Long-Term Epigenetic Regulation of Foxo3 Expression in Neonatal Valproate-Exposed Rat Hippocampus with Sex-Related Differences
Abstract
1. Introduction
2. Results
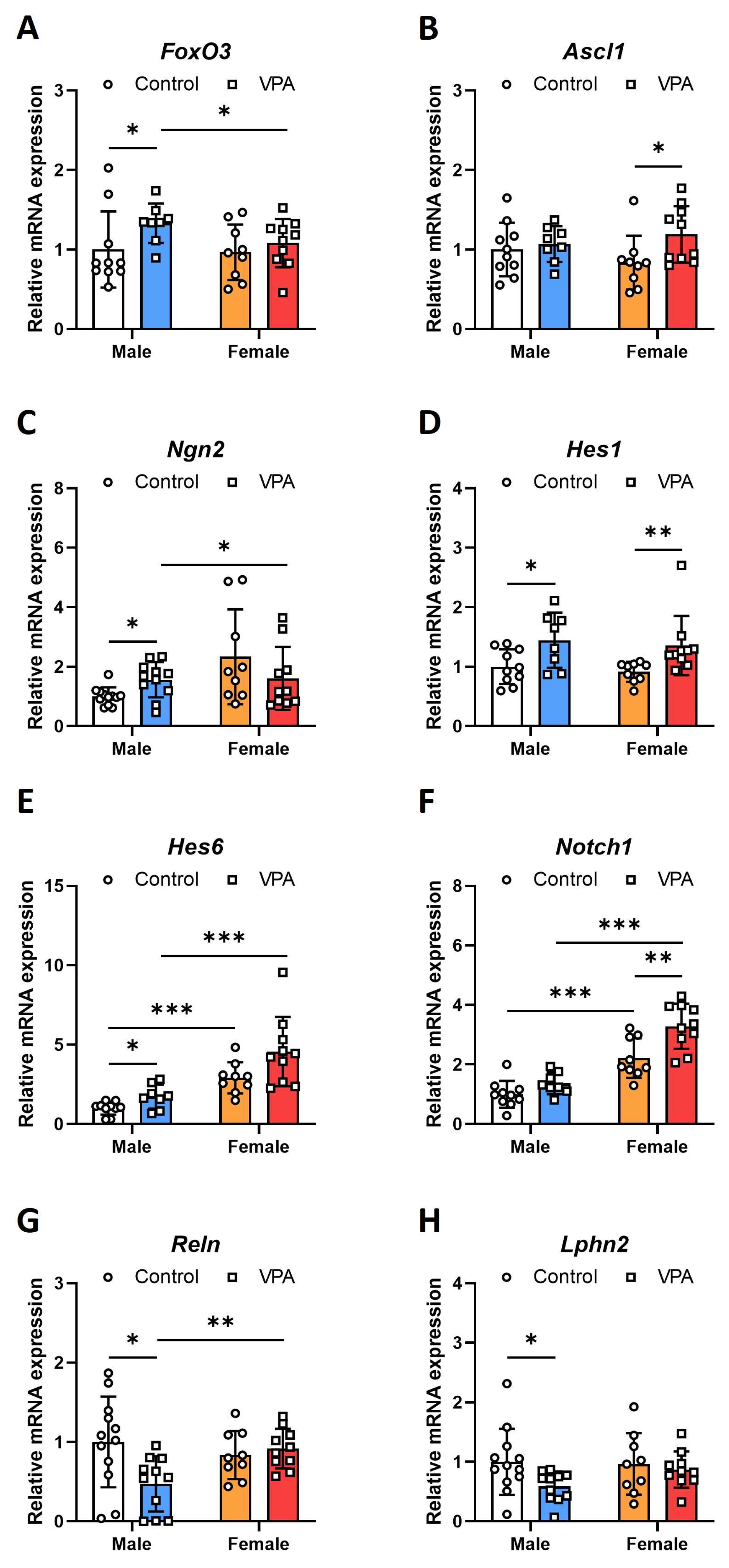
2.1. Neonatal VPA Exposure Upregulated mRNA Expression of the Foxo3 Gene in the 4-Week-Old Rat Hippocampus with Sex-Related Differences
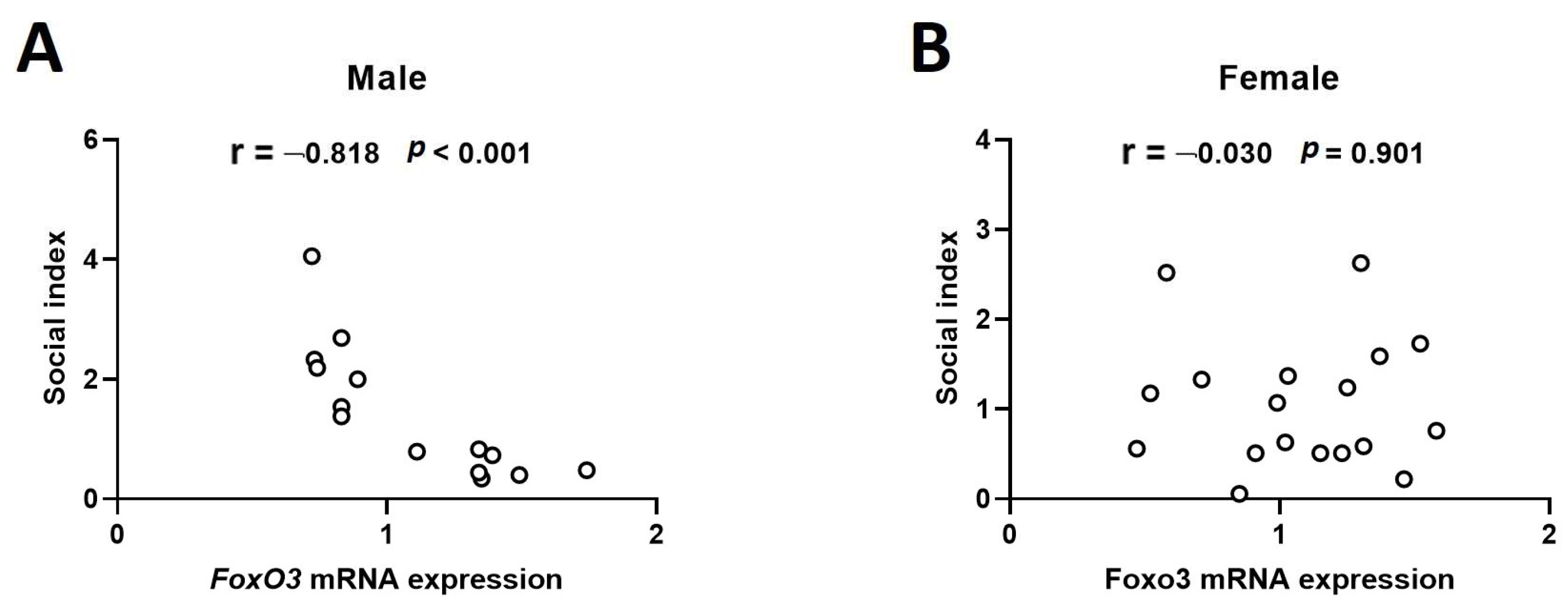
2.2. Hippocampal mRNA Expression of Foxo3 Shows a Negative Correlation with Sociality Index in the 4-Week-Old Male Rats
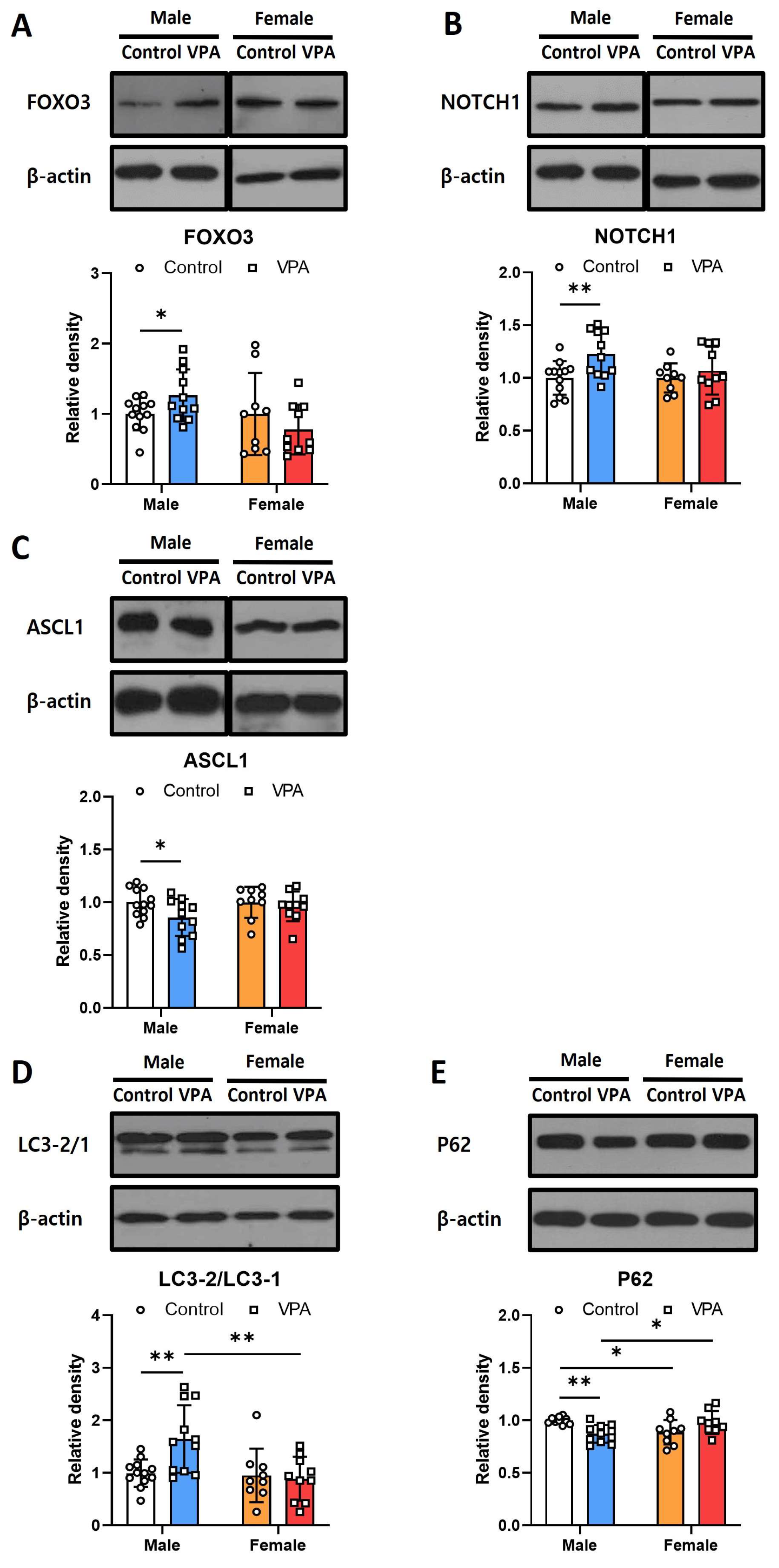
2.3. Neonatal VPA Exposure Had Long-Term Effects on Foxo3/Ascl1/Notch Protein Expression and Autophagy Signaling in the 4-Week-Old Male Hippocampus
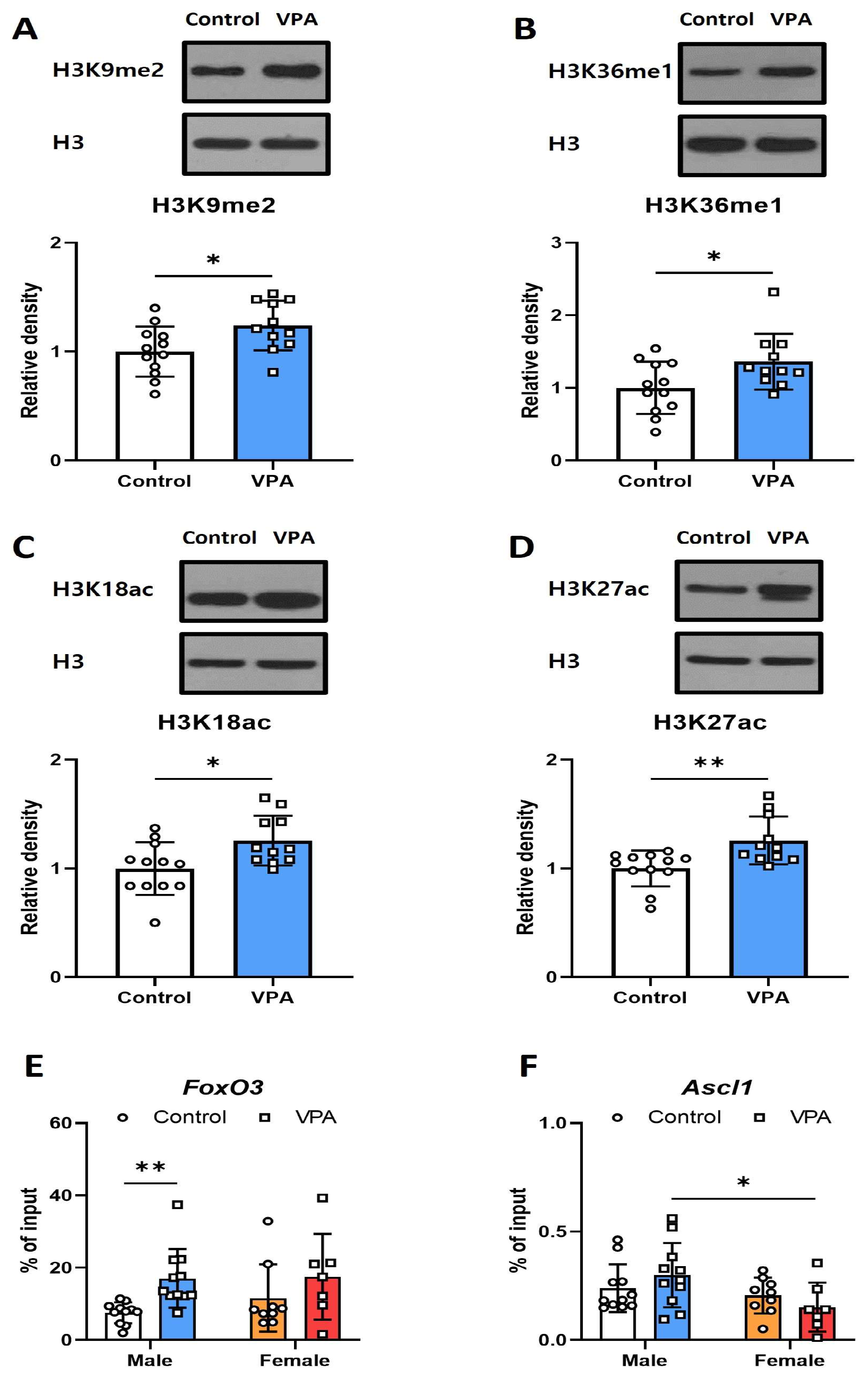
2.4. Neonatal VPA Exposure Led to Increased H3K27ac Levels at the Foxo3 Promoter in the Hippocampi of 4-Week-Old Male Rats
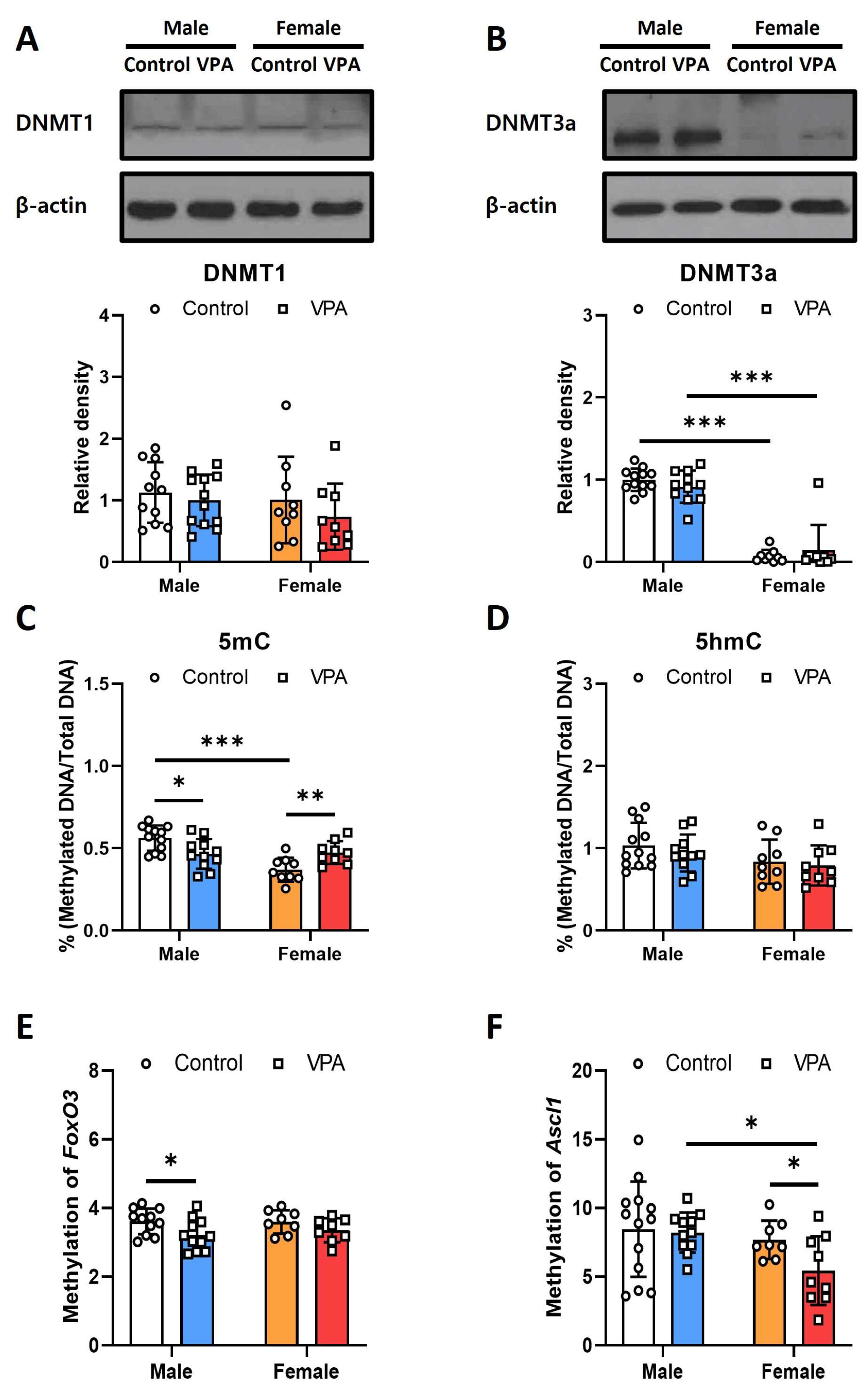
2.5. Neonatal VPA Exposure Decreased DNA Methylation at the Promoter Regions of Foxo3 and Ascl1 in the 4-Week-Old Rats’ Hippocampi with Sex-Related Differences
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Social Interaction Test
4.3. Real-Time Quantitative Polymerase Chain Reaction
4.4. Histone Extraction and Western Blot
4.5. Chromatin Immunoprecipitation-Quantitative Polymerase Chain Reaction (ChIP-qPCR)
4.6. Global DNA Methylation and Hydroxymethylation Assay
4.7. Methylation-Sensitive Restriction Enzymes (MSRE)-Based qPCR Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Lancet 2018, 392, 508. [Google Scholar] [CrossRef]
- Tordjman, S.; Somogyi, E.; Coulon, N.; Kermarrec, S.; Cohen, D.; Bronsard, G.; Bonnot, O.; Weismann-Arcache, C.; Botbol, M.; Lauth, B.; et al. Gene × Environment Interactions in Autism Spectrum Disorders: Role of Epigenetic Mechanisms. Front. Psychiatry 2014, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Chaliha, D.; Albrecht, M.; Vaccarezza, M.; Takechi, R.; Lam, V.; Al-Salami, H.; Mamo, J. A Systematic Review of the Valproic-Acid-Induced Rodent Model of Autism. Dev. Neurosci. 2020, 42, 12–48. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.S.; Gonzales, E.L.; Kim, K.C.; Yang, S.M.; Kim, J.-W.; Mabunga, D.F.; Cheong, J.H.; Han, S.-H.; Bahn, G.H.; Shin, C.Y. The Transgenerational Inheritance of Autism-like Phenotypes in Mice Exposed to Valproic Acid during Pregnancy. Sci. Rep. 2016, 6, 36250. [Google Scholar] [CrossRef] [PubMed]
- Milutinovic, S.; Detich, N.; Szyf, M. Valproate Induces Widespread Epigenetic Reprogramming Which Involves Demethylation of Specific Genes. Carcinogenesis 2007, 28, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Lunke, S.; Maxwell, S.; Khurana, I.; KN, H.; Okabe, J.; Al-Hasani, K.; El-Osta, A. Epigenetic Evidence of an Ac/Dc Axis by VPA and SAHA. Clin. Epigenet. 2021, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mony, T.J.; Lee, J.W.; Dreyfus, C.; DiCicco-Bloom, E.; Lee, H.J. Valproic Acid Exposure during Early Postnatal Gliogenesis Leads to Autistic-like Behaviors in Rats. Clin. Psychopharmacol. Neurosci. 2016, 14, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Weinstein-Fudim, L.; Ergaz, Z.; Turgeman, G.; Yanai, J.; Szyf, M.; Ornoy, A. Gender Related Changes in Gene Expression Induced by Valproic Acid in A Mouse Model of Autism and the Correction by S-Adenosyl Methionine. Does It Explain the Gender Differences in Autistic Like Behavior? Int. J. Mol. Sci. 2019, 20, 5278. [Google Scholar] [CrossRef] [PubMed]
- Dana, H.; Tahtasakal, R.; Sener, E.F. Animal Models of Autism: A Perspective from Autophagy Mechanisms. J. Transl. Genet. Genom. 2020, 4, 251–262. [Google Scholar] [CrossRef]
- Audesse, A.J.; Dhakal, S.; Hassell, L.A.; Gardell, Z.; Nemtsova, Y.; Webb, A.E. FOXO3 Directly Regulates an Autophagy Network to Functionally Regulate Proteostasis in Adult Neural Stem Cells. PLoS Genet. 2019, 15, e1008097. [Google Scholar] [CrossRef]
- Renault, V.M.; Rafalski, V.A.; Morgan, A.A.; Salih, D.A.M.; Brett, J.O.; Webb, A.E.; Villeda, S.A.; Thekkat, P.U.; Guillerey, C.; Denko, N.C.; et al. FoxO3 Regulates Neural Stem Cell Homeostasis. Cell Stem Cell 2009, 5, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.X.; Zhang, Q.L. PI3K/AKT/MTOR-Mediated Autophagy in the Development of Autism Spectrum Disorder. Brain Res. Bull. 2016, 125, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.E.; Pollina, E.A.; Vierbuchen, T.; Urbán, N.; Ucar, D.; Leeman, D.S.; Martynoga, B.; Sewak, M.; Rando, T.A.; Guillemot, F.; et al. FOXO3 Shares Common Targets with ASCL1 Genome-Wide and Inhibits ASCL1-Dependent Neurogenesis. Cell Rep. 2013, 4, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Sueda, R.; Imayoshi, I.; Harima, Y.; Kageyama, R. High Hes1 Expression and Resultant Ascl1 Suppression Regulate Quiescent vs. Active Neural Stem Cells in the Adult Mouse Brain. Genes Dev. 2019, 33, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Oproescu, A.M.; Han, S.; Schuurmans, C. New Insights Into the Intricacies of Proneural Gene Regulation in the Embryonic and Adult Cerebral Cortex. Front. Mol. Neurosci. 2021, 14, 642016. [Google Scholar] [CrossRef] [PubMed]
- Aydin, B.; Kakumanu, A.; Rossillo, M.; Moreno-Estellés, M.; Garipler, G.; Ringstad, N.; Flames, N.; Mahony, S.; Mazzoni, E.O. Proneural Factors Ascl1 and Neurog2 Contribute to Neuronal Subtype Identities by Establishing Distinct Chromatin Landscapes. Nat. Neurosci. 2019, 22, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Dennis, D.J.; Han, S.; Schuurmans, C. BHLH Transcription Factors in Neural Development, Disease, and Reprogramming. Brain Res. 2019, 1705, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Zimmer, C.; Waclaw, R.R.; Mattar, P.; Shaker, T.; Kovach, C.; Logan, C.; Campbell, K.; Guillemot, F.; Schuurmans, C. Ascl1 Participates in Cajal–Retzius Cell Development in the Neocortex. Cereb. Cortex 2011, 21, 2599–2611. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.R.; Maxeiner, S.; Sando, R.; Tsetsenis, T.; Malenka, R.C.; Südhof, T.C. Postsynaptic Adhesion GPCR Latrophilin-2 Mediates Target Recognition in Entorhinal-Hippocampal Synapse Assembly. J. Cell Biol. 2017, 216, 3831–3846. [Google Scholar] [CrossRef]
- Anstötz, M.; Huang, H.; Marchionni, I.; Haumann, I.; MacCaferri, G.; Lübke, J.H.R. Developmental Profile, Morphology, and Synaptic Connectivity of Cajal–Retzius Cells in the Postnatal Mouse Hippocampus. Cereb. Cortex 2016, 26, 855–872. [Google Scholar] [CrossRef]
- Jang, E.-H.; Lee, J.-H.; Kim, S.-A. Acute Valproate Exposure Induces Mitochondrial Biogenesis and Autophagy with FOXO3a Modulation in SH-SY5Y Cells. Cells 2021, 10, 2522. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Kim, S.A. Acute Valproate Exposure Affects Proneural Factor Expression by Increasing FOXO3 in the Hippocampus of Juvenile Mice with a Sex-Based Difference. Neurosci. Lett. 2023, 806, 137226. [Google Scholar] [CrossRef] [PubMed]
- Lützner, N.; Kalbacher, H.; Krones-Herzig, A.; Rösl, F. FOXO3 Is a Glucocorticoid Receptor Target and Regulates LKB1 and Its Own Expression Based on Cellular AMP Levels via a Positive Autoregulatory Loop. PLoS ONE 2012, 7, e42166. [Google Scholar] [CrossRef]
- Körholz, K.; Ridinger, J.; Krunic, D.; Najafi, S.; Gerloff, X.F.; Frese, K.; Meder, B.; Peterziel, H.; Vega-Rubin-de-celis, S.; Witt, O.; et al. Broad-Spectrum HDAC Inhibitors Promote Autophagy through FOXO Transcription Factors in Neuroblastoma. Cells 2021, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nada, S.; Kimura, H.; Tajima, S.; Takahashi, Y.; Kitamura, A.; Oneyama, C.; Okada, M. The MTOR Pathway Controls Cell Proliferation by Regulating the FoxO3a Transcription Factor via SGK1 Kinase. PLoS ONE 2014, 9, e88891. [Google Scholar] [CrossRef] [PubMed]
- Fueta, Y.; Sekino, Y.; Yoshida, S.; Kanda, Y.; Ueno, S. Prenatal Exposure to Valproic Acid Alters the Development of Excitability in the Postnatal Rat Hippocampus. Neurotoxicology 2018, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Juliandi, B.; Tanemura, K.; Igarashi, K.; Tominaga, T.; Furukawa, Y.; Otsuka, M.; Moriyama, N.; Ikegami, D.; Abematsu, M.; Sanosaka, T.; et al. Reduced Adult Hippocampal Neurogenesis and Cognitive Impairments Following Prenatal Treatment of the Antiepileptic Drug Valproic Acid. Stem Cell Rep. 2015, 5, 996–1009. [Google Scholar] [CrossRef]
- Daitoku, H.; Sakamaki, J.I.; Fukamizu, A. Regulation of FoxO Transcription Factors by Acetylation and Protein-Protein Interactions. Biochim. Biophys. Acta Mol. Cell Res. 2011, 1813, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Oh, H.; Santo, E.; Kim, D.-Y.; Chen, J.W.; Bronson, R.T.; Locasale, J.W.; Na, Y.; Lee, J.; Reed, S.; et al. FOXO Protects against Age-Progressive Axonal Degeneration. Aging Cell 2018, 17, e12701. [Google Scholar] [CrossRef]
- Ludikhuize, M.C.; Rodríguez Colman, M.J. Metabolic Regulation of Stem Cells and Differentiation: A Forkhead Box O Transcription Factor Perspective. Antioxid. Redox Signal 2021, 34, 1004–1024. [Google Scholar] [CrossRef]
- Sakamaki, J.I.; Daitoku, H.; Yoshimochi, K.; Miwa, M.; Fukamizu, A. Regulation of FOXO1-Mediated Transcription and Cell Proliferation by PARP-1. Biochem. Biophys. Res. Commun. 2009, 382, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.S.; Kuno, A.; Hosoda, R.; Horio, Y. Regulation of FOXOs and P53 by SIRT1 Modulators under Oxidative Stress. PLoS ONE 2013, 8, e73875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO–Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Olmos, Y.; Sánchez-Gómez, F.J.; Wild, B.; García-Quintans, N.; Cabezudo, S.; Lamas, S.; Monsalve, M. SirT1 Regulation of Antioxidant Genes Is Dependent on the Formation of a FoxO3a/PGC-1α Complex. Antioxid. Redox Signal 2013, 19, 1507. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fleming, A.; Ricketts, T.; Pavel, M.; Virgin, H.; Menzies, F.M.; Rubinsztein, D.C. Autophagy Regulates Notch Degradation and Modulates Stem Cell Development and Neurogenesis. Nat. Commun. 2016, 7, 10533. [Google Scholar] [CrossRef] [PubMed]
- Lasky, J.L.; Wu, H. Notch Signaling, Brain Development, and Human Disease. Pediatr. Res. 2005, 57, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiang, Z.; Jia, Y.; He, X.; Wang, L.; Cui, W. The Notch Signaling Pathway Inhibitor Dapt Alleviates Autism-like Behavior, Autophagy and Dendritic Spine Density Abnormalities in a Valproic Acid-Induced Animal Model of Autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 94, 109644. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; Liu, P.H.; Chen, T.C.; Lee, R.A.; New, J.; Zhang, D.; Lei, C.; Chau, A.; Tang, Y.; Cheung, E.; et al. Transcriptional Regulation of FoxO3 Gene by Glucocorticoids in Murine Myotubes. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E572–E585. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Chai, J.H.; Chang, S.Y.; Kim, S.A. Acute Valproate Exposure Induces Sex-Specific Changes in Steroid Hormone Metabolism in the Cerebral Cortex of Juvenile Mice. Neurochem. Res. 2020, 45, 2044–2051. [Google Scholar] [CrossRef]
- Kim, S.-A.; Jang, E.-H.; Lee, J.; Cho, S.-H. Neonatal Exposure to Valproate Induces Long-Term Alterations in Steroid Hormone Levels in the Brain Cortex of Prepubertal Rats. Int. J. Mol. Sci. 2023, 24, 6681. [Google Scholar] [CrossRef]
- Juliandi, B.; Abematsu, M.; Nakashima, K. Epigenetic Regulation in Neural Stem Cell Differentiation. Dev. Growth Differ. 2010, 52, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, Y.; Campbell, S.L.; Le, T.; Li, E.; Sweatt, J.D.; Silva, A.J.; Fan, G. Dnmt1 and Dnmt3a Maintain DNA Methylation and Regulate Synaptic Function in Adult Forebrain Neurons. Nat. Neurosci. 2010, 13, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.J.; Gonzales, E.L.; Mabunga, D.F.N.; Valencia, S.T.; Kim, D.G.; Kim, Y.; Adil, K.J.L.; Shin, D.; Park, D.; Shin, C.Y. Sex-specific Behavioral Features of Rodent Models of Autism Spectrum Disorder. Exp. Neurobiol. 2018, 27, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Bódi, V.; Májer, T.; Kelemen, V.; Világi, I.; Szűcs, A.; Varró, P. Alterations of the Hippocampal Networks in Valproic Acid-Induced Rat Autism Model. Front. Neural Circuits 2022, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Seung, H.; Kwon, K.J.; Ko, M.J.; Lee, E.J.; Oh, H.A.; Choi, C.S.; Kim, K.C.; Gonzales, E.L.; You, J.S.; et al. Subchronic Treatment of Donepezil Rescues Impaired Social, Hyperactive, and Stereotypic Behavior in Valproic Acid-Induced Animal Model of Autism. PLoS ONE 2014, 9, e104927. [Google Scholar] [CrossRef]
- Niknazar, S.; Nahavandi, A.; Peyvandi, A.A.; Peyvandi, H.; Akhtari, A.S.; Karimi, M. Comparison of the Adulthood Chronic Stress Effect on Hippocampal BDNF Signaling in Male and Female Rats. Mol. Neurobiol. 2015, 53, 4026–4033. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.-H.; Kim, S.-A. Long-Term Epigenetic Regulation of Foxo3 Expression in Neonatal Valproate-Exposed Rat Hippocampus with Sex-Related Differences. Int. J. Mol. Sci. 2024, 25, 5287. https://doi.org/10.3390/ijms25105287
Jang E-H, Kim S-A. Long-Term Epigenetic Regulation of Foxo3 Expression in Neonatal Valproate-Exposed Rat Hippocampus with Sex-Related Differences. International Journal of Molecular Sciences. 2024; 25(10):5287. https://doi.org/10.3390/ijms25105287
Chicago/Turabian StyleJang, Eun-Hye, and Soon-Ae Kim. 2024. "Long-Term Epigenetic Regulation of Foxo3 Expression in Neonatal Valproate-Exposed Rat Hippocampus with Sex-Related Differences" International Journal of Molecular Sciences 25, no. 10: 5287. https://doi.org/10.3390/ijms25105287
APA StyleJang, E.-H., & Kim, S.-A. (2024). Long-Term Epigenetic Regulation of Foxo3 Expression in Neonatal Valproate-Exposed Rat Hippocampus with Sex-Related Differences. International Journal of Molecular Sciences, 25(10), 5287. https://doi.org/10.3390/ijms25105287





