Lincoln, NE, USA). We performed densitometric analysis on the appropriate bands of interest.
4.8. Immunohistological Analysis of Olfactory Bulb Sections
Immunolabeling was carried out on OB sections from the perfused brains described in Section 4.3. On the day of staining, stored sections were rinsed in PBS and blocked with 2% bovine serum albumin, 0.5% Triton X-100, and 0.05% Tween-20 in PBS for 1 h at RT. After blocking, the sections were incubated with the appropriate primary antibody overnight at RT. Following primary antibody incubation, the sections were washed with PBS several times (5× for 5 min each) and incubated with the appropriate secondary antibody for 90 min at RT. After this, the sections were washed with PBS again and incubated with 10 µg/mL Hoechst 33342 for 5 min at RT to stain the nucleus. The stained sections were carefully mounted on poly-L-lysine-coated slides with the DPX organic solvent, followed by dehydration through being kept for one min each in water, 70% ethanol, 95% ethanol, 100% ethanol, and Xylene in that order. The sections were imaged using an inverted fluorescence microscope (model TE-2000U; Nikon, Tokyo, Japan), and 2×, 20×, 30×, or 60× images were captured with a SPOT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI, USA) using MetaMorph, version 5.0 (Molecular Devices, Sunnyvale, CA, USA).
4.9. Measurement of Metal Levels
Mn and V levels in the STR were measured using ICP-MS in a similar method to that which we previously used to measure essential metal concentrations in N27 cells [37]. Striatal tissues dissected from treated mice were used for the analysis. We used ICP-MS to determine the metal concentrations at m/z 51 in each sample. To resolve the isotopes of interest from any interferences, we used the ELEMENT 1 ICP-MS device (Thermo Finnigan, San Jose, CA, USA), which is a high-resolution double-focusing instrument operated at medium resolution (m/∆m = 4000) [159]. The samples were each placed in an acid-washed 5-mL Teflon vial and digested in 150 L of high-purity nitric acid (Ultrex II, J.T. Baker). After digestion, the samples were diluted to 5 mL with 18.2 MΩ of deionized water to achieve a final acid concentration of approximately 3% nitric acid. The supernatant was then analyzed with the ICP-MS to obtain the metal concentrations. An internal standard method was used for quantification, as described previously [37,160]. The results for every sample were calculated using the integrated average background-subtracted peak intensities from 20 consecutive scans. To correct for differences in elemental ionization efficiency in the ICP-MS, a multi-element standard was used to derive normalization factors for V and Mn. Concentrations for V and Mn were subsequently calculated for each sample.
4.10. Data Analysis
Data analysis was performed using Prism 4.0 (GraphPad, San Diego, CA, USA). For all experiments, raw data were analyzed using one-way ANOVA. Statistically significant differences between the control and treatment groups or between treatment groups are indicated in figures by asterisks (* p ≤ 0.05, ** p < 0.01, and *** p < 0.001) or hashtags (# p ≤ 0.05, ## p < 0.01, and ### p < 0.001), respectively.
Author Contributions
Conceptualization, H.A.N. and A.G.K.; Methodology, H.A.N.; Software, H.A.N.; Validation, H.A.N.; Formal analysis, H.A.N.; Investigation, H.A.N.; Data curation, H.A.N.; Writing—original draft, H.A.N.; Writing—review & editing, H.A.N., A.B.-C., H.J., V.A., A.K. and A.G.K.; Visualization, H.A.N.; Supervision, A.G.K.; Project administration, A.G.K.; Funding acquisition, A.G.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health (NIH) R01 Grants ES010586, ES026892 and ES034196. This work was also supported in part by The U.S. Army Medical Research Material Command endorsed by the US Army through the Parkinson’s Research Program (PRP), Investigator-Initiated Research Award (IIRA), Program Announcement Funding Opportunity Announcement Number W81XWH-17-PRP-IIRA, under the award No. W81XWH1810106. The Isakson Endowed Chair, Georgia Research Alliance, W. Eugene and Linda Lloyd Endowed Chair to AGK are also acknowledged.
Institutional Review Board Statement
The animal study protocol was approved in October 2011 by the Institutional Animal Care and Use Committee (IACUC protocols 12-5-6042, 18-309, 18-321) at Iowa State University (Ames, IA, USA).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Travis White and Robert Houk for their assistance in the metal measurement using the ICP-MS device, which was provided by the U. S. Department of Energy, Nuclear Nonproliferation and Basic Energy Sciences Programs. We thank Gary Zenitsky for proofreading the manuscript.
Conflicts of Interest
A.G.K. and V.A. have an equity interest in PK Biosciences Corporation and Probiome Therapeutics located in Ames, Iowa. The terms of this arrangement have been reviewed and approved by Iowa State University and the University of Georgia in accordance with their conflicts-of-interest policies. Other authors declare no actual or potential competing financial interests.
Abbreviations
Vanadium pentoxide, V2O5; vanadium, V; manganese, Mn; olfactory bulb, OB; substantia nigra, SN; cadmium, Cd; copper, Cu; Parkinson’s disease, PD; striatum, STR; dopamine, DA; central nervous system, CNS; tyrosine hydroxylase, TH; immunohistochemistry, IHC; 3,4-dihydroxyphenylacetic acid, DOPAC; 4-hydroxynonenal, 4HNE; glial fibrillary acidic protein, GFAP; iron, Fe; lead, Pb; zinc, Zn; arsenic, As; blood-brain barrier, BBB; room temperature, RT; inductively coupled plasma mass spectrometry, ICP-MS.
References
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The essential metals for humans: A brief overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Carmona, A.; Roudeau, S.; Ortega, R. Molecular Mechanisms of Environmental Metal Neurotoxicity: A Focus on the Interactions of Metals with Synapse Structure and Function. Toxics 2021, 9, 198. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Hastings, L. Neurotoxic exposure and olfactory impairment. Clin. Occup. Environ. Med. 2001, 1, 547–575. [Google Scholar]
- Antunes, M.B.; Bowler, R.; Doty, R.L. San Francisco/Oakland Bay bridge welder study: Olfactory function. Neurology 2007, 69, 1278–1284. [Google Scholar] [CrossRef]
- Mascagni, P.; Consonni, D.; Bregante, G.; Chiappino, G.; Toffoletto, F. Olfactory Function in Workers Exposed to Moderate Airborne Cadmium Levels. NeuroToxicology 2003, 24, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.R. Differential impact of heavy metals on neurotoxicity during development and in aging central nervous system. Curr. Opin. Toxicol. 2021, 26, 33–38. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Willis, A.W.; Evanoff, B.A.; Lian, M.; Galarza, A.; Wegrzyn, A.; Schootman, M.; Racette, B.A. Metal Emissions and Urban Incident Parkinson Disease: A Community Health Study of Medicare Beneficiaries by Using Geographic Information Systems. Am. J. Epidemiol. 2010, 172, 1357–1363. [Google Scholar] [CrossRef]
- Mortimer, J.A.; Borenstein, A.R.; Nelson, L.M. Associations of welding and manganese exposure with Parkinson disease: Review and meta-analysis. Neurology 2012, 79, 1174–1180. [Google Scholar] [CrossRef]
- Aschner, M.; Erikson, K.M.; Hernández, E.H.; Tjalkens, R. Manganese and its role in Parkinson’s disease: From transport to neuropathology. Neuromolecular Med. 2009, 11, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, A.; Au, C.; Aschner, M. Manganese-Induced Dopaminergic Neurodegeneration: Insights into Mechanisms and Genetics Shared with Parkinson’s Disease. Chem. Rev. 2009, 109, 4862–4884. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Tan, X.; Luo, Y.; Kanda, H. Relationship between Blood Levels of Heavy Metals and Parkinson’s Disease in China. Neuroepidemiology 2009, 34, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Criswell, S.R.; Lundin, J.I.; Hobson, A.; Seixas, N.; Kotzbauer, P.T.; Evanoff, B.A.; Perlmutter, J.S.; Zhang, J.; Sheppard, L.; et al. Increased risk of parkinsonism associated with welding exposure. NeuroToxicology 2012, 33, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Neal, A.P.; Guilarte, T.R. Mechanisms of lead and manganese neurotoxicity. Toxicol. Res. 2013, 2, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Hutchens, S.; Liu, C.; Jursa, T.; Shawlot, W.; Aschner, M.; Smith, D.R.; Mukhopadhyay, S. SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J. Biol. Chem. 2019, 294, 1860–1876. [Google Scholar] [CrossRef]
- O’neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Mergler, D. Neurotoxic Effects of Low Level Exposure to Manganese in Human Populations. Environ. Res. 1999, 80, 99–102. [Google Scholar] [CrossRef]
- Pal, P.K.; Samii, A.; Calne, D.B. Manganese neurotoxicity: A review of clinical features, imaging and pathology. Neurotoxicology 1999, 20, 227–238. [Google Scholar] [PubMed]
- Chillrud, S.N.; Epstein, D.; Ross, J.M.; Sax, S.N.; Pederson, D.; Spengler, J.D.; Kinney, P.L. Elevated airborne exposures of teenagers to manganese, chromium, and iron from steel dust and New York City’s subway system. Environ. Sci. Technol. 2004, 38, 732–737. [Google Scholar] [CrossRef]
- Mattison, D.R.; Milton, B.; Krewski, D.; Levy, L.; Dorman, D.C.; Aggett, P.J.; Roels, H.A.; Andersen, M.E.; Karyakina, N.A.; Shilnikova, N.; et al. Severity scoring of manganese health effects for categorical regression. NeuroToxicology 2016, 58, 203–216. [Google Scholar] [CrossRef]
- Milton, B.; Krewski, D.; Mattison, D.R.; Karyakina, N.A.; Ramoju, S.; Shilnikova, N.; Birkett, N.; Farrell, P.J.; McGough, D. Modeling U-shaped dose-response curves for manganese using categorical regression. NeuroToxicology 2017, 58, 217–225. [Google Scholar] [CrossRef]
- Peres, T.V.; Parmalee, N.L.; Martinez-Finley, E.J.; Aschner, M. Untangling the Manganese-α-Synuclein Web. Front. Neurosci. 2016, 10, 364. [Google Scholar] [CrossRef]
- Chen, P.; Culbreth, M.; Aschner, M. Exposure, epidemiology, and mechanism of the environmental toxicant manganese. Environ. Sci. Pollut. Res. 2016, 23, 13802–13810. [Google Scholar] [CrossRef]
- Caito, S.; Aschner, M. Neurotoxicity of metals. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 169–189. [Google Scholar] [CrossRef]
- Brenneman, K.A.; Cattley, R.C.; Ali, S.F.; Dorman, D.C. Manganese-induced developmental neurotoxicity in the CD rat: Is oxidative damage a mechanism of action? Neurotoxicology 1999, 20, 477–487. [Google Scholar] [PubMed]
- Calne, D.B.; Chu, N.S.; Huang, C.C.; Lu, C.S.; Olanow, W. Manganism and idiopathic parkinsonism: Similarities and differences. Neurology 1994, 44, 1583. [Google Scholar] [CrossRef]
- Eriksson, H.; Tedroff, J.; Thuomas, K.; Aquilonius, S.-M.; Hartvig, P.; Fasth, K.-J.; Bjurling, P.; Långström, B.; Hedström, K.-G.; Heilbronn, E. Manganese induced brain lesions inMacaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch. Toxicol. 1992, 66, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Nagatomo, S.; Umehara, F.; Hanada, K.; Nobuhara, Y.; Takenaga, S.; Arimura, K.; Osame, M. Manganese intoxication during total parenteral nutrition: Report of two cases and review of the literature. J. Neurol. Sci. 1999, 162, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Bowman, A.B.; Kwakye, G.F.; Hernández, E.H.; Aschner, M. Role of manganese in neurodegenerative diseases. J. Trace Elem. Med. Biol. 2011, 25, 191–203. [Google Scholar] [CrossRef]
- Verity, M.A. Manganese neurotoxicity: A mechanistic hypothesis. Neurotoxicology 1999, 20, 489–497. [Google Scholar]
- Parenti, M.; Flauto, C.; Parati, E.; Vescovi, A.; Groppetti, A. Manganese neurotoxicity: Effects ofl-DOPA and pargyline treatments. Brain Res. 1986, 367, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, A.; Facheris, L.; Zaffaroni, A.; Malanca, G.; Parati, E. Dopamine metabolism alterations in a manganese-treated pheochromocytoma cell line (PC12). Toxicology 1991, 67, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Dorman, D.C.; Struve, M.F.; James, R.; Marshall, M.W.; Parkinson, C.U.; Wong, B.A. Influence of Particle Solubility on the Delivery of Inhaled Manganese to the Rat Brain: Manganese Sulfate and Manganese Tetroxide Pharmacokinetics Following Repeated (14-Day) Exposure. Toxicol. Appl. Pharmacol. 2001, 170, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Lyon, F. Cobalt in Hard Metals and Cobalt Sulfate, Gallium Arsenide, Indium Phosphide and Vanadium Pentoxide. IARC Monogr. Eval. Carcinog. Risks Hum. 2006, 86, 227–292. [Google Scholar]
- Kiviluoto, M.; Pyy, L.; Pakarinen, A. Serum and urinary vanadium of vanadium-exposed workers. Scand. J. Work. Environ. Health 1979, 5, 362–367. [Google Scholar] [CrossRef]
- Ngwa, H.A.; Kanthasamy, A.; Anantharam, V.; Song, C.; Witte, T.; Houk, R.; Kanthasamy, A.G. Vanadium induces dopaminergic neurotoxicity via protein kinase Cdelta dependent oxidative signaling mechanisms: Relevance to etiopathogenesis of Parkinson’s disease. Toxicol. Appl. Pharmacol. 2009, 240, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, H.A.; Kanthasamy, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.G. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. NeuroToxicology 2013, 43, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Avila-Costa, M.R.; Colín-Barenque, L.; Zepeda-Rodríguez, A.; Antuna, S.B.; Saldivar, L.; Espejel-Maya, G.; Mussali-Galante, P.; del Carmen Avila-Casado, M.; Reyes-Olivera, A.; Anaya-Martinez, V.; et al. Ependymal epithelium disruption after vanadium pentoxide inhalation: A mice experimental model. Neurosci. Lett. 2005, 381, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Avila-Costa, M.R.; Fortoul, T.I.; Niño-Cabrera, G.; Colín-Barenque, L.; Bizarro-Nevares, P.; Gutiérrez-Valdez, A.L.; Ordóñez-Librado, J.L.; Rodríguez-Lara, V.; Mussali-Galante, P.; Díaz-Bech, P.; et al. Hippocampal cell alterations induced by the inhalation of vanadium pentoxide (V2O5) promote memory deterioration. NeuroToxicology 2006, 27, 1007–1012. [Google Scholar] [CrossRef]
- Avila-Costa, M.R.; Flores, E.M.; Colin-Barenque, L.; Ordoñez, J.L.; Gutiérrez, A.L.; Niño-Cabrera, H.G.; Mussali-Galante, P.; Fortoul, T.I. Nigrostriatal Modifications After Vanadium Inhalation: An Immunocytochemical and Cytological Approach. Neurochem. Res. 2004, 29, 1365–1369. [Google Scholar] [CrossRef]
- Fatola, O.I.; Olaolorun, F.A.; Olopade, F.E.; Olopade, J.O. Trends in vanadium neurotoxicity. Brain Res. Bull. 2019, 145, 75–80. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, J.D.; Heal, M.R.; Beverland, I.J.; Howe, A.; Gibson, M.D.; Hibbs, L.R.; MacNee, W.; Donaldson, K. Soluble transition metals cause the pro-inflammatory effects of welding fumes in vitro. Toxicol. Appl. Pharmacol. 2004, 196, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Nemery, B. Metal toxicity and the respiratory tract. Eur. Respir. J. 1990, 3, 202–219. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, J.A.; Du, H. Re: Vanadium exposure-induced neurobehavioral alterations among Chinese workers Li et al. (2013). NeuroToxicology 2014, 44, 369–370. [Google Scholar] [CrossRef] [PubMed]
- Adrian, H. A mechanism for effect of vanadium on hardenability of medium carbon manganese steel. Mater. Sci. Technol. 1999, 15, 366–378. [Google Scholar] [CrossRef]
- Latchoumycandane, C.; Anantharam, V.; Kitazawa, M.; Yang, Y.; Kanthasamy, A.; Kanthasamy, A.G. Protein kinase Cδ is a key downstream mediator of manganese-induced apoptosis in dopaminergic neuronal cells. J. Pharmacol. Exp. Ther. 2005, 313, 46–55. [Google Scholar] [CrossRef]
- Davila, N.G.; Blakemore, L.J.; Trombley, P.Q. Dopamine Modulates Synaptic Transmission Between Rat Olfactory Bulb Neurons in Culture. J. Neurophysiol. 2003, 90, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Johansson, O.; Goldstein, M.; Halász, N.; Hökfelt, T.; Ljungdahl, Å. Immunohistochemical identification of two types of dopamine neuron in the rat olfactory bulb as seen by serial sectioning. J. Neurocytol. 1981, 10, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Adam, Y.; Mizrahi, A. Long-Term Imaging Reveals Dynamic Changes in the Neuronal Composition of the Glomerular Layer. J. Neurosci. 2011, 31, 7967–7973. [Google Scholar] [CrossRef]
- Hsia, A.Y.; Vincent, J.-D.; Lledo, P.-M.; Wang, Z.-J.; Hu, S.S.-J.; Bradshaw, H.B.; Sun, L.; Mackie, K.; Straiker, A.; Heinbockel, T.; et al. Dopamine Depresses Synaptic Inputs Into the Olfactory Bulb. J. Neurophysiol. 1999, 82, 1082–1085. [Google Scholar] [CrossRef]
- Tillerson, J.L.; Caudle, W.M.; Parent, J.M.; Gong, C.; Schallert, T.; Miller, G.W. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav. Brain Res. 2006, 172, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Dopaminergic Modulation of Glomerular Circuits in the Mouse Olfactory Bulb. Front. Cell. Neurosci. 2020, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, A.G.; Kitazawa, M.; Kanthasamy, A.; Anantharam, V. Role of proteolytic activation of protein kinase Cδ in oxidative stress-induced apoptosis. Antioxid. Redox Signal. 2003, 5, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Whitton, P.S. Inflammation as a causative factor in the aetiology of Parkinson’s disease. Br. J. Pharmacol. 2007, 150, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Morris, H.R. Pathogenetic insights into young-onset Parkinson disease. Nat. Rev. Neurol. 2020, 16, 245–246. [Google Scholar] [CrossRef]
- Schneider, S.A.; Hizli, B.; Alcalay, R.N. Emerging Targeted Therapeutics for Genetic Subtypes of Parkinsonism. Neurotherapeutics 2020, 17, 1378–1392. [Google Scholar] [CrossRef]
- Racette, B.A.; McGee-Minnich, L.; Moerlein, S.M.; Mink, J.W.; Videen, T.O.; Perlmutter, J.S. Welding-related parkinsonism: Clinical features, treatment, and pathophysiology. Neurology 2001, 56, 8–13. [Google Scholar] [CrossRef]
- Park, R.M.; Schulte, P.A.; Bowman, J.D.; Walker, J.T.; Bondy, S.C.; Yost, M.G.; Touchstone, J.A.; Dosemeci, M. Potential occupational risks for neurodegenerative diseases. Am. J. Ind. Med. 2005, 48, 63–77. [Google Scholar] [CrossRef]
- Guilarte, T.R. Manganese and Parkinson’s disease: A critical review and new findings. Environ. Health Perspect. 2010, 118, 1071–1080. [Google Scholar] [CrossRef]
- Živančević, K.; Baralić, K.; Jorgovanović, D.; Djordjević, A.B.; Ćurčić, M.; Miljaković, E.A.; Antonijević, B.; Bulat, Z.; Đukić-Ćosić, D. Elucidating the influence of environmentally relevant toxic metal mixture on molecular mechanisms involved in the development of neurodegenerative diseases: In silico toxicogenomic data-mining. Environ. Res. 2021, 194, 110727. [Google Scholar] [CrossRef] [PubMed]
- Bunting, R.M. Vanadium: How market developments affect the titanium industry. Strategic minerals corporation. In Proceedings of the Titanium 2006, International Titanium Association Conference, San Diego, CA, USA, 3 October 2006. [Google Scholar]
- Imtiaz, M.; Rizwan, M.S.; Xiong, S.; Li, H.; Ashraf, M.; Shahzad, S.M.; Shahzad, M.; Rizwan, M.; Tu, S. Vanadium, recent advancements and research prospects: A review. Environ. Int. 2015, 80, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Han, M.J.; Ozaki, T.; Yu, J. Electronic structure and magnetic properties of small manganese oxide clusters. J. Chem. Phys. 2005, 123, 34306. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lee, C.Y.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. AgxVOPO4: A demonstration of the dependence of battery-related electrochemical properties of silver vanadium phosphorous oxides on Ag/V ratios. J. Power Sources 2011, 196, 3325–3330. [Google Scholar] [CrossRef] [PubMed]
- Marschilok, A.C.; Kozarsky, E.S.; Tanzil, K.; Zhu, S.; Takeuchi, K.J.; Takeuchi, E.S. Electrochemical reduction of silver vanadium phosphorous oxide, Ag2VO2PO4: Silver metal deposition and associated increase in electrical conductivity. J. Power Source 2010, 195, 6839–6846. [Google Scholar] [CrossRef] [PubMed]
- Hossain, H.; Abdullah, N.; Tan, K.H.; Saidur, R.; Radzi, M.A.M.; Shafie, S. Evolution of Vanadium Redox Flow Battery in Electrode. Chem. Rec. 2023, 24, e202300092. [Google Scholar] [CrossRef] [PubMed]
- Mousavihashemi, S.; Murcia-López, S.; Rodriguez-Olguin, M.A.; Gardeniers, H.; Andreu, T.; Morante, J.R.; Arce, A.S.; Flox, C. Overcoming Voltage Losses in Vanadium Redox Flow Batteries Using WO3 as a Positive Electrode. ChemCatChem 2022, 14, e202201106. [Google Scholar] [CrossRef]
- Ye, Z.; Chen, N.; Zheng, Z.; Xiong, L.; Chen, D. Preparation of Sulfonated Poly (arylene ether)/SiO2 Composite Membranes with Enhanced Proton Selectivity for Vanadium Redox Flow Batteries. Molecules 2023, 28, 3130. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yan, J.; Hong, Y.; Yu, Z.; Chen, J.; Zheng, J. Ultrahigh-rate and ultralong-life aqueous batteries enabled by special pair-dancing proton transfer. Sci. Adv. 2023, 9, eadf4589. [Google Scholar] [CrossRef]
- Dobson, A.W.; Erikson, K.M.; Aschner, M. Manganese Neurotoxicity. Ann. N. Y. Acad. Sci. 2004, 1012, 115–128. [Google Scholar] [CrossRef]
- Lucchini, R.; Tieu, K. Manganese-Induced Parkinsonism: Evidence from Epidemiological and Experimental Studies. Biomolecules 2023, 13, 1190. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.S.; Puntel, R.L.; Aschner, M. Manganese in health and disease. In Interrelations between Essential Metal Ions and Human Diseases; Sigel, A., Sigel, H., Sigel, R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 199–227. [Google Scholar] [CrossRef]
- Pajarillo, E.; Nyarko-Danquah, I.; Digman, A.; Multani, H.K.; Kim, S.; Gaspard, P.; Aschner, M.; Lee, E. Mechanisms of manganese-induced neurotoxicity and the pursuit of neurotherapeutic strategies. Front. Pharmacol. 2022, 13, 1011947. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Gubert, P.; Boas, G.R.V.; Paes, M.M.; Santamaría, A.; Lee, E.; Tinkov, A.A.; Bowman, A.B.; Aschner, M. Manganese-induced neurodegenerative diseases and possible therapeutic approaches. Expert Rev. Neurother. 2020, 20, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.M.; Santos, M.L.; Batoréu, M.C.; Aschner, M. Prolactin is a peripheral marker of manganese neurotoxicity. Brain Res. 2011, 1382, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, W.W.; Nelson, G.; Nielsen, S.S.; Racette, B.A. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. Am. J. Ind. Med. 2019, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Criswell, S.R.M.; Nielsen, S.S.; Warden, M.N.; Flores, H.P.; Lenox-Krug, J.; Racette, S.; Sheppard, L.; Checkoway, H.; Racette, B.A. MRI Signal Intensity and Parkinsonism in Manganese-Exposed Workers. J. Occup. Environ. Med. 2019, 61, 641–645. [Google Scholar] [CrossRef]
- Criswell, S.R.; Perlmutter, J.S.; Huang, J.L.; Golchin, N.; Flores, H.P.; Hobson, A.; Aschner, M.; Erikson, K.M.; Checkoway, H.; Racette, B.A. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup. Environ. Med. 2012, 69, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Powers, K.M.; Smith-Weller, T.; Franklin, G.M.; Longstreth, W.T., Jr.; Swanson, P.D.; Checkoway, H. Parkinson’s disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 2003, 60, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Richardson, R.J. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology 1998, 50, 1346–1350. [Google Scholar] [CrossRef]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Kortsha, G.X.; Brown, G.G.; Richardson, R.J. Occupational exposure to manganese, copper, lead, iron, mercury and zinc and the risk of Parkinson’s disease. Neurotoxicology 1999, 20, 239–247. [Google Scholar]
- Gorell, J.M.; Johnson, C.C.; Rybicki, B.A.; Peterson, E.L.; Kortsha, G.X.; Brown, G.G.; Richardson, R.J. Occupational exposures to metals as risk factors for Parkinson’s disease. Neurology 1997, 48, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Jenner, P.; Schapira, A.H.V.; Marsden, C.D. The Royal Kings and Queens Parkinson’s Disease Research Group Alterations in levels of iron, ferritin, and other trace metals in neurodegenerative diseases affecting the basal ganglia. Ann. Neurol. 1992, 32, S94–S100. [Google Scholar] [CrossRef] [PubMed]
- Dexter, D.T.; Wells, F.R.; Lee, A.J.; Agid, F.; Agid, Y.; Jenner, P.; Marsden, C.D. Increased Nigral Iron Content and Alterations in Other Metal Ions Occurring in Brain in Parkinson’s Disease. J. Neurochem. 1989, 52, 1830–1836. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.V.; Ali, M.M.; Saxena, D.K.; Murthy, R.C. Behavioral and neurochemical changes in rats simultaneously exposed to manganese and lead. Arch. Toxicol. 1981, 49, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.V.; Murthy, R.; Saxena, D.; Lal, B. Effects of pre- and postnatal combined exposure to Pb and Mn on brain development in rats. Ind. Health 1983, 21, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Mejía, J.; Díaz-Barriga, F.; Calderón, J.; Ríos, C.; Jiménez-Capdeville, M. Effects of Lead–Arsenic Combined Exposure on Central Monoaminergic Systems. Neurotoxicol. Teratol. 1997, 19, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Kalia, K.; Murthy, R.; Chandra, S.V. Tissue disposition of 54Mn in lead pretreated rats. Ind. Health 1984, 22, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Baccarelli, A. Metals and Neurotoxicology. J. Nutr. 2007, 137, 2809–2813. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, V.M.; Dufour, L.; Carrizales, L.; Díaz-Barriga, F.; Jiménez-Capdeville, M.E. Effects of oral exposure to mining waste on in vivo dopamine release from rat striatum. Environ. Health Perspect. 1998, 106, 487–491. [Google Scholar]
- Kanthasamy, A.; Jin, H.; Mehrotra, S.; Mishra, R.; Kanthasamy, A.; Rana, A. Novel cell death signaling pathways in neurotoxicity models of dopaminergic degeneration: Relevance to oxidative stress and neuroinflammation in Parkinson’s disease. NeuroToxicology 2010, 31, 555–561. [Google Scholar] [CrossRef]
- Kaul, S.; Ananrharam, V.; Kanthasamy, A. Low dose oxidative insult promotes apoptosis in dopaminergic cells via caspase-3 dependent proteolytic activation of PKC delta: Relevance to environmental factors and Parkinson’s disease. Toxicol. Sci. 2003, 72, 79. [Google Scholar]
- Kitazawa, M.; Anantharam, V.; Kanthasamy, A.G. Translocation of protein kinase CS to mitochondria promotes proteolytic inactivation of Bcl-2 during MMT-induced apoptosis in dopaminergic cells. Neurotoxicology 2003, 24, 305–306. [Google Scholar]
- Yoritaka, A.; Hattori, N.; Uchida, K.; Tanaka, M.; Stadtman, E.R.; Mizuno, Y. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc. Natl. Acad. Sci. USA 1996, 93, 2696–2701. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.K. Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 2004, 10 (Suppl. 7), S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Kanthasamy, A.; Kitazawa, M.; Kaul, S.; Anantharam, V.; Kanthasamy, A.G. Methylcyclopentadienyl manganese tricarbonyl (MMT) induces apoptosis by PKC delta dependent activation of NF-kappa B in mesencephalic dopaminergic neuronal cells. Toxicol. Sci. 2003, 72, 353. [Google Scholar]
- Reyland, M.E.; Jones, D.N. Multifunctional roles of PKCδ: Opportunities for targeted therapy in human disease. Pharmacol. Ther. 2016, 165, 1–13. [Google Scholar] [CrossRef]
- Brodie, C.; Blumberg, P.M. Regulation of cell apoptosis by protein kinase c δ. Apoptosis 2003, 8, 19–27. [Google Scholar] [CrossRef]
- Steinberg, S.F. Distinctive activation mechanisms and functions for protein kinase Cδ. Biochem. J. 2004, 384, 449–459. [Google Scholar] [CrossRef]
- Gordon, R.; Singh, N.; Lawana, V.; Ghosh, A.; Harischandra, D.S.; Jin, H.; Hogan, C.; Sarkar, S.; Rokad, D.; Panicker, N.; et al. Protein kinase Cδ upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson’s disease. Neurobiol. Dis. 2016, 93, 96–114. [Google Scholar] [CrossRef]
- Anantharam, V.; Kitazawa, M.; Wagner, J.; Kaul, S.; Kanthasamy, A.G. Caspase-3-dependent proteolytic cleavage of protein kinase Cδ is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J. Neurosci. 2002, 22, 1738–1751. [Google Scholar] [CrossRef]
- Kanthasamy, A.G.; Kitazawa, M.; Kaul, S.; Yang, Y.; Lahiri, D.K.; Anantharam, V.; Kanthasamy, A. Proteolytic Activation of Proapoptotic Kinase PKCδ Is Regulated by Overexpression of Bcl-2: Implications for Oxidative Stress and Environmental Factors in Parkinson’s Disease. Ann. N. Y. Acad. Sci. 2003, 1010, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Mesholam, R.I.; Moberg, P.J.; Mahr, R.N.; Doty, R.L. Olfaction in neurodegenerative disease: A meta-analysis of olfactory functioning in Alzheimer’s and Parkinson’s diseases. Arch. Neurol. 1998, 55, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Doty, R.L.; Stern, M.B.; Pfeiffer, C.; Gollomp, S.M.; Hurtig, H.I. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1992, 55, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Tissingh, G.; Booij, J.; Bergmans, P.; Winogrodzka, A.; Janssen, A.G.; van Royen, E.A.; Wolters, E.C. Iodine-123-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) tropane SPECT in healthy controls and early-stage, drug-naive Parkinson’s disease. J. Nucl. Med. 1998, 39, 1143–1148. [Google Scholar] [PubMed]
- Ward, C.D.; Hess, W.A.; Calne, D.B. Olfactory impairment in Parkinson’s disease. Neurology 1983, 33, 943. [Google Scholar] [CrossRef] [PubMed]
- Rombaux, P.; Mouraux, A.; Bertrand, B.; Nicolas, G.; Duprez, T.; Hummel, T. Olfactory Function and Olfactory Bulb Volume in Patients with Postinfectious Olfactory Loss. Laryngoscope 2006, 116, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Yousem, D.M.; Geckle, R.J.; Bilker, W.B.; Kroger, H.; Doty, R.L. Posttraumatic smell loss: Relationship of psychophysica tests and volumes of the olfactory bulbs and tracts and the temporal lobes. Acad. Radiol. 1999, 6, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.; Choi, W.; Jeong, H.-Y.; Lee, J.; Kim, J. MR Imaging–Based Evaluations of Olfactory Bulb Atrophy in Patients with Olfactory Dysfunction. Am. J. Neuroradiol. 2017, 39, 532–537. [Google Scholar] [CrossRef]
- Wang, J.; You, H.; Liu, J.-F.; Ni, D.-F.; Zhang, Z.-X.; Guan, J. Association of Olfactory Bulb Volume and Olfactory Sulcus Depth with Olfactory Function in Patients with Parkinson Disease. Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef]
- Rombaux, P.; Huart, C.; Deggouj, N.; Duprez, T.; Hummel, T. Prognostic Value of Olfactory Bulb Volume Measurement for Recovery in Postinfectious and Posttraumatic Olfactory Loss. Otolaryngol. Neck Surg. 2012, 147, 1136–1141. [Google Scholar] [CrossRef]
- Huisman, E.; Uylings, H.B.; Hoogland, P.V. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson’s disease patients. Mov. Disord. 2008, 23, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Steur, E.N.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.J.; Stimson, E.; Henderson, J.M.; Halliday, G.M. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 2002, 125, 2431–2445. [Google Scholar] [CrossRef]
- Pearce, R.K.B.; Hawkes, C.H.; Daniel, S.E. The anterior olfactory nucleus in Parkinson’s disease. Mov. Disord. 1995, 10, 283–287. [Google Scholar] [CrossRef]
- Silveira-Moriyama, L.; Holton, J.L.; Kingsbury, A.; Ayling, H.; Petrie, A.; Sterlacci, W.; Poewe, W.; Maier, H.; Lees, A.J.; Revesz, T. Regional differences in the severity of Lewy body pathology across the olfactory cortex. Neurosci. Lett. 2009, 453, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.W.; Petrovitch, H.; Abbott, R.D.; Tanner, C.M.; Popper, J.; Masaki, K.; Launer, L.; White, L.R. Association of olfactory dysfunction with risk for future Parkinson’s disease. Ann. Neurol. 2008, 63, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Atianjoh, F.E.; Ladenheim, B.; Krasnova, I.N.; Cadet, J.L. Amphetamine causes dopamine depletion and cell death in the mouse olfactory bulb. Eur. J. Pharmacol. 2008, 589, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ladenheim, B.; Jayanthi, S.; Cadet, J.L. Methamphetamine Administration Causes Death of Dopaminergic Neurons in the Mouse Olfactory Bulb. Biol. Psychiatry 2007, 61, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.; Kawano, T.; Margolis, F.; Joh, T. Transneuronal regulation of tyrosine hydroxylase expression in olfactory bulb of mouse and rat. J. Neurosci. 1983, 3, 69–78. [Google Scholar] [CrossRef]
- Lledo, P.-M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Schröder, H.; Moser, N.; Huggenberger, S. The Mouse Olfactory System. In Neuroanatomy of the Mouse: An Introduction; Schröder, H., Moser, N., Huggenberger, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 319–331. [Google Scholar]
- Doty, R.L.; Risser, J.M. Influence of the D-2 dopamine receptor agonist quinpirole on the odor detection performance of rats before and after spiperone administration. Psychopharmacology 1989, 98, 310–315. [Google Scholar] [CrossRef]
- Duchamp-Viret, P.; Coronas, V.; Delaleu, J.-C.; Moyse, E.; Duchamp, A. Dopaminergic modulation of mitral cell activity in the frog olfactory bulb: A combined radioligand binding–electrophysiological study. Neuroscience 1997, 79, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Koster, N.; Norman, A.; Richtand, N.; Nickell, W.; Puche, A.; Pixley, S.; Shipley, M. Olfactory receptor neurons express D2 dopamine receptors. J. Comp. Neurol. 1999, 411, 666–673. [Google Scholar] [CrossRef]
- Wilson, D.; Sullivan, R. The D2 antagonist spiperone mimics the effects of olfactory deprivation on mitral/tufted cell odor response patterns. J. Neurosci. 1995, 15, 5574–5581. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, C. Olfaction in neurodegenerative disorder. Mov. Disord. 2003, 18, 364–372. [Google Scholar] [CrossRef]
- Salehi, F.; Krewski, D.; Mergler, D.; Normandin, L.; Kennedy, G.; Philippe, S.; Zayed, J. Bioaccumulation and locomotor effects of manganese phosphate/sulfate mixture in Sprague-Dawley rats following subchronic (90 days) inhalation exposure. Toxicol. Appl. Pharmacol. 2003, 191, 264–271. [Google Scholar] [CrossRef]
- Tapin, D.; Kennedy, G.; Lambert, J.; Zayed, J. Bioaccumulation and locomotor effects of manganese sulfate in Sprague–Dawley rats following subchronic (90 days) inhalation exposure. Toxicol. Appl. Pharmacol. 2006, 211, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Normandin, L.; Beaupré, L.A.; Salehi, F.; Pierre, A.S.; Kennedy, G.; Mergler, D.; Butterworth, R.F.; Philippe, S.; Zayed, J. Manganese distribution in the brain and neurobehavioral changes following inhalation exposure of rats to three chemical forms of manganese. Neurotoxicology 2004, 25, 433–441. [Google Scholar] [CrossRef]
- Normandin, L.; Carrier, G.; Gardiner, P.F.; Kennedy, G.; Hazell, A.S.; Mergler, D.; Butterworth, R.F.; Philippe, S.; Zayed, J. Assessment of bioaccumulation, neuropathology, and neurobehavior following subchronic (90 days) inhalation in Sprague–Dawley rats exposed to manganese phosphate. Toxicol. Appl. Pharmacol. 2002, 183, 135–145. [Google Scholar] [CrossRef]
- Vezér, T.; Kurunczi, A.; Náray, M.; Papp, A.; Nagymajtényi, L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am. J. Ind. Med. 2007, 50, 841–852. [Google Scholar] [CrossRef]
- Dorman, D.C.; Brenneman, K.A.; McElveen, A.M.; Lynch, S.E.; Roberts, K.C.; Wong, B.A. Olfactory Transport: A Direct Route Of Delivery Of Inhaled Manganese Phosphate To The Rat Brain. J. Toxicol. Environ. Health Part A 2002, 65, 1493–1511. [Google Scholar] [CrossRef] [PubMed]
- Dorman, D.C.; Struve, M.F.; Vitarella, D.; Byerly, F.L.; Goetz, J.; Miller, R. Neurotoxicity of manganese chloride in neonatal and adult CD rats following subchronic (21-day) high-dose oral exposure. J. Appl. Toxicol. 2000, 20, 179–187. [Google Scholar]
- Done, A.K. Of metals and chelation. Emer. Med. 1979, 11, 186–218. [Google Scholar]
- WHO. Vanadium. In Chapter 6.12; WHO Regional Office for Europe: Copenhagen, Denmark, 2000; p. 9. [Google Scholar]
- Hashimoto, M.; Masliah, E. Alpha-synuclein in Lewy Body Disease and Alzheimer’s Disease. Brain Pathol. 1999, 9, 707–720. [Google Scholar] [CrossRef]
- Takeda, A.; Hashimoto, M.; Mallory, M.; Sundsumo, M.; Hansen, L.; Sisk, A.; Masliah, E. Abnormal distribution of the non-Abeta component of Alzheimer’s disease amyloid precursor/alpha-synuclein in Lewy body disease as revealed by proteinase K and formic acid pretreatment. Lab. Investig. J. Tech. Methods Pathol. 1998, 78, 1169–1177. [Google Scholar]
- Soto, C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003, 4, 49–60. [Google Scholar] [CrossRef]
- Miraglia, F.; Betti, L.; Palego, L.; Giannaccini, G. Parkinson’s Disease and Alpha-Synucleinopathies: From Arising Pathways to Therapeutic Challenge. Cent. Nerv. Syst. Agents Med. Chem. 2015, 15, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Chesselet, M.F. In vivo alpha-synuclein overexpression in rodents: A useful model of Parkinson’s disease? Exp. Neurol. 2008, 209, 22–27. [Google Scholar] [CrossRef]
- Breydo, L.; Wu, J.W.; Uversky, V.N. α-Synuclein misfolding and Parkinson’s disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2012, 1822, 261–285. [Google Scholar] [CrossRef]
- Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain 2015, 138, 1642–1657. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Chandran, K.; Kalivendi, S.V.; Joseph, J.; Antholine, W.E.; Hillard, C.J.; Kanthasamy, A.; Kanthasamy, A.; Kalyanaraman, B. Neuroprotection by a mitochondria-targeted drug in a Parkinson’s disease model. Free. Radic. Biol. Med. 2010, 49, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Neuroprotective effect of protein kinase Cδ inhibitor rottlerin in cell culture and animal models of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2007, 322, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Langley, M.R.; Ghaisas, S.; Ay, M.; Luo, J.; Palanisamy, B.N.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Manganese exposure exacerbates progressive motor deficits and neurodegeneration in the MitoPark mouse model of Parkinson’s disease: Relevance to gene and environment interactions in metal neurotoxicity. NeuroToxicology 2018, 64, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Saminathan, H.; Kanthasamy, A.; Anantharam, V.; Jin, H.; Sondarva, G.; Harischandra, D.S.; Qian, Z.; Rana, A.; Kanthasamy, A.G. The peptidyl-prolyl isomerase Pin1 up-regulation and proapoptotic function in dopaminergic neurons: Relevance to the pathogenesis of Parkinson disease. J. Biol. Chem. 2013, 288, 21955–21971. [Google Scholar] [CrossRef] [PubMed]
- Graff, C.L.; Pollack, G.M. Nasal Drug Administration: Potential for Targeted Central Nervous System Delivery. J. Pharm. Sci. 2005, 94, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Tetreault, N.A.; Mulligan, C.K.; Hutson, C.B.; Masliah, E.; Chesselet, M. Olfactory deficits in mice overexpressing human wildtype α-synuclein. Eur. J. Neurosci. 2008, 28, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lussier, S.; Rane, A.; Choi, S.; Andersen, J. Inducible dopaminergic glutathione depletion in an alpha-synuclein transgenic mouse model results in age-related olfactory dysfunction. Neuroscience 2011, 172, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.; Donohoe, S.; Thody, A. The role of estrogen and progesterone in the control of preputial gland sex attractant odors in the female rat. Physiol. Behav. 1982, 28, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.G.; Clancy, A.N.; Macrides, F.; Agosta, W.C.; Bronson, F.H. Chemical Properties of a Female Mouse Pheromone that Stimulates Gonadotropin Secretion in Males1. Biol. Reprod. 1988, 38, 193–199. [Google Scholar] [CrossRef]
- Kitazawa, M.; Anantharam, V.; Kanthasamy, A.G. Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells. Free. Radic. Biol. Med. 2001, 31, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Kaul, S.; Kanthasamy, A.; Kitazawa, M.; Anantharam, V.; Kanthasamy, A.G. Caspase-3 dependent proteolytic activation of protein kinase Cδ mediates and regulates 1-methyl-4-phenylpyridinium (MPP+)-induced apoptotic cell death in dopaminergic cells: Relevance to oxidative stress in dopaminergic degeneration. Eur. J. Neurosci. 2003, 18, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Anantharam, V.; Sun, F.; Kanthasamy, A.G. Environmental Neurotoxic Pesticide Increases Histone Acetylation to Promote Apoptosis in Dopaminergic Neuronal Cells: Relevance to Epigenetic Mechanisms of Neurodegeneration. Mol. Pharmacol. 2010, 77, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Shum, S.C.K.; Neddersen, R.; Houk, R.S. Elemental speciation by liquid chromatography–inductively coupled plasma mass spectrometry with direct injection nebulization. Analyst 1992, 117, 577–582. [Google Scholar] [CrossRef]
- Choi, C.J.; Anantharam, V.; Saetveit, N.J.; Houk, R.S.; Kanthasamy, A.G. Normal Cellular Prion Protein Protects against Manganese-Induced Oxidative Stress and Apoptotic Cell Death. Toxicol. Sci. 2007, 98, 495–509. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).