Porcine Placenta Peptide Inhibits UVB-Induced Skin Wrinkle Formation and Dehydration: Insights into MAPK Signaling Pathways from In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Results
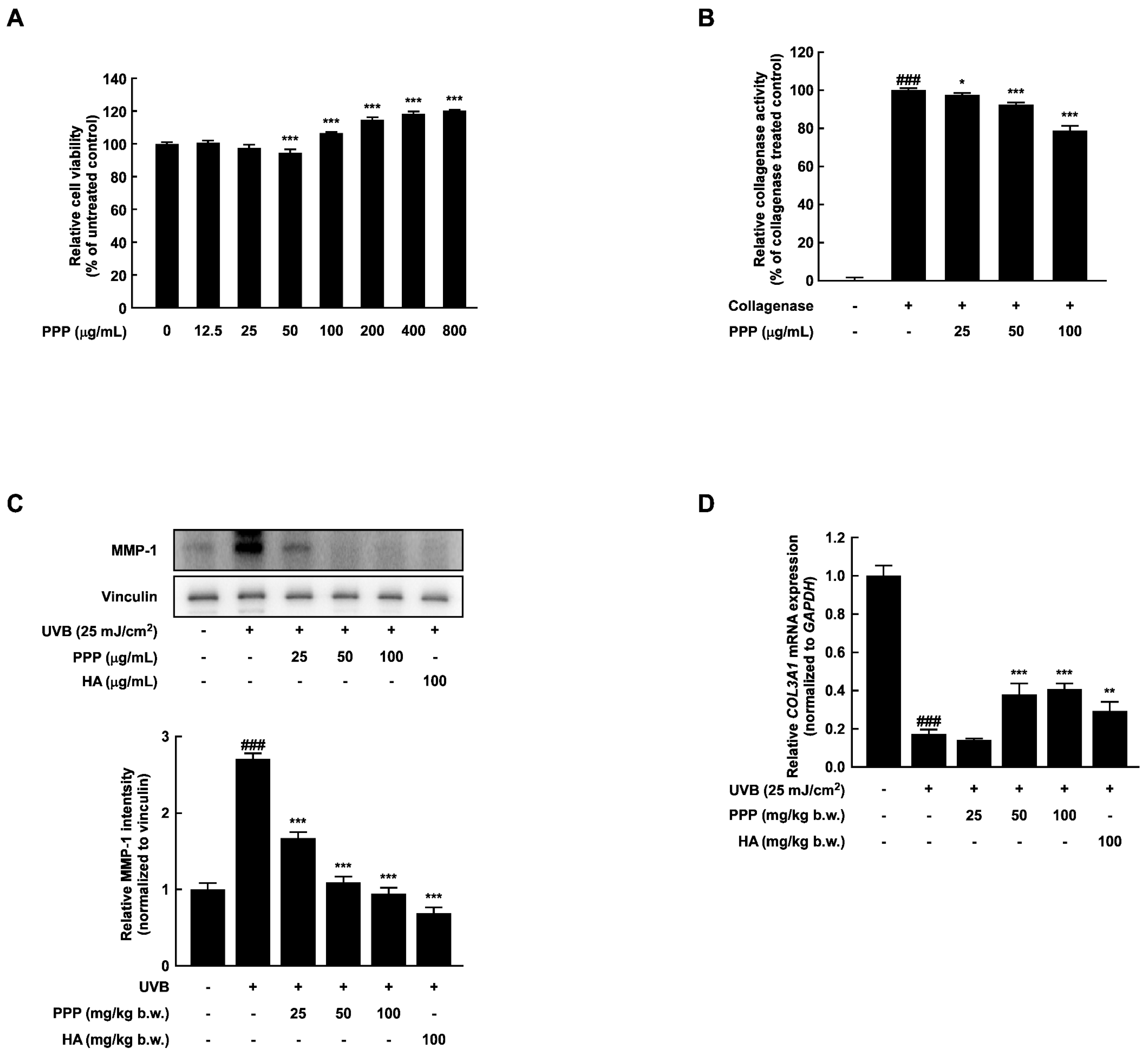
2.1. PPP Inhibits UVB-Induced Skin Wrinkle Formation by Suppressing MMPs Expression
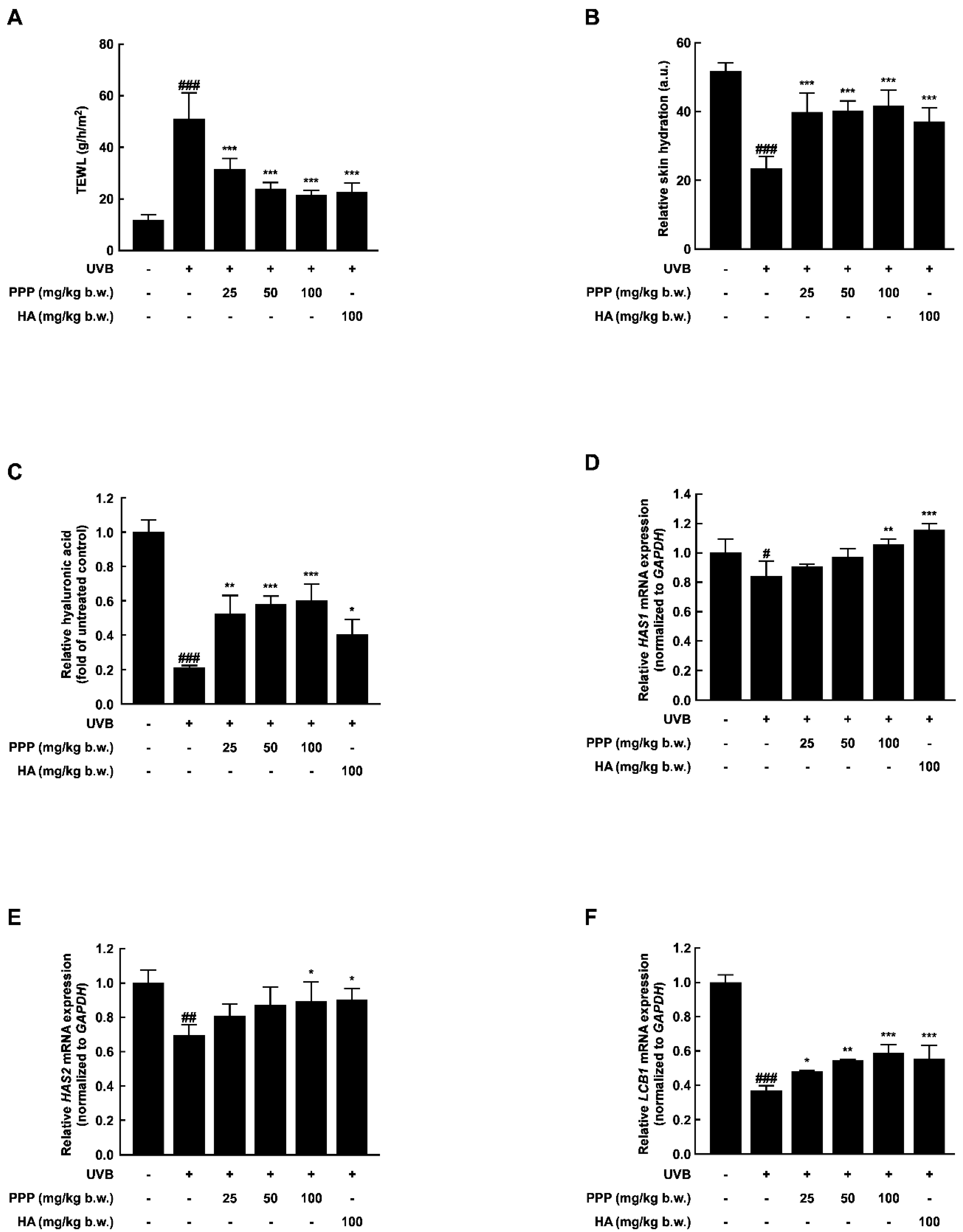
2.2. PPP Prevents UVB-Induced Skin Dehydration by Elevating HAS Transcription Level
2.3. PPP Reduces UVB-Induced Skin Inflammation by Increasing the Antioxidant Enzyme Transcriptional Level
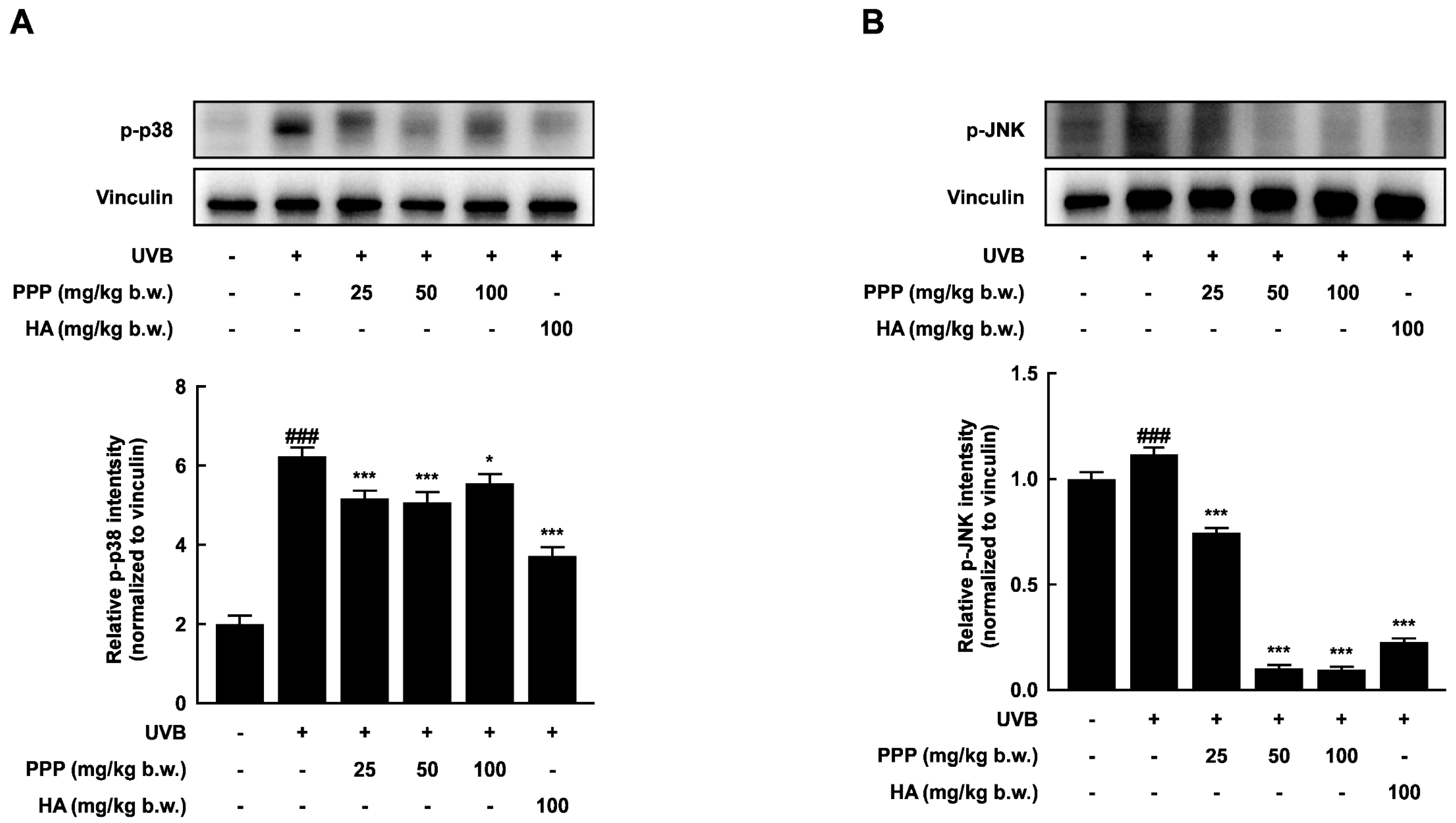
2.4. PPP Suppresses UVB-Induced Skin Damage through p38 MAPK Kinase and JNK Signaling Pathways
2.5. PPP Enhances Expression of Collagen in HDF Cells Irradiated with UVB
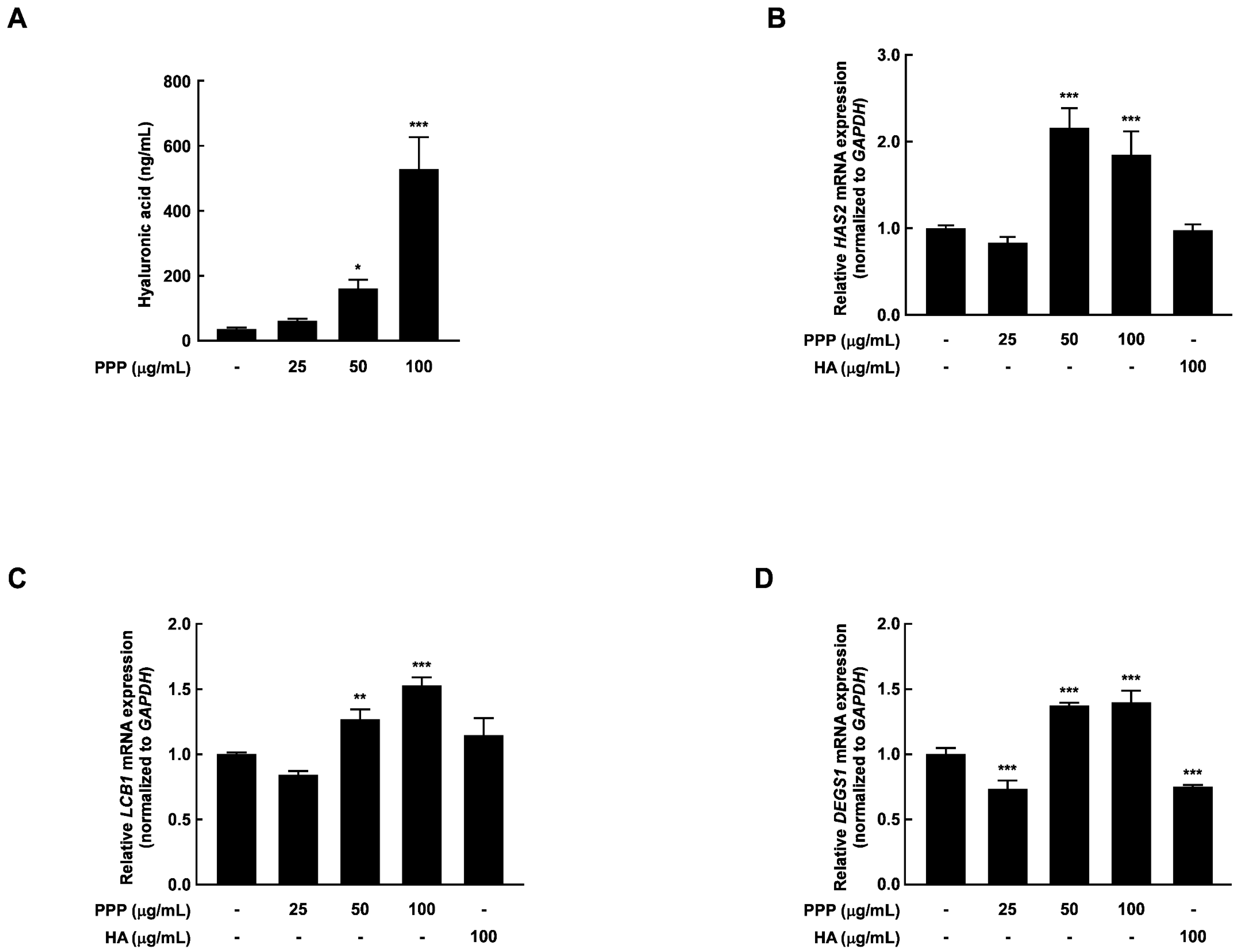
2.6. PPP Increases Hyaluronic Acid Contents by Upregulating Transcriptional Factors Associated with Skin Hydration in HDF Cells
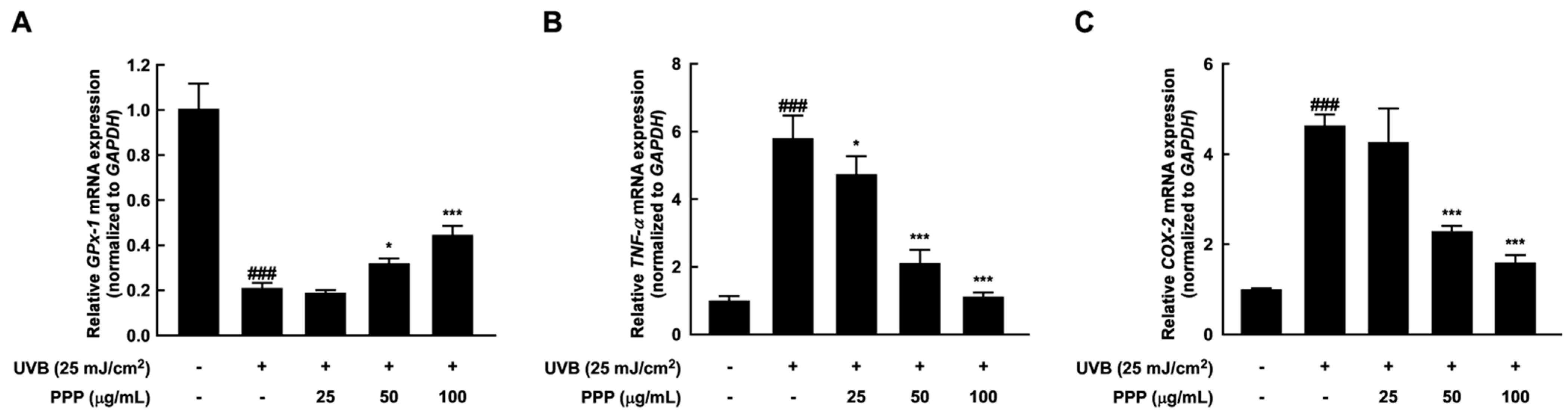
2.7. Anti-Inflammatory Effect of PPP by Upregulating mRNA Levels of Antioxidant Enzyme
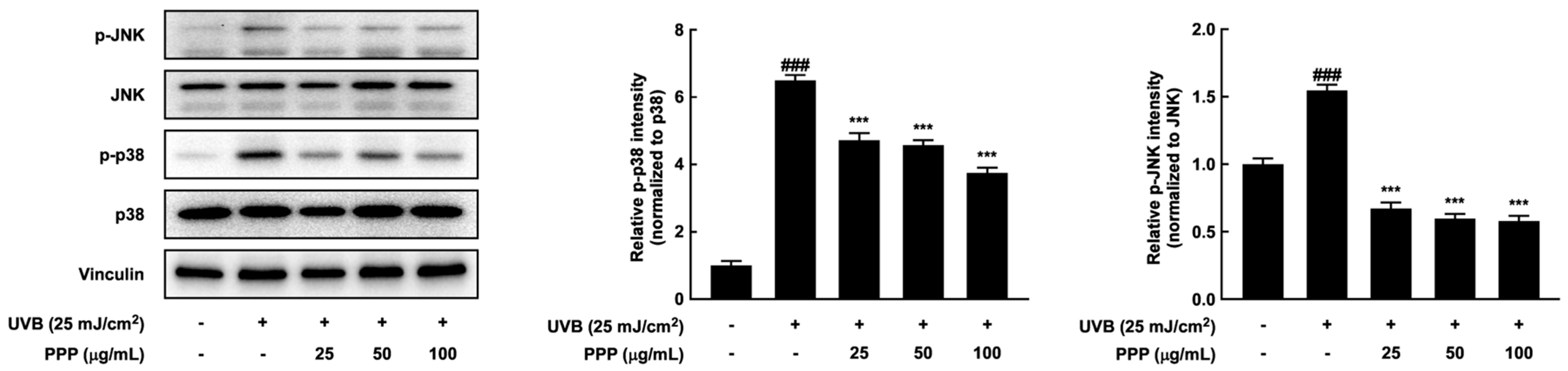
2.8. Effects of PPP on p38 MAPK Kinase and JNK Signaling Pathways in HDF Cells
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Animal Experiments
4.3. Wrinkle Depth Analysis
4.4. Masson’s Trichrome Staining
4.5. Immunohistochemistry
4.6. Hematoxylin and Eosin Staining
4.7. Western Blotting Analysis
4.8. Hyaluronic Acid ELISA Assay
4.9. Quantitative Real-Time Polymerase Chain Reaction
4.10. Cell Culture and Sample Treatment
4.11. Treatment of the PPP
4.12. Collagenase Activity Assay
4.13. MTS Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, O.K.; Kim, D.; Lee, M.; Park, S.H.; Yamada, W.; Eun, S.; Lee, J. Standardized Edible Bird’s Nest Extract Prevents UVB Irradiation-Mediated Oxidative Stress and Photoaging in the Skin. Antioxidants 2021, 10, 1452. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Jia, X.; Fu, C.; Yu, H.; Wang, Y. Antioxidant peptides, the guardian of life from oxidative stress. Med. Res. Rev. 2024, 44, 275–364. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Grootveld, M.; Bahorun, T. Free radicals in biology and medicine: From inflammation to biotechnology. Biofactors 2006, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Na, H.W.; Jang, Y.; Shin, D.Y.; Choi, H.; Kim, H.J.; Seo, Y.R. Network analysis to understand side effects of UVB on skin through transcriptomic approach. Mol. Cell. Toxicol. 2022, 18, 647. [Google Scholar] [CrossRef]
- Rijken, F.; Kiekens, R.C.; van den Worm, E.; Lee, P.L.; van Weelden, H.; Bruijnzeel, P.L. Pathophysiology of photoaging of human skin: Focus on neutrophils. Photochem. Photobiol. Sci. 2006, 5, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, Z.F.; Xu, C.; Jiang, M.J. Shikonin alleviates testosterone-induced benign prostatic hyperplasia in rats via the Nrf2-ARE and NF-κB pathway. Mol. Cell. Toxicol. 2022. [Google Scholar] [CrossRef]
- Li, Y.K.; Yin, R.; Liang, M.; Chen, C. Nrf2 suppresses erastin-induced ferroptosis through activating system Xc(-) in ovarian cancer. Mol. Cell. Toxicol. 2022. [Google Scholar] [CrossRef]
- Begum, R.; Kim, C.S.; Fadriquela, A.; Bajgai, J.; Jing, X.Y.; Kim, D.H.; Kim, S.K.; Lee, K.J. Molecular hydrogen protects against oxidative stress-induced RAW 264.7 macrophage cells through the activation of Nrf2 and inhibition of MAPK signaling pathway. Mol. Cell. Toxicol. 2020, 16, 103–118. [Google Scholar] [CrossRef]
- McArdle, F.; Rhodes, L.E.; Parslew, R.; Jack, C.I.; Friedmann, P.S.; Jackson, M.J. UVR-induced oxidative stress in human skin in vivo: Effects of oral vitamin C supplementation. Free Radic. Biol. Med. 2002, 33, 1355–1362. [Google Scholar] [CrossRef]
- Munro, H.N.; Pilistine, S.J.; Fant, M.E. The placenta in nutrition. Annu. Rev. Nutr. 1983, 3, 97–124. [Google Scholar] [CrossRef]
- Hong, J.W.; Lee, W.J.; Hahn, S.B.; Kim, B.J.; Lew, D.H. The effect of human placenta extract in a wound healing model. Ann. Plast. Surg. 2010, 65, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Bhattacharyya, D. Cell adhesion by aqueous extract of human placenta used as wound healer. Indian J. Exp. Biol. 2007, 45, 732–738. [Google Scholar] [PubMed]
- Jash, A.; Kwon, H.K.; Sahoo, A.; Lee, C.G.; So, J.S.; Kim, J.; Oh, Y.K.; Kim, Y.B.; Im, S.H. Topical application of porcine placenta extract inhibits the progression of experimental contact hypersensitivity. J. Ethnopharmacol. 2011, 133, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, S.K.; Shukla, D.; Tripathi, A.K.; Agrawal, S.; Singh, M.K.; Shukla, V.K. Effect of placental-extract gel and cream on non-healing wounds. J. Wound Care 2006, 15, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Park, H.J.; Seo, H.G.; Kim, J.H.; Lim, G.S.; Lee, W.Y.; Kim, N.H.; Kim, J.H.; Lee, J.H.; Jung, H.S.; et al. Immune modulation effect of porcine placenta extracts in weaned the pig. J. Anim. Sci. 2013, 91, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Nagae, M.; Nagata, M.; Teramoto, M.; Yamakawa, M.; Matsuki, T.; Ohnuki, K.; Shimizu, K. Effect of Porcine Placenta Extract Supplement on Skin Condition in Healthy Adult Women: A Randomized, Double-Blind Placebo-Controlled Study. Nutrients 2020, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Kim, S.W.; Kim, B.; Lee, H.N.; Kim, S.J.; Song, M.; Kim, S.; Kim, J.; Kim, Y.B.; Kim, J.H.; et al. Alpha-fetoprotein, identified as a novel marker for the antioxidant effect of placental extract, exhibits synergistic antioxidant activity in the presence of estradiol. PLoS ONE 2014, 9, e99421. [Google Scholar] [CrossRef]
- Hong, K.B.; Park, Y.; Kim, J.H.; Kim, J.M.; Suh, H.J. Effects of Porcine Placenta Extract Ingestion on Ultraviolet B-induced Skin Damage in Hairless Mice. Korean J. Food Sci. Anim. Resour. 2015, 35, 413–420. [Google Scholar] [CrossRef]
- Choi, S.Y.; Ko, E.J.; Lee, Y.H.; Kim, B.G.; Shin, H.J.; Seo, D.B.; Lee, S.J.; Kim, B.J.; Kim, M.N. Effects of collagen tripeptide supplement on skin properties: A prospective, randomized, controlled study. J. Cosmet. Laser Ther. 2014, 16, 132–137. [Google Scholar] [CrossRef]
- Chavoshnejad, P.; Foroughi, A.H.; Dhandapani, N.; German, G.K.; Razavi, M.J. Effect of collagen degradation on the mechanical behavior and wrinkling of skin. Phys. Rev. E 2021, 104, 034406. [Google Scholar] [CrossRef]
- Shaheen, A.E.; Gebreel, H.M.; Moussa, L.A.; Zakaria, A.E.; Nemr, W.A. Photoprotection Against UV-Induced Skin Damage Using Hyaluronic Acid Produced by Lactiplantibacillus plantarum and Enterococcus durans. Curr. Microbiol. 2023, 80, 262. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.J.; Lee, S.J.; Lee, M.S. Effects of Hahella chejuensis-Derived Prodigiosin on UV-Induced ROS Production, Inflammation and Cytotoxicity in HaCaT Human Skin Keratinocytes. J. Microbiol. Biotechnol. 2021, 31, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Dang, Y.; Gao, W.; Zhang, Y.; Xu, P.; Gu, J.; Ye, X. P38 and JNK signal pathways are involved in the regulation of phlorizin against UVB-induced skin damage. Exp. Dermatol. 2015, 24, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Durr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R. Acute effects of UVR on human eyes and skin. Prog. Biophys. Mol. Biol. 2006, 92, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 10474. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.A.; Yoo, T.; Hwang, J.S.; Kang, E.S.; Paek, K.S.; Park, C.; Kim, J.H.; Do, J.T.; Seo, H.G. Peroxisome proliferator-activated receptor delta modulates MMP-2 secretion and elastin expression in human dermal fibroblasts exposed to ultraviolet B radiation. J. Dermatol. Sci. 2014, 76, 44–50. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron Inhibits UVB-Induced AP-1 Binding Sites Transcriptional Activation on MMP-1 and MMP-3 Promoters by MAPK Signaling Pathway in Human Dermal Fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Jeong, S.; Yoon, S.; Kim, S.; Jung, J.; Kor, M.; Shin, K.; Lim, C.; Han, H.S.; Lee, H.; Park, K.Y.; et al. Anti-Wrinkle Benefits of Peptides Complex Stimulating Skin Basement Membrane Proteins Expression. Int. J. Mol. Sci. 2019, 21, 73. [Google Scholar] [CrossRef]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu Kassim, N.H.; Czernuszka, J.; Rahman, M.T. Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin. Skin Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Zych, M.; Urbisz, K.; Kimsa-Dudek, M.; Kamionka, M.; Dudek, S.; Raczak, B.K.; Waclawek, S.; Chmura, D.; Kaczmarczyk-Zebrowska, I.; Stebel, A. Effects of Water-Ethanol Extracts from Four Sphagnum Species on Gene Expression of Selected Enzymes in Normal Human Dermal Fibroblasts and Their Antioxidant Properties. Pharmaceuticals 2023, 16, 1076. [Google Scholar] [CrossRef] [PubMed]
- Stachowitz, S.; Alessandrini, F.; Abeck, D.; Ring, J.; Behrendt, H. Permeability barrier disruption increases the level of serine palmitoyltransferase in human epidermis. J. Investig. Dermatol. 2002, 119, 1048–1052. [Google Scholar] [PubMed]
- Afaq, F.; Adhami, V.M.; Mukhtar, H. Photochemoprevention of ultraviolet B signaling and photocarcinogenesis. Mutat. Res. 2005, 571, 153–173. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.W.; Kleszczynski, K.; Hardkop, L.H.; Kruse, N.; Zillikens, D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2’-deoxyguanosine) in ex vivo human skin. J. Pineal Res. 2013, 54, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Jo, S.; Jung, Y.S.; Cho, Y.R.; Seo, J.W.; Lim, W.C.; Nam, T.G.; Lim, T.G.; Byun, S. Oral Administration of Rosa gallica Prevents UVB-Induced Skin Aging through Targeting the c-Raf Signaling Axis. Antioxidants 2021, 10, 1663. [Google Scholar] [CrossRef]
- Stasiewicz, A.; Conde, T.; Gegotek, A.; Domingues, M.R.; Domingues, P.; Skrzydlewska, E. Prevention of UVB Induced Metabolic Changes in Epidermal Cells by Lipid Extract from Microalgae Nannochloropsis oceanica. Int. J. Mol. Sci. 2023, 24, 11302. [Google Scholar] [CrossRef]
- Long, Y.; Wang, W.; Zhang, Y.; Du, F.; Zhang, S.; Li, Z.; Deng, J.; Li, J. Photoprotective Effects of Dendrobium nobile Lindl. Polysaccharides against UVB-Induced Oxidative Stress and Apoptosis in HaCaT Cells. Int. J. Mol. Sci. 2023, 24, 6120. [Google Scholar] [CrossRef]
- Kim, A.L.; Labasi, J.M.; Zhu, Y.; Tang, X.; McClure, K.; Gabel, C.A.; Athar, M.; Bickers, D.R. Role of p38 MAPK in UVB-induced inflammatory responses in the skin of SKH-1 hairless mice. J. Investig. Dermatol. 2005, 124, 1318–1325. [Google Scholar] [CrossRef]
- Choi, K.S.; Kundu, J.K.; Chun, K.S.; Na, H.K.; Surh, Y.J. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch. Biochem. Biophys. 2014, 559, 38–45. [Google Scholar] [CrossRef]


| Gene | Organism | Primer Sequences (5’ → 3’) | Accession Number |
|---|---|---|---|
| GAPDH | M | F: CAT CAC TGC CAC CCA GAA GAC TG R: ATG CCA GTG AGC TTC CCG TTC AG | NM_001411843.1 |
| COL7A1 | M | F: CAG GCA TTG GTG CCA GTG AAC A R: TGG ACC TCC TAC CTC ACA GTC A | NM_007738.4 |
| HAS1 | M | F: GTG CGA GTG TTG GAT GAA GAC C R: CCA CAT TGA AGG CTA CCC AGT ATC | NM_008215.2 |
| HAS2 | M | F: GCC ATT TTC CGA ATC CAA ACA GAC R: CCT GCC ACA CTT ATT GAT GAG AAC C | NM_008216.3 |
| LCB1 | M | F: AGC GCC TGG CAA AGT TTA TG R: GTG GAG AAG CCG TAC GTG TAA AT | NM_009269.2 |
| Gpx-1 | M | F: CCC ACT GCG CTC ATG A R: GGC ACA CCG GAG ACC AAA | NM_001329528.1 |
| TNF- α | M | F: GGT GCC TAT GTC TCA GCC TCT T R: GCC ATA GAA CTG ATG AGA GGG AG | NM_001278601.1 |
| IL-1α | M | F: TCG CAG CAG GGT TTT CTA GG R: CAG CTT TAA GGA CGG GAG GG | NM_010554.4 |
| GAPDH | H | F: GTC TCC TCT GAC TTC AAC AGC G R: ACC ACC CTG TTG CTG TAG CCA A | NM_001357943.2 |
| COL3A1 | H | F: TGG TGC CCC TGG TCC TTG CT R: TAC GGG GCA AAA CCG CCA G | NM_000090.4 |
| HAS2 | H | F: TCG CAA CAC GTA ACG CAA T R: ACT TCT CTT TTT CCA CCC CAT TT | NM_005328.3 |
| LCB1 | H | F: CCA TGG AGT GGC CTG AAA GA R: CTG ACA CCA TTT GGT AAC AAT CCT A | NM_001281303.2 |
| DEGS1 | H | F: GCT GAT GGC GTC GAT GTA GA R: TGA AAG CGG TAC AGA AGA ACC A | NM_001321542.2 |
| Gpx-1 | H | F: TTC CCG TGC AAC CAG TTT G R: GGA CGT ACT TGA GGG AAT TCA GA | NM_001329503.2 |
| TNF -α | H | F: ATC CTG GGG GAC CCA ATG TA R: AAA AGA AGG CAC AGA GGC CA | NM_000594.4 |
| COX-2 | H | F: CGG TGA AAC TCT GGC TAG ACA G R: GCA AAC CGT AGA TGC TCA GGG | NM_000963.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, W.-J.; Kim, J.; Baek, K.-S.; Lim, W.; Lim, T.-G. Porcine Placenta Peptide Inhibits UVB-Induced Skin Wrinkle Formation and Dehydration: Insights into MAPK Signaling Pathways from In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2024, 25, 83. https://doi.org/10.3390/ijms25010083
Sim W-J, Kim J, Baek K-S, Lim W, Lim T-G. Porcine Placenta Peptide Inhibits UVB-Induced Skin Wrinkle Formation and Dehydration: Insights into MAPK Signaling Pathways from In Vitro and In Vivo Studies. International Journal of Molecular Sciences. 2024; 25(1):83. https://doi.org/10.3390/ijms25010083
Chicago/Turabian StyleSim, Woo-Jin, Jinhak Kim, Kwang-Soo Baek, Wonchul Lim, and Tae-Gyu Lim. 2024. "Porcine Placenta Peptide Inhibits UVB-Induced Skin Wrinkle Formation and Dehydration: Insights into MAPK Signaling Pathways from In Vitro and In Vivo Studies" International Journal of Molecular Sciences 25, no. 1: 83. https://doi.org/10.3390/ijms25010083
APA StyleSim, W.-J., Kim, J., Baek, K.-S., Lim, W., & Lim, T.-G. (2024). Porcine Placenta Peptide Inhibits UVB-Induced Skin Wrinkle Formation and Dehydration: Insights into MAPK Signaling Pathways from In Vitro and In Vivo Studies. International Journal of Molecular Sciences, 25(1), 83. https://doi.org/10.3390/ijms25010083








