Integrated Bulk Segregant Analysis, Fine Mapping, and Transcriptome Revealed QTLs and Candidate Genes Associated with Drought Adaptation in Wild Watermelon
Abstract
1. Introduction
2. Results
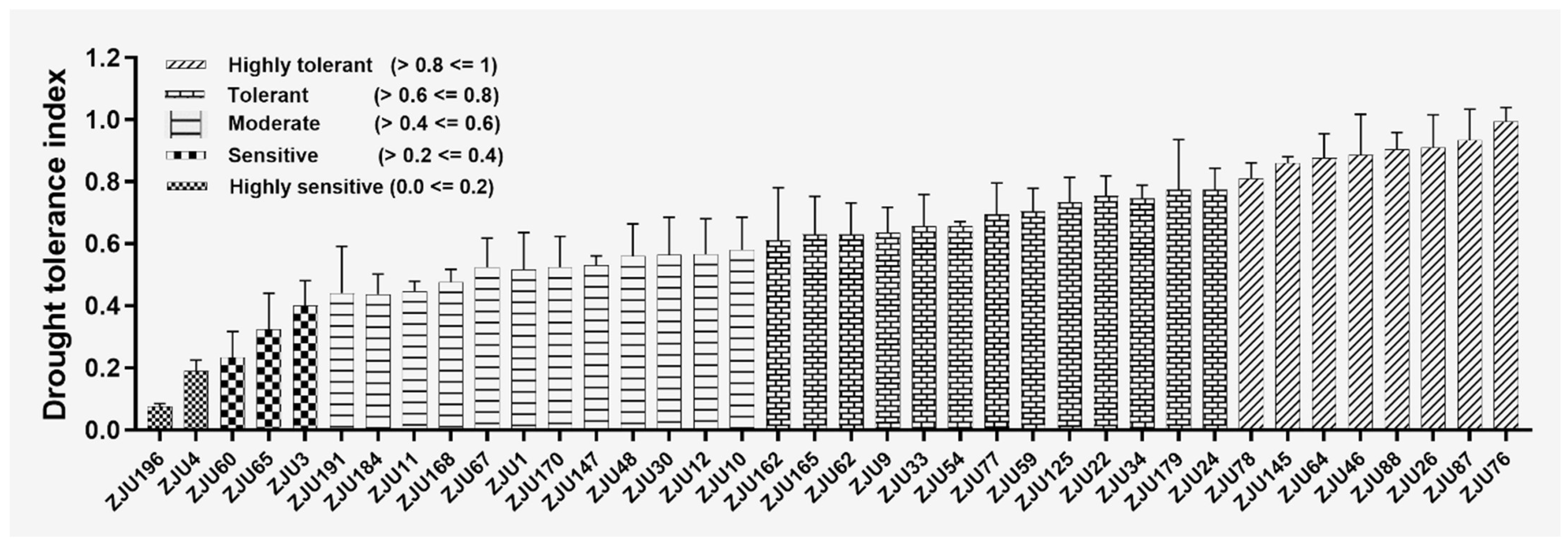
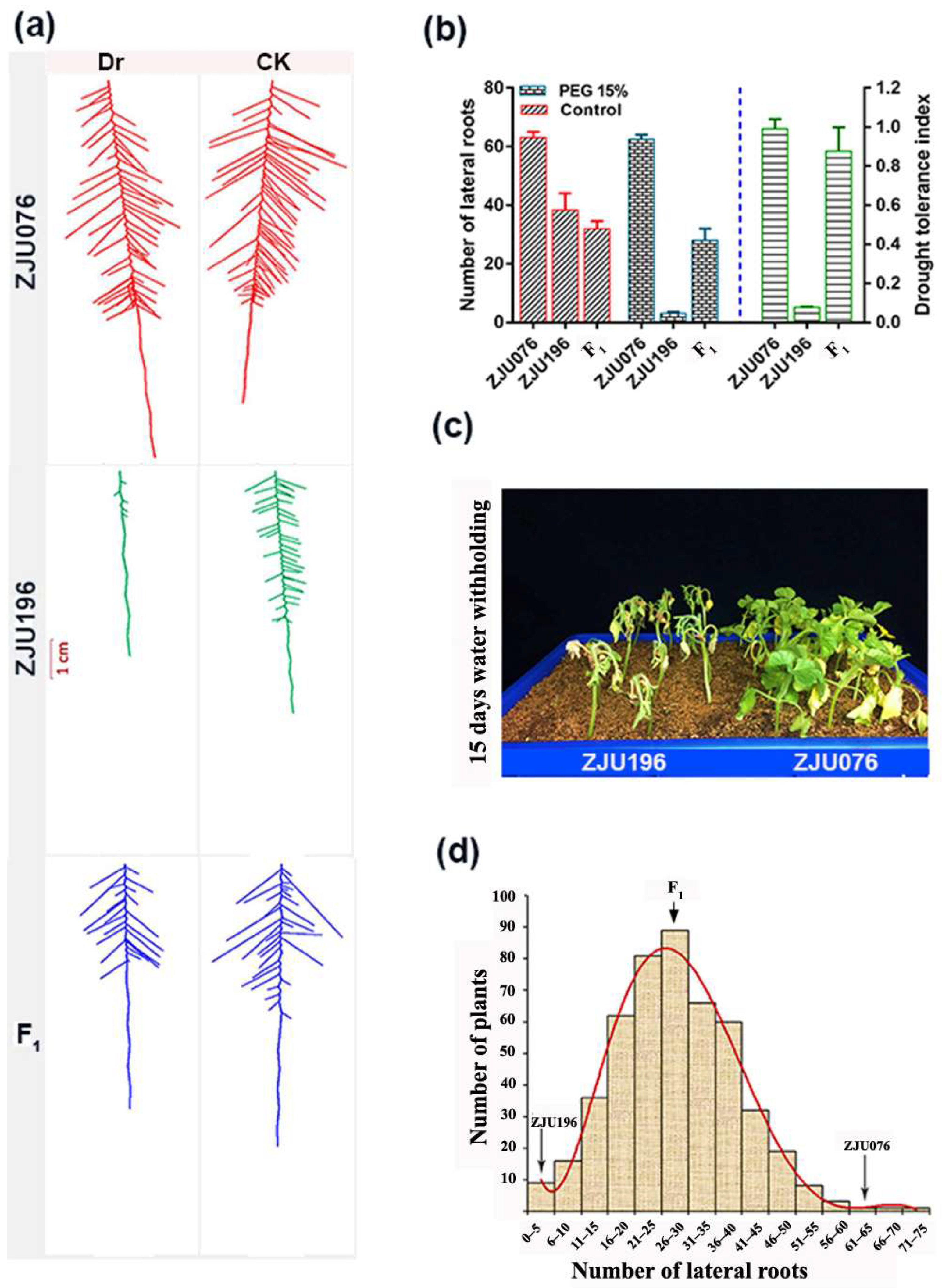
2.1. Root Phenotyping of Watermelon Accessions, Parental Line Selection, and Mapping Population Screening under Drought Stress
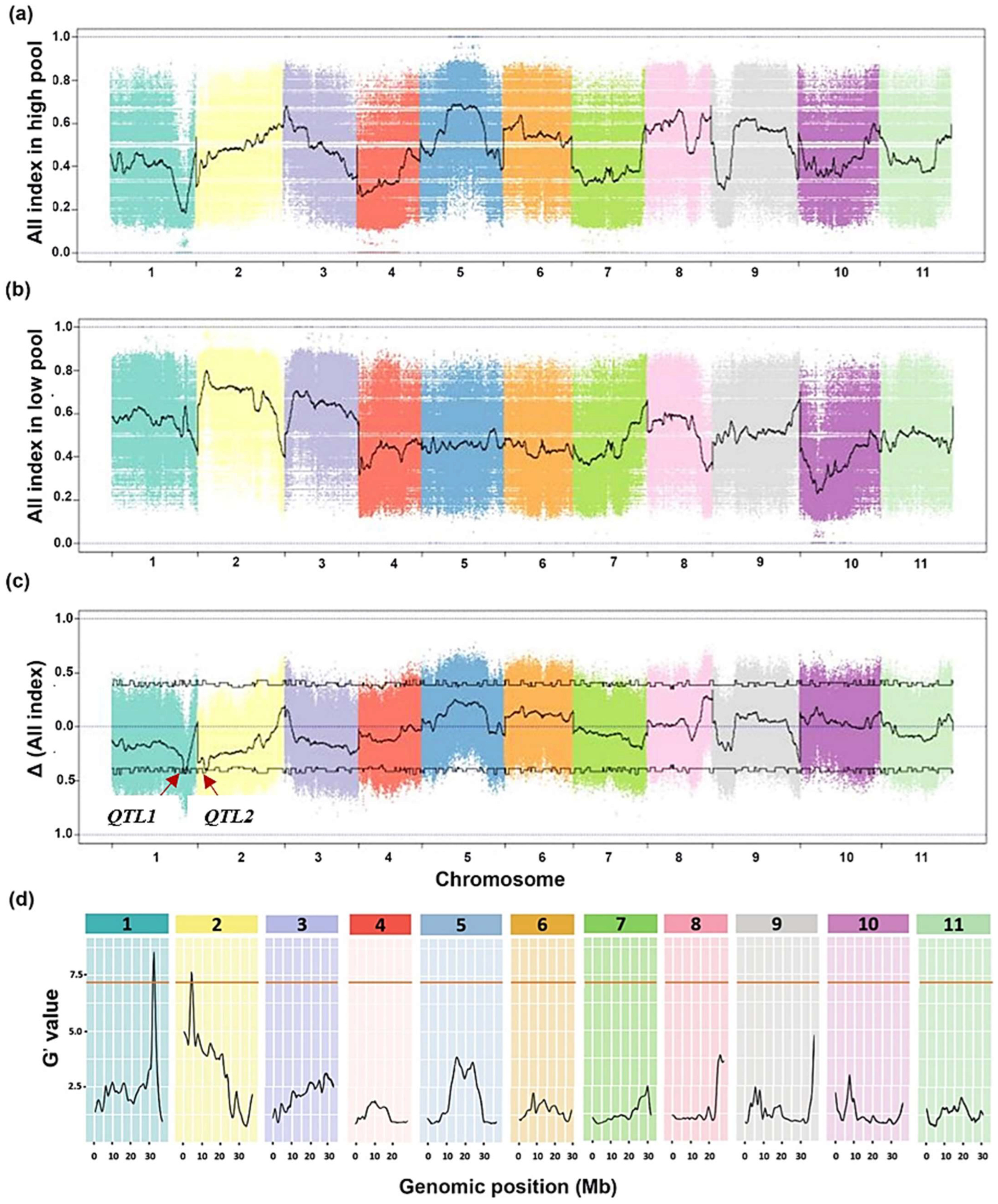
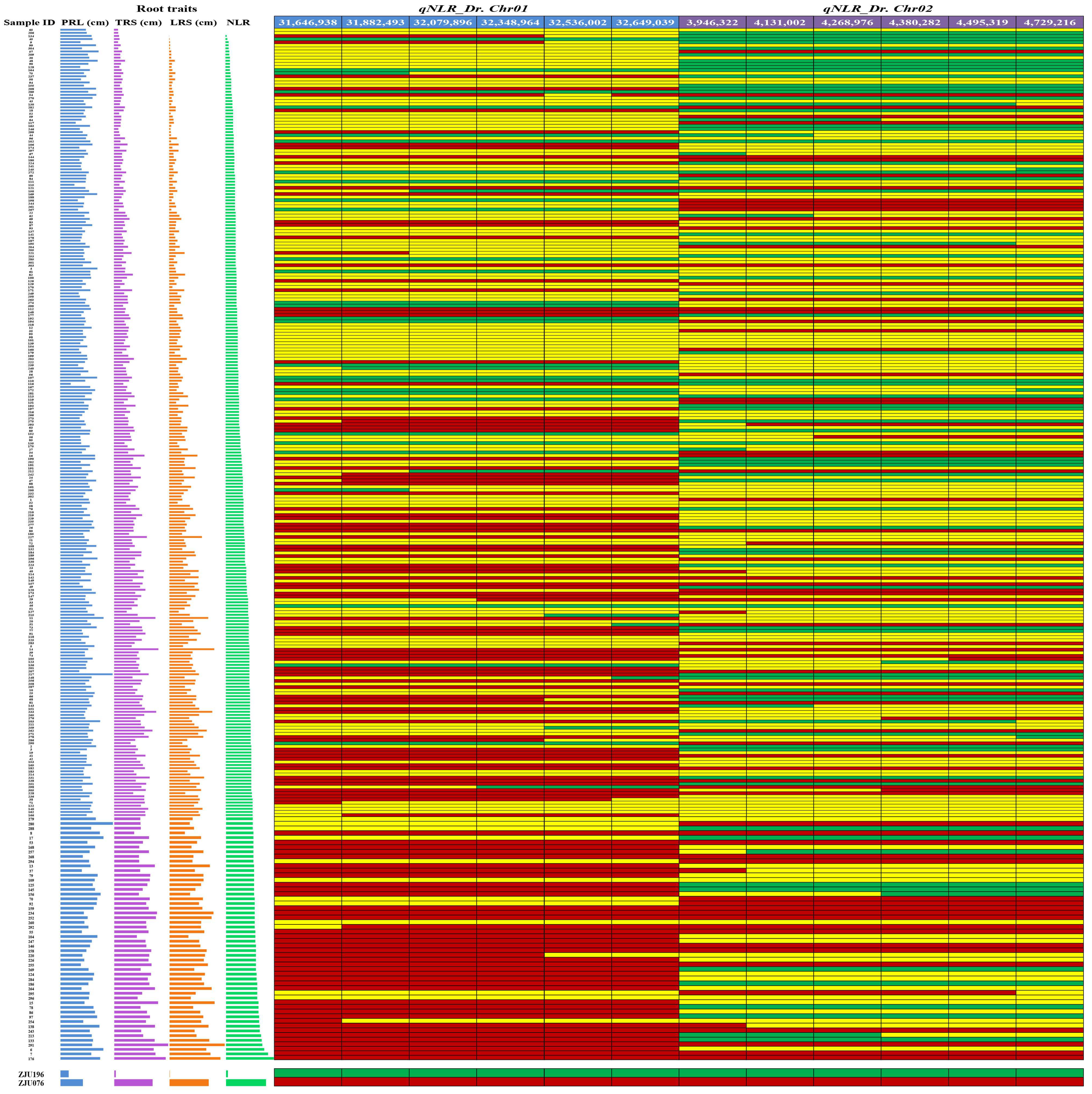
2.2. Identification and Fine-Mapping of the QTLs Associated with the Drought Adaptation in Watermelon
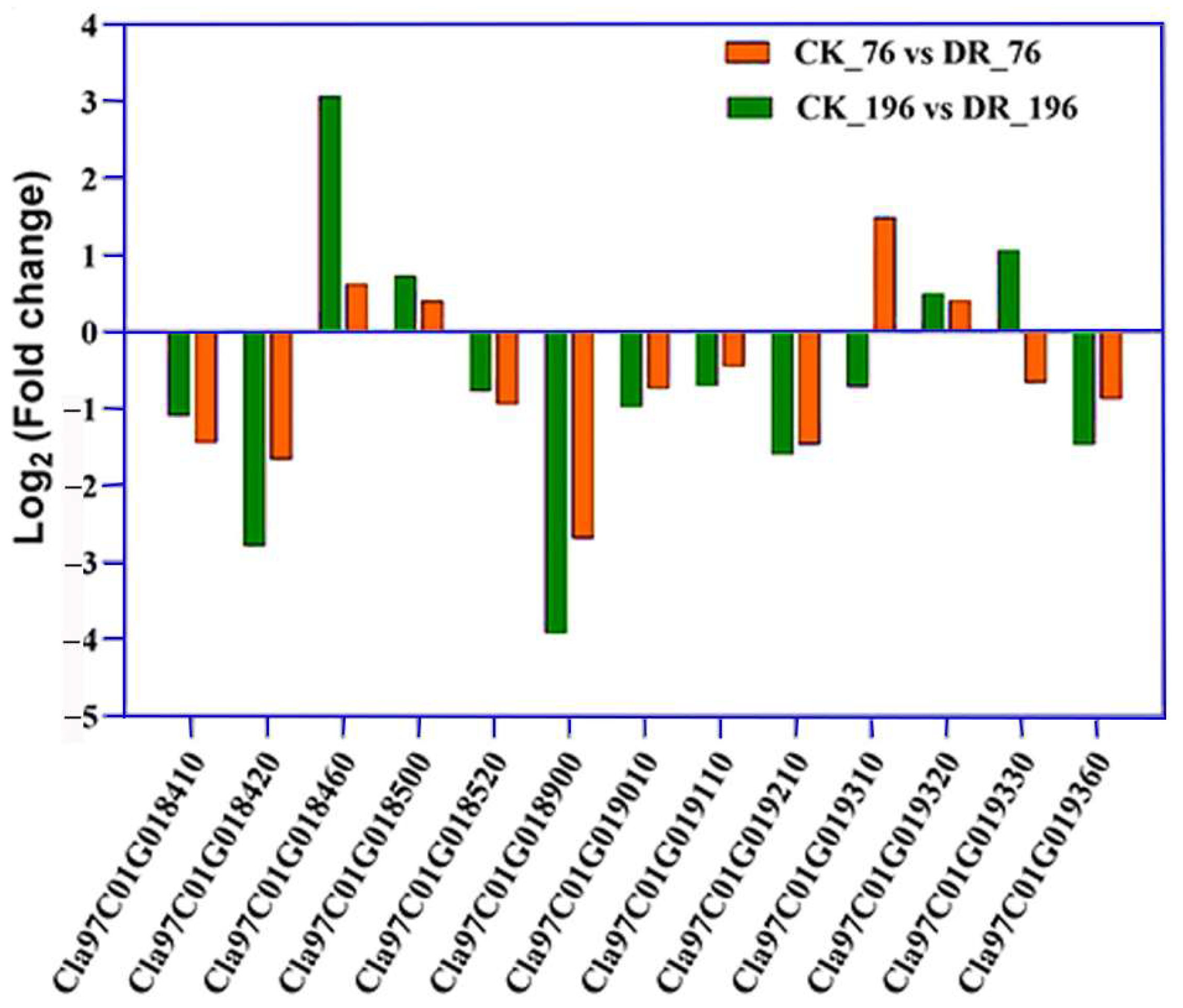
2.3. Transcriptome Analysis of the Watermelon Root under Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Experimental Design, and Phenotyping Procedures
4.2. Population Construction, Offspring Screening, and Sampling
4.3. DNA Extraction, Quality Detection, and Library Construction
4.4. Bulk Segregant Analysis Pipelines
4.5. Haplotype Analysis for QTL Validation and Fine Mapping
4.6. RNA-Seq Methods
4.6.1. Sample Preparation, Library Construction, and Sequencing
4.6.2. RNA Data Analysis
4.7. Detection of the Candidate Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regan, P.M.; Kim, H.; Maiden, E. Climate change, adaptation, and agricultural output. Reg. Environ. Chang. 2018, 19, 113–123. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://faostat.fao.org (accessed on 16 August 2022).
- Mall, M.; Kumar, R.; Akhtar, M.Q. Chapter 1—Horticultural crops and abiotic stress challenges. In Stress Tolerance in Horticultural Crops; Chandra Rai, A., Rai, A., Kumar Rai, K., Rai, V.P., Kumar, A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 1–19. [Google Scholar]
- Li, H.; Mo, Y.; Cui, Q.; Yang, X.; Guo, Y.; Wei, C.; Yang, J.; Zhang, Y.; Ma, J.; Zhang, X. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and -susceptible watermelon genotypes. Plant Sci. 2019, 278, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Lammerts van Bueren, E.T.; Struik, P.C.; van Eekeren, N.; Nuijten, E. Towards resilience through systems-based plant breeding. A review. Agron. Sustain. Dev. 2018, 38, 42. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef] [PubMed]
- Gaur, P.M.; Krishnamurthy, L.; Kashiwagi, J. Improving Drought-Avoidance Root Traits in Chickpea (Cicer arietinum L.)—Current Status of Research at ICRISAT. Plant Prod. Sci. 2015, 11, 3–11. [Google Scholar] [CrossRef]
- Satbhai, S.B.; Ristova, D.; Busch, W. Underground tuning: Quantitative regulation of root growth. J. Exp. Bot. 2015, 66, 1099–1112. [Google Scholar] [CrossRef]
- Sofi, P.A.; Rehman, K.; Gull, M.; Kumari, J.; Djanaguiraman, M.; Prasad, P.V.V. Integrating root architecture and physiological approaches for improving drought tolerance in common bean (Phaseolus vulgaris L.). Plant Physiol. Rep. 2021, 26, 4–22. [Google Scholar] [CrossRef]
- Chen, Y.; Rengel, Z.; Palta, J.; Siddique, K.H.M. Efficient root systems for enhancing tolerance of crops to water and phosphorus limitation. Indian. J. Plant Physiol. 2018, 23, 689–696. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, R.G.; Mao, G.; Koczan, J.M. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic Acid. Plant Physiol. 2006, 142, 1065–1074. [Google Scholar] [CrossRef]
- Maurel, C.; Nacry, P. Root architecture and hydraulics converge for acclimation to changing water availability. Nat. Plants 2020, 6, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Wasaya, A.; Zhang, X.; Fang, Q.; Yan, Z. Root phenotyping for drought tolerance: A review. Agronomy 2018, 8, 241. [Google Scholar] [CrossRef]
- Katuuramu, D.N.; Wechter, W.P.; Washington, M.L.; Horry, M.; Cutulle, M.A.; Jarret, R.L.; Levi, A. Phenotypic Diversity for Root Traits and Identification of Superior Germplasm for Root Breeding in Watermelon. HortScience 2020, 55, 1272–1279. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Leon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. J. Exp. Bot. 2021, 72, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Roorkiwal, M.; Valliyodan, B.; Zhou, L.; Chen, P.; Varshney, R.K.; Nguyen, H.T. Genetic diversity of root system architecture in response to drought stress in grain legumes. J. Exp. Bot. 2018, 69, 3267–3277. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.B.; Kwon, S.-W.; Ham, T.-H.; Kim, S.; Thomson, M.; Hechanova, S.L.; Jena, K.K.; Park, Y. Marker-Assisted Breeding. In Current Technologies in Plant Molecular Breeding: A Guide Book of Plant Molecular Breeding for Researchers; Koh, H.-J., Kwon, S.-Y., Thomson, M., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 95–144. [Google Scholar]
- Paris, H.S. Origin and emergence of the sweet dessert watermelon. Citrullus Lanatus. Ann. Bot. 2015, 116, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Dube, J.; Ddamulira, G.; Maphosa, M. Watermelon production in Africa: Challenges and opportunities. Int. J. Veg. Sci. 2020, 27, 211–219. [Google Scholar] [CrossRef]
- Mahmoud, A.; Qi, R.; Zhao, H.; Yang, H.; Liao, N.; Ali, A.; Malangisha, G.K.; Ma, Y.; Zhang, K.; Zhou, Y.; et al. An allelic variant in the ACS7 gene promotes primary root growth in watermelon. Theor. Appl. Genet. 2022, 135, 3357–3373. [Google Scholar] [CrossRef]
- Yoshimura, K.; Masuda, A.; Kuwano, M.; Yokota, A.; Akashi, K. Programmed proteome response for drought avoidance/tolerance in the root of a C(3) xerophyte (wild watermelon) under water deficits. Plant Cell Physiol. 2008, 49, 226–241. [Google Scholar] [CrossRef]
- Kawasaki, S.; Miyake, C.; Kohchi, T.; Fujii, S.; Uchida, M.; Yokota, A. Responses of wild watermelon to drought stress: Accumulation of an ArgE homologue and citrulline in leaves during water deficits. Plant Cell Physiol. 2000, 41, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Takahara, K.; Akashi, K.; Yokota, A. Purification and characterization of glutamate N-acetyltransferase involved in citrulline accumulation in wild watermelon. FEBS J. 2005, 272, 5353–5364. [Google Scholar] [CrossRef] [PubMed]
- Mandizvo, T.; Odindo, A.O.; Mashilo, J. Citron Watermelon Potential to Improve Crop Diversification and Reduce Negative Impacts of Climate Change. Sustainability 2021, 13, 2269. [Google Scholar] [CrossRef]
- Malambane, G.; Batlang, U.; Ramolekwa, K.; Tsujimoto, H.; Akashi, K. Growth chamber and field evaluation of physiological factors of two watermelon genotypes. Plant Stress. 2021, 2, 100017–100027. [Google Scholar] [CrossRef]
- Akashi, K.; Yoshimura, K.; Kajikawa, M.; Hanada, K.; Kosaka, R.; Kato, A.; Katoh, A.; Nanasato, Y.; Tsujimoto, H.; Yokota, A. Potential involvement of drought-induced Ran GTPase CLRan1 in root growth enhancement in a xerophyte wild watermelon. Biosci. Biotechnol. Biochem. 2016, 80, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Sanda, S.; Yoshida, K.; Kuwano, M.; Kawamura, T.; Munekage, Y.N.; Akashi, K.; Yokota, A. Responses of the photosynthetic electron transport system to excess light energy caused by water deficit in wild watermelon. Physiol. Plant 2011, 142, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Nanasato, Y.; Miyake, C.; Takahara, K.; Kohzuma, K.; Munekage, Y.N.; Yokota, A.; Akashi, K. Chapter 23 Mechanisms of Drought and High Light Stress Tolerance Studied in a Xerophyte, Citrullus lanatus (Wild Watermelon). In The Chloroplast: Basics and Applications; Rebeiz, C.A., Benning, C., Bohnert, H.J., Daniell, H., Hoober, J.K., Lichtenthaler, H.K., Portis, A.R., Tripathy, B.C., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 363–378. [Google Scholar]
- Akashi, K.; Yoshimura, K.; Nanasato, Y.; Takahara, K.; Munekage, Y.; Yokota, A. Wild plant resources for studying molecular mechanisms of drought/strong light stress tolerance. Plant Biotechnol. J. 2008, 25, 257–263. [Google Scholar] [CrossRef][Green Version]
- Nanasato, Y.; Akashi, K.; Yokota, A. Co-expression of cytochrome b561 and ascorbate oxidase in leaves of wild watermelon under drought and high light conditions. Plant Cell Physiol. 2005, 46, 1515–1524. [Google Scholar] [CrossRef]
- Akashi, K.; Nishimura, N.; Ishida, Y.; Yokota, A. Potent hydroxyl radical-scavenging activity of drought-induced type-2 metallothionein in wild watermelon. Biochem. Biophys. Res. Commun. 2004, 323, 72–78. [Google Scholar] [CrossRef]
- Yokota, A.; Kawasaki, S.; Iwano, M.; Nakamura, C.; Miyake, C.; Akashi, K. Citrulline and DRIP-1 Protein (ArgE Homologue) in Drought Tolerance of Wild Watermelon. Ann. Bot. 2002, 89, 825–832. [Google Scholar] [CrossRef]
- Akashi, K.; Miyake, C.; Yokota, A. Citrulline, a novel compatible solute in drought-tolerant wild watermelon leaves, is an efficient hydroxyl radical scavenger. FEBS Lett. 2001, 508, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Cui, Q.; Zhang, Z.; Yan, X.; Ahammed, G.J.; Yang, X.; Yang, J.; Wei, C.; Zhang, X. Alkanes (C29 and C31)-Mediated Intracuticular Wax Accumulation Contributes to Melatonin- and ABA-Induced Drought Tolerance in Watermelon. J. Plant Growth Regul. 2020, 39, 1441–1450. [Google Scholar] [CrossRef]
- Yang, Y.; Mo, Y.; Yang, X.; Zhang, H.; Wang, Y.; Li, H.; Wei, C.; Zhang, X. Transcriptome Profiling of Watermelon Root in Response to Short-Term Osmotic Stress. PLoS ONE 2016, 11, e0166314. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.-l.; Zheng, J.-x.; Yang, R.-p.; Liu, C.-m.; Gu, X.-r.; Zhang, X.; Wei, C.-h. Physiological responses and tolerance to drought stress of different watermelon genotypes. Yingyong Shengtai Xuebao 2016, 27, 1942–1952. [Google Scholar] [PubMed]
- Mo, Y.; Yang, R.; Liu, L.; Gu, X.; Yang, X.; Wang, Y.; Zhang, X.; Li, H. Growth, photosynthesis and adaptive responses of wild and domesticated watermelon genotypes to drought stress and subsequent re-watering. Plant Growth Regul. 2016, 79, 229–241. [Google Scholar] [CrossRef]
- Mo, Y.; Wang, Y.; Yang, R.; Zheng, J.; Liu, C.; Li, H.; Ma, J.; Zhang, Y.; Wei, C.; Zhang, X. Regulation of Plant Growth, Photosynthesis, Antioxidation and Osmosis by an Arbuscular Mycorrhizal Fungus in Watermelon Seedlings under Well-Watered and Drought Conditions. Front. Plant Sci. 2016, 7, 644. [Google Scholar] [CrossRef]
- Guzzon, F.; Müller, J.V.; do Nascimento Araujo, M.; Cauzzi, P.; Orsenigo, S.; Mondoni, A.; Abeli, T. Drought avoidance adaptive traits in seed germination and seedling growth of Citrullus amarus landraces. S. Afr. J. Bot. 2017, 113, 382–388. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, G.; Guo, S.; Ren, Y.; Xu, Y.; Ling, K.-S. Screening the USDA watermelon germplasm collection for drought tolerance at the seedling stage. HortScience 2011, 46, 1245–1248. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, J.; Yang, Y. Genome-Wide Identification and Expression Analysis of NF-Y Transcription Factor Families in Watermelon (Citrullus lanatus). J. Plant Growth Regul. 2017, 36, 590–607. [Google Scholar] [CrossRef]
- Song, Q.; Joshi, M.; DiPiazza, J.; Joshi, V. Functional Relevance of Citrulline in the Vegetative Tissues of Watermelon During Abiotic Stresses. Front. Plant Sci. 2020, 11, 512. [Google Scholar] [CrossRef]
- Erez, M.E.; İnal, B.; Karipçin, M.Z.; Altıntaş, S. Physiological and Gene-Expression Variations in Watermelon (Citrullus lanatus L.) Cultivars Exposed to Drought Stress. Acta Soc. Bot. Pol. 2020, 89, 8921. [Google Scholar] [CrossRef]
- Chung, J.-S.; Park, S.-H.; Min, J.-H.; Min, K.-H.; Lee, S.; Lee, K.-H.; Kim, C.S. Reduced Expression of Gongdae Ring Zinc Finger 1 (GdRZF1) Enhances Drought Stress Tolerance in Watermelon (Citrullus lanatus). Korean J. Hortic. Sci. Technol. 2017, 35, 637–646. [Google Scholar] [CrossRef]
- Lema, M. Marker Assisted Selection in Comparison to Conventional Plant Breeding: Review Article. Agric. Res. J. 2018, 14, 555913. [Google Scholar] [CrossRef]
- Jiang, G.-L. Molecular Marker-Assisted Breeding: A Plant Breeder’s Review. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 431–472. [Google Scholar]
- Jamann, T.M.; Balint-Kurti, P.J.; Holland, J.B. QTL Mapping Using High-Throughput Sequencing. In Plant Functional Genomics: Methods and Protocols; Alonso, J.M., Stepanova, A.N., Eds.; Springer: New York, NY, USA, 2015; Volume 1284, pp. 257–285. [Google Scholar]
- Varshney, R.K.; Singh, V.K.; Hickey, J.M.; Xun, X.; Marshall, D.F.; Wang, J.; Edwards, D.; Ribaut, J.M. Analytical and Decision Support Tools for Genomics-Assisted Breeding. Trends Plant Sci. 2016, 21, 354–363. [Google Scholar] [CrossRef] [PubMed]
- de Dorlodot, S.; Forster, B.; Pages, L.; Price, A.; Tuberosa, R.; Draye, X. Root system architecture: Opportunities and constraints for genetic improvement of crops. Trends Plant Sci. 2007, 12, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.; van Eeuwijk, F.A.; Hammer, G.L.; Podlich, D.W.; Messina, C. Modeling QTL for complex traits: Detection and context for plant breeding. Curr. Opin. Plant Biol. 2009, 12, 231–240. [Google Scholar] [CrossRef]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Idrissi, O.; Udupa, S.M.; De Keyser, E.; McGee, R.J.; Coyne, C.J.; Saha, G.C.; Muehlbauer, F.J.; Van Damme, P.; De Riek, J. Identification of Quantitative Trait Loci Controlling Root and Shoot Traits Associated with Drought Tolerance in a Lentil (Lens culinaris Medik.) Recombinant Inbred Line Population. Front. Plant Sci. 2016, 7, 1174. [Google Scholar] [CrossRef]
- Liang, H.; Yu, Y.; Yang, H.; Xu, L.; Dong, W.; Du, H.; Cui, W.; Zhang, H. Inheritance and QTL mapping of related root traits in soybean at the seedling stage. Theor. Appl. Genet. 2014, 127, 2127–2137. [Google Scholar] [CrossRef]
- Johnson, W.C.; Jackson, L.E.; Ochoa, O.; van Wijk, R.; Peleman, J.; Clair, D.A.S.; Michelmore, R.W. Lettuce, a shallow-rooted crop, and Lactuca serriola, its wild progenitor, differ at QTL determining root architecture and deep soil water exploitation. Theor. Appl. Genet. 2000, 101, 1066–1073. [Google Scholar] [CrossRef]
- Khan, A.; Pan, X.; Najeeb, U.; Tan, D.K.Y.; Fahad, S.; Zahoor, R.; Luo, H. Coping with drought: Stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 2018, 51, 47. [Google Scholar] [CrossRef]
- Ngwepe, R.M.; Mashilo, J.; Shimelis, H. Progress in genetic improvement of citron watermelon (Citrullus lanatus var. citroides): A review. Genet. Resour. Crop. Evol. 2019, 66, 735–758. [Google Scholar]
- Wang, Z.; Hu, H.; Goertzen, L.R.; McElroy, J.S.; Dane, F. Analysis of the Citrullus colocynthis transcriptome during water deficit stress. PLoS ONE 2014, 9, e104657. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, L.; Liu, D.; Zeng, J.; Cao, S.; Xia, X.; Yan, J.; Song, X.; He, Z.; Zhang, Y. Fine mapping and validation of a major QTL for grain weight on chromosome 5B in bread wheat. Theor. Appl. Genet. 2021, 13, 3731–3741. [Google Scholar] [CrossRef]
- Lei, L.; Zheng, H.; Bi, Y.; Yang, L.; Liu, H.; Wang, J.; Sun, J.; Zhao, H.; Li, X.; Li, J.; et al. Identification of a Major QTL and Candidate Gene Analysis of Salt Tolerance at the Bud Burst Stage in Rice (Oryza sativa L.) Using QTL-Seq and RNA-Seq. Rice 2020, 13, 55. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, H.; Chu, S.; Li, H.; Chi, Y.; Triebwasser-Freese, D.; Lv, H.; Yu, D. Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol. Biol. 2017, 93, 137–150. [Google Scholar] [CrossRef]
- Ma, Y.; Min, L.; Wang, J.; Li, Y.; Wu, Y.; Hu, Q.; Ding, Y.; Wang, M.; Liang, Y.; Gong, Z.; et al. A combination of genome-wide and transcriptome-wide association studies reveals genetic elements leading to male sterility during high temperature stress in cotton. New Phytol. 2021, 231, 165–181. [Google Scholar] [CrossRef]
- Wang, T.; Hou, L.; Jian, H.; Di, F.; Li, J.; Liu, L. Combined QTL mapping, physiological and transcriptomic analyses to identify candidate genes involved in Brassica napus seed aging. Mol. Genet. Genom. 2018, 293, 1421–1435. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Cai, X.; Zhou, Z.; Agong, S.G.; Wang, K.; Liu, F. Identification of QTLs and candidate genes for physiological traits associated with drought tolerance in cotton. J. Cotton Res. 2020, 3, 3. [Google Scholar] [CrossRef]
- Diouf, I.; Albert, E.; Duboscq, R.; Santoni, S.; Bitton, F.; Gricourt, J.; Causse, M. Integration of QTL, Transcriptome and Polymorphism Studies Reveals Candidate Genes for Water Stress Response in Tomato. Genes. 2020, 11, 900. [Google Scholar] [CrossRef]
- Sheng, C.; Song, S.; Zhou, R.; Li, D.; Gao, Y.; Cui, X.; Tang, X.; Zhang, Y.; Tu, J.; Zhang, X.; et al. QTL-Seq and Transcriptome Analysis Disclose Major QTL and Candidate Genes Controlling Leaf Size in Sesame (Sesamum indicum L.). Front. Plant Sci. 2021, 12, 580846. [Google Scholar] [CrossRef]
- Di, F.; Wang, T.; Ding, Y.; Chen, X.; Wang, H.; Li, J.; Liu, L. Genetic Mapping Combined with a Transcriptome Analysis to Screen for Candidate Genes Responsive to Abscisic Acid Treatment in Brassica napus Embryos During Seed Germination. DNA Cell Biol. 2020, 39, 533–547. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, H.; Guo, H.; Zhou, C.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Ding, Y.; Liu, L. Identification of the vernalization gene VRN-B1 responsible for heading date variation by QTL mapping using a RIL population in wheat. BMC Plant Biol. 2020, 20, 331. [Google Scholar] [CrossRef]
- Gelli, M.; Konda, A.R.; Liu, K.; Zhang, C.; Clemente, T.E.; Holding, D.R.; Dweikat, I.M. Validation of QTL mapping and transcriptome profiling for identification of candidate genes associated with nitrogen stress tolerance in sorghum. BMC Plant Biol. 2017, 17, 123. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Liu, X.; Wang, W.; Liu, D.; Teng, Z.; Fang, X.; Tan, Z.; Tang, S.; Yang, J.; et al. Fine mapping and RNA-Seq unravels candidate genes for a major QTL controlling multiple fiber quality traits at the T1 region in upland cotton. BMC Genom. 2016, 17, 295. [Google Scholar] [CrossRef]
- Diouf, I.A.; Derivot, L.; Bitton, F.; Pascual, L.; Causse, M. Water Deficit and Salinity Stress Reveal Many Specific QTL for Plant Growth and Fruit Quality Traits in Tomato. Front. Plant Sci. 2018, 9, 279. [Google Scholar] [CrossRef]
- Yue, Z.; Ma, R.; Cheng, D.; Yan, X.; He, Y.; Wang, C.; Pan, X.; Yin, L.; Zhang, X.; Wei, C. Candidate gene analysis of watermelon stripe pattern locus ClSP ongoing recombination suppression. Theor. Appl. Genet. 2021, 134, 3263–3277. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Yang, W.; Pasriga, R.; Wu, Y.; Hong, W.J.; Bureau, C.; Wi, S.J.; Zhang, T.; Wang, R.; et al. Homeobox transcription factor OsZHD2 promotes root meristem activity in rice by inducing ethylene biosynthesis. J. Exp. Bot. 2020, 71, 5348–5364. [Google Scholar] [CrossRef]
- Zhang, Y.; Mitsuda, N.; Yoshizumi, T.; Horii, Y.; Oshima, Y.; Ohme-Takagi, M.; Matsui, M.; Kakimoto, T. Two types of bHLH transcription factor determine the competence of the pericycle for lateral root initiation. Nat. Plants 2021, 7, 633–643. [Google Scholar] [CrossRef]
- Yin, Y.; Zhu, Q.; Dai, S.; Lamb, C.; Beachy, R.N. RF2a, a bZIP transcriptional activator of the phloem-specific rice tungro bacilliform virus promoter, functions in vascular development. EMBO J. 1997, 16, 5247–5259. [Google Scholar] [CrossRef]
- Iven, T.; Strathmann, A.; Böttner, S.; Zwafink, T.; Heinekamp, T.; Guivarc’h, A.; Roitsch, T.; Dröge-Laser, W. Homo- and heterodimers of tobacco bZIP proteins counteract as positive or negative regulators of transcription during pollen development. Plant J. 2010, 63, 155–166. [Google Scholar] [CrossRef]
- Toh, S.; McCourt, P.; Tsuchiya, Y. HY5 is involved in strigolactone-dependent seed germination in Arabidopsis. Plant Signal Behav. 2012, 7, 556–558. [Google Scholar] [CrossRef]
- Izawa, T.; Foster, R.; Nakajima, M.; Shimamoto, K.; Chua, N.H. The rice bZIP transcriptional activator RITA-1 is highly expressed during seed development. Plant Cell 1994, 6, 1277–1287. [Google Scholar]
- Guan, Y.; Ren, H.; Xie, H.; Ma, Z.; Chen, F. Identification and characterization of bZIP-type transcription factors involved in carrot (Daucus carota L.) somatic embryogenesis. Plant J. 2009, 60, 207–217. [Google Scholar] [CrossRef]
- Huang, X.; Ouyang, X.; Yang, P.; Lau, O.S.; Li, G.; Li, J.; Chen, H.; Deng, X.W. Arabidopsis FHY3 and HY5 Positively Mediate Induction of COP1 Transcription in Response to Photomorphogenic UV-B Light. Plant Cell 2012, 24, 4590–4606. [Google Scholar] [CrossRef]
- Chen, H.; Chen, W.; Zhou, J.; He, H.; Chen, L.; Chen, H.; Deng, X.W. Basic leucine zipper transcription factor OsbZIP16 positively regulates drought resistance in rice. Plant Sci. 2012, 193–194, 8–17. [Google Scholar] [CrossRef]
- Yoshida, T.; Fujita, Y.; Sayama, H.; Kidokoro, S.; Maruyama, K.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J. 2010, 61, 672–685. [Google Scholar] [CrossRef]
- Yamamoto, A.; Bhuiyan, M.N.; Waditee, R.; Tanaka, Y.; Esaka, M.; Oba, K.; Jagendorf, A.T.; Takabe, T. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and Arabidopsis plants. J. Exp. Bot. 2005, 56, 1785–1796. [Google Scholar] [CrossRef]
- Stevens, R.; Truffault, V.; Baldet, P.; Gautier, H. Ascorbate Oxidase in Plant Growth, Development, and Stress Tolerance. In Ascorbic Acid in Plant Growth, Development and Stress Tolerance; Hossain, M.A., Munné-Bosch, S., Burritt, D.J., Diaz-Vivancos, P., Fujita, M., Lorence, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 273–295. [Google Scholar]
- Pignocchi, C.; Fletcher, J.M.; Wilkinson, J.E.; Barnes, J.D.; Foyer, C.H. The function of ascorbate oxidase in tobacco. Plant Physiol. 2003, 132, 1631–1641. [Google Scholar] [CrossRef]
- Kerk, N.M.; Jiang, K.; Feldman, L.J. Auxin Metabolism in the Root Apical Meristem1. Plant Physiol. 2000, 122, 925–932. [Google Scholar] [CrossRef]
- Mei, Y.; Gao, H.B.; Yuan, M.; Xue, H.W. The Arabidopsis ARCP protein, CSI1, which is required for microtubule stability, is necessary for root and anther development. Plant Cell 2012, 24, 1066–1080. [Google Scholar] [CrossRef]
- Coates, J.C.; Laplaze, L.; Haseloff, J. Armadillo-related proteins promote lateral root development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2006, 103, 1621–1626. [Google Scholar] [CrossRef]
- Kinoshita, A.; ten Hove, C.A.; Tabata, R.; Yamada, M.; Shimizu, N.; Ishida, T.; Yamaguchi, K.; Shigenobu, S.; Takebayashi, Y.; Iuchi, S.; et al. A plant U-box protein, PUB4, regulates asymmetric cell division and cell proliferation in the root meristem. Development 2015, 142, 444–453. [Google Scholar] [CrossRef]
- Sivamani, E.; Bahieldin, A.; Wraith, J.M.; Al-Niemi, T.; Dyer, W.E.; Ho, T.-H.D.; Qu, R. Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. Plant Sci. 2000, 155, 1–9. [Google Scholar] [CrossRef]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.; Wu, R. Expression of a Late Embryogenesis Abundant Protein Gene, HVA1, from Barley Confers Tolerance to Water Deficit and Salt Stress in Transgenic Rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef]
- Shahzad, Z.; Kellermeier, F.; Armstrong, E.M.; Rogers, S.; Lobet, G.; Amtmann, A.; Hills, A. EZ-Root-VIS: A Software Pipeline for the Rapid Analysis and Visual Reconstruction of Root System Architecture. Plant Physiol. 2018, 177, 1368–1381. [Google Scholar] [CrossRef]
- Armengaud, P.; Zambaux, K.; Hills, A.; Sulpice, R.; Pattison, R.J.; Blatt, M.R.; Amtmann, A. EZ-Rhizo: Integrated software for the fast and accurate measurement of root system architecture. Plant J. 2009, 57, 945–956. [Google Scholar] [CrossRef]
- Álvarez, S.; Bañón, S.; Sánchez-Blanco, M.J. Regulated deficit irrigation in different phenological stages of potted geranium plants: Water consumption, water relations and ornamental quality. Acta Physiol. Plant 2013, 35, 1257–1267. [Google Scholar] [CrossRef]
- CIMMYT. Laboratory Protocols: CIMMYT Applied Molecular Genetics Laboratory, 3rd ed.; CIMMYT: Méx, Mexico, 2005; iii+92p. [Google Scholar]
- Liao, N.; Hu, Z.; Li, Y.; Hao, J.; Chen, S.; Xue, Q.; Ma, Y.; Zhang, K.; Mahmoud, A.; Ali, A.; et al. Ethylene-responsive factor 4 is associated with the desirable rind hardness trait conferring cracking resistance in fresh fruits of watermelon. Plant Biotechnol. J. 2019, 18, 1066–1077. [Google Scholar] [CrossRef]
- Ai, Y.; Zhang, Q.; Wang, W.; Zhang, C.; Cao, Z.; Bao, M.; He, Y. Transcriptomic Analysis of Differentially Expressed Genes during Flower Organ Development in Genetic Male Sterile and Male Fertile Tagetes erecta by Digital Gene-Expression Profiling. PLoS ONE 2016, 11, e0150892. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Heger, A.; Sudbery, I. UMI-tools: Modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 2017, 27, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2014, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Haynes, W. Benjamini–Hochberg Method. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, 2013; p. 78. [Google Scholar]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Mao, X.; Cai, T.; Olyarchuk, J.G.; Wei, L. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics 2005, 21, 3787–3793. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.; Qi, R.; Chi, X.; Liao, N.; Malangisha, G.K.; Ali, A.; Moustafa-Farag, M.; Yang, J.; Zhang, M.; Hu, Z. Integrated Bulk Segregant Analysis, Fine Mapping, and Transcriptome Revealed QTLs and Candidate Genes Associated with Drought Adaptation in Wild Watermelon. Int. J. Mol. Sci. 2024, 25, 65. https://doi.org/10.3390/ijms25010065
Mahmoud A, Qi R, Chi X, Liao N, Malangisha GK, Ali A, Moustafa-Farag M, Yang J, Zhang M, Hu Z. Integrated Bulk Segregant Analysis, Fine Mapping, and Transcriptome Revealed QTLs and Candidate Genes Associated with Drought Adaptation in Wild Watermelon. International Journal of Molecular Sciences. 2024; 25(1):65. https://doi.org/10.3390/ijms25010065
Chicago/Turabian StyleMahmoud, Ahmed, Rui Qi, Xiaolu Chi, Nanqiao Liao, Guy Kateta Malangisha, Abid Ali, Mohamed Moustafa-Farag, Jinghua Yang, Mingfang Zhang, and Zhongyuan Hu. 2024. "Integrated Bulk Segregant Analysis, Fine Mapping, and Transcriptome Revealed QTLs and Candidate Genes Associated with Drought Adaptation in Wild Watermelon" International Journal of Molecular Sciences 25, no. 1: 65. https://doi.org/10.3390/ijms25010065
APA StyleMahmoud, A., Qi, R., Chi, X., Liao, N., Malangisha, G. K., Ali, A., Moustafa-Farag, M., Yang, J., Zhang, M., & Hu, Z. (2024). Integrated Bulk Segregant Analysis, Fine Mapping, and Transcriptome Revealed QTLs and Candidate Genes Associated with Drought Adaptation in Wild Watermelon. International Journal of Molecular Sciences, 25(1), 65. https://doi.org/10.3390/ijms25010065





