Regulation of Glutathione S-Transferase Omega 1 Mediated by Cysteine Residues Sensing the Redox Environment
Abstract
1. Introduction
2. Results
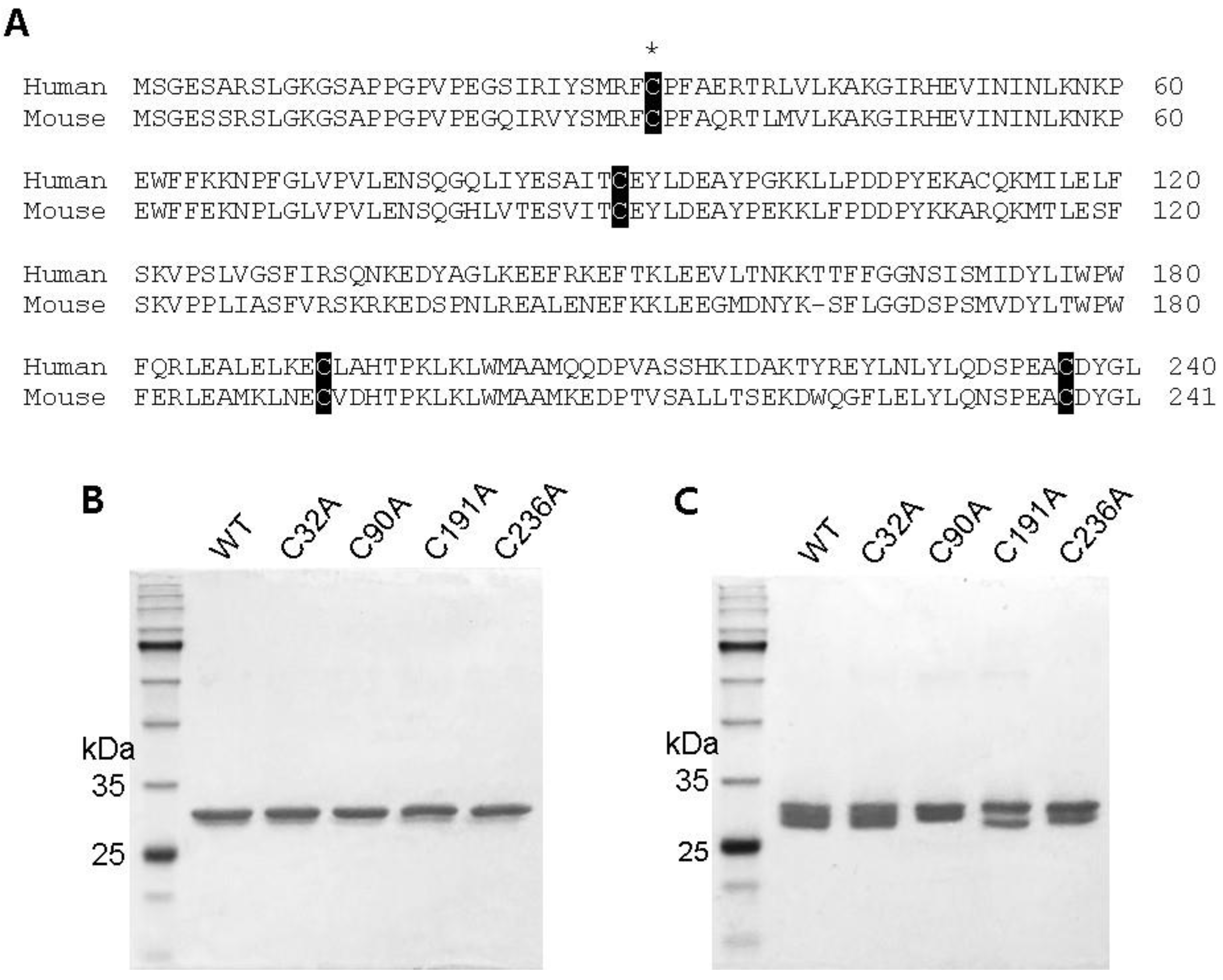
2.1. Preparation of Cysteine Mutant GstO1 and Characterization
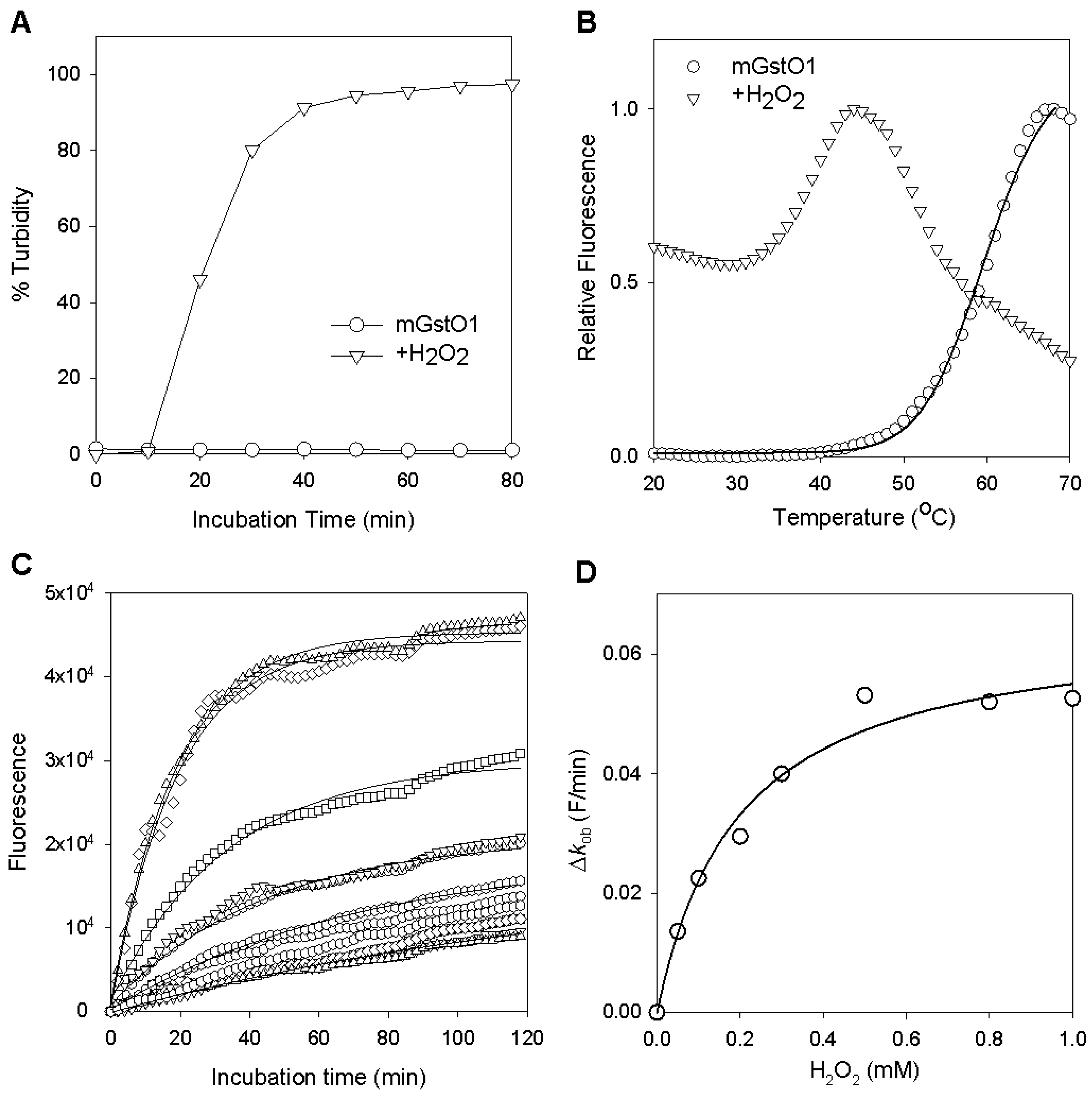
2.2. Changes in GstO1 Stability Dependent on the Redox Environment
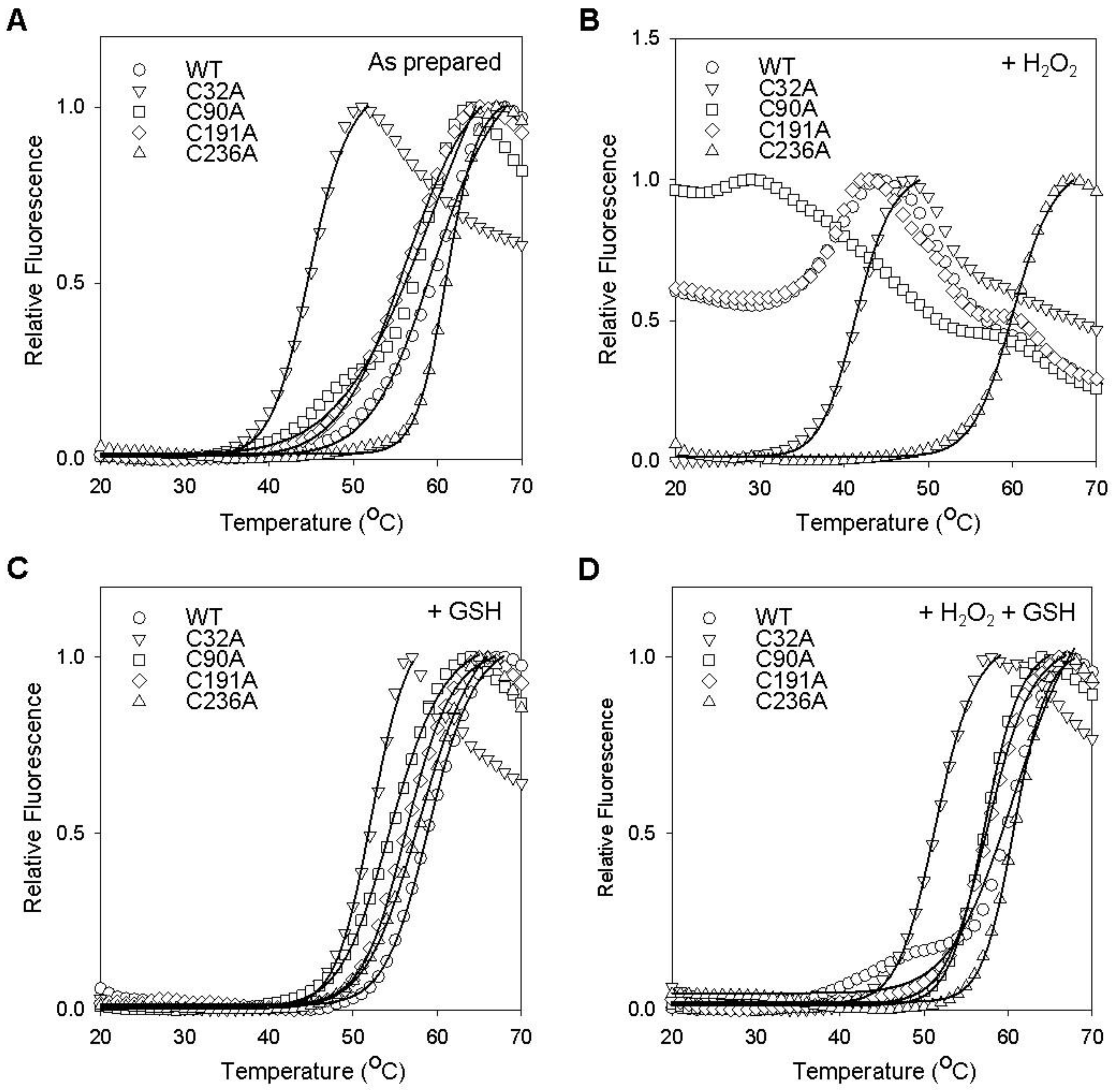
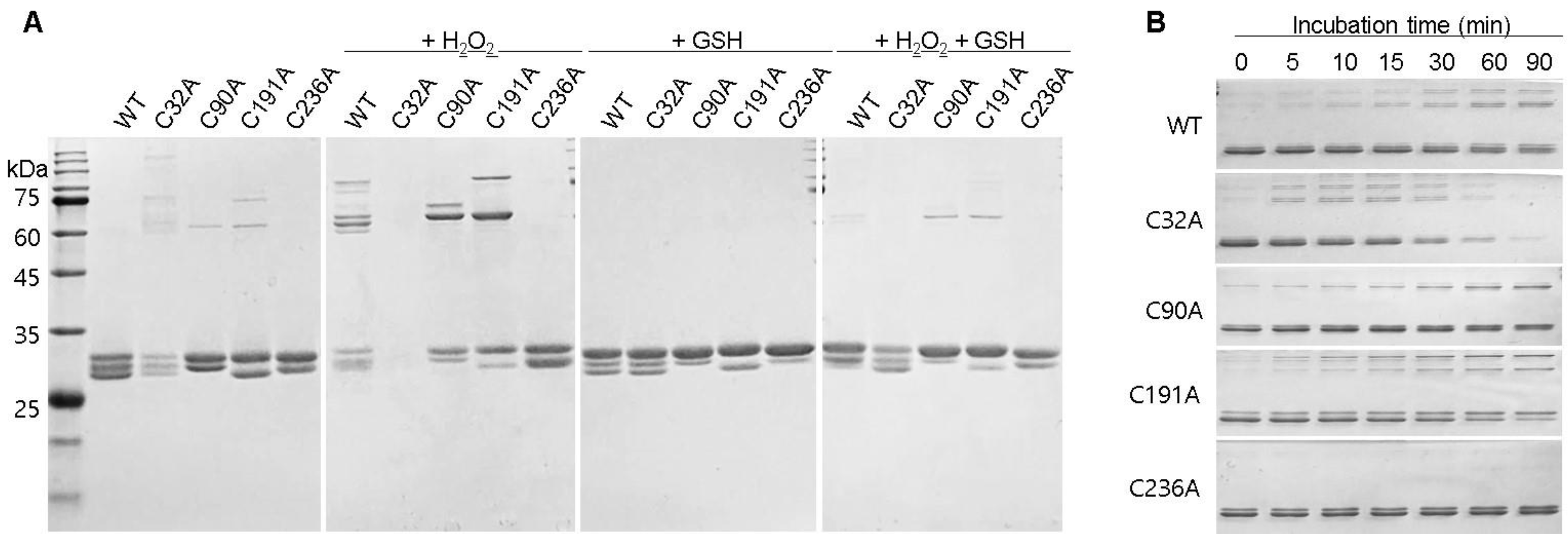
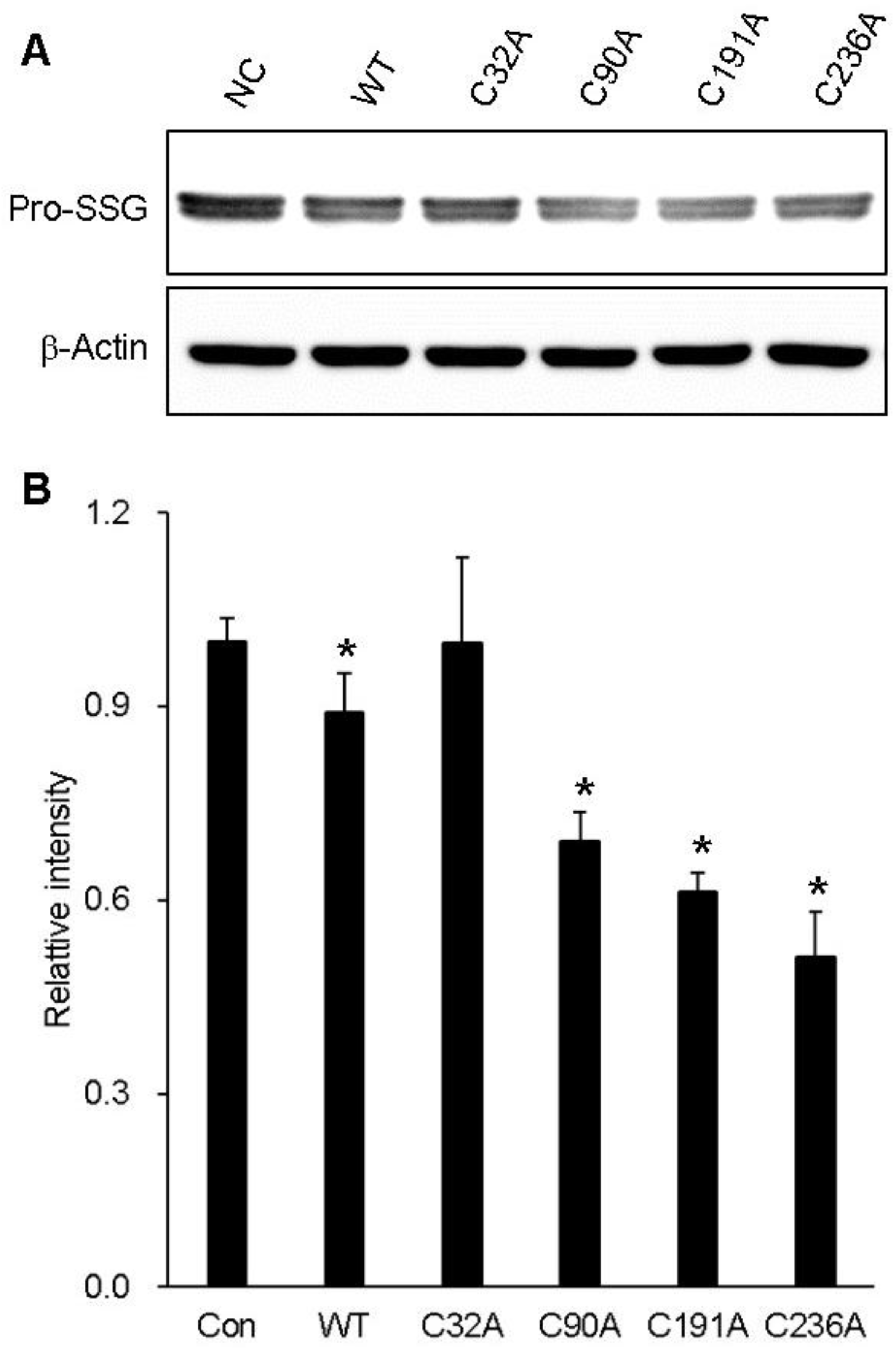
2.3. Cysteine Residues Critical for the Redox-Dependent Stability of GstO1
2.4. Redox-Dependent Changes in the Conformation of GstO1
2.5. Cysteine Residues Critical for the Deglutathionylation Activity of GstO1
3. Discussion
4. Materials and Methods
4.1. Protein Preparation
4.2. Enzyme Activity Assays
4.3. Thermal Stability Assays
4.4. Western Blot Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Board, P.G. The omega-class glutathione transferases: Structure, function, and genetics. Drug Metab. Rev. 2011, 43, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Marcos, R.; Creus, A.; Coggan, M.; Oakley, A.J.; Board, P.G. Polymorphism of glutathione transferase Omega 1 in a population exposed to a high environmental arsenic burden. Pharmacogenet. Genom. 2008, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Coggan, M.; Cappello, J.; Zhou, H.; Oakley, A.J.; Anders, M.W. S-(4-Nitrophenacyl)glutathione is a specific substrate for glutathione transferase omega 1-1. Anal. Biochem. 2008, 374, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Anders, M.W. Glutathione transferase omega 1 catalyzes the reduction of S-(phenacyl)glutathiones to acetophenones. Chem. Res. Toxicol. 2007, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Board, P.G.; Coggan, M.; Chelvanayagam, G.; Easteal, S.; Jermiin, L.S.; Schulte, G.K.; Danley, D.E.; Hoth, L.R.; Griffor, M.C.; Kamath, A.V.; et al. Identification, characterization, and crystal structure of the Omega class glutathione transferases. J. Biol. Chem. 2000, 275, 24798–24806. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Board, P.G. A role for glutathione transferase Omega 1 (GSTO1-1) in the glutathionylation cycle. J. Biol. Chem. 2013, 288, 25769–25779. [Google Scholar] [CrossRef]
- Board, P.G.; Menon, D. Structure, function and disease relevance of Omega-class glutathione transferases. Arch. Toxicol. 2016, 90, 1049–1067. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, K.; Samanta, S.; Kyani, A.; Yang, S.H.; Tamura, S.; Ziemke, E.; Stuckey, J.A.; Li, S.; Chinnaswamy, K.; Otake, H.; et al. Mechanistic evaluation and transcriptional signature of a glutathione S-transferase omega 1 inhibitor. Nat. Commun. 2016, 7, 13084. [Google Scholar] [CrossRef]
- Adam, G.C.; Sorensen, E.J.; Cravatt, B.F. Proteomic profiling of mechanistically distinct enzyme classes using a common chemotype. Nat. Biotechnol. 2002, 20, 805–809. [Google Scholar] [CrossRef]
- Djukic, T.; Simic, T.; Pljesa-Ercegovac, M.; Matic, M.; Suvakov, S.; Coric, V.; Dragicevic, D.; Savic-Radojevic, A. Upregulated glutathione transferase omega-1 correlates with progression of urinary bladder carcinoma. Redox Rep. 2017, 22, 486–492. [Google Scholar] [CrossRef]
- Menon, D.; Coll, R.; O’Neill, L.A.J.; Board, P.G. GSTO1-1 modulates metabolism in macrophages activated through the LPS and TLR4 pathway. J. Cell Sci. 2015, 128, 1982–1990. [Google Scholar] [CrossRef] [PubMed]
- Menon, D.; Innes, A.; Oakley, A.J.; Dahlstrom, J.E.; Jensen, L.M.; Brustle, A.; Tummala, P.; Rooke, M.; Casarotto, M.G.; Baell, J.B.; et al. GSTO1-1 plays a pro-inflammatory role in models of inflammation, colitis and obesity. Sci. Rep. 2017, 7, 17832. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.; Mashaal, A.; Go, S.; Kim, J. Inhibition of glutathione S-transferase omega 1-catalyzed protein deglutathionylation suppresses adipocyte differentiation. BMB Rep. 2023, 56, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Djukic, T.; Stevanovic, G.; Coric, V.; Bukumiric, Z.; Pljesa-Ercegovac, M.; Matic, M.; Jerotic, D.; Todorovic, N.; Asanin, M.; Ercegovac, M.; et al. GSTO1, GSTO2 and ACE2 Polymorphisms Modify Susceptibility to Developing COVID-19. J. Pers. Med. 2022, 12, 458. [Google Scholar] [CrossRef]
- Radic, T.M.; Coric, V.M.; Pljesa-Ercegovac, M.S.; Basta-Jovanovic, G.M.; Radojevic-Skodric, S.M.; Dragicevic, D.P.; Matic, M.G.; Bogdanovic, L.M.; Dzamic, Z.M.; Simic, T.P.; et al. Concomitance of Polymorphisms in Glutathione Transferase Omega Genes Is Associated with Risk of Clear Cell Renal Cell Carcinoma. Tohoku J. Exp. Med. 2018, 246, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Hollman, A.L.; Tchounwou, P.B.; Huang, H.C. The Association between Gene-Environment Interactions and Diseases Involving the Human GST Superfamily with SNP Variants. Int. J. Environ. Res. Public Health 2016, 13, 379. [Google Scholar] [CrossRef]
- Piaggi, S.; Marchi, E.; Carnicelli, V.; Zucchi, R.; Griese, M.; Hector, A.; Sorio, C.; Pompella, A.; Corti, A. Airways glutathione S-transferase omega-1 and its A140D polymorphism are associated with severity of inflammation and respiratory dysfunction in cystic fibrosis. J. Cyst. Fibros. 2021, 20, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, D.; Potgieter, M.; Ambele, M.A.; Adam, L.; Durandt, C.; Pepper, M.S. The Role of Reactive Oxygen Species in Adipogenic Differentiation. Adv. Exp. Med. Biol. 2018, 1083, 125–144. [Google Scholar] [PubMed]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
- Nobusue, H.; Onishi, N.; Shimizu, T.; Sugihara, E.; Oki, Y.; Sumikawa, Y.; Chiyoda, T.; Akashi, K.; Saya, H.; Kano, K. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat. Commun. 2014, 5, 3368. [Google Scholar] [CrossRef]
- Chen, L.; Hu, H.; Qiu, W.; Shi, K.; Kassem, M. Actin depolymerization enhances adipogenic differentiation in human stromal stem cells. Stem Cell Res. 2018, 29, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Watanabe, K.; Fujioka, D.; Nakamura, K.; Nakamura, T.; Uematsu, M.; Bachschmid, M.M.; Matsui, R.; Kugiyama, K. Protein S-glutathionylation stimulate adipogenesis by stabilizing C/EBPbeta in 3T3L1 cells. FASEB J. 2020, 34, 5827–5837. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, Y.J.; Choi, H.; Ko, E.H.; Kim, J.W. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J. Biol. Chem. 2009, 284, 10601–10609. [Google Scholar] [CrossRef]
- Jeong, J.; Park, J.; Lee, D.Y.; Kim, J. C-terminal truncation of a bovine B(12) trafficking chaperone enhances the sensitivity of the glutathione-regulated thermostability. BMB Rep. 2013, 46, 169–174. [Google Scholar] [CrossRef]

| Thiol Transferase (mU/mg) | Deglutathionylation (mU/mg) | DHA Reductase (mU/mg) | Glutathione S-Transferase (mU/mg) | 4-NPG Reductase (U/mg) | |
|---|---|---|---|---|---|
| WT | 116 ± 11 | 3.0 ± 0.1 | 132 ± 31 | ND | 9.1 ± 0.2 |
| C32A | ND | ND | ND | 40 ± 4 | ND |
| C90A | 101 ± 18 | 3.1 ± 0.1 | 133 ± 31 | ND | 10.4 ± 0.6 |
| C191A | 111 ± 13 | 3.1 ± 0.1 | 146 ± 10 | ND | 10.6 ± 0.8 |
| C236A | 150 ± 22 | 3.5 ± 0.1 | 166 ± 19 | ND | 6.1 ± 0.6 |
| Control | H2O2 | GSH | H2O2 + GSH | |
|---|---|---|---|---|
| WT | 60.0 ± 0.3 | <20 * | 59.0 ± 0.2 | 61.0 ± 0.8 |
| C32A | 45.0 ± 0.3 | 41.0 ± 0.8 | 52.0 ± 0.5 | 51.0 ± 0.8 |
| C90A | 57.0 ± 1.5 | <20 * | 54.0 ± 0.7 | 57.0 ± 0.4 |
| C191A | 56.0 ± 0.7 | <20 * | 56.0 ± 0.6 | 58.0 ± 0.7 |
| C236A | 61.0 ± 0.2 | 60.0 ± 0.4 | 58.0 ± 0.4 | 61.0 ± 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Choi, J.; Iram, S.; Kim, J. Regulation of Glutathione S-Transferase Omega 1 Mediated by Cysteine Residues Sensing the Redox Environment. Int. J. Mol. Sci. 2024, 25, 5279. https://doi.org/10.3390/ijms25105279
Kim K, Choi J, Iram S, Kim J. Regulation of Glutathione S-Transferase Omega 1 Mediated by Cysteine Residues Sensing the Redox Environment. International Journal of Molecular Sciences. 2024; 25(10):5279. https://doi.org/10.3390/ijms25105279
Chicago/Turabian StyleKim, Kwonyoung, Jeongin Choi, Sana Iram, and Jihoe Kim. 2024. "Regulation of Glutathione S-Transferase Omega 1 Mediated by Cysteine Residues Sensing the Redox Environment" International Journal of Molecular Sciences 25, no. 10: 5279. https://doi.org/10.3390/ijms25105279
APA StyleKim, K., Choi, J., Iram, S., & Kim, J. (2024). Regulation of Glutathione S-Transferase Omega 1 Mediated by Cysteine Residues Sensing the Redox Environment. International Journal of Molecular Sciences, 25(10), 5279. https://doi.org/10.3390/ijms25105279






