Novel Alleles from Cicer reticulatum L. for Genetic Improvement of Cultivated Chickpeas Identified through Genome Wide Association Analysis
Abstract
1. Introduction
2. Results
2.1. Variability of Yield and Selected Yield Contributing Traits of Chickpeas
2.2. Correlation among the Yield and Yield Contributing Traits of Chickpeas
2.3. Path Coefficient Analysis
2.4. Effects of Genotype, Environment and their Interaction on Seed Yield and Yield Contributing Traits of Chickpeas
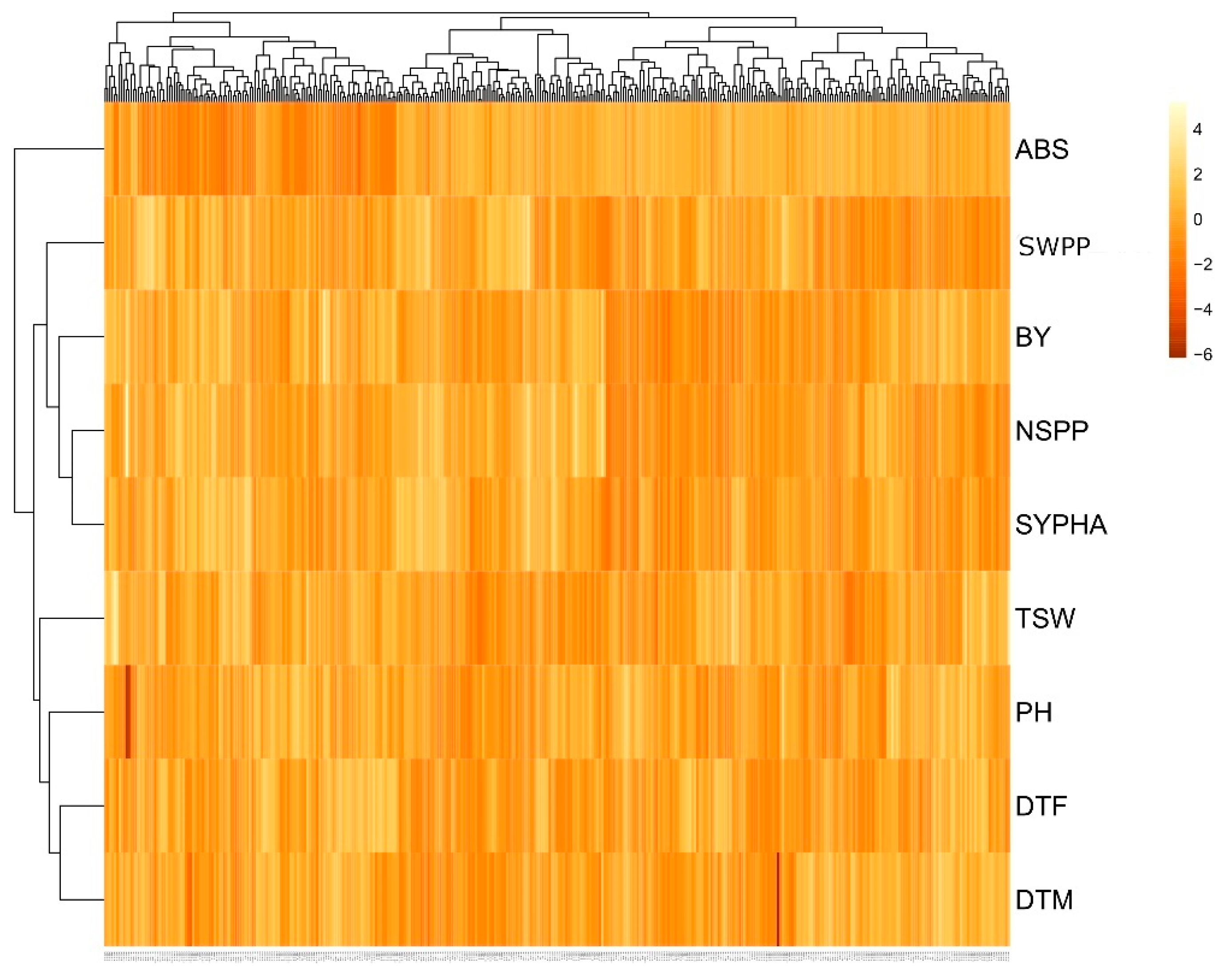
2.5. Cluster Analysis Based on Agronomic and Yield Traits
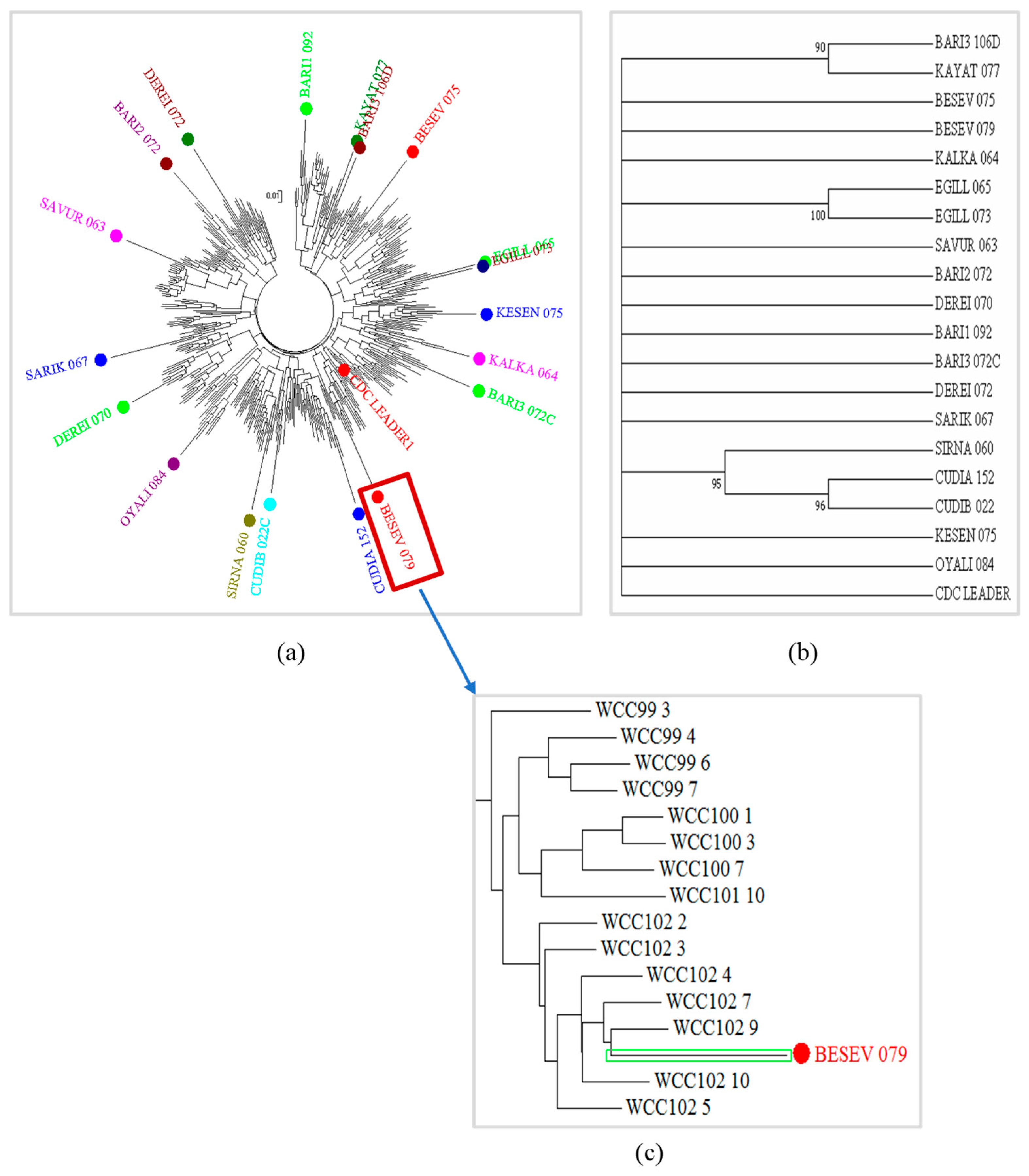
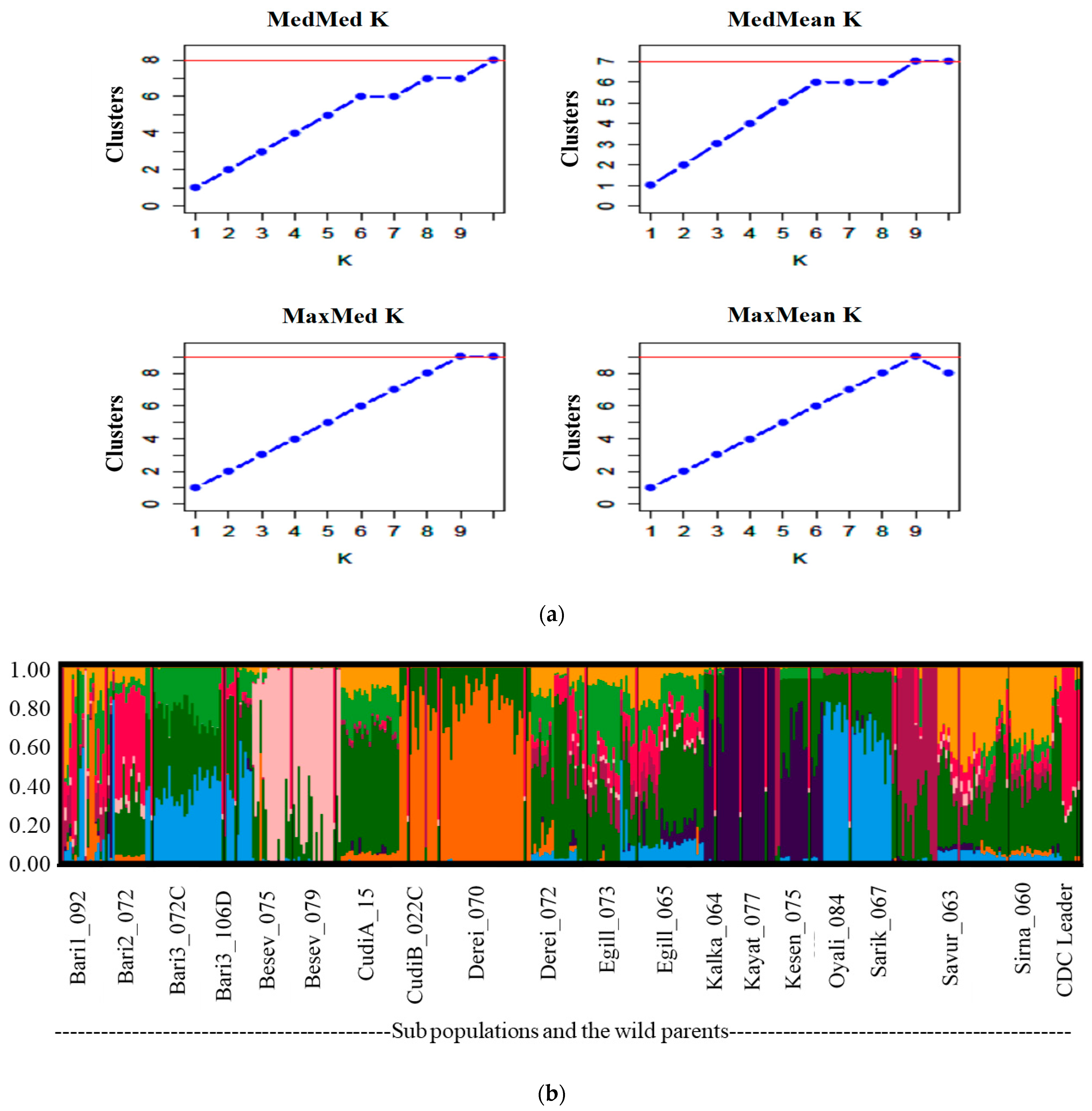
2.6. Genetic Diversity and Population Structure Analyses
2.7. Association Analysis and Potential Candidate Genes
3. Discussion
3.1. Variability and Performance of the Interspecific Lines
3.2. Interrelationships among the Yield and Yield Contributing Traits for Efficient Selection
3.3. Genotype by Environment Interaction, and Broad Sense Heritability
3.4. Clustering of the Chickpea Lines Based on the Phenotypic Traits
3.5. Genetic Diversity in the Chickpea Lines Using SNPs
3.6. Association Mapping of the Studied Traits
4. Materials and Methods
4.1. Source of Germplasm
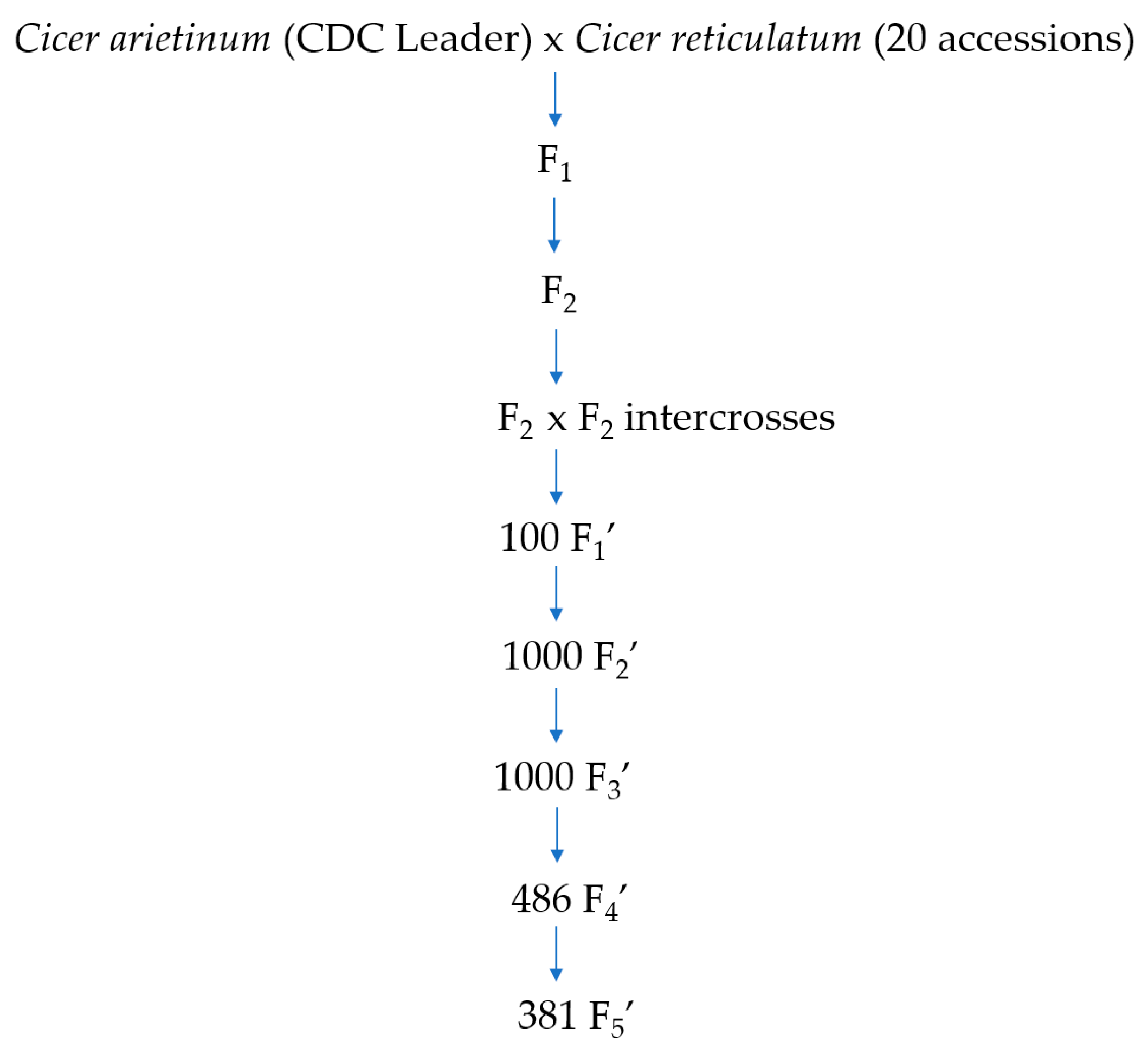
4.2. Development of Chickpea Lines from Interspecific Crosses of C. arietinum x C. reticulatum
4.3. Experimental Setup, Data Collection, and Management
4.4. Genotyping of Chickpea Populations and Data Analysis
4.5. Statistical Analyses
4.6. Genetic Diversity and Population Structure Analyses
4.7. Genome-Wide Association Study (GWAS)
5. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Sharma, P.; Varshney, R.K.; Sharma, S.K.; Singh, N.K. Chickpea improvement. Role of wild species and Genetic Markers. Biotech. Genet. Eng. Rev. 2008, 25, 267–314. [Google Scholar] [CrossRef] [PubMed]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Merga, B.; Jema, H. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Clarke, H.; Siddique, K. Response of chickpea genotypes to low temperature stress during reproductive development. Field Crops Res. 2004, 90, 323–334. [Google Scholar] [CrossRef]
- Pande, S.; Siddique, K.; Kishore, G.; Bayaa, B.; Gaur, P.; Gowda, C.; Bretag, T.; Crouch, J. Ascochyta blight of chickpea (Cicer arietinum L.): A review of biology, pathogenicity, and disease management. Crop Pasture Sci. 2005, 56, 317–332. [Google Scholar] [CrossRef]
- Anbessa, Y.; Warkentin, T.; Vandenberg, A.; Ball, R. Inheritance of time to flowering in chickpea in a short-season temperate environment. J. Hered. 2006, 97, 55–61. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef]
- Sudupak, A.; Akkaya, S.; Kence, A. Analysis of genetic relationships among perennial and annual Cicer species growing in Turkey using RAPD markers. Theor. Appl. Genet. 2002, 105, 1220–1228. [Google Scholar] [CrossRef]
- Jaiswal, H.K.; Singh, B.D.; Singh, A.K.; Singh, R.M. Introgression of genes for yield and yield traits from C. reticulatum into C. arietinum. Int. Chickpea Newsletter. 1986, 14, 5–8. [Google Scholar]
- Singh, M.; Kumar, K.; Bisht, I.S.; Dutta, M.; Rana, M.K.; Rana, J.C.; Bansal, K.C.; Sarker, A. Exploitation of wild annual Cicer species for widening the gene pool of chickpea cultivars. Plant Breed. 2015, 134, 186–192. [Google Scholar] [CrossRef]
- Robertson, L.D.; Ocampo, B.; Singh, K.B. Morphological variation in wild annual Cicer species in comparison to the cultigen. Euphytica 1997, 95, 309–319. [Google Scholar] [CrossRef]
- Harlan, J.R. Genetic resources in wild relatives of crops. Crop Sci. 1976, 16, 329–333. [Google Scholar] [CrossRef]
- McCouch, S. Diversifying selection in plant breeding. PLoS Biol. 2004, 2, e347. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Upadhyaya, H.D.; Stalker, H.T.; Blair, M.W.; Bertioli, D.J.; Nielen, S.; Ortiz, R. Enhancing crop gene pools with beneficial traits using wild relatives. Plant Breed. Rev. 2008, 30, 179–230. [Google Scholar]
- Van Rheenen, H.A.; Pundir, R.P.S.; Miranda, J.H. How to accelerate the genetic improvement of a recalcitrant crop species such as chickpea. Curr. Sci. 1993, 65, 414–417. [Google Scholar]
- Ahmad, F.; Gaur, P.M.; Croser, J. Chickpea (Cicer arietinum L.). In Genetic Resources, Chromosome Engineering and Crop Improvement-Grain Legumes; Singh, R.J., Jauhar, P.P., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 187–217. [Google Scholar]
- Collard, B.C.Y.; Pang, E.C.K.; Ades, P.K.; Taylor, P.Y.J. Preliminary investigation of QTLs associated with seedlings resistance to ascochyta blight from Cicer echinospermum, a wild relative of chickpea. Theor. Appl. Genet. 2003, 107, 719–729. [Google Scholar] [CrossRef]
- Sharma, S.; Upadhyaya, H.D.; Varshney, R.K.; Gowda, C.L. Pre-breeding for diversification of primary gene pool and genetic enhancement of grain legumes. Front. Plant Sci. 2013, 4, 309–314. [Google Scholar] [CrossRef]
- Mason, A.S. Polyploidy and Hybridization for Crop Improvement; CBC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2016. [Google Scholar]
- Aggarwal, H.; Rao, A.; Rana, J.S.; Singh, J.; Kumar, A.; Chhokar, V.; Beniwal, V. Inter simple sequence repeats reveal significant genetic diversity among chickpea (Cicer arietinum L.) cultivars. J. Plant Sci. 2011, 6, 202–212. [Google Scholar] [CrossRef]
- Gupta, P.K.; Varshney, R.K. Cereal genomics: An overview. In Cereal Genomics; Gupta, P.K., Varshney, R.K., Eds.; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2004; pp. 1–18. [Google Scholar]
- Kujur, A.; Bajaj, D.; Saxena, M.S.; Tripathi, S.; Upadhyaya, H.D.; Gowda, C.L.L.; Singh, S.; Jain, M.; Tyagi, A.K.; Parida, S.K. Functionally relevant microsatellite markers from chickpea transcription factor genes for efficient genotyping applications and trait association mapping. DNA Res. 2013, 20, 355–374. [Google Scholar] [CrossRef]
- Bajaj, D.; Das, S.; Badoni, S.; Kumar, V.; Singh, M.; Bansal, K.C.; Tyagi, A.K.; Parida, S.K. Genome-wide high-throughput SNP discovery and genotyping for understanding natural (functional) allelic diversity and domestication patterns in wild chickpea. Sci. Rep. 2015, 5, 12468. [Google Scholar] [CrossRef]
- Varshney, R.K.; Song, C.; Saxena, R.K.; Azam, S.; Yu, S.; Sharpe, A.G.; Cannon, S.; Baek, J.; Rosen, B.D.; Tar’An, B.; et al. Draft genome sequence of chickpea (Cicer arietinum) provides a resource for trait improvement. Nat. Biotechnol. 2013, 31, 240–246. [Google Scholar] [CrossRef]
- Jones, E.S.; Sullivan, H.; Bhattramakki, D.; Smith, J.S.C. A comparison of simple sequence repeat and single nucleotide polymorphism marker technologies for the genotypic analysis of maize (Zea mays L.). Theor. Appl. Genet. 2007, 115, 361–371. [Google Scholar] [CrossRef]
- Hall, A.; Bastow, R.M.; Davis, S.J.; Hanano, S.; McWatters, H.G.; Hibberd, V.; Doyle, M.R.; Sung, S.; Halliday, K.J.; Amasino, R.M.; et al. The TIME FOR COFFEE gene maintains the amplitude and timing of Arabidopsis circadian clocks. Plant Cell 2003, 15, 2719–2729. [Google Scholar] [CrossRef]
- Rieu, I.; Ruiz-Rivero, O.; Fernandez-Garcia, N.; Griffiths, J.; Powers, S.J.; Gong, F.; Linhartova, T.; Eriksson, S.; Nilsson, O.; Thomas, S.G.; et al. The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J. 2008, 53, 488–504. [Google Scholar] [CrossRef]
- Onai, K.; Ishiura, M. PHYTOCLOCK 1 encoding a novel GARP protein essential for the Arabidopsis circadian clock. Genes Cells 2005, 10, 963–972. [Google Scholar] [CrossRef]
- Peng, P.; Yan, Z.; Zhu, Y.; Li, J. Regulation of the Arabidopsis GSK3-like kinase BRASSINOSTEROID-INSENSITIVE 2 through proteasome-mediated protein degradation. Mol. Plant. 2008, 1, 338–346. [Google Scholar] [CrossRef]
- Weigel, M.; Varotto, C.; Pesaresi, P.; Finazzi, G.; Rappaport, F.; Salamini, F.; Leister, D. Plastocyanin is indispensable for photosynthetic electron flow in Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 31286–31289. [Google Scholar] [CrossRef]
- Friso, G.; Giacomelli, L.; Ytterberg, A.J.; Peltier, J.B.; Rudella, A.; Sun, Q.; Wijk, K.J. In-depth analysis of the thylakoid membrane proteome of Arabidopsis thaliana chloroplasts: New proteins, new functions, and a plastid proteome database. Plant Cell 2004, 16, 478–499. [Google Scholar] [CrossRef]
- Siddique, K.H.M.; Brindsmead, R.B.; Knight, R.; Knights, E.J.; Paull, J.G.; Rose, I.A. Adaptation of chickpea and faba bean to Australia. In Linking Research and Marketing Opportunities for Pulses in the 21st Century; Knights, R., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 289–303. [Google Scholar]
- Kantar, M.B.; Nashoba, A.R.; Anderson, J.E.; Blackman, B.K.; Rieseberg, L.H. The Genetics and genomics of plant domestication. BioScience 2017, 67, 971–982. [Google Scholar] [CrossRef]
- Singh, K.B.; Ocampo, B. Interspecific hybridization in annual Cicer species. J. Genet. Breed. 1993, 47, 199–204. [Google Scholar]
- Singh, M.; Rani, S.; Malhotra, N.; Katna, G.; Sarker, A. Transgressive segregations for agronomic improvement using interspecific crosses between C. arietinum L. × C. reticulatum L. and C. arietinum L. × C. echinospermum Davis species. PLoS ONE 2018, 13, e0203082. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Sharma, H.C.; Upadhyaya, H.D. Exploitation of wild relatives of pigeon pea and chickpea for resistance to Helicoverpa armigera. J. SAT Agric. Res. 2007, 3, 4. [Google Scholar]
- von Wettberg, E.J.B.; Chang, P.L.; Başdemir, F.; Carrasquila-Garcia, N.; Korbu, L.B.; Moenga, S.M.; Bedada, G.; Greenlon, A.; Moriuchi, K.S.; Singh, V.; et al. Ecology and genomics of an important crop wild relative as a prelude to agricultural innovation. Nat. Commun. 2018, 9, 12. [Google Scholar] [CrossRef]
- Gaur, P.M.; Gowda, C.L.L.; Knights, E.J.; Warkentin, T.D.; Acikgoz, N. Breeding Achievements. In Chickpea Breeding and Management; Yadav, S.S., Redden, B., Chen, W., Sharma, B., Eds.; CABI: Wallingford, UK, 2007; pp. 391–416. [Google Scholar]
- Kaloki, P.; Devasirvatham, V.; Tan, D.K.Y. Chickpea abiotic stresses: Combating drought, heat and cold, abiotic and biotic stress in plants. In Alexandre Bosco de Oliveira; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, M.; Bajaj, D.; Parida, S.K. A high-resolution InDel (insertion-deletion) markers-anchored consensus genetic map identifies major QTLs governing pod number and seed yield in chickpea. Front. Plant Sci. 2016, 7, 1362. [Google Scholar] [CrossRef]
- Kozak, M.; Krzanowski, W.; Tartanus, M. Use of the correlation coefficient in agricultural sciences: Problems, pitfalls and how to deal with them. Acad. Bras. De Ciências 2012, 84, 1147–1156. [Google Scholar] [CrossRef]
- Patane, C. Variation and relationships among some nutritional traits in Sicilian genotypes of chickpea (Cicer arietinum L.). J. Food Qual. 2006, 29, 282–293. [Google Scholar] [CrossRef]
- Hu, X.; Carver, B.F.; Powers, C.; Yan, L.; Zhu, L.; Chen, C. Effectiveness of genomic selection by response to selection for winter wheat variety improvement. Plant Genome. 2019, 12, 180090. [Google Scholar] [CrossRef]
- Qureshi, A.S.; Shaukat, A.; Bakhsh, A.; Arshad, M.; Ghafoor, A. An assessment of variability for economically important traits in chickpea (Cicer Arietinum L.). Pak. J. Bot. 2004, 36, 779–785. [Google Scholar]
- Ahmad, Z.; Mumtaz, A.S.; Nisar, M.; Khan, N. Diversity Analysis of Chickpea (Cicer arietinum L.) Germplasm and its Implications for Conservation and Crop Breeding. Agric. Sci. 2012, 3, 723–731. [Google Scholar]
- Belete, T.; Mekbib, F.; Eshete, M. Assessment of genetic improvement in grain yield potential and related traits of kabuli type chickpea (Cicer arietinum L.) varieties in Ethiopia (1974–2009). Adv. Crop. Sci. Tech. 2017, 5, 284. [Google Scholar] [CrossRef]
- Jha, U.C.; Singh, S.P.; Lavanya, G.R. Assessment of genetic variability and correlation of important yield related traits in chickpea (Cicer arientinum L.). Legume Res. 2012, 35, 341–344. [Google Scholar]
- Chongo, G.; Buchwaldt, L.; Gossen, B.D.; Lafond, G.P.; May, W.E.; Johnson, E.N.; Hogg, T. Foliar fungicides to manage ascochyta blight (Ascochyta rabiei) of chickpea in Canada. Can. J. Plant Pathol. 2003, 25, 135–142. [Google Scholar] [CrossRef]
- Tadesse, M.; Turoop, L.; Ojiewo, C.O. Survey of Chickpea (Cicer arietinum L.) Ascochyta Blight (Ascochyta rabiei Pass.) Disease status in production regions of Ethiopia. Plant 2017, 5, 23–30. [Google Scholar] [CrossRef]
- Frimpong, A.; Sinha, A.; Tar’an, B.; Warkentin, T.D.; Gossen, B.D.; Chibbar, R.N. Genotype and growing environment influence chickpea (Cicer arietinum L.) seed composition. J. Sci. Food Agric. 2009, 89, 2052–2063. [Google Scholar] [CrossRef]
- Crossa, J. From genotype x environment interaction to gene × environment interaction. Curr. Genomics. 2012, 13, 225–244. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Gaur, P.M. Rapid screening technique for canopy temperature status and its relevance to drought tolerance improvement in chickpea. SAT ejournal 2008, 6, 1–4. [Google Scholar]
- Available online: http://www.icrisat.org/journal/volume6/chickpea_pigeonpea/kashiwagil.pdf (accessed on 1 October 2019).
- Varshney, R.K.; Thudi, M.; Nayak, S.N.; Gaur, P.M.; Kashiwagi, J.; Krishnamurthy, L.; Jaganathan, D.; Koppolu, J.; Bohra, A.; Tripathi, S.; et al. Genetic dissection of drought tolerance in chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2014, 127, 445–462. [Google Scholar] [CrossRef]
- Toker, C. Estimates of broad-sense heritability for seed yield and yield criteria in faba bean (Vicia faba L.). Hereditas 2004, 140, 222–225. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, I.S.; Yadav, C.K. Stability analysis of yield and related traits in chickpea (Cicer arietinum L.). Legume Res. 2014, 37, 641–645. [Google Scholar] [CrossRef]
- Abdi, K.D. Stability Analysis in Chickpea Genotype Sets as Tool for Breeding Germplasm Structuring Strategy and Adaptability Scoping. Aust. J. Crop Sci. 2018, 6, 2072–8506. [Google Scholar]
- Khan, R.; Farhatullah, K.H. Dissection of genetic variability and heritability estimates of chickpea germplasm for various morphological markers and quantitative traits. Sarhad J. Agric. 2011, 27, 67–72. [Google Scholar]
- Mba, C.; Guimaraes, E.P.; Ghosh, K. Re-orienting crop improvement for the changing climatic conditions of the 21st century. Agric. Food Secur. 2012, 1, 7. [Google Scholar] [CrossRef]
- Addisu, F.; Shumet, T. Variability, heritability and genetic advance for some yield and yield related traits in barley (Hordeum vulgare L.) landraces in Ethiopia. Int. J. Plant Breed. Genet. 2015, 9, 68–76. [Google Scholar] [CrossRef]
- Yirgu, M. A review on heritable improvement in yield potential and morphological traits of cereal and pulse crops in Ethiopia. Glob. J. Agric. Res. Rev. 2017, 5, 239–243. [Google Scholar]
- Zali, H.; Farshadfar, E.; Sabaghpour, S.H. Genetic variability and interrelationships among agronomic traits in chickpea (Cicer arietinum L.) genotypes. Crop. Breed. J. 2011, 1, 127–132. [Google Scholar]
- Nath, U.K.; Rani, S.; Paul, M.R.; Alam, M.N.; Horneburg, B. Selection of superior lentil (Lens esculenta M.) genotypes by assessing character association and genetic diversity. Sci. World J. 2014, 2014, 372–405. [Google Scholar] [CrossRef]
- Sharifi, P.; Astereki, H.; Pouresmael, M. Evaluation of variations in chickpea (Cicer arietinum L.) yield and yield components by multivariate technique. Ann. Agrar. Sci. 2018, 16, 136–142. [Google Scholar] [CrossRef]
- Admas, S.; Abeje, G. Phenotypic diversity studies in chickpea (Cicer arietinum L.) Germplasm of Ethiopian Collections. Int. J. Curr. Res. 2017, 9, 48506–48512. [Google Scholar]
- Sachdeva, S.; Bharadwaj, C.; Sharma, V.; Patil, B.S.; Soren, K.R.; Roorkiwal, M.; Varshney, R.; Bhat, K.V. Molecular and phenotypic diversity among chickpea (Cicer arietinum) genotypes as a function of drought tolerance. Crop. Pasture Sci. 2018, 69, 142–153. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Dwivedi, S.L.; Baum, M.; Varshney, R.K.; Udupa, S.M.; Gowda, C.L.; Hoisington, D.; Singh, S. Genetic structure, diversity, and allelic richness in composite collection and reference set in chickpea (Cicer arietinum L.). BMC Plant Biol. 2008, 8, 106. [Google Scholar] [CrossRef]
- Sharma, S. Pre-breeding using wild species for genetic enhancement of grain legumes at ICRISAT. Crop Sci. 2017, 57, 1132–1144. [Google Scholar] [CrossRef]
- Adak, A.; Sari, D.; Sari, H.; Toker, C. Gene effects of Cicer reticulatum on qualitative and quantitative traits in the cultivated chickpea. Plant Breed. 2017, 136, 939–947. [Google Scholar] [CrossRef]
- Verma, M.M.; Sandhu, J.S.; Brar, H.S.; Brar, J.S. Crossability studies in different species of Cicer (L.). Crop. Improv. 1990, 17, 179–181. [Google Scholar]
- Varshney, R.K.; Nayak, S.; Jayashree, B.; Eshwar, K.; Upadhyaya, H.D.; Hoisington, D.A. Development of cost-effective SNP assays for chickpea genome analysis and breeding. J. SAT Agric. Res. 2007, 3, 29–31. [Google Scholar]
- Yang, J.; Manolio, T.A.; Pasquale, L.R.; Boerwinkle, E.; Caporaso, N.; Cunningham, J.M.; de Andrade, M.; Feenstra, B.; Feingold, E.; Hayes, M.G.; et al. Genome partitioning of genetic variation for complex traits using common SNPs. Nat. Genet. 2011, 43, 519–525. [Google Scholar] [CrossRef]
- Caruana, B.M.; Pembleton, L.; Constable, W.; Rodoni, F.; Slater, A.T.; Cogan, N.O.I. Validation of genotyping by sequencing using transcriptomics for diversity and application of genomic selection in tetraploid potato. Front. Plant Sci. 2019, 10, 670. [Google Scholar] [CrossRef]
- Cobos, M.J.; Fernandez, M.J.; Rubio, J.; Kharrat, M.; Moreno, M.T.; Gil, J.; Millan, T. A linkage map of chickpea (Cicer arietinum L.) based on populations from Kabuli x Desi cross; location of genes for resistance to fusarium wilt race 0. Theor. Appl. Genet. 2005, 110, 1347–1353. [Google Scholar] [CrossRef]
- Huang, X.H.; Han, B. Natural variations and genome-wide association studies in crop plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef]
- Long, L.; Peng, Z.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genome-wide association study reveals genomic regions controlling root and shoot traits at late growth stages in wheat. Ann. Bot. 2019, 124, 993–1006. [Google Scholar] [CrossRef]
- Skotte, L.; Korneliussen, T.S.; Albrechtsen, A. Estimating individual admixture proportions from next generation sequencing data. Genetics 2013, 195, 693–702. [Google Scholar] [CrossRef]
- Farahani, S.; Maleki, M.; Mehrabi, R.; Kanouni, H.; Scheben, A.; Batley, J.; Talebi, R. Whole Genome Diversity, Population Structure, and Linkage Disequilibrium Analysis of Chickpea (Cicer arietinum L.) Genotypes Using Genome-Wide DArTseq-Based SNP Markers. Genes 2019, 10, 676. [Google Scholar] [CrossRef]
- Ott, A.; Liu, S.; Schnable, J.C.; Yeh, C.-T.E.; Wang, K.-S.; Schnable, P.S. tGBS® genotyping-by-sequencing enables reliable genotyping of heterozygous loci. Nucleic Acids Res. 2017, 45, e178. [Google Scholar] [CrossRef]
- Zhao, K.; Tung, C.W.; Eizenga, G.C.; Wright, M.H.; Ali, M.L.; Price, A.H.; Norton, G.J.; Islam, M.R.; Reynolds, A.; Mezey, J.; et al. Genome-wide association mapping reveals a rich genetic architecture of complex traits in Oryza sativa. Nat. Commun. 2011, 2, 467. [Google Scholar] [CrossRef]
- Saxena, M.S.; Bajaj, D.; Kujur, A.; Das, S.; Badoni, S.; Kumar, V.; Singh, M.; Bansal, K.C.; Tyagi, A.K.; Parida, S.K. Natural allelic diversity, genetic structure and linkage disequilibrium pattern in wild chickpea. PLoS ONE 2014, 9, e107484. [Google Scholar] [CrossRef][Green Version]
- Nguyen, N.H.; Premachandra, H.K.A.; Kilian, A.; Knibb, W. Genomic prediction using DArT-Seq technology for yellowtail kingfish Seriola lalandi. BMC Genom. 2018, 19, 107. [Google Scholar] [CrossRef]
- Basu, U.; Srivastava, R.; Bajaj, D.; Thakro, V.; Daware, A.; Malik, N.; Upadhyaya, H.D.; Parida, S.K. Genome-wide generation and genotyping of informative SNPs to scan molecular signatures for seed yield in chickpea. Sci. Rep. 2018, 8, 13240. [Google Scholar] [CrossRef]
- Roorkiwal, M.; von Wettberg, E.J.; Upadhyaya, H.D.; Warschefsky, E.; Rathore, A.; Varshney, R.K. Exploring germplasm diversity to understand the domestication process in Cicer spp. using SNP and DArT Markers. PLoS ONE 2013, 9, e102016. [Google Scholar] [CrossRef]
- Winkler, L.R.; Bonman, J.M.; Chao, S.; Yimer, B.A.; Bockelman, H.; Klos, K.E. Population structure and genotype-phenotype associations in a collection of oat landraces and historic cultivars. Front. Plant Sci. 2016, 7, 1077. [Google Scholar] [CrossRef]
- Fu, Z.; Epstein, B.; Kelley, J.L.; Zheng, Q.; Bergland, A.O.; Carrillo, C.I.C.; Jensen, A.S.; Dahan, J.; Karasev, A.V.; Snyder, W.E. Using NextRAD sequencing to infer movement of herbivores among host plants. PLoS ONE 2017, 12, e0177742. [Google Scholar] [CrossRef]
- Azhaguvel, P.; Vidya, S.D.; Sharma, A.; Varshney, R.K. Methodological advancement in molecular markers to delimit the gene(s) for crop improvement. In Floriculture, Ornamental and Plant Biotechnology Advances and Tropical Issues; Texiera da Silva, J., Ed.; Global Science Books: London, UK, 2006; pp. 460–499. [Google Scholar]
- Abbo, S.; Molina, C.; Jungmann, R.; Grusak, M.; Berkovitch, Z.; Reifen, R.; Kahl, G.; Winter, P. Quantitative trait loci governing carotenoid concentration and weight in seeds of chickpea (Cicer arietinum L.). Theor. Appl. Genet. 2005, 111, 185–195. [Google Scholar] [CrossRef]
- Thudi, M.; Upadhyaya, H.D.; Rathore, A.; Gaur, P.M.; Krishnamurthy, L.; Roorkiwal, M.; Nayak, S.N.; Chaturvedi, S.K.; Basu, P.S.; Gangarao, N.V.P.R.; et al. Genetic dissection of drought and heat tolerance in chickpea through genome-wide and candidate gene-based association mapping approaches. PLoS ONE 2014, 9, e96758. [Google Scholar] [CrossRef]
- Ridge, S.; Deokar, A.; Lee, R.; Daba, K.; Macknight, R.C.; Weller, J.L.; Tar’an, B. The chickpea early flowering 1 (Efl1) locus is an ortholog of Arabidopsis ELF3. Plant Physiol. 2017, 175, 802–875. [Google Scholar] [CrossRef]
- Upadhyaya, H.D.; Bajaj, D.; Das, S.; Saxena, M.S.; Badoni, S.; Kumar, V.; Tripathi, S.; Gowda, C.L.L.; Sharma, S.; Tyagi, A.K.; et al. A genome-scale integrated approach aids in genetic dissection of complex flowering time trait in chickpea. Plant Mol. Biol. 2015, 89, 403–420. [Google Scholar] [CrossRef]
- Daba, K.; Warkentin, T.D.; Bueckert, R.; Todd, C.D.; Tar’an, B. Determination of Photoperiod-Sensitive Phase in Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 478. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.N.; Li, G.J.; Wang, X.; Cheng, L.G.; Zhang, Y.M. Mapping quantitative trait loci for seed size traits in soybean (Glycine max L. Merr.). Theor. Appl. Genet. 2011, 122, 581–594. [Google Scholar] [CrossRef]
- Danehloueipour, N.; Yan, G.; Clarke, H.J.; Siddique, K.H.M. Successful stem cutting propagation of chickpea, its wild relatives and their interspecific hybrids. Aust. J. Exp. Agric. 2006, 46, 1349–1354. [Google Scholar] [CrossRef]
- Federer, W.T. Augmented (or hoonuiku) designs. Hawaii Plant. Rec. 1956, 55, 191–208. [Google Scholar]
- Reddy, M.V.; Singh, K.B. Evaluation of a world collection of chickpea germplasm accessions for resistance to ascochyta blight. Plant Dis. 1984, 68, 900–901. [Google Scholar] [CrossRef]
- Wu, T.D.; Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 2010, 26, 873–881. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Singh, M.; Ceccarelli, S.; Hamblin, J. Estimation of heritability from varietal trials data. Theor. Appl. Genet. 1993, 86, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.H.; Lange, K. Enhancements to the ADMIXTURE algorithm for individual ancestry estimation. BMC Bioinform. 2011, 12, 246. [Google Scholar] [CrossRef]
- Li, Y.L.; Liu, J.X. Structure Selector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Res. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Xavier, A.; Xu, S.; Muir, W.M.; Rainey, K.M. NAM: Association studies in multiple populations. Bioinformatics 2015, 31, 3862–3864. [Google Scholar] [CrossRef]


| Traits | Saskatoon-2017 | Moose Jaw-2017 | ||||||
|---|---|---|---|---|---|---|---|---|
| F4 Lines | CDC Leader | F4 Lines | CDC Leader | |||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | |
| Days to flowering | 47.0–63.0 | 53.0 (5.00) | 49.0–54.0 | 52.0 (2.00) | 43.0–58.0 | 50.0 (5.00) | 50.0–53.0 | 52.0 (2.00) |
| Days to maturity | 72.0–99.0 | 89.0 (7.40) | 87.0–93.0 | 90.0 (2.00) | 70.0–99.0 | 88.0 (8.00) | 89.0–92.0 | 91.0 (2.00) |
| Plant height (cm) | 20.0–64.0 | 31.9 (19.0) | 32.0–35.0 | 33.0 (1.73) | 18.0–50.0 | 28.9 (15.0) | 30.0–36.0 | 32.7 (3.06) |
| Ascochyta blight score | 4.00–8.00 | 6.22 (1.40) | 0.0 | 0.0 | 4.00–9.00 | 5.39 (2.30) | 0.0 | 0.0 |
| Biomass yield per plant (g) | 10.5–346 | 53.1 (99.0) | 10.7–20.9 | 14.6 (5.51) | 1.30–98.8 | 13.5 (88.0) | 9.28–15.6 | 13.1 (3.37) |
| Number of seeds per plant | 1.00–180 | 43.0 (77.0) | 21.0–42.0 | 33.0 (11.0) | 1.00–53.0 | 18.0 (51.0) | 14.0–49.0 | 27.0 (19.0) |
| Thousand seed weight (g) | 101–695 | 361 (27.0) | 211–250 | 227 (20.3) | 121–453 | 246 (21.0) | 239–268 | 256 (15.1) |
| Seed weight per plant (g) | 0.10–96.0 | 10.7 (75.0) | 4.44–8.74 | 7.00 (2.28) | 0.10–14.2 | 4.37 (54.0) | 8.86–11.7 | 10.1 (1.47) |
| Seed yield (kg/ha) | - | - | - | - | 100–6000 | 1820 (55.0) | 2392–2676 | 2563 (150) |
| Harvest index | 0.02–0.84 | 0.31 (37.0) | 0.48–0.56 | 0.50 (0.04) | 0.01–0.69 | 0.40 (39.0) | 0.35–0.56 | 0.40 (0.12) |
| Traits | Limerick-2018 | Lucky Lake-2018 | ||||||
|---|---|---|---|---|---|---|---|---|
| F5 Lines | CDC Leader | F5 Lines | CDC Leader | |||||
| Range | Mean | Range | Mean | Range | Mean | Range | Mean | |
| Days to flowering | 42.0–57.0 | 49.0 (4.80) | 55.0–57.0 | 56.0 (1.00) | 31.0–53.0 | 45.0 (5.50) | 50.0–54.0 | 52.0 (2.00) |
| Days to maturity | 82.0–96.0 | 90.0 (2.70) | 88.0–93.0 | 91.0 (3.00) | 75.0–95.0 | 88.0 (2.90) | 89.0–93.0 | 91.0 (2.00) |
| Plant height (cm) | 22.0–45.0 | 32.0 (17.0) | 34.0–38.0 | 36.0 (2.08) | 16.7–35.0 | 27.4 (13.0) | 28.0–32.0 | 30.0 (2.00) |
| Ascochyta blight score | 4.00–9.00 | 6.90 (32.0) | - | - | - | - | - | - |
| Biomass yield per plant (g) | 1.00–37.2 | 13.9 (19.0) | 15.0–18.0 | 13.0 (3.66) | 3.20–57.6 | 17.5 (17.0) | 13.1–19.7 | 15.8 (3.50) |
| Number of seeds per plant | 2.00–45.0 | 15.0 (16.0) | 44.0–51.0 | 47.0 (4.00) | 3.00–103 | 26.0 (16.0) | 16.0–31.0 | 21.0 (8.00) |
| Thousand seed weight (g) | 136–467 | 236 (15.0) | 262–281 | 270 (9.00) | 135–576 | 261 (11.0) | 271–358 | 335 (57.0) |
| Seed weight per plant (g) | 0.40–10.6 | 3.49 (32.0) | 11.8–14.3 | 12.8 (1.34) | 1.70–14.6 | 6.73 (30.0) | 7.17–11.1 | 8.70 (2.01) |
| Seed yield (kg/ha) | 20–4400 | 1460 (28.0) | 2623–2807 | 2703 (95) | 100–3900 | 1740 (28.0) | 3302–3533 | 3395 (122) |
| Harvest index | 0.02–0.71 | 0.27 (29.0) | 0.77–0.80 | 0.78 (0.01) | 0.13–0.66 | 0.38 (18.0) | 0.54–0.56 | 0.50 (0.01) |
| Traits | DTM | PH | ABS | BY | NSPP | TSW | HI | SWPP |
|---|---|---|---|---|---|---|---|---|
| DTF | 0.02 ns | 0.04 ns | −0.04 ns | 0.01 ** | −0.13 * | 0.08 ns | −0.28 *** | −0.11 * |
| DTM | −0.03 ns | 0.07 ns | −0.07 ns | −0.15 ** | 0.11 * | −0.25 *** | −0.13 * | |
| PH | −0.18 *** | 0.63 *** | 0.50 *** | 0.16 *** | −0.18 *** | 0.51 *** | ||
| ABS | −0.17 *** | −0.22 *** | 0.01 ns | −0.05 ns | −0.24 *** | |||
| BY | 0.78 *** | 0.26 *** | −0.20 *** | 0.82 *** | ||||
| NSPP | −0.05 ns | 0.21 *** | 0.95 *** | |||||
| TSW | −0.24 *** | 0.09 ns | ||||||
| HI | 0.22 *** |
| Traits | DTM | PH | ABS | BY | SWPP | NSPP | TSW | HI | SY |
|---|---|---|---|---|---|---|---|---|---|
| DTF | 0.19 *** | 0.21 *** | 0.03 ns | 0.15 *** | 0.05 ns | 0.01 ns | 0.14 ** | −0.18 *** | 0.08 ns |
| DTM | 0.12 * | 0.09 ns | 0.29 *** | 0.07 ns | 0.07 ns | 0.08 ns | −0.35 *** | 0.10 * | |
| PH | −0.01 ns | 0.33 *** | 0.29 *** | 0.23 *** | 0.16 *** | −0.03 ns | 0.28 *** | ||
| ABS | 0.07 ns | 0.04 ns | 0.04 ns | 0.03 ns | −0.06 ns | 0.02 ns | |||
| BY | 0.80 *** | 0.75 *** | 0.22 *** | −0.21 *** | 0.76 *** | ||||
| SWPP | 0.90 *** | 0.37 *** | 0.35 *** | 0.99 *** | |||||
| NSPP | −0.04 ns | 0.27 *** | 0.88 *** | ||||||
| TSW | 0.26 *** | 0.36 *** | |||||||
| HI | 0.33 *** |
| Traits | DTM | PH | ABS | BY | SWPP | NSPP | TSW | HI | SY |
|---|---|---|---|---|---|---|---|---|---|
| DTF | 0.28 *** | 0.23 *** | −0.16 ** | 0.22 *** | 0.01 ns | −0.01 ns | 0.11 ** | −0.15 *** | 0.00 ns |
| DTM | 0.29 *** | 0.12 ** | 0.21 *** | −0.07 ns | −0.06 ns | 0.10 * | −0.15 *** | −0.07 ns | |
| PH | −0.03 ns | 0.37 *** | −0.05 ns | −0.05 ns | 0.03 ns | −0.21 *** | −0.04 ns | ||
| ABS | −0.33 *** | −0.07 ns | −0.01 ns | −0.15 *** | 0.13 * | −0.06 ns | |||
| BY | 0.07 ns | 0.05 ns | 0.11 * | −0.33 *** | 0.08 ns | ||||
| SWPP | 0.92 *** | 0.17 *** | 0.69 *** | 0.99 *** | |||||
| NSPP | −0.13 ** | 0.69 *** | 0.91 *** | ||||||
| TSW | 0.04 ns | 0.18 *** | |||||||
| HI | 0.67 *** |
| Traits | DTM | PH | BY | SWPP | NSPP | TSW | HI | SY |
|---|---|---|---|---|---|---|---|---|
| DTF | 0.53 *** | 0.28 *** | 0.25 *** | 0.01 ns | 0.04 ns | 0.17 *** | −0.14 *** | 0.03 ns |
| DTM | 0.31 *** | 0.37 *** | −0.07 ns | 0.14 *** | 0.16 *** | −0.16 *** | 0.05 ns | |
| PH | 0.18 *** | 0.06 ns | 0.02 ns | 0.16 *** | −0.04 ns | 0.13 ** | ||
| BY | 0.03 ns | 0.80 *** | 0.17 *** | −0.20 *** | 0.34 ns | |||
| SWPP | 0.27 *** | 0.16 *** | 0.39 *** | 0.54 *** | ||||
| NSPP | −0.16 *** | 0.48 *** | 0.42 *** | |||||
| TSW | 0.30 *** | 0.31 *** | ||||||
| HI | 0.57 *** |
| Pathway | Direct Effect | |||
|---|---|---|---|---|
| Saskatoon-2017 | Moose Jaw-2017 | |||
| Standardized Estimates | Standard Error | Standardized Estimates | Standard Error | |
| BY→ NSPP | 0.85 *** | 0.03 | 0.17 *** | 0.00 |
| BY→ SWPP | 0.11 ** | 0.01 | 0.01 ns | 0.00 |
| BY→ HI | −0.98 *** | 0.00 | −0.63 *** | 0.00 |
| TSW→ NSPP | −0.27 *** | 0.00 | −0.01 ns | 0.01 |
| TSW→ SWPP | 0.10 ** | 0.00 | 0.36 *** | 0.00 |
| TSW→ HI | −0.06 ns | 0.00 | 0.24 *** | 0.00 |
| NSPP→ SWPP | 0.87 *** | 0.01 | 0.91 *** | 0.00 |
| NSPP→ HI | 0.01 ns | 0.00 | 0.48 *** | 0.00 |
| SWPP→ HI | 0.98 *** | 0.00 | 0.07 ns | 0.01 |
| Indirect effect | ||||
| BY→ HI (Through NSPP) | 0.00 | - | 0.08 | - |
| BY→ HI (Through SWPP) | 0.11 | - | 0.00 | - |
| TSW→ HI (Through NSPP) | −0.00 | - | −0.00 | - |
| TSW→ HI (Through SWPP) | −0.01 | - | 0.03 | - |
| NSPP→ HI (Through SWPP) | 0.00 | - | 0.43 | - |
| Pathway | Direct Effect | |||
|---|---|---|---|---|
| Limerick-2018 | Lucky Lake-2018 | |||
| Standardized Estimates | Standard Error | Standardized Estimates | Standard Error | |
| BY→ NSPP | 0.06 ns | 0.07 | 0.75 *** | 0.06 |
| BY→ SWPP | −0.00 ns | 0.00 | 0.01 ns | 0.03 |
| BY→ HI | −0.38 *** | 0.00 | −0.59 *** | 0.00 |
| TSW→ NSPP | −0.14 ** | 0.01 | −0.22 *** | 0.01 |
| TSW→ SWPP | 0.30 *** | 0.00 | 0.07 ns | 0.00 |
| TSW→ HI | 0.14 ** | 0.00 | 0.50 *** | 0.00 |
| NSPP→ SWPP | 0.96 *** | 0.00 | 0.02 ns | 0.02 |
| NSPP→ HI | 0.62 *** | 0.00 | 0.98 *** | 0.00 |
| SWPP→ HI | 0.13 * | 0.01 | 0.06 ns | 0.00 |
| Indirect effect | ||||
| BY→ HI (Through NSPP) | 0.04 | - | 0.74 | - |
| BY→ HI (Through SWPP) | −0.00 | - | 0.00 | - |
| TSW→ HI (Through NSPP) | −0.09 | - | 0.22 | - |
| TSW→ HI (Through SWPP) | 0.04 | - | 0.00 | - |
| NSPP→ HI (Through SWPP) | 0.12 | - | 0.00 | - |
| Traits | F Values of the Effects | H2 | ||
|---|---|---|---|---|
| G | E | G × E | ||
| Days to flowering | 6.36 *** | 919 *** | 1.03 ns | 0.54 |
| Days to maturity | 4.39 *** | 262 *** | 1.15 * | 0.35 |
| Plant height (cm) | 2.06 *** | 479 *** | 0.93 ns | 0.15 |
| Biomass weight per plant (g) | 1.93 *** | 39.8 *** | 1.03 ns | 0.14 |
| Number of seeds per plant | 2.78 *** | 681 *** | 1.56 *** | 0.18 |
| Thousand seed weight (g) | 6.47 *** | 263 *** | 6.16 *** | 0.08 |
| Seed weight per plant (g) | 3.04 *** | 710 *** | 1.43 *** | 0.45 |
| Traits | Cluster-I (67) | Cluster-II (104) | Cluster-III (68) | Cluster-IV (72) | Cluster-V (42) | Cluster-VI (28) |
|---|---|---|---|---|---|---|
| Days to flowering | 47.0 ± 2.0 | 47.0 ± 3.0 | 46.0 ± 2.0 | 50.0 ± 3.0 | 51.0 ± 2.0 | 45.0 ± 2.0 |
| Days to maturity | 88.0 ± 2.0 | 90.0 ± 2.0 | 87.0 ± 2.0 | 91.0 ± 2.0 | 89.0 ± 2.0 | 87.0 ± 1.0 |
| Plant height (cm) | 29.2 ± 3.6 | 28.8 ± 2.4 | 28.2 ± 2.7 | 33.3 ± 2.4 | 28.9 ± 2.1 | 29.0 ± 2.4 |
| Ascochyta blight score | 6.64 ± 1.6 | 7.81 ± 0.7 | 8.00 ± 0.7 | 6.83 ± 1.4 | 5.48 ± 1.5 | 4.65 ± 0.9 |
| Biomass yield per plant (g) | 17.6 ± 4.0 | 14.7 ± 3.3 | 10.8 ± 5.5 | 20.5 ± 4.3 | 16.4 ± 3.8 | 13.6 ± 4.3 |
| Number of seeds per plant | 28.0 ± 7.0 | 19.0 ± 6.0 | 17.0 ± 6.0 | 22.0 ± 6.0 | 21.0 ± 5.0 | 18.0 ± 4.0 |
| Thousand seed weight (g) | 253 ± 28 | 238 ± 31 | 234 ± 34 | 268 ± 43 | 245 ± 31 | 260 ± 45 |
| Seed weight per plant (g) | 6.87 ± 1.4 | 4.20 ± 1.1 | 5.32 ± 1.5 | 4.90 ± 1.4 | 4.65 ± 1.1 | 4.96 ± 1.4 |
| Seed yield (kg/ha) | 2310 ± 394 | 1332 ± 384 | 1360 ± 432 | 1680 ± 394 | 1598 ± 300 | 1650 ± 528 |
| Traits | Field Sites | Chromosome | Most Significant SNP | Number of SNPs | −log10 p Value |
|---|---|---|---|---|---|
| Days to flowering | Combined | VI | Ca6_V1_P-46744160 | 1 | 5.24 |
| Biomass | Combined | III | Ca3_V1_P-31624927 | 1 | 6.26 |
| Number of seeds per plant | Saskatoon | II | Ca2_V1_P-33400910 | 2 | 10.2 |
| IV | Ca4_V1_P-27008886 | 2 | 6.63 | ||
| V | Ca5_V1_P-28287194 | 1 | 6.04 | ||
| Moose Jaw | VI | Ca6_V1_P-22032893 | 2 | 5.09 | |
| Thousand seed weight (g) | Moose Jaw | I | Ca1_V1_P-710760 | 1 | 5.30 |
| IV | Ca4_V1_P-9707182 | 5 | 6.60 | ||
| Seed weight per plant (g) | Saskatoon | I | Ca1_V1_P-25733193 | 1 | 5.01 |
| II | Ca2_V1_P-30049933 | 2 | 6.32 | ||
| IV | Ca4_V1_P-40127929 | 1 | 5.12 | ||
| V | Ca5_V1_P-41263716 | 3 | 6.94 | ||
| VI | Ca6_V1_P-41147990 | 3 | 9.32 | ||
| VII | Ca7_V1_P-45085937 | 3 | 5.19 |
| Traits | Field Sites | Chromosome | Most Significant SNP | Number of SNPs | −log10 p Value |
|---|---|---|---|---|---|
| Days to flowering | Combined | IV | Ca4_V1_P-13022400 | 9 | 10.4 |
| Days to maturity | Lucky Lake | VIII | Ca8_V1_P-957257 | 1 | 5.08 |
| Biomass | Combined | VII | Ca7_V1_P-34285390 | 2 | 12.1 |
| Number of seeds per plant | Lucky Lake | II | Ca2_V1_P-15088105 | 1 | 5.02 |
| III | Ca3_V1_P-14460088 | 1 | 5.14 | ||
| VII | Ca7_V1_P-34285390 | 2 | 6.79 | ||
| VIII | Ca8_V1_P-310610 | 1 | 8.95 | ||
| Thousand seed weight (g) | Limerick | I | Ca1_V1_P-14313744 | 1 | 6.54 |
| II | Ca2_V1_P-17609263 | 2 | 6.28 | ||
| V | Ca5_V1_P-32629686 | 1 | 5.32 | ||
| Lucky Lake | V | Ca5_V1_P-29349635 | 1 | 5.23 | |
| VI | Ca6_V1_P-16130634 | 1 | 11.2 |
| Gene Id | Chromosome | Start | End | Description | Gene Function | Reference |
|---|---|---|---|---|---|---|
| Related to flowering | ||||||
| Ca_TIC | IV | 13836536 | 13844034 | protein (tic) | Early flowering | [26] |
| Ca_GA20OX2 | IV | 13002067 | 13004480 | gibberellin 20 oxidase 2 | Associated with flowering time | [27] |
| Ca_PCL1 | VI | 54242622 | 54245220 | transcription factor PCL1-like | Associated with flowering time | [28] |
| Related to yield | ||||||
| Ca_10265 | II | 32585905 | 32594820 | Protein kinase | Regulates photophosp-horylation activity | [29] |
| Ca_10221 | II | 33011956 | 33016412 | Protein kinase | Regulates photophosp-horylation activity | [29] |
| Ca_10204 | II | 33172919 | 33174836 | Plastocyanin-like | Involved in electrons to photosystem I | [30] |
| Ca_14921 | IV | 39853369 | 39854663 | Photosystem I PsaG/PsaK protein | Involved in photosynthesis | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.W.; Deokar, A.A.; Lindsay, D.; Tar’an, B. Novel Alleles from Cicer reticulatum L. for Genetic Improvement of Cultivated Chickpeas Identified through Genome Wide Association Analysis. Int. J. Mol. Sci. 2024, 25, 648. https://doi.org/10.3390/ijms25010648
Rahman MW, Deokar AA, Lindsay D, Tar’an B. Novel Alleles from Cicer reticulatum L. for Genetic Improvement of Cultivated Chickpeas Identified through Genome Wide Association Analysis. International Journal of Molecular Sciences. 2024; 25(1):648. https://doi.org/10.3390/ijms25010648
Chicago/Turabian StyleRahman, Mohammad Waliur, Amit A. Deokar, Donna Lindsay, and Bunyamin Tar’an. 2024. "Novel Alleles from Cicer reticulatum L. for Genetic Improvement of Cultivated Chickpeas Identified through Genome Wide Association Analysis" International Journal of Molecular Sciences 25, no. 1: 648. https://doi.org/10.3390/ijms25010648
APA StyleRahman, M. W., Deokar, A. A., Lindsay, D., & Tar’an, B. (2024). Novel Alleles from Cicer reticulatum L. for Genetic Improvement of Cultivated Chickpeas Identified through Genome Wide Association Analysis. International Journal of Molecular Sciences, 25(1), 648. https://doi.org/10.3390/ijms25010648






