Abstract
Podocyte injury can disrupt the glomerular filtration barrier (GFB), leading to podocytopathies that emphasize podocytes as the glomerulus’s key organizer. The coordinated cytoskeleton is essential for supporting the elegant structure and complete functions of podocytes. Therefore, cytoskeleton rearrangement is closely related to the pathogenesis of podocytopathies. In podocytopathies, the rearrangement of the cytoskeleton refers to significant alterations in a string of slit diaphragm (SD) and focal adhesion proteins such as the signaling node nephrin, calcium influx via transient receptor potential channel 6 (TRPC6), and regulation of the Rho family, eventually leading to the disorganization of the original cytoskeletal architecture. Thus, it is imperative to focus on these proteins and signaling pathways to probe the cytoskeleton rearrangement in podocytopathies. In this review, we describe podocytopathies and the podocyte cytoskeleton, then discuss the molecular mechanisms involved in cytoskeleton rearrangement in podocytopathies and summarize the effects of currently existing drugs on regulating the podocyte cytoskeleton.
1. Introduction
Podocytopathies are kidney diseases caused by podocyte injury, characterized by proteinuria. Despite the lack of precise epidemiological data, it is evident that the prevalence of podocytopathies is increasing globally, and they are the leading cause of end-stage renal disease (ESRD). However, even with the high prevalence and various complications, the current treatment for podocytopathies is mainly symptomatic and poorly effective, such as immunosuppressive drugs, renin–angiotensin system antagonists, and diuretics. Moreover, the disease often relapses after treatment with these drugs [1].
Podocytes are terminally differentiated cells that, together with the glomerular basement membrane (GBM) and endothelial cells, constitute the glomerular filtration barrier (GFB) [2]. Podocytes comprise the cell body, primary processes, and secondary foot processes (FPs), forming an octopus-like structure [3]. The interdigitating FPs of adjacent podocytes build a special connection, namely the slit diaphragm (SD) [4]. As the critical component of the kidney filtration units, podocytes maintain their unique cellular structure through an intricate and coordinated network of cytoskeletons [5]. Direct or indirect podocyte injury perturbs the cytoskeleton, especially the actin filaments (AFs), resulting in foot process effacement (FPE), proteinuria, and ultimately chronic kidney disease (CKD). Podocyte injury poses a threat to the glomerulus and is the determinant of podocytopathies, such as focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), membranous nephropathy (MN), and diabetic kidney disease (DKD) [6].
Actin cytoskeleton dysfunction is the principal mechanism of FPE in podocytes [7]. Podocytes are extremely motile, particularly after injury, and the morphology of podocytes is highly dependent on the actin cytoskeleton, which is regulated by cytoskeleton-associated proteins [8]. To adapt to various stresses, such as genetic mutations and significant mechanical forces, podocytes rearrange the cytoskeleton to maintain its distinct architecture and intact filtration characteristic [9]. A series of SD proteins are regulated and altered to participate in podocyte cytoskeleton rearrangement [9,10,11]. These studies are of great significance for elucidating the mechanism of podocyte cytoskeleton rearrangement in podocytopathies. Accordingly, targeting the podocyte cytoskeleton holds significant therapeutic promise.
In this review, we discuss recent findings shedding new insight into the cytoskeleton rearrangement in podocytopathies, summarize the effects of currently available drugs on the cytoskeleton, and explore more potential therapeutic approaches.
2. Overview of Podocytopathies
Podocytopathies are glomerular disorders characterized by podocyte injury with proteinuria and can ultimately progress to CKD [1]. The primary forms of podocytopathies usually refer to FSGS and MCD [12]. Podocytopathies can also be secondary to systemic diseases of a broad array of etiologies, including genetic variants and environmental triggers such as immune, metabolic, infectious, toxic, and hemodynamic factors [13]. The immune system plays a pivotal role in podocyte injury, for example, in MN and lupus nephritis (LN). Long-term, poorly controlled diabetes mellitus leads to DKD accompanied by hemodynamic changes, resulting in glomerular hyperfiltration [1]. The concept of podocytopathies localizes the damage to podocytes. FPE is the primary morphological feature in podocytopathies. Persistent and severe injury induces the detachment of podocytes from the GBM, leading to the loss of podocytes and consequently glomerulosclerosis. These changes are tightly associated with excessive proteinuria [1,14].
3. Composition and Structure of the Podocyte Cytoskeleton
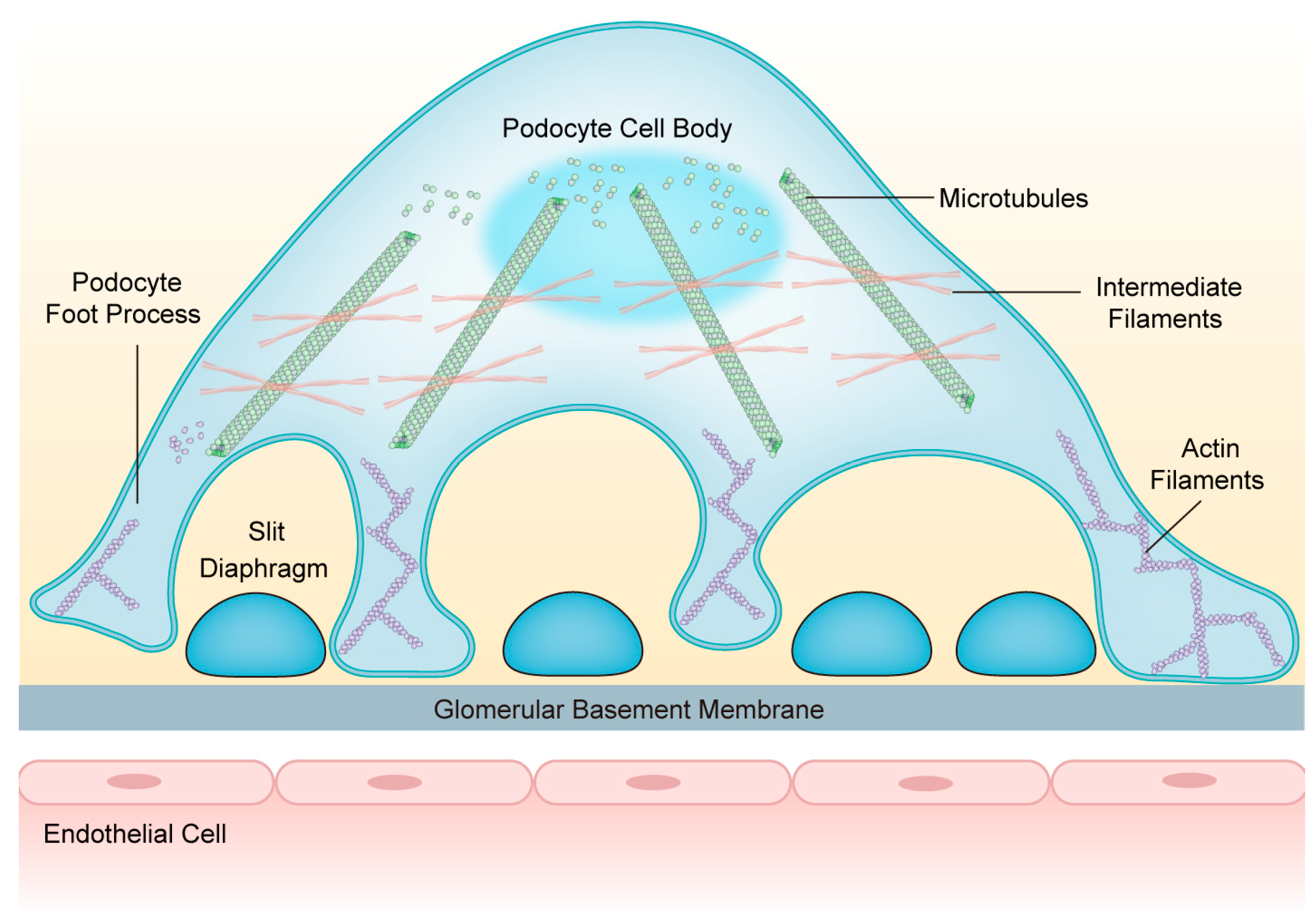
Podocytes are equipped with a well-orchestrated cytoskeletal backbone consisting of the actin cytoskeleton, microtubules (MTs), and intermediate filaments (IFs). It is indispensable for maintaining GFB integrity and regulating cell structure, stability, motility, cell adhesion, and SD insertion [15]. The cell body and major processes of podocytes are based on MTs and IFs, while the FPs mainly rely on the actin cytoskeleton [16] (Figure 1). Under physiological conditions, the podocyte cytoskeleton is in dynamic equilibrium to preserve highly organized cellular function [17].
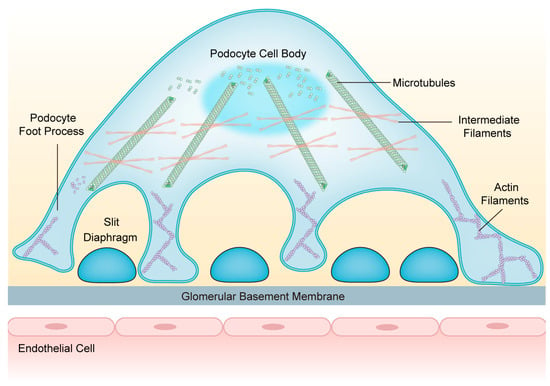
Figure 1.
The structure of podocytes and the network of podocyte cytoskeletons. Podocytes consist of cell bodies, primary processes, and secondary foot processes; adjacent foot processes form the slit diaphragm. The cell bodies and primary processes are based on microtubules and intermediate filaments, while the foot processes mainly depend on the actin filaments. Abbreviations: FPs: foot processes; SD: slit diaphragm; GBM: glomerular basement membrane; MTs: microtubules; IFs: intermediate filaments; AFs: actin filaments.
3.1. Actin Cytoskeleton
The actin cytoskeleton is the core component of podocytes and is mainly abundant in the FPs. It plays a dominant role in establishing and maintaining the distinct architecture of podocytes [7]. The composition of the actin cytoskeleton in the FPs contains two groups: central actin bundles above the SD and a network of cortical actin fibers beneath the curved plasma membrane of the FPs [18]. The AFs are composed of globular actin monomers and are in a highly dynamic state, constantly polymerizing and depolymerizing, mediated by the interplay between multiple actin-associated proteins [19]. Many biochemical factors contribute to the regulation of AFs assembly and disassembly, such as the actin-related proteins 2/3 (Arp2/3) complex, cofilin, and transgelin. In addition, mechanical forces can also influence the assembly and disassembly of AFs [10]. The actin cytoskeleton is strongly sensitive and responsive to the mechanical stress that podocytes face [20]. The AFs provide a structural and functional basis, thus building the cytoarchitecture of podocytes and enhancing adhesion to the extracellular matrix [7,16]. The central actin bundles are engaged in generating tensile forces, whereas the cortical actin network is necessary to uphold the elaborate morphology of FPs [15].
3.2. Microtubules
Podocytes contain a sophisticated and ordered microtubule network, one of the principal cytoskeletal elements within the cell body, and the primary processes of podocytes [21]. MTs are highly dynamic polymers that assemble from α- and β-tubulin heterodimers with an uneven polarity, which refers to fast-growing (plus) ends and slow-growing (minus) poles in coexistence [22]. MTs actively participate in the maintenance of cellular architecture and intracellular transport of proteins and organelles within podocytes, particularly playing a pivotal role in the morphogenesis of podocytes and process growth, branching, and elongation [23]. Podocyte process formation and bipolar orientation mechanically depend on the correct assembly of MTs [24]. The dynamic balance of MT assembly and disassembly is closely regulated by microtubule-associated proteins (MAPs) such as MAP3 and MAP4 [25].
3.3. Intermediate Filaments
IFs are composed of heterogeneous proteins with an intermediate size [26]. Three IF proteins, including vimentin, nestin, and desmin, are abundantly expressed in the cell body and primary processes of podocytes [27]. The first established role of IFs is to provide mechanical support for podocytes, thus stabilizing the cellular biomechanics [28]. IFs significantly modulate the elasticity of the glomerulus and communicate constantly with actin [29]. In addition, IFs impact the organization of MTs and microfilaments and the control of cell cycle, cell survival, and apoptosis [26]. As a sensitive marker of podocyte injury, desmin is upregulated in response to mechanical stresses [27]. Nestin contributes to the metanephric mesenchyme-derived structures of the kidney [30].
4. Cytoskeleton Rearrangement in Podocytopathies and Podocyte-Centric Diseases
4.1. Focal Segmental Glomerulosclerosis
FSGS is a histological pattern of diverse clinical–pathologic syndromes characterized by focal and segmental glomerular scarring, podocyte injury, and proteinuria [31]. Studies have provided clues that podocyte cytoskeleton rearrangement is involved in the pathogenesis of FSGS [32]. During the development of FSGS, the expression or distribution of cardinal SD proteins altered, and the podocyte cytoskeleton was rearranged [33].
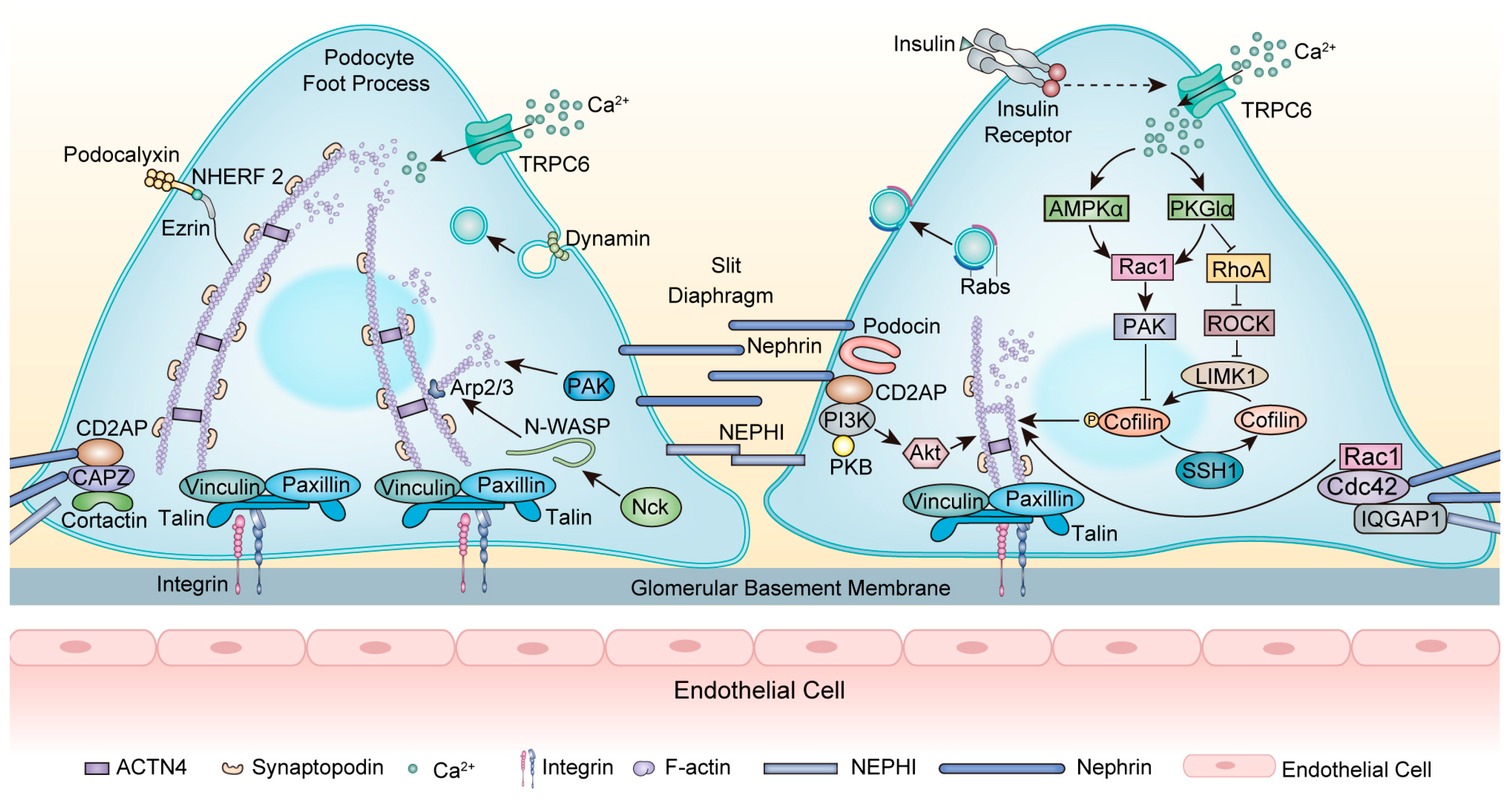
As a transmembrane protein, nephrin forms a zipper-like structure in the SD and acts as a signaling hub [34] (Figure 2). Nephrin (encoded by NPHS1) is essential for maintaining cytoskeleton stability. NPHS1 mutations typically cause congenital nephrotic syndrome (NS) of the Finnish type and also FSGS in adults [35,36]. Nephrin directly interacts with podocin (encoded by NPHS2) to transport nephrin to the plasma membrane [37]. NPHS2 mutations lead to the misfolding and mislocalization of podocin and interrupt the proper trafficking of nephrin, causing FSGS [38]. In addition, nephrin interacts with CD2AP with a subunit of PI3K and subsequently activates the Akt kinase pathway, which is necessary for regulating the actin cytoskeleton [39]. CD2AP mutations are related to primary FSGS, and mutant CD2AP displays a significantly decreased F-actin binding efficiency [40]. Moreover, the actin crosslinking protein ACTN4 interacts with nephrin [41]. Mutations in ACTN4 caused irreversible reductions in the actin cytoskeleton as well as stiffer actin dynamics, causing an autosomal dominant FSGS [42]. After nephrin phosphorylation, the adaptor protein Nck binds to phosphorylated nephrin and neuronal Wiskott–Aldrich syndrome protein (N-WASP), then stimulates the Arp2/3 complex, which triggers actin polymerization and accelerates AFs assembly [43]. Nck1 and Nck2 cooperate to control podocyte adhesion and actin cytoskeleton rearrangement [44]. Podocyte-specific deletion of Nck1/2 in mice results in the FSGS phenotype [45].
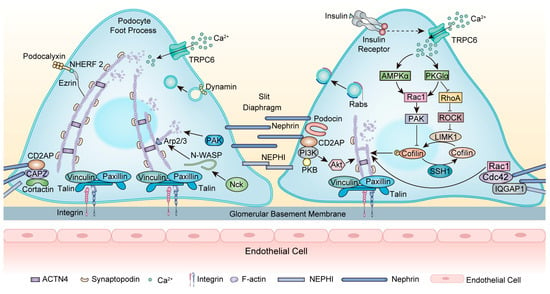
Figure 2.
Modulation of the actin cytoskeleton within the foot processes. At the SD, nephrin acts as a signaling hub to interact with other proteins. Nephrin interacts with CD2AP with a subunit of PI3K and then activates the Akt kinase pathway, leading to actin cytoskeleton rearrangement. The adaptor protein Nck binds to phosphorylated nephrin and N-WASP, stimulating the Arp2/3 complex, which triggers actin polymerization. The scaffolding protein IQGAP1 interacts with nephrin and regulates actin dynamin. In DKD, insulin modulates actin cytoskeleton rearrangement by activating TRPC6, affecting AMPKα and PKGIα, and then regulating RhoA and Rac1. These interactions can regulate the PAK/cofilin-dependent signaling pathway. Dynamin functions in endocytosis and regulates the scission and release of vesicles. Rabs are localized in close proximity to vesicles within FPs and serve as predominant mediators of vesicle trafficking while closely interacting with cytoskeleton proteins. Abbreviations: FPs: foot processes; SD: slit diaphragm; GBM: glomerular basement membrane; AFs: actin filaments; TRPC6: Transient receptor potential channel 6; CD2AP: CD2-associated protein; CAPZ: F-actin capping protein; PAK: p21-activated kinases; Nck: Non-catalytic region of tyrosine kinase; N-WASP: neuronal Wiskott–Aldrich syndrome protein; Arp2/3: actin-related proteins 2/3; ACTN4: α-actinin-4; NEPH1: nephrin-like protein 1; NHEFR 2: Na+/H+ exchange regulatory cofactor 2; IR: insulin receptor; AMPKα: AMP-activated protein kinase α; PKGIα: protein kinase G type Iα; ROCK: Rho-associated protein kinase; IQGAP1: IQ domain GTPase-activating protein 1; PI3K: phosphoinositide 3-kinase; PKB: protein kinase B; Ca2+: calcium; F-actin: filamentous actin.
The coordination of RhoA and Rac1/Cdc42 activity is vital to maintaining the actin cytoskeleton. Increased activity in RhoA and Rac1 and decreased activity in Cdc42 induce actin cytoskeleton disruption, FPE, and FSGS [46]. Synaptopodin can regulate RhoA and Rac1, therefore modulating actin dynamics [47]. Loss of synaptopodin decreases RhoA activation and increases Rac1 activation, thus altering the actin cytoskeleton [48]. Li and colleagues recently identified an unconventional myosin, Myo9A, as a novel podocyte cytoskeletal component that regulates RhoA activity. Myo9AR701X/D mutant mice developed FSGS and showed increased RhoA activity and decreased Myo9A–actin—calmodulin interaction in podocytes. Therefore, Myo9A mutations may underlie a novel form of human autosomal dominant FSGS [49]. Contrary to the aforementioned, RhoA deficiency can also contribute to podocyte cytoskeleton dysfunction in FSGS. The expression and activity of RhoA were significantly diminished in ADR-induced podocyte injury. Mechanistically, inadequate RhoA disturbed the cytoskeleton of podocytes and triggered podocyte apoptosis by inhibiting the Yes-associated protein (YAP)/dendrin signal [50]. In addition, as a crucial regulator of the actin cytoskeleton dynamics, deficient Cdc42 and its downstream effector N-WASP downregulated YAP, thereby facilitating podocyte apoptosis [51].
Rabs, another group of small GTPases, play a predominant role in intracellular vesicle trafficking and are closely associated with cytoskeleton components, functioning in kidney biology [52,53] (Figure 2). The Rab family encompasses over 70 GTPases. Within this extensive family, the expression of Rab3A and its effector Rabphillin-3A (Rph3A) has been identified in podocytes surrounding vesicles within FPs. In patients with FSGS, there is an upregulation of Rab3A-Rph3A expression, potentially leading to cytoskeleton modifications [54,55].
Transient receptor potential channel 6 (TRPC6) is calcium-permeable, and increased calcium influx through TRPC6 is one of the major determinants of podocyte injury in FSGS [56]. Calcium entry via TRPC6 downregulates nephrin and synaptopodin and stimulates the activation of RhoA, leading to actin cytoskeleton rearrangement [57]. Interestingly, feedback from actin cytoskeleton rearrangement modulates calcium entry through TRPC6 [58]. Mutations of the TRPC6 gene are related to autosomal dominant inherited FSGS [59]. TRPC6 knockout podocytes displayed an altered actin cytoskeleton, presenting decreased motility due to a loss of calpain activity. The regulation of calpain activity by TRPC6 was independent of its channel activity, indicating a new mechanism for the pathogenesis of FSGS [60].
Additionally, recent studies have revealed a few molecules that can regulate podocyte cytoskeleton rearrangement in FSGS. A soluble factor of permeability, calcium/calmodulin-dependent serine protein kinase (CASK), is newly found to cause recurrent FSGS after transplantation. Recombinant CASK induces actin cytoskeleton rearrangement in immortalized podocytes, redistributing the junction proteins ZO-1 and synaptopodin [61]. Given the secretion of circulating CASK into the sera of patients with recurrent FSGS, it holds promise as a novel biomarker or therapeutic target for recurrent FSGS, thereby conveniently reflecting renal lesions and potentially reducing the frequency of renal biopsy. However, further comprehensive studies are warranted to validate its feasibility and practicality. Chen and colleagues revealed a FAM40A gene mutation in data from 54 FSGS patients. Mutant FAM40A (p521M > T) altered the mouse podocyte morphology and cytoskeleton with disordered F-actin, loss of FPs, and nephrin, indicating that mutant FAM40A may be associated with familial FSGS [62]. In the adriamycin (ADR) murine model and patients with FSGS, a complement C3 convertase regulator decay-accelerating factor (DAF) was lost, leading to the activation of complement and the formation of C3a. Then C3a/C3aR ligations on podocytes initiated autocrine IL-1β/IL-1R1 signaling, which induced nephrin reduction and cytoskeleton rearrangement, finally leading to glomerulosclerosis [63]. Additionally, the expression of plectin was downregulated in ADR-induced podocyte injury, thereby resulting in cytoskeleton rearrangement and ultimately facilitating podocyte apoptosis through the activation of the integrin α6β4/focal adhesion kinase (FAK)/p38 signaling pathway [64].
Asparagine endopeptidase (AEP) is an endosomal and lysosomal cysteine protease recognized for its proteolytic action to degrade substrates [65]. AEP exhibits high expression in the kidney, and numerous studies have established its indispensability for renal physiology and homeostasis [66,67,68]. Impairment of lysosomal function disrupted the regulation of SD proteins, nephrin, and podocin, leading to FPE [69]. Our recent research revealed that AEP exerted a renoprotective effect in animal models of FSGS by regulating podocyte cytoskeleton rearrangement through its enzymatic activity. In ADR-induced nephropathy, AEP was abundantly expressed in podocytes. AEP cleaved the actin-binding protein transgelin at the N150 site, removing the C terminus of transgelin. The fragment of transgelin was capable of protecting against podocyte injury and restoring F-actin and paxillin, a focal adhesion protein. Collectively, AEP modulates podocyte cytoskeleton rearrangement by exerting a proteolytic role on the actin regulatory protein transgelin. The protective effect of AEP on podocytes provides a potential strategy for the treatment of FSGS [70].
4.2. Minimal Change Disease
Minimal change disease (MCD) is one of the primary forms of podocytopathies, and it is the most common NS in children [71].
Alterations in nephrin play a critical role in the pathogenesis of MCD [72]. IQ domain GTPase-activating protein 1 (IQGAP1), a scaffolding protein linking the actin cytoskeleton to the SD, was found to interact with nephrin and regulate the podocyte actin cytoskeleton [73] (Figure 2). IQGAP1 expression and its interaction with nephrin in podocytes were vastly decreased in PAN-induced rats, an animal model of MCD. In podocytes, IQGAP1 knockout significantly reduced the colocalization of IQGAP1 with nephrin and exacerbated the disruption of F-actin. These effects were dramatically restored by IQGAP1 overexpression [74].
In addition, nephrin phosphorylation is known to decrease in patients with MCD [72]. Phosphorylated nephrin was available to bind to the nonreceptor tyrosine kinase c-Abl in the SH2/SH3-dependent form, and c-Abl recruitment depended on the phosphorylation of nephrin. Moreover, nephrin-c-Abl signaling was shown to modulate podocyte cytoskeleton rearrangement. This observation revealed that c-Abl was involved in podocyte cytoskeleton rearrangement, depending on nephrin. It indicated that c-Abl was a novel downstream effector of nephrin in maintaining podocyte cytoskeleton stability. Compared with control patients, nephrin-c-Abl colocalization decreased in the glomeruli of patients with MCD, consistent with previous results in animal models and cultured podocytes [75]. These findings suggested that nephrin-c-Abl signal transduction dynamically regulated the podocyte cytoskeleton and participated in podocyte injury during MCD.
In addition to nephrin, other SD proteins such as podocin, CD2AP, and ACTN4 can be modulated in MCD. Administration of IL-13 substantially downregulated synaptopodin, ACTN4, CD2AP, and ZO-1 in podocytes and thus rearranged the cytoskeleton of podocytes. IL-13 may be involved in the pathogenesis of MCD, and specifically targeting IL-13 could be a promising therapeutic option for MCD [76]. In addition, serum- and glucocorticoid-inducible kinase 3 (SGK3) is a downstream mediator of PI3K, which is essential for maintaining the intact function of podocytes. In PAN-induced podocyte injury in vitro, the activity of SGK3 was decreased, leading to reduced glycogen synthase kinase-3 (GSK3) phosphorylation, and then GSK3 was activated. Consequently, GSK3 activation resulted in decreased expression of podocin, which damaged the podocyte cytoskeleton [77]. Angiopoietin-like protein 4 (ANGPTL4) is a glycoprotein belonging to the angiopoietin-like protein family. ANGPTL4 was markedly increased in the glomerulus in a PAN-induced injury. ANGPTL4 participated in the podocyte cytoskeleton rearrangement by observably downmodulating podocin and ACTN4, thereby promoting FPE and proteinuria [78]. In addition, ANGPTL4 downregulated the expression of synaptopodin, induced podocyte cytoskeleton rearrangement, and promoted podocyte apoptosis in MCD [79].
Recent studies have found the role of autoantibodies in the pathogenesis of MCD. Autoantibodies against nephrin play a vital role in cytoskeleton rearrangement. By evaluating sera from patients with MCD, Watts and colleagues reported that circulating nephrin autoantibodies were present in patients with MCD and were associated with heavy proteinuria. Moreover, the subtle IgG colocalized with nephrin specifically. Based on these observations, the integrity of the SD and the cytoskeleton organization may be disrupted by the binding of nephrin to autoantibodies [80]. In addition, Annexin A2 (ANXA2), a calcium-regulated phospholipid-binding protein, can interact with F-actin and other cytoskeleton-associated proteins. ANXA2 regulates the podocyte cytoskeleton by modulating Rho signaling [81]. The level of ANXA2 autoantibodies was much higher in children with primary MCD, which significantly induced proteinuria. As for the mechanism, the autoantibodies against ANXA2 activated the Cdc42 and Rac1/2/3 Rho pathways by facilitating ANXA2 phosphorylation at Tyr24, thereby resulting in the cytoskeleton rearrangement of podocytes and inducing proteinuria even in primary NS [82]. In brief, ANXA2 was expressed in podocytes and served as the antigen for the autoantibody in patients with primary MCD. It was involved in the pathogenesis of MCD and may be a potential therapeutic target.
4.3. Diabetic Kidney Disease
Diabetic kidney disease (DKD) is one of the major complications of diabetes mellitus and is the leading cause of ESRD worldwide [83]. Podocyte cytoskeleton rearrangement is one of the major contributors to the pathogenesis of DKD [84].
The structure of the podocyte cytoskeleton is highly dependent on ATP availability [85]. Podocytes are metabolically active, and their function relies on efficient glucose oxidation for energy production [84]. Chronic hyperglycemia leads to progressive impairment of glucose metabolism in podocytes, greatly hindering mitochondrial ATP production [86]. This failure of ATP synthesis by mitochondria significantly disrupts the balance between cytoskeleton assembly and disassembly, particularly affecting the actin cytoskeleton and MTs, ultimately resulting in the cytoskeleton rearrangement [87,88,89]. Additionally, GTP plays a crucial role in regulating the structure and function of the podocyte cytoskeleton, primarily through its interaction with Rho GTPases [90]. GTP biosynthesis enzymes dynamically regulate the activity of Rho GTPases, which undergo cycling between an inactive GDP-bound state and an active GTP-bound conformation [91]. Moreover, podocytes incubated with HG exhibited a significant increase in Cdc42-GTP level, which correlated with cytoskeleton remodeling [92].
Insulin is a hormone that plays a pivotal role in glucose metabolism and can regulate podocyte cytoskeleton rearrangement, mainly through the modulation of the Rho family (Figure 2). The importance of insulin signaling has been highlighted in the pathogenesis of DKD [93]. Insulin-induced actin cytoskeleton rearrangement occurs via activation of TRPC6 channels, and this activation increases AMPKα phosphorylation and the colocalization of TRPC6 with AMPKα2 subunits. The activation of both TRPC6 and AMPKα2 was necessary to activate Rac1 [94]. Moreover, insulin can regulate Rac1 and RhoA in a protein kinase G type Iα (PKGIα)-dependent manner [95]. PKGI is a type of PKG that belongs to the family of serine/threonine kinases. PKGI is primarily a mediator of vasodilation and was later found to be a major modulator of filtration barrier permeability [96]. The activation of PKGI induced actin cytoskeleton rearrangement and consequently increased podocyte filtration barrier permeability via activation of Rac1 and inhibition of RhoA. This mechanism was disrupted in diabetic circumstances [95]. The appropriate PKGIα-Rac1 interplay is also a major regulator of the PAK/cofilin-dependent signaling pathway in podocytes. The downregulation of PKGIα or Rac1 expression abolished this effect [97]. Accordingly, PKGIα–Rac1–RhoA crosstalk is necessary to properly organize the podocyte cytoskeleton and stabilize glomerular architecture and function (Figure 2).
The role of Rab3A has also been investigated in DKD. Rab3A knockout mice fed with the HG diet exhibited renal lesions similar to those in DKD patients, along with profound alterations in actin cytoskeleton plasticity and proteinuria [98]. Furthermore, the Rab3A/Rab27A system is a core player in podocyte injury and vesicle transport in DKD. Silencing RAB3A/RAB27A in podocytes exposed to HG-induced cytoskeleton disorganization and cellular apoptosis [99]. Considering the crucial involvement of Rabs in vesicle transport and their close association with the podocyte cytoskeleton, detecting the expression of Rabs in urine exosomes holds promise for non-invasive assessment of cytoskeleton disorders and kidney lesions. This approach could potentially reduce the need for repeated kidney biopsy; however, further studies are required to confirm the highly specific association between Rabs and podocyte cytoskeleton disorders as well as renal lesions.
Podocyte cytoskeleton rearrangement is tightly associated with the progress of cellular apoptosis in DKD. The downregulation of RhoA in DKD results in the disruption of the podocyte cytoskeleton, thereby promoting apoptosis by inhibiting YAP. Thus, RhoA/YAP signaling is a promising target for treating DKD [100]. Additionally, tyrosine-protein kinase receptor (TYRO3) expression was largely suppressed in DKD patients and HG-treated podocytes. Knockdown of TYRO3-induced podocyte cytoskeleton rearrangement facilitates cellular apoptosis and exacerbates proteinuria in DKD mice. Mechanistically, TYRO3 protected podocytes from cytoskeleton disturbances and therefore ameliorated apoptosis through the JNK/c-jun-p53 pathway [101]. Under diabetic conditions, ANGPTL4 expression was upregulated, resulting in actin cytoskeleton rearrangement and podocyte apoptosis via activating the integrin-β1/FAK pathway [102]. The expression of brain acid-soluble protein 1 (BASP1), a critical regulator of the actin cytoskeleton, was upregulated in DKD. This upregulation contributed to the rearrangement of the actin cytoskeleton and subsequently promoted podocyte apoptosis by activating the p53 apoptotic pathway through modulation of Wilms’ tumor 1 transcription factor (WT1) [103]. The aforementioned signaling pathways possess the potential to mitigate podocyte cytoskeleton disorders and apoptosis, thereby offering therapeutic prospects for DKD.
A number of molecules have been implicated in the regulation of SD proteins and actin cytoskeleton rearrangement in DKD. The protein tyrosine phosphatase SHP-1 has been shown to modulate SD protein and podocyte cytoskeleton rearrangement. Its expression was increased in the podocytes of diabetic mice and patients with diabetes. Podocyte-specific deletion of SHP-1 prevented FPE and restored the expression of podocin. In terms of the mechanism, SHP-1 reduced SUMO2 post-translational modification of podocin, and inhibition of SHP-1 rescued reduced podocin in diabetic conditions [104]. The expression of MYH9 was significantly decreased in the glomeruli of diabetic patients and mice. In DKD, the reduction of MYH9 induced podocyte injury and actin cytoskeleton rearrangement, which manifested as a decreased expression of nephrin, synaptopodin, ZO-1, and β1 integrin, a focal adhesion protein [105]. In addition, dipeptidyl peptidase-4 (DPP-4) activity was observed in nodular lesions in DKD, which induced degradation of synaptopodin and reduction of RhoA, destroying the podocyte cytoskeleton [106]. Slit–Robo GTP activating protein 2a (SRGAP2a), belonging to the large family of Rho-GTPase, primarily localizes at podocytes and largely colocalizes with synaptopodin. SRGAP2a was downregulated in DKD patients and db/db mice exhibiting disrupted podocyte cytoskeletons, and SRGAP2a knockdown in podocytes rearranged the cytoskeleton by inactivating RhoA/Cdc42 [107]. In addition, by performing RNA sequencing in kidneys isolated from DKD patients, Hu and colleagues reported that the novel lncRNA ENST00000436340 was upregulated in DKD and contributed to cytoskeleton rearrangement with collapsed AFs, upregulation of desmin, and downregulation of ZO-1, ultimately leading to podocyte injury and DKD [108].
Cathepsin L, a lysosomal cysteine protease, was involved in the pathogenesis of DKD [109]. This enzyme cleaves the large GTPase dynamin, CD2AP, and synaptopodin, which are associated with actin cytoskeleton rearrangement of podocytes, FPE, and proteinuria [110,111,112]. Our research group recently discovered that AEP exhibits protective effects against podocyte injury by regulating the cytoskeleton dynamics in DKD. AEP was highly expressed in diabetic kidney podocyte injury and podocytes exposed to hyperglycemia. Mechanistically, AEP directly cleaved the actin-binding protein cofilin-1 at the N138 site. The resulting cofilin-1–1–138 fragment was capable of preventing podocyte cytoskeleton disarrangement and ameliorating podocyte injury in DKD [113].
The discovery of these mechanisms provides a strong rationale for targeting podocyte cytoskeleton rearrangement in DKD.
4.4. Membranous Nephropathy
Membranous nephropathy (MN) is a kind of autoimmune glomerular disease typically associated with autoantibodies against the phospholipase A2 receptor (PLA2R) and characterized by apparent thickening of the glomerular capillary walls owing to immune complex deposition [114]. The immune-related mechanisms of MN have been extensively studied, and increasing evidence implicates that podocyte cytoskeleton rearrangement is closely related to the pathogenesis of MN [115].
Ubiquitin C-terminal hydrolase-L1 (UCH-L1), a member of the de-ubiquitinating enzyme families of the ubiquitin–proteasome pathway, was found to express in podocytes. The expression of UCH-L1 was aberrantly increased in the podocytes of patients with MN and the passive Heymann nephritis (PHN) rats, an experimental model of MN [116]. The conspicuous upregulation of UCH-L1 in podocytes resulted in a massive accumulation of p27Kip1, which contacted cytoskeleton-associated proteins including RhoA and Rac, therefore regulating the podocyte cytoskeleton [117]. Moreover, upregulation of UCH-L1 could lead to cytoskeleton rearrangement of podocytes with decreased synaptopodin and RhoA [118,119]. However, UCH-L1 damaged the podocyte cytoskeleton and caused MN by suppressing plakoglobin instead of directly acting on synaptopodin and RhoA. Upregulation of UCH-L1 was relevant to a prominent downregulation of plakoglobin, which takes close participation in rearranging podocyte cytoskeleton and maintaining podocyte cytoarchitecture [119]. This finding sheds new insight into the treatment of MN.
In addition, the levels of several molecules are altered in MN, thereby regulating podocyte cytoskeleton rearrangement. The glomerular expression of ANGPTL4 was significantly increased in PHN rats and MN patients. Intriguingly, it was tightly connected with podocyte injury and excessive proteinuria [120]. A subsequent study observed that overexpression of ANGPTL4 resulted in cytoskeleton rearrangement of podocytes, exhibiting decreased expression of synaptopodin. This study provided a potential therapeutic target for MN [79]. In addition, the expression of glomerular Matriptase was dramatically increased in patients with MN. Matriptase directly cleaved podocin, thus disrupting the cytoskeleton of podocytes and the integrity of SD. This finding implies that inhibiting Matriptase could be a potential therapeutic target for podocytopathies [121]. In addition, as mentioned above in MCD, the nephrin-c-Abl colocalization was suppressed in the glomeruli of patients with MN [75].
In 2009, PLA2R was identified as the primary targeted autoantigen in MN. The presence of autoantibodies against PLA2R is highly specific to MN and serves as a valuable biomarker for the diagnosis of MN [114]. Haddad and colleagues recently revealed that in primary MN, antibodies against PLA2R1 combined with podocyte PLA2R1 and thereby activated the complement system, which was promoted by the activation of the lectin pathway in a glycosylation-dependent manner. Subsequently, these effects induced the proteolysis of two podocyte cytoskeleton proteins, synaptopodin and NEPH1, disorganizing the podocyte cytoskeleton [115].
4.5. Lupus Nephritis
LN is a kidney disease that primarily injures podocytes. It is characterized by the deposition of autoantibodies, intrinsic and adaptive immune activation, and the production of local mediators associated with renal inflammation and fibrosis [122].
Recently, MiR-26a was identified as a multifunctional mediator of kidney diseases. It dynamically modulates podocyte cytoskeleton organization and is extremely important for podocyte function [123]. MiR-26a expression was significantly decreased in patients with LN as well as in the glomerulus of B6.MRLc1 mice, a mouse model of autoimmune glomerulonephritis. Decreased MiR-26a was tightly correlated with reduced actin proteins and the IFs protein vimentin, indicating the disruption and rearrangement of the podocyte cytoskeleton [124]. MicroRNAs can be released into urine, and several urinary microRNAs have been identified as potential biomarkers for kidney diseases. Moreover, exosome-derived microRNAs in urine have garnered significant attention [125]. Therefore, further exploration is warranted to assess the utility of exosomal MiR-26a in predicting cytoskeleton disruption and determining therapeutic efficacy, and extensive, robust clinical studies are necessary to validate the clinical value of MiR-26a.
Neutrophils are implicated as a causal regulator in LN and can directly interact with podocytes. Cytokines released by podocytes recruit neutrophils and promote the release of neutrophil granule components, consequently disrupting the actin cytoskeleton. These changes contribute to renal abnormalities and proteinuria in animal models of LN [126]. In addition to MN, the conspicuous upregulation of UCH-L1 was also observed in LN and led to podocyte cytoskeleton rearrangement by decreasing the expression of plakoglobin in podocytes [119].
5. Therapeutic Implications
Considering the determinant role of podocytes in glomerular filtration function and the fact that podocyte injury is the hallmark of proteinuric diseases, it is logical to treat podocytes as a specific target for developing novel therapeutic strategies. Additionally, a series of cellular and signaling mechanisms indicate that treatment focusing on cytoskeleton rearrangement may be beneficial for podocytopathies. Currently, existing therapeutic options focus on renal pathophysiology, which has poor specificity, limited therapeutic efficacy, and is often accompanied by significant side effects. However, several studies have confirmed their effects on the podocyte cytoskeleton and the treatment of podocytopathies, which are summarized next to provide potential therapeutic options [127,128] (Table 1).
5.1. Glucocorticoids
Glucocorticoids (GCs) are the cornerstone medication for proteinuric diseases, and they exert well-known effects via immunosuppressive and anti-inflammatory responses [129]. An increasing number of studies have indicated that the therapeutic benefits of GCs can be attributed to the direct target of podocytes, attenuating podocyte cytoskeleton rearrangement [130,131].
GCs therapy can regulate the expression or distribution of nephrin and therefore maintain the podocyte actin cytoskeleton and filtration function. Dexamethasone (DEX) promoted process formation and cellular maturation with a striking upregulation of nephrin in human cultured podocytes [132]. Under glucose starvation-induced rapid endoplasmic reticulum (ER) stress, nephrin was not sufficiently glycosylated and remained in the ER. DEX facilitated the synthesis of fully glycosylated nephrin and rescued the disturbed transport of nephrin to the plasma membrane [133]. Prednisone realized antiproteinuric effects through alterations in the expression and distribution of nephrin and other SD proteins such as podocin, CD2AP, and ACTN4 [134]. LN mice with early treatment with GCs exhibited increased expression of nephrin and podocin and preserved histological morphology [135]. Additionally, DEX increased the phosphorylation of nephrin by activating Fyn with the cooperation of Nck [136]. Further studies revealed that GCs also enhanced nephrin phosphorylation by activating serum and glucocorticoid-regulated kinase 1 (SGK1) [137].
In addition to nephrin, GCs can regulate other actin-associated proteins, including TRPC6, ACTN4, Rac1, and synaptopodin. In PAN rats or cultured podocytes, DEX can rescue remarkably reduced processes and maintain SD integrity by blocking TRPC6 signaling and stabilizing TRPC6 expression [138]. DEX can interact with ACTN4 and maintain the balance of ACTN4 expression in cultured murine podocytes; it significantly stabilizes the actin cytoskeleton and prevents ADR-induced actin cytoskeleton rearrangement [139]. GCs were found to decrease the overactivity of Rac1, which was related to proteinuria, and this Rac1 inhibition had therapeutic efficacy and protected podocyte filtration function [140]. In a retrospective study with 17 FSGS patients, reduced synaptopodin expression was connected with an unsatisfactory response to GC treatment [141]. A subsequent study found that GCs increased synaptopodin expression in PAN-injured glomeruli [142]. Additionally, in immortalized mouse podocytes stimulated with vasoactive factors, DEX modulated cyclic guanosine monophosphate (cGMP)-mediated actin rearrangement and podocyte motility [143]. Overexpression of Krüppel-like factor 15 (KLF15), a downstream target crucial for podocyte restoration upon GCs treatment, prevented the disassembly of the actin cytoskeleton and enhanced actin stress fiber formation under cell stress in cultured human podocytes [144,145,146].
5.2. Rituximab
Rituximab (RTX), located on the surface of B cells, is a chimeric monoclonal antibody selective against CD20. This drug has been approved for B cell lymphoma and various autoimmune diseases and is also applied for podocytopathies such as FSGS and MCD [147,148].
RTX can directly affect the actin cytoskeleton and prevent the reduced expression of nephrin and podocin [149]. In an in vitro PAN model, treatment with RTX dramatically prevented disruption of the actin cytoskeleton and increased podocyte actin stress fibers, which was related to a substantial enhancement in cell adhesion [150].
In addition to CD20, Perosa and colleagues found a potential reactivity of RTX to sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b), a putative acid-sphingomyelinase (ASMase) [151]. A later study reported that RTX could directly target podocytes in recurrent FSGS by preserving SMPDL-3b, which modulates actin rearrangement. The total number of SMPDL3b-positive cells was decreased in kidney biopsies from patients with recurrent FSGS after transplantation. In the study, podocytes were exposed to the recurrent FSGS sera in vitro and displayed a striking disruption of the actin cytoskeleton. Surprisingly, pretreatment with RTX reversed these adverse changes by stabilizing SMPDL-3b and ASMase to regulate actin cytoskeleton rearrangement in podocytes [152]. Previously, remission of recurrent FSGS with relapsing proteinuria after transplantation by using RTX has been reported in many instances [153,154,155,156]. As seen in both clinical and experimental contexts, SMPDL-3b is a direct target of RTX and a regulator of the podocyte cytoskeleton. These findings provide a solid basis for RTX to prevent recurrent FSGS after kidney transplantation.
5.3. Calcineurin Inhibitor
Calcineurin inhibitors (CNIs), including cyclosporine A (CsA) and tacrolimus (TAC, also known as FK506), are widely used to treat proteinuric kidney disease [157]. CNIs are commonly known to mediate immunosuppressive effects by inhibiting calcineurin and suppressing T cell activation via regulating the nuclear factor of activated T cells (NFAT) signaling [158]. Also, the benefits of CNIs on proteinuria can be attributed to their direct effects on the podocyte actin cytoskeleton beyond their effects on T cells [159].
It has been revealed that in podocytes, activation of calcineurin leads to dephosphorylation of synaptopodin, making it more susceptible to the deleterious degradation by cathepsin L, which influences the actin cytoskeleton and induces FPE and proteinuria [160]. CsA in a non-immune form protected podocytes by blocking the calcineurin-mediated dephosphorylation of synaptopodin and consequently maintaining synaptopodin. This mechanism stabilized the actin cytoskeleton, improved foot process motility, and protected against proteinuria [161]. TAC was shown to have similar effects to CsA [162]. Since synaptopodin is a regulator of RhoA and Cdc42 during cytoskeleton rearrangement, it can be concluded that CsA eventually protects the podocyte actin cytoskeleton by regulating RhoA and Cdc42 signaling [163].
Activation of calcineurin tightly requires TRPC6-mediated calcium influx [164]. Constitutive activation of NFAT in podocytes increased TRPC6 expression and induced severe proteinuria [165]. Downregulating TRPC6 and blocking TRPC6-mediated NFAT activation through calcineurin inhibition contributed to the antiproteinuric effect of CNIs [166]. Another study showed that pretreatment with CNIs could reduce insulin-stimulated TRPC6 expression dependent on the calcineurin pathway in DKD [167]. Calcineurin also directly regulates WAVE1, a key regulator of Arp2/3, playing an essential role in regulating cytoskeleton organization. CsA regulated WAVE1 phosphorylation and significantly decreased WAVE1 expression. By this mechanism, the disorganized actin arrangement was partially rescued both in vivo and in vitro [168]. Recent studies have unveiled novel mechanisms by which CNIs affect the podocyte cytoskeleton in podocytopathies. Shen and colleagues found that overexpression of ANGPTL4 directly induced podocyte cytoskeleton rearrangement and reduced synaptopodin expression. CNIs ameliorated PAN-induced expression of ANGPTL4 by targeting the Calcineurin/NFAT signaling pathway [169]. TAC remarkably reduced ANGPTL4 expression, accompanied by improved FPs and a reduction of proteinuria in PHN rats [120] and ADR rats [170]. Yasuda and colleagues demonstrated that TAC alleviated podocyte damage by increasing the expression of a binding protein of TAC FKBP12, which was expressed along the actin cytoskeleton and maintained process formation in ADR-induced podocyte injury. TAC also enhanced the interaction of FKBP12 with actin-associated proteins 14-3-3β and synaptopodin to maintain F-actin [171]. Moreover, TAC significantly improved the expression of nephrin and podocin in diabetic rats [172] and maintained the microtubule-associated protein 1 light chain 3 alpha (LC3) expression in PAN-induced podocyte injury [173]. In clinical settings, CNIs were effective in FSGS [174] and lupus podocytopathy cases [175].
5.4. Abatacept
B7-1 usually expresses on activated B cells and other antigen-presenting cells, and it provides a co-stimulatory signal for T cell activation and survival [176]. Podocytes can be induced to express B7-1 in various animal models, and B7-1 is crucial in the orchestration of the podocyte cytoskeleton and is associated with proteinuria [177]. Abatacept is an inhibitor that targets the co-stimulatory molecule B7-1 [178]. It has been approved for the treatment of rheumatoid arthritis [179] and juvenile idiopathic arthritis [180] and is being explored for treating other autoimmune diseases.
Yu and colleagues proposed abatacept targeting podocytes as a novel therapy for proteinuric diseases. In vitro data showed that B7-1 disrupted β1 integrin activation by specifically interfering with its association with talin, which was blocked by abatacept. Administration of abatacept induces partial or complete remission in patients with B7-1-positive primary FSGS or recurrent FSGS after transplantation [178]. However, the validity was questioned due to the small patient number, concurrent treatment regimens, and so on. In several other cases, patients with FSGS or MCD were either responsive or unresponsive to abatacept [181,182,183,184]. These conflicting findings suggest that targeting B7-1 requires more evidence to prove the efficacy of abatacept in podocytopathies. A pilot study (NCT02592798) to evaluate the safety and efficacy of abatacept (given every 28 days for four cycles) in patients with MCD or FSGS and active proteinuria (implying steroid resistance) has been completed. We excitedly expect the results for publication [185].
In DKD, podocyte B7-1 expression was upregulated. Podocytes cultured in vitro with HG exhibited PI3K-mediated B7-1 upregulation, which was related to the degradation of synaptopodin and the loss of activated α3β1 integrin in deranged AFs. Importantly, abatacept prevented cytoskeleton disruption and adhesion abnormalities. In diabetic mice, in vivo, podocyte B7-1 was induced as well, and abatacept ameliorated proteinuria [186]. Nevertheless, it was reported that B7-1 was not induced in humans and experimental DKD [187,188], and abatacept did not show renoprotective effects in STZ-induced DKD [189]. B7-1 expression was also induced in the podocytes of mice and patients with LN, which was associated with the severity of proteinuria [177,178]. A meta-analysis showed that abatacept was effective for the management of LN patients [190], while two clinical trials failed to prove the beneficial effects of abatacept in patients with LN [191,192].
Above all, B7-1 staining in podocytopathies and the effect of abatacept via targeting podocyte B7-1 remain particularly controversial. Administration of abatacept to treat podocytopathies requires more reliable clinical data.
5.5. Others
Dynamin is characterized by its role in endocytosis, which modulates the dynamics of the actin cytoskeleton and vesicle transport [193]. In podocytes, dynamin plays a vital role in maintaining cellular structure and function through its oligomerization to regulate the actin cytoskeleton by stimulating actin polymerization and cross-interacting with AFs [194]. Bis-T-23 promoted actin-dependent dynamin oligomerization and thus facilitated actin stress fiber formation and focal adhesion maturation in a dynamin-dependent manner, resulting in a reduction of proteinuria in cultured mouse podocytes [195,196]. Ono and colleagues recently found that Bis-T-23 prevented proteinuria and podocyte injury in a podocyte-specific O-linked β-N-acetylglucosamine transferase knockout model (Podo-Ogty/−) by the same mechanism [197]. An ex vivo treatment of podocytes that detached from the GBM in two patients with FSGS and MN, respectively, was responsive to Bis-T-23, accompanied by restored cytoskeleton and focal adhesion [198]. These findings implicate the feasibility of modulating dynamin-dependent actin dynamics as a potential therapeutic approach for podocytopathies.
Fasudil is the only RhoA kinase inhibitor approved for clinical use, and it has been reported to exert protective roles in kidney diseases with well-established effectiveness and safety [199]. It attenuated angiotensin II (Ang II)-induced cytoskeleton rearrangement and preserved podocyte mobility by modulating SD proteins, including nephrin and CD2AP [200]. Tian and colleagues recently clarified that in MRL/lpr mice (an animal model of LN), Fasudil sustained podocyte actin cytoskeleton by inhibiting CaMK4/RhoA signaling and inducing the release of YAP from the 14-3-3β (a chaperonin protein for YAP) to make up for the podocyte damage [201]. Another Rho kinase inhibitor, Y27632, notably attenuated podocyte actin cytoskeleton rearrangement in Ang II-treated rats [202]. It also stabilized the MTs and promoted FP formation [203].
Moreover, two RAS inhibitors, angiotensin-converting enzyme (ACEI) and Ang II type 1 receptor blocker (ARB), are well-known for hemodynamic effects. They significantly attenuated the reduction of nephrin and proteinuria in PAN nephropathy [204] and in the glomeruli of type 2 diabetic rats [205]. Given that AngII can directly increase the expression of TRPC6, which leads to proteinuria [206], ACEI/ARB may inhibit TRPC6 expression in an AngII-dependent manner and reduce proteinuria [207]. The mTOR-inhibitor everolimus (EV) stabilized the podocyte actin cytoskeleton by preventing PAN-induced inhibition of the RhoA–ROCK–MLC pathway in vitro [208]. It also regulated MT stability through increasing tubulin beta 2B (TUBB2B) and doublecortin domain containing 2 (DCDC2) expression [209].
5.6. New Potential Therapeutic Strategies
In recent years, studies have identified several potential therapeutic targets to modulate podocyte cytoskeleton rearrangement in podocytopathies.
The melanocortin-1 receptor (MC1R) is one of the adrenocorticotropic hormone (ACTH) receptors and is the most abundant ACTH receptor in the glomeruli. Glomerular MC1R expression was increased in patients and rat models of MN and FSGS, and MC1R exerted a renoprotective effect in podocytes by stabilizing the actin cytoskeleton. This mechanism was realized by ERK1/2-dependent phosphorylation and inhibition of EGFR signaling, accompanied by the stabilization of synaptopodin and the formation of stress fibers in podocytes [210]. Moreover, MC5R agonists also attenuated proteinuria and prevented PAN-induced disruption of the podocyte actin cytoskeleton with the restoration of synaptopodin [211]. Melanocortin therapy was demonstrated to protect podocytes and reduce proteinuria; it may be promising to be a beneficial agent to treat patients with podocytopathies [212,213].
In addition, the angiotensin II type-2 receptor (AT2R) was shown to protect against podocyte injury in FSGS. AT2R agonist compound 21 (C21) stabilized the podocyte actin cytoskeleton and restored the expression of synaptopodin by inhibiting cathepsin L, thus maintaining the integrity of podocyte structure and filtration function [214]. Bardoxolone-methyl (CDDO-Me) is a synthetic triterpenoid known for its anti-inflammatory, antioxidant, and anti-apoptosis effects. In ADR-induced nephropathy, CDDO-Me can modulate the cytoskeleton rearrangement of podocytes. By activating the NRF2 pathway, CDDO-Me enhanced SRGAP2a expression to stabilize the podocyte cytoskeleton [215].
A DPP-4 inhibitor is a kind of drug used to treat type 2 diabetes mellitus, and recently it was found to protect podocytes independent of its hypoglycemic role. DPP-4 activity was detected in the podocytes of DKD and can be considered a biomarker of kidney disease. Treatment with the DPP-4 inhibitor saxagliptin dramatically reduced proteinuria and stabilized the expression of nephrin, synaptopodin, and RhoA [106]. DPP-4 inhibition prevented the reduced expression of podocin as well [216]. Metformin is another drug widely used to treat type 2 diabetes. Recently, it was observed to modulate cytoskeleton dynamics by increasing the expression of nephrin and stabilizing the expression of the TRPC6 channel dependent on AMPKα1 activation and regulation of Rho-family small GTPase activity in diabetic conditions. Eventually, metformin treatment reduced glomerular filtration barrier permeability and proteinuria [217]. These findings may broaden the use of these anti-diabetic drugs and provide more options for the treatment of DKD.
Klotho, primarily identified as the anti-aging gene, was recently demonstrated to function in kidney diseases. Klotho administration attenuated actin cytoskeleton disruption and improved podocyte filtration function. It restored the expression of nephrin and ZO-1 and attenuated the increased TRPC6 expression [218]. A subsequent study found that klotho protected against podocyte cytoskeleton disruption and podocyte injury in DKD by significantly inhibiting the expression of TRPC6 [219]. These studies provide evidence that klotho is a potential therapeutic strategy for treating DKD by preserving the podocyte cytoskeleton.
Moreover, catalpol, as an ingredient from the traditional Chinese medicinal herb Rehmannia glutinosa, could ameliorate podocyte injury in DKD by stabilizing the podocyte cytoskeleton. Treatment with catalpol markedly improved the decrease in nephrin and synaptopodin expression. It also rescued disrupted podocyte cytoskeletons by inhibiting RhoA and Cdc42 activation [220]. A compound phloretin from a natural plant was capable of restoring the expression of nephrin and podocin in DKD, thus protecting the podocyte cytoskeleton and the integrity of SD [221]. In PAN-induced podocyte injury, the expression of ACTN4 and CD2AP was significantly reduced, and Shenqi granule was able to upregulate the expression of ACTN4 and CD2AP [222].
In addition to serving as a diagnostic tool for cytoskeleton disruption and kidney diseases, recent studies have suggested that exosomes can also be utilized as a therapeutic means. Wang and colleagues hypothesized that intramuscular administration of miR-29 encapsulated in exosomes could counteract renal fibrosis induced by unilateral ureteral obstruction (UUO) [125]. Therefore, supplementation of exosomes carrying proteins or microRNAs related to podocyte cytoskeleton rearrangement is anticipated to represent a novel strategy for alleviating cytoskeleton disorganization and renal lesions. Nevertheless, it is worth noting that the isolation, purification, and storage of exosomes remain formidable challenges. The specificity, efficacy, and potential side effects of exosomes still require large clinical studies for validation [223,224].

Table 1.
Agents that can modulate podocyte cytoskeleton rearrangement.
Table 1.
Agents that can modulate podocyte cytoskeleton rearrangement.
| Agents | Medicine | Mechanisms/Pathways | References |
|---|---|---|---|
| GCs | DEX | Upregulating nephrin and increasing nephrin phosphorylation | [132,136,137] |
| Facilitating nephrin synthesis and transporting nephrin to the plasma membrane | [133] | ||
| Blocking TRPC6 signaling and stabilizing TRPC6 expression | [138] | ||
| Stabilizing ACTN4 expression | [139] | ||
| Inhibiting Rac1 overactivity | [140] | ||
| Increasing synaptopodin expression | [142] | ||
| / | RTX | Preventing reduced expression of nephrin and podocin | [149] |
| Stabilizing SMPDL-3b | [152] | ||
| CNIs | CsA | Maintaining synaptopodin by blocking the calcineurin-mediated dephosphorylation of synaptopodin | [161] |
| Downregulating TRPC6 expression | [166] | ||
| Decreasing WAVE1 expression | [168] | ||
| TAC | Reducing Angptl4 expression | [120,170] | |
| Increasing FKBP12 expression and enhancing the interaction of FKBP12 with 14-3-3β and synaptopodin | [171] | ||
| Increasing nephrin and podocin expression and maintaining MAP1 LC3 | [172,173] | ||
| / | Abatacept | Stabilizing β1 integrin activation and its association with talin | [178] |
| / | Bis-T-23 | Promoting actin-dependent dynamin oligomerization | [195,196,197] |
| Rho kinase inhibitor | Fasudi | Modulating nephrin and CD2AP | [200] |
| Inhibiting CaMK4/RhoA signaling and inducing the release of YAP | [201] | ||
| Y27632 | Stabilizing actin cytoskeleton and MTs | [202,203] | |
| RAS inhibitor | ACEI/ARB | Stabilizing nephrin expression | [204,205] |
| Inhibiting TRPC6 expression | [207] | ||
| mTOR-inhibitor | EV | Preventing inhibition of the RhoA–ROCK–MLC pathway Stabilizing MTs | [208,209] |
6. Conclusions and Perspectives
In the last few years, the coordination of the podocyte cytoskeleton to sustain intact podocyte structure and function has been strongly emphasized. A large number of mechanisms contributing to cytoskeleton rearrangement in podocytopathies emerged. Nevertheless, it is worth noting that the pathophysiology of podocytes is an evolving scientific issue, so there is an urgent need to constantly explore more valuable mechanisms and improve our understanding of the pathogenesis. Although several drugs have been identified to act on the podocyte cytoskeleton in podocytopathies, the specific mechanisms and the clinical efficacy remain controversial and ambiguous. Therefore, a more in-depth perspective on elaborate signaling cascades or molecules governing podocyte cytoskeleton rearrangement will pave novel avenues for specifically and precisely treating podocytopathies and podocyte-centric diseases.
Author Contributions
Conceptualization, C.Z.; writing—original draft preparation, S.M.; writing—review and editing, S.M., Y.Q. and C.Z.; supervision, C.Z.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is funded by the National Natural Science Foundation of China (82370728, 81974096, 82170773, 81974097, 82100729, 82200808, 82200841, 82300843, and 82300786), the National Key Research and Development Program of China (2021YFC2500200), and the Key Research and Development Program of Hubei Province (2023BCB034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kopp, J.B.; Anders, H.-J.; Susztak, K.; Podestà, M.A.; Remuzzi, G.; Hildebrandt, F.; Romagnani, P. Podocytopathies. Nat. Rev. Dis. Primers 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Fogo, A.B. The Targeted Podocyte. J. Clin. Investig. 2011, 121, 2142–2145. [Google Scholar] [CrossRef] [PubMed]
- Imasawa, T.; Rossignol, R. Podocyte Energy Metabolism and Glomerular Diseases. Int. J. Biochem. Cell Biol. 2013, 45, 2109–2118. [Google Scholar] [CrossRef] [PubMed]
- Durvasula, R.V.; Shankland, S.J. Podocyte Injury and Targeting Therapy: An Update. Curr. Opin. Nephrol. Hypertens. 2006, 15, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Dylewski, J. Regulation of the Actin Cytoskeleton in Podocytes. Cells 2020, 9, 1700. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Altintas, M.M. Podocytes. F1000Research 2016, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Lan, B.; Liu, H.; Persson, P.B.; Lai, E.Y.; Mao, J. A Critical Role of the Podocyte Cytoskeleton in the Pathogenesis of Glomerular Proteinuria and Autoimmune Podocytopathies. Acta Physiol. 2022, 235, e13850. [Google Scholar] [CrossRef]
- Endlich, N.; Siegerist, F.; Endlich, K. Are Podocytes Motile? Pflug. Arch. Eur. J. Physiol. 2017, 469, 951–957. [Google Scholar] [CrossRef]
- Perico, L.; Conti, S.; Benigni, A.; Remuzzi, G. Podocyte—Actin Dynamics in Health and Disease. Nat. Rev. Nephrol. 2016, 12, 692–710. [Google Scholar] [CrossRef]
- Lappalainen, P.; Kotila, T.; Jégou, A.; Romet-Lemonne, G. Biochemical and Mechanical Regulation of Actin Dynamics. Nat. Rev. Mol. Cell Biol. 2022, 23, 836–852. [Google Scholar] [CrossRef]
- Welsh, G.I.; Saleem, M.A. The Podocyte Cytoskeleton—Key to a Functioning Glomerulus in Health and Disease. Nat. Rev. Nephrol. 2012, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.; Bomback, A.S. Approach to Diagnosis and Management of Primary Glomerular Diseases Due to Podocytopathies in Adults: Core Curriculum 2020. Am. J. Kidney Dis. 2020, 75, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, L.; Lugli, G.; Raglianti, V.; Ravaglia, F.; Buti, E.; Landini, S.; Becherucci, F. Defining Diagnostic Trajectories in Patients with Podocytopathies. Clin. Kidney J. 2022, 15, 2006–2019. [Google Scholar] [CrossRef] [PubMed]
- Barisoni, L.; Schnaper, H.W.; Kopp, J.B. A Proposed Taxonomy for the Podocytopathies: A Reassessment of the Primary Nephrotic Diseases. Clin. J. Am. Soc. Nephrol. 2007, 2, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Schell, C.; Huber, T.B. The Evolving Complexity of the Podocyte Cytoskeleton. J. Am. Soc. Nephrol. 2017, 28, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Ishibe, S. Targeting the Podocyte Cytoskeleton: From Pathogenesis to Therapy in Proteinuric Kidney Disease. Nephrol. Dial. Transplant. 2016, 31, 1577–1583. [Google Scholar] [CrossRef]
- Pollard, T.D.; Goldman, R.D. Overview of the Cytoskeleton from an Evolutionary Perspective. Cold Spring Harb. Perspect. Biol. 2018, 10, a030288. [Google Scholar] [CrossRef]
- Muraleedharan, S.; Sam, A.; Skaer, H.; Inamdar, M.S. Networks That Link Cytoskeletal Regulators and Diaphragm Proteins Underpin Filtration Function in Drosophila Nephrocytes. Exp. Cell Res. 2018, 364, 234–242. [Google Scholar] [CrossRef]
- Ahmadian, E.; Eftekhari, A.; Atakishizada, S.; Valiyeva, M.; Ardalan, M.; Khalilov, R.; Kavetskyy, T. Podocytopathy: The Role of Actin Cytoskeleton. Biomed. Pharmacother. 2022, 156, 113920. [Google Scholar] [CrossRef]
- Qu, C.; Roth, R.; Puapatanakul, P.; Loitman, C.; Hammad, D.; Genin, G.M.; Miner, J.H.; Suleiman, H.Y. Three-Dimensional Visualization of the Podocyte Actin Network Using Integrated Membrane Extraction, Electron Microscopy, and Machine Learning. J. Am. Soc. Nephrol. 2022, 33, 155–173. [Google Scholar] [CrossRef]
- Xu, W.; Ge, Y.; Liu, Z.; Gong, R. Glycogen Synthase Kinase 3β Orchestrates Microtubule Remodeling in Compensatory Glomerular Adaptation to Podocyte Depletion. J. Biol. Chem. 2015, 290, 1348–1363. [Google Scholar] [CrossRef] [PubMed]
- Gödel, M.; Temerinac, D.; Grahammer, F.; Hartleben, B.; Kretz, O.; Riederer, B.M.; Propst, F.; Kohl, S.; Huber, T.B. Microtubule Associated Protein 1b (MAP1B) Is a Marker of the Microtubular Cytoskeleton in Podocytes but Is Not Essential for the Function of the Kidney Filtration Barrier in Mice. PLoS ONE 2015, 10, e0140116. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Mundel, P. A Role of Microtubules during the Formation of Cell Processes in Neuronal and Non-Neuronal Cells. Cell Tissue Res. 1998, 291, 163–174. [Google Scholar] [CrossRef]
- Kobayashi, N.; Gao, S.; Chen, J.; Saito, K.; Miyawaki, K.; Li, C.; Pan, L.; Saito, S.; Terashita, T.; Matsuda, S. Process Formation of the Renal Glomerular Podocyte: Is There Common Molecular Machinery for Processes of Podocytes and Neurons? Anat. Sci. Int. 2004, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Heid, H.W.; Sakai, T.; Kriz, W.; Huber, G.; Mundel, P. Molecular Characterization Reveals Identity of Microtubule-Associated Proteins MAP3 and MAP4. Biochem. Biophys. Res. Commun. 2000, 268, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, A.; Bucci, C. Role of Intermediate Filaments in Vesicular Traffic. Cells 2016, 5, 20. [Google Scholar] [CrossRef]
- Zou, J.; Yaoita, E.; Watanabe, Y.; Yoshida, Y.; Nameta, M.; Li, H.; Qu, Z.; Yamamoto, T. Upregulation of Nestin, Vimentin, and Desmin in Rat Podocytes in Response to Injury. Virchows Arch. 2006, 448, 485–492. [Google Scholar] [CrossRef]
- Ge, X.; Zhang, T.; Yu, X.; Muwonge, A.N.; Anandakrishnan, N.; Wong, N.J.; Haydak, J.C.; Reid, J.M.; Fu, J.; Wong, J.S.; et al. LIM-Nebulette Reinforces Podocyte Structural Integrity by Linking Actin and Vimentin Filaments. J. Am. Soc. Nephrol. 2020, 31, 2372–2391. [Google Scholar] [CrossRef]
- Embry, A.E.; Mohammadi, H.; Niu, X.; Liu, L.; Moe, B.; Miller-Little, W.A.; Lu, C.Y.; Bruggeman, L.A.; McCulloch, C.A.; Janmey, P.A.; et al. Biochemical and Cellular Determinants of Renal Glomerular Elasticity. PLoS ONE 2016, 11, e0167924. [Google Scholar] [CrossRef]
- Chen, J.; Boyle, S.; Zhao, M.; Su, W.; Takahashi, K.; Davis, L.; DeCaestecker, M.; Takahashi, T.; Breyer, M.D.; Hao, C.-M. Differential Expression of the Intermediate Filament Protein Nestin during Renal Development and Its Localization in Adult Podocytes. J. Am. Soc. Nephrol. 2006, 17, 1283–1291. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Kaskel, F.J.; Falk, R.J. Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2011, 365, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- Veissi, S.T.; Smeets, B.; van Wijk, J.A.E.; Classens, R.; van der Velden, T.J.A.M.; Jeronimus-Klaasen, A.; Veltkamp, F.; Nienhuis, E.M.; Morello, W.; Montini, G.; et al. Circulating Permeability Factors in Focal Segmental Glomerulosclerosis: In Vitro Detection. Kidney Int. Rep. 2022, 7, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Massengill, S.; Trachtman, H. Genetic Spectrum of Nephrotic Syndrome: Impact of Podocytopathy in Adult Life. Adv. Chronic Kidney Dis. 2022, 29, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Patrakka, J.; Tryggvason, K. Nephrin—A Unique Structural and Signaling Protein of the Kidney Filter. Trends Mol. Med. 2007, 13, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Butt, L.; Unnersjö-Jess, D.; Höhne, M.; Edwards, A.; Binz-Lotter, J.; Reilly, D.; Hahnfeldt, R.; Ziegler, V.; Fremter, K.; Rinschen, M.M.; et al. A Molecular Mechanism Explaining Albuminuria in Kidney Disease. Nat. Metab. 2020, 2, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Santín, S.; García-Maset, R.; Ruíz, P.; Giménez, I.; Zamora, I.; Peña, A.; Madrid, Á.; Camacho, J.A.; Fraga, G.; Sánchez-Moreno, A.; et al. Nephrin Mutations Cause Childhood- and Adult-Onset Focal Segmental Glomerulosclerosis. Kidney Int. 2009, 76, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Nishibori, Y.; Liu, L.; Hosoyamada, M.; Endou, H.; Kudo, A.; Takenaka, H.; Higashihara, E.; Bessho, F.; Takahashi, S.; Kershaw, D.; et al. Disease-Causing Missense Mutations in NPHS2 Gene Alter Normal Nephrin Trafficking to the Plasma Membrane. Kidney Int. 2004, 66, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Riguetti, M.T.P.; Varela, P.; Fernandes, D.E.; Polito, M.G.; Casimiro, F.M.; Pesquero, J.B.; Mastroianni-Kirsztajn, G. Familial Focal Segmental Glomerulosclerosis With Late-Onset Presentation and R229Q/R291W Podocin Mutations. Front. Genet. 2020, 11, 533373. [Google Scholar] [CrossRef]
- Park, H.-Y.; Seong, S.-B.; Min, S.-Y.; Ha, T.-S. CD2-Associated Protein/Phosphoinositide 3-Kinase Signaling Has a Preventive Role in Angiotensin II-Induced Podocyte Apoptosis. Int. J. Biochem. Cell Biol. 2016, 79, 370–381. [Google Scholar] [CrossRef]
- Takano, T.; Bareke, E.; Takeda, N.; Aoudjit, L.; Baldwin, C.; Pisano, P.; Matsuda, J.; El Andalousi, J.; Muhtadie, L.; Bernard, C.; et al. Recessive Mutation in CD2AP Causes Focal Segmental Glomerulosclerosis in Humans and Mice. Kidney Int. 2019, 95, 57–61. [Google Scholar] [CrossRef]
- Michaud, J.-L.R.; Hosseini-Abardeh, M.; Farah, K.; Kennedy, C.R.J. Modulating α-Actinin-4 Dynamics in Podocytes. Cell Motil. Cytoskelet. 2009, 66, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Odenthal, J.; Dittrich, S.; Ludwig, V.; Merz, T.; Reitmeier, K.; Reusch, B.; Höhne, M.; Cosgun, Z.C.; Hohenadel, M.; Putnik, J.; et al. Modeling of ACTN4-Based Podocytopathy Using Drosophila Nephrocytes. Kidney Int. Rep. 2023, 8, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Blasutig, I.M.; Eremina, V.; Ruston, J.M.; Bladt, F.; Li, H.; Huang, H.; Larose, L.; Li, S.S.-C.; Takano, T.; et al. Nck Adaptor Proteins Link Nephrin to the Actin Cytoskeleton of Kidney Podocytes. Nature 2006, 440, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; Phippen, N.J.; Keyvani Chahi, A.; Tilak, M.; Banerjee, S.L.; Lu, P.; New, L.A.; Williamson, C.R.; Platt, M.J.; Simpson, J.A.; et al. Complementary Nck1/2 Signaling in Podocytes Controls α Actinin-4–Mediated Actin Organization, Adhesion, and Basement Membrane Composition. J. Am. Soc. Nephrol. 2022, 33, 1546–1567. [Google Scholar] [CrossRef] [PubMed]
- Akchurin, O.; Reidy, K.J. Genetic Causes of Proteinuria and Nephrotic Syndrome: Impact on Podocyte Pathobiology. Pediatr. Nephrol. 2015, 30, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Brähler, S.; Yu, H.; Suleiman, H.; Krishnan, G.M.; Saunders, B.T.; Kopp, J.B.; Miner, J.H.; Zinselmeyer, B.H.; Shaw, A.S. Intravital and Kidney Slice Imaging of Podocyte Membrane Dynamics. J. Am. Soc. Nephrol. 2016, 27, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Yanagida-Asanuma, E.; Asanuma, K.; Kim, K.; Donnelly, M.; Young Choi, H.; Hyung Chang, J.; Suetsugu, S.; Tomino, Y.; Takenawa, T.; Faul, C.; et al. Synaptopodin Protects Against Proteinuria by Disrupting Cdc42:IRSp53:Mena Signaling Complexes in Kidney Podocytes. Am. J. Pathol. 2007, 171, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.; Suleiman, H.Y.; Miner, J.H. Synaptopodin Is Dispensable for Normal Podocyte Homeostasis but Is Protective in the Context of Acute Podocyte Injury. J. Am. Soc. Nephrol. 2020, 31, 2815–2832. [Google Scholar] [CrossRef]
- Li, Q.; Gulati, A.; Lemaire, M.; Nottoli, T.; Bale, A.; Tufro, A. Rho-GTPase Activating Protein Myosin MYO9A Identified as a Novel Candidate Gene for Monogenic Focal Segmental Glomerulosclerosis. Kidney Int. 2021, 99, 1102–1117. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Chen, Y.; Zhang, H.; Yu, C.; Zhou, F.; Zhang, Z.; Jiang, L.; Li, R.; Ma, J.; et al. RhoA Deficiency Disrupts Podocyte Cytoskeleton and Induces Podocyte Apoptosis by Inhibiting YAP/Dendrin Signal. BMC Nephrol. 2016, 17, 66. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Chen, Y.; Zhang, H.; Zhang, Q.; Li, R.; Ma, J.; Li, Z.; Yu, C.; Lai, Y.; et al. Cdc42 Deficiency Induces Podocyte Apoptosis by Inhibiting the Nwasp/Stress Fibers/YAP Pathway. Cell Death Dis. 2016, 7, e2142. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Arroyo, O.; Selma-Soriano, E.; Ortega, A.; Cortes, R.; Redon, J. Small Rab GTPases in Intracellular Vesicle Trafficking: The Case of Rab3A/Raphillin-3A Complex in the Kidney. Int. J. Mol. Sci. 2021, 22, 7679. [Google Scholar] [CrossRef] [PubMed]
- Rastaldi, M.P.; Armelloni, S.; Berra, S.; Li, M.; Pesaresi, M.; Poczewski, H.; Langer, B.; Kerjaschki, D.; Henger, A.; Blattner, S.M.; et al. Glomerular Podocytes Possess the Synaptic Vesicle Molecule Rab3A and Its Specific Effector Rabphilin-3a. Am. J. Pathol. 2003, 163, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Selma-Soriano, E.; Llamusi, B.; Fernández-Costa, J.M.; Ozimski, L.L.; Artero, R.; Redón, J. Rabphilin Involvement in Filtration and Molecular Uptake in Drosophila Nephrocytes Suggests a Similar Role in Human Podocytes. Dis. Models Mech. 2020, 13, dmm041509. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Staruschenko, A. TRPC6 Channel as an Emerging Determinant of the Podocyte Injury Susceptibility in Kidney Diseases. Am. J. Physiol.-Ren. Physiol. 2015, 309, F393–F397. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, J.; Tsai, H.; Li, L.; Feng, Q.; Miao, J.; Fan, Q. Over-Expressing Transient Receptor Potential Cation Channel 6 in Podocytes Induces Cytoskeleton Rearrangement through Increases of Intracellular Ca2+ and RhoA Activation. Exp. Biol. Med. 2011, 236, 184–193. [Google Scholar] [CrossRef]
- Shalygin, A.; Shuyskiy, L.S.; Bohovyk, R.; Palygin, O.; Staruschenko, A.; Kaznacheyeva, E. Cytoskeleton Rearrangements Modulate TRPC6 Channel Activity in Podocytes. Int. J. Mol. Sci. 2021, 22, 4396. [Google Scholar] [CrossRef]
- Riehle, M.; Büscher, A.K.; Gohlke, B.-O.; Kaßmann, M.; Kolatsi-Joannou, M.; Bräsen, J.H.; Nagel, M.; Becker, J.U.; Winyard, P.; Hoyer, P.F.; et al. TRPC6 G757D Loss-of-Function Mutation Associates with FSGS. J. Am. Soc. Nephrol. 2016, 27, 2771–2783. [Google Scholar] [CrossRef]
- Farmer, L.K.; Rollason, R.; Whitcomb, D.J.; Ni, L.; Goodliff, A.; Lay, A.C.; Birnbaumer, L.; Heesom, K.J.; Xu, S.-Z.; Saleem, M.A.; et al. TRPC6 Binds to and Activates Calpain, Independent of Its Channel Activity, and Regulates Podocyte Cytoskeleton, Cell Adhesion, and Motility. J. Am. Soc. Nephrol. 2019, 30, 1910–1924. [Google Scholar] [CrossRef]
- Beaudreuil, S.; Zhang, X.; Herr, F.; Harper, F.; Candelier, J.J.; Fan, Y.; Yeter, H.; Dudreuilh, C.; Lecru, L.; Vazquez, A.; et al. Circulating CASK Is Associated with Recurrent Focal Segmental Glomerulosclerosis after Transplantation. PLoS ONE 2019, 14, e0219353. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, Y.; Zhao, X. FAM40A Alters the Cytoskeleton of Podocytes in Familial Focal and Segmental Glomerulosclerosis by Regulating F-Actin and Nephrin. Arch. Med. Sci. 2019, 15, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, A.; Cantarelli, C.; Petrosyan, A.; Andrighetto, S.; Budge, K.; D’Agati, V.D.; Hartzell, S.; Malvi, D.; Donadei, C.; Thurman, J.M.; et al. Loss of Decay-Accelerating Factor Triggers Podocyte Injury and Glomerulosclerosis. J. Exp. Med. 2020, 217, e20191699. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wang, X.; Yin, X.; Li, Y.; Liu, X.; Wang, H.; Liu, X.; Zhang, J.; Gao, H.; Shi, B.; et al. Plectin Protects Podocytes from Adriamycin-induced Apoptosis and F-actin Cytoskeletal Disruption through the Integrin A6β4/FAK/P38 MAPK Pathway. J. Cell. Mol. Medi 2018, 22, 5450–5467. [Google Scholar] [CrossRef] [PubMed]
- Solberg, R.; Lunde, N.N.; Forbord, K.M.; Okla, M.; Kassem, M.; Jafari, A. The Mammalian Cysteine Protease Legumain in Health and Disease. Int. J. Mol. Sci. 2022, 23, 15983. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Matthews, S.P.; Reinheckel, T.; Fleming, S.; Watts, C. Asparagine Endopeptidase Is Required for Normal Kidney Physiology and Homeostasis. FASEB J. 2011, 25, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Yamasaki, M.; Fukui, T. Degradation of Acetoacetyl-CoA Synthetase, a Ketone Body-Utilizing Enzyme, by Legumain in the Mouse Kidney. Biochem. Biophys. Res. Commun. 2014, 453, 631–635. [Google Scholar] [CrossRef]
- Martínez-Fábregas, J.; Prescott, A.; Van Kasteren, S.; Pedrioli, D.L.; McLean, I.; Moles, A.; Reinheckel, T.; Poli, V.; Watts, C. Lysosomal Protease Deficiency or Substrate Overload Induces an Oxidative-Stress Mediated STAT3-Dependent Pathway of Lysosomal Homeostasis. Nat. Commun. 2018, 9, 5343. [Google Scholar] [CrossRef]
- Yamamoto-Nonaka, K.; Koike, M.; Asanuma, K.; Takagi, M.; Oliva Trejo, J.A.; Seki, T.; Hidaka, T.; Ichimura, K.; Sakai, T.; Tada, N.; et al. Cathepsin D in Podocytes Is Important in the Pathogenesis of Proteinuria and CKD. J. Am. Soc. Nephrol. 2016, 27, 2685–2700. [Google Scholar] [CrossRef]
- Qiu, Y.; Lei, C.; Zeng, J.; Xie, Y.; Cao, Y.; Yuan, Q.; Su, H.; Zhang, Z.; Zhang, C. Asparagine Endopeptidase Protects Podocytes in Adriamycin-Induced Nephropathy by Regulating Actin Dynamics through Cleaving Transgelin. Mol. Ther. 2023, 31, 3337–3354. [Google Scholar] [CrossRef]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Uchida, K.; Uchida, S.; Sasaki, S.; Nitta, K. Dexamethasone Increases the Phosphorylation of Nephrin in Cultured Podocytes. Clin. Exp. Nephrol. 2011, 15, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Kilpeläinen, P.; Hellman, U.; Sun, Y.; Wartiovaara, J.; Morgunova, E.; Pikkarainen, T.; Yan, K.; Jonsson, A.P.; Tryggvason, K. Characterization of the Interactions of the Nephrin Intracellular Domain: Evidence that the Scaffolding Protein IQGAP1 Associates with Nephrin. FEBS J. 2004, 272, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liang, W.; Yang, Y.; Pan, Y.; Yang, Q.; Chen, X.; Singhal, P.C.; Ding, G. IQGAP1 Regulates Actin Cytoskeleton Organization in Podocytes through Interaction with Nephrin. Cell. Signal. 2015, 27, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, Q.; Zhong, Z.; Liang, W.; Zhang, L.; Yang, Y.; Ding, G. Role of C-Abl and Nephrin in Podocyte Cytoskeletal Remodeling Induced by Angiotensin II. Cell Death Dis. 2018, 9, 185. [Google Scholar] [CrossRef]
- Ha, T.-S.; Nam, J.A.; Seong, S.-B.; Saleem, M.A.; Park, S.J.; Shin, J.I. Montelukast Improves the Changes of Cytoskeletal and Adaptor Proteins of Human Podocytes by Interleukin-13. Inflamm. Res. 2017, 66, 793–802. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, H.; Liu, S.; Yuan, Y.; Yuan, C.; Mwamunyi, M.; Pearce, D.; Yao, L. Lack of Serum- and Glucocorticoid-inducible Kinase 3 Leads to Podocyte Dysfunction. FASEB J. 2018, 32, 576–587. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Deng, H.; Liu, M.; Lin, X.; Zhang, M.; Li, G.; Yue, S.; Gao, X. ANGPTL4 Promotes Nephrotic Syndrome by Downregulating Podocyte Expression of ACTN4 and Podocin. Biochem. Biophys. Res. Commun. 2023, 639, 176–182. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, Y.; Lin, C.; Weng, C.; Wang, Y.; Feng, S.; Wang, C.; Shao, X.; Lin, W.; Li, B.; et al. Calcineurin Inhibitors Ameliorate PAN -induced Podocyte Injury through the NFAT–Angptl4 Pathway. J. Pathol. 2020, 252, 227–238. [Google Scholar] [CrossRef]
- Watts, A.J.B.; Keller, K.H.; Lerner, G.; Rosales, I.; Collins, A.B.; Sekulic, M.; Waikar, S.S.; Chandraker, A.; Riella, L.V.; Alexander, M.P.; et al. Discovery of Autoantibodies Targeting Nephrin in Minimal Change Disease Supports a Novel Autoimmune Etiology. J. Am. Soc. Nephrol. 2022, 33, 238–252. [Google Scholar] [CrossRef]
- Lin, L.; Hu, K. Annexin A2 and Kidney Diseases. Front. Cell Dev. Biol. 2022, 10, 974381. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zhang, Y.; Zhuang, J.; Bi, Y.; Xu, H.; Shen, Q.; Liu, J.; Fu, H.; Wang, J.; Feng, C.; et al. The Important Roles and Molecular Mechanisms of Annexin A2 Autoantibody in Children with Nephrotic Syndrome. Ann. Transl. Med. 2021, 9, 1452. [Google Scholar] [CrossRef]
- Thomas, M.C.; Brownlee, M.; Susztak, K.; Sharma, K.; Jandeleit-Dahm, K.A.M.; Zoungas, S.; Rossing, P.; Groop, P.-H.; Cooper, M.E. Diabetic Kidney Disease. Nat. Rev. Dis. Primers 2015, 1, 15018. [Google Scholar] [CrossRef] [PubMed]
- Mohandes, S.; Doke, T.; Hu, H.; Mukhi, D.; Dhillon, P.; Susztak, K. Molecular Pathways That Drive Diabetic Kidney Disease. J. Clin. Investig. 2023, 133, e165654. [Google Scholar] [CrossRef] [PubMed]
- Bartolák-Suki, E.; Imsirovic, J.; Nishibori, Y.; Krishnan, R.; Suki, B. Regulation of Mitochondrial Structure and Dynamics by the Cytoskeleton and Mechanical Factors. Int. J. Mol. Sci. 2017, 18, 1812. [Google Scholar] [CrossRef] [PubMed]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial Dysfunction in Diabetic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.; Rajendran, M.; Rostovtseva, T.; Hool, L. How Cytoskeletal Proteins Regulate Mitochondrial Energetics in Cell Physiology and Diseases. Phil. Trans. R. Soc. B 2022, 377, 20210324. [Google Scholar] [CrossRef]
- Oosterheert, W.; Klink, B.U.; Belyy, A.; Pospich, S.; Raunser, S. Structural Basis of Actin Filament Assembly and Aging. Nature 2022, 611, 374–379. [Google Scholar] [CrossRef]
- Plastino, J.; Blanchoin, L. Dynamic Stability of the Actin Ecosystem. J. Cell Sci. 2019, 132, jcs219832. [Google Scholar] [CrossRef]
- Wolff, D.W.; Bianchi-Smiraglia, A.; Nikiforov, M.A. Compartmentalization and Regulation of GTP in Control of Cellular Phenotypes. Trends Mol. Med. 2022, 28, 758–769. [Google Scholar] [CrossRef]
- Matsuda, J.; Asano-Matsuda, K.; Kitzler, T.M.; Takano, T. Rho GTPase Regulatory Proteins in Podocytes. Kidney Int. 2021, 99, 336–345. [Google Scholar] [CrossRef]
- Shen, J.; Wang, R.; He, Z.; Huang, H.; He, X.; Zhou, J.; Yan, Y.; Shen, S.; Shao, X.; Shen, X.; et al. NMDA Receptors Participate in the Progression of Diabetic Kidney Disease by Decreasing Cdc42-GTP Activation in Podocytes: NMDARs in DKD Podocytes. J. Pathol. 2016, 240, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Anderson, M.; Dryer, S.E. Insulin Increases Surface Expression of TRPC6 Channels in Podocytes: Role of NADPH Oxidases and Reactive Oxygen Species. Am. J. Physiol.-Ren. Physiol. 2012, 302, F298–F307. [Google Scholar] [CrossRef] [PubMed]
- Rachubik, P.; Szrejder, M.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Angielski, S.; Piwkowska, A. The TRPC6-AMPK Pathway Is Involved in Insulin-Dependent Cytoskeleton Reorganization and Glucose Uptake in Cultured Rat Podocytes. Cell Physiol. Biochem. 2018, 51, 393–410. [Google Scholar] [CrossRef]
- Rachubik, P.; Szrejder, M.; Rogacka, D.; Typiak, M.; Audzeyenka, I.; Kasztan, M.; Pollock, D.M.; Angielski, S.; Piwkowska, A. Insulin Controls Cytoskeleton Reorganization and Filtration Barrier Permeability via the PKGIα-Rac1-RhoA Crosstalk in Cultured Rat Podocytes. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2022, 1869, 119301. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A. Role of Protein Kinase G and Reactive Oxygen Species in the Regulation of Podocyte Function in Health and Disease: PODOCYTE AND PKGIα-DEPENDENT SIGNALING. J. Cell. Physiol. 2017, 232, 691–697. [Google Scholar] [CrossRef]
- Audzeyenka, I.; Rogacka, D.; Rachubik, P.; Typiak, M.; Rychłowski, M.; Angielski, S.; Piwkowska, A. The PKGIα–Rac1 Pathway Is a Novel Regulator of Insulin-dependent Glucose Uptake in Cultured Rat Podocytes. J. Cell Physiol. 2021, 236, 4655–4668. [Google Scholar] [CrossRef]
- Armelloni, S.; Calvaresi, N.; Ikehata, M.; Corbelli, A.; Mattinzoli, D.; Giardino, L.A.; Li, M.; Messa, P.; Rastaldi, M.P. Proteinuria and Glomerular Damage in Rab3A Knockout Mice Chronically Fed a High-Glucose Diet. Nephron Exp. Nephrol. 2012, 120, e69–e80. [Google Scholar] [CrossRef]
- Martinez-Arroyo, O.; Flores-Chova, A.; Sanchez-Garcia, B.; Redon, J.; Cortes, R.; Ortega, A. Rab3A/Rab27A System Silencing Ameliorates High Glucose-Induced Injury in Podocytes. Biology 2023, 12, 690. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, Y.; Yu, H.; Yu, X.; Zhou, J.; Xiao, J. RhoA Protects the Podocytes against High Glucose-Induced Apoptosis through YAP and Plays Critical Role in Diabetic Nephropathy. Biochem. Biophys. Res. Commun. 2018, 504, 949–956. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, S.; Shi, J.; Xu, X.; Wang, L.; Zhai, X.; Hou, Q.; Qin, W.; Chen, Z. TYRO3 Protects Podocyte via JNK/c-Jun-P53 Pathway. Arch. Biochem. Biophys. 2023, 739, 109578. [Google Scholar] [CrossRef]
- Guo, K.; Pan, P.; Wu, M.; Ma, Y.; Lu, J.; Chen, H. Hyposialylated Angiopoietin-like-4 Induces Apoptosis of Podocytes via Β1 Integrin/FAK Signaling in Diabetic Nephropathy. Mol. Cell. Endocrinol. 2020, 505, 110730. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, C.; Ye, Q.; Tong, L.; Jiang, H.; Zhu, X.; Huang, L.; Lin, W.; Fu, H.; Wang, J.; et al. Podocyte Apoptosis in Diabetic Nephropathy by BASP1 Activation of the P53 Pathway via WT1. Acta Physiol. 2021, 232. [Google Scholar] [CrossRef] [PubMed]
- Lizotte, F.; Rousseau, M.; Denhez, B.; Lévesque, D.; Guay, A.; Liu, H.; Moreau, J.; Higgins, S.; Sabbagh, R.; Susztak, K.; et al. Deletion of Protein Tyrosine Phosphatase SHP-1 Restores SUMOylation of Podocin and Reverses the Progression of Diabetic Kidney Disease. Kidney Int. 2023, 104, 787–802. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Lee, S.J.; Lee, J.-H.; Kim, J.-H.; Son, S.S.; Cha, S.-K.; Lee, E.S.; Chung, C.H.; Lee, E.Y. Angiotensin II-Mediated MYH9 Downregulation Causes Structural and Functional Podocyte Injury in Diabetic Kidney Disease. Sci. Rep. 2019, 9, 7679. [Google Scholar] [CrossRef] [PubMed]
- Kubo, A.; Hidaka, T.; Nakayama, M.; Sasaki, Y.; Takagi, M.; Suzuki, H.; Suzuki, Y. Protective Effects of DPP-4 Inhibitor on Podocyte Injury in Glomerular Diseases. BMC Nephrol. 2020, 21, 402. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, S.; Hou, Q.; Qiu, D.; Shi, J.; Wang, L.; Chen, Z.; Zhang, M.; Duan, A.; Qin, W.; et al. Dissection of Glomerular Transcriptional Profile in Patients with Diabetic Nephropathy: SRGAP2a Protects Podocyte Structure and Function. Diabetes 2018, 67, 717–730. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Q.; Fan, X.; Zhen, J.; Wang, C.; Chen, H.; Liu, Y.; Zhou, P.; Zhang, T.; Huang, T.; et al. Long Noncoding RNA ENST00000436340 Promotes Podocyte Injury in Diabetic Kidney Disease by Facilitating the Association of PTBP1 with RAB3B. Cell Death Dis. 2023, 14, 130. [Google Scholar] [CrossRef]
- Garsen, M.; Rops, A.L.W.M.M.; Dijkman, H.; Willemsen, B.; Van Kuppevelt, T.H.; Russel, F.G.; Rabelink, T.J.; Berden, J.H.M.; Reinheckel, T.; Van Der Vlag, J. Cathepsin L Is Crucial for the Development of Early Experimental Diabetic Nephropathy. Kidney Int. 2016, 90, 1012–1022. [Google Scholar] [CrossRef]
- Yaddanapudi, S.; Altintas, M.M.; Kistler, A.D.; Fernandez, I.; Möller, C.C.; Wei, C.; Peev, V.; Flesche, J.B.; Forst, A.-L.; Li, J.; et al. CD2AP in Mouse and Human Podocytes Controls a Proteolytic Program that Regulates Cytoskeletal Structure and Cellular Survival. J. Clin. Investig. 2011, 121, 3965–3980. [Google Scholar] [CrossRef]
- Sever, S.; Altintas, M.M.; Nankoe, S.R.; Möller, C.C.; Ko, D.; Wei, C.; Henderson, J.; Del Re, E.C.; Hsing, L.; Erickson, A.; et al. Proteolytic Processing of Dynamin by Cytoplasmic Cathepsin L Is a Mechanism for Proteinuric Kidney Disease. J. Clin. Investig. 2007, 117, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Adair, B.; Reinheckel, T. Specialized Roles for Cysteine Cathepsins in Health and Disease. J. Clin. Investig. 2010, 120, 3421–3431. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Li, M.; Qiu, Y.; Xie, Y.; Hao, Z.; Yin, X.; Zhang, Z.; Su, H.; Yang, L.; Lin, J.; et al. Asparaginyl Endopeptidase Protects against Podocyte Injury in Diabetic Nephropathy through Cleaving Cofilin-1. Cell Death Dis. 2022, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Ronco, P.; Beck, L.; Debiec, H.; Fervenza, F.C.; Hou, F.F.; Jha, V.; Sethi, S.; Tong, A.; Vivarelli, M.; Wetzels, J. Membranous Nephropathy. Nat. Rev. Dis. Primers 2021, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- Haddad, G.; Lorenzen, J.M.; Ma, H.; De Haan, N.; Seeger, H.; Zaghrini, C.; Brandt, S.; Kölling, M.; Wegmann, U.; Kiss, B.; et al. Altered Glycosylation of IgG4 Promotes Lectin Complement Pathway Activation in Anti-PLA2R1–Associated Membranous Nephropathy. J. Clin. Investig. 2021, 131, e140453. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Schwesinger, C.; Meyer, T.N.; Sievert, H.; Hoxha, E.; Sachs, M.; Klupp, E.-M.; Münster, S.; Balabanov, S.; Carrier, L.; Helmchen, U.; et al. Ubiquitin C-Terminal Hydrolase-L1 Activity Induces Polyubiquitin Accumulation in Podocytes and Increases Proteinuria in Rat Membranous Nephropathy. Am. J. Pathol. 2011, 178, 2044–2057. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, F.; Sachs, M.; Meyer, T.N.; Sievert, H.; Lindenmeyer, M.T.; Wiech, T.; Cohen, C.D.; Balabanov, S.; Stahl, R.A.K.; Meyer-Schwesinger, C. UCH-L1 Induces Podocyte Hypertrophy in Membranous Nephropathy by Protein Accumulation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 945–958. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, W.; Sun, Y.; Qiao, Y.; Zhang, L.; Zhao, Z.; Lv, S. Wnt/β-Catenin Signaling Mediated-UCH-L1 Expression in Podocytes of Diabetic Nephropathy. Int. J. Mol. Sci. 2016, 17, 1404. [Google Scholar] [CrossRef]
- Fang, Y.; Li, F.; Qi, C.; Mao, X.; Xu, Y.; Zhao, Z.; Wu, H.; Zhang, Z. Plakoglobin Is Involved in Cytoskeletal Rearrangement of Podocytes under the Regulation of UCH-L1. Biochem. Biophys. Res. Commun. 2020, 529, 112–118. [Google Scholar] [CrossRef]
- Peng, L.; Ma, J.; Cui, R.; Chen, X.; Wei, S.-Y.; Wei, Q.-J.; Li, B. The Calcineurin Inhibitor Tacrolimus Reduces Proteinuria in Membranous Nephropathy Accompanied by a Decrease in Angiopoietin-Like-4. PLoS ONE 2014, 9, e106164. [Google Scholar] [CrossRef]
- Ozawa, S.; Matsubayashi, M.; Nanaura, H.; Yanagita, M.; Mori, K.; Asanuma, K.; Kajiwara, N.; Hayashi, K.; Ohashi, H.; Kasahara, M.; et al. Proteolytic Cleavage of Podocin by Matriptase Exacerbates Podocyte Injury. J. Biol. Chem. 2020, 295, 16002–16012. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Li, P.; Dang, X.; Zhang, X.; Mao, Y.; Chen, X. Lupus Nephritis: New Progress in Diagnosis and Treatment. J. Autoimmun. 2022, 132, 102871. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pan, X.; Fu, X.; Yang, Y.; Chen, J.; Lin, W. MicroRNA-26a: An Emerging Regulator of Renal Biology and Disease. Kidney Blood Press. Res. 2019, 44, 287–297. [Google Scholar] [CrossRef]
- Ichii, O.; Otsuka-Kanazawa, S.; Horino, T.; Kimura, J.; Nakamura, T.; Matsumoto, M.; Toi, M.; Kon, Y. Decreased miR-26a Expression Correlates with the Progression of Podocyte Injury in Autoimmune Glomerulonephritis. PLoS ONE 2014, 9, e110383. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, B.; Zhang, A.; Hassounah, F.; Seow, Y.; Wood, M.; Ma, F.; Klein, J.D.; Price, S.R.; Wang, X.H. Exosome-Mediated miR-29 Transfer Reduces Muscle Atrophy and Kidney Fibrosis in Mice. Mol. Ther. 2019, 27, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Caster, D.J.; Korte, E.A.; Tan, M.; Barati, M.T.; Tandon, S.; Creed, T.M.; Salant, D.J.; Hata, J.L.; Epstein, P.N.; Huang, H.; et al. Neutrophil Exocytosis Induces Podocyte Cytoskeletal Reorganization and Proteinuria in Experimental Glomerulonephritis. Am. J. Physiol.-Ren. Physiol. 2018, 315, F595–F606. [Google Scholar] [CrossRef]
- Mathieson, P.W. The Podocyte as a Target for Therapies—New and Old. Nat. Rev. Nephrol. 2012, 8, 52–56. [Google Scholar] [CrossRef]
- Lee, H.W.; Arif, E.; Altintas, M.M.; Quick, K.; Maheshwari, S.; Plezia, A.; Mahmood, A.; Reiser, J.; Nihalani, D.; Gupta, V. High-Content Screening Assay-Based Discovery of Paullones as Novel Podocyte-Protective Agents. Am. J. Physiol.-Ren. Physiol. 2018, 314, F280–F292. [Google Scholar] [CrossRef]
- Ponticelli, C.; Locatelli, F. Glucocorticoids in the Treatment of Glomerular Diseases: Pitfalls and Pearls. Clin. J. Am. Soc. Nephrol. 2018, 13, 815–822. [Google Scholar] [CrossRef]
- Wang, H.; Duan, A.; Zhang, J.; Wang, Q.; Xing, Y.; Qin, Z.; Liu, Z.; Yang, J. Glucocorticoid Receptor Wields Chromatin Interactions to Tune Transcription for Cytoskeleton Stabilization in Podocytes. Commun. Biol. 2021, 4, 675. [Google Scholar] [CrossRef]
- van den Broek, M.; Smeets, B.; Schreuder, M.F.; Jansen, J. The Podocyte as a Direct Target of Glucocorticoids in Nephrotic Syndrome. Nephrol. Dial. Transplant. 2022, 37, 1808–1815. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.-Y.; Saleem, M.A.; Coward, R.J.; Ni, L.; Witherden, I.R.; Mathieson, P.W. Direct Effects of Dexamethasone on Human Podocytes. Kidney Int. 2006, 70, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Khoshnoodi, J.; Takenaka, H.; Hosoyamada, M.; Nakajo, A.; Bessho, F.; Kudo, A.; Takahashi, S.; Arimura, Y.; Yamada, A.; et al. The Effect of Dexamethasone on Defective Nephrin Transport Caused by ER Stress: A Potential Mechanism for the Therapeutic Action of Glucocorticoids in the Acquired Glomerular Diseases. Kidney Int. 2006, 69, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Ding, J.; Fan, Q.; Guan, N. Diversities of Podocyte Molecular Changes Induced by Different Antiproteinuria Drugs. Exp. Biol. Med. 2006, 231, 585–593. [Google Scholar] [CrossRef]
- Moysiadis, D.; Perysinaki, G.; Bertsias, G.; Stratakis, S.; Kyriacou, K.; Nakopoulou, L.; Boumpas, D.; Daphnis, E. Early Treatment with Glucocorticoids or Cyclophosphamide Retains the Slit Diaphragm Proteins Nephrin and Podocin in Experimental Lupus Nephritis. Lupus 2012, 21, 1196–1207. [Google Scholar] [CrossRef]
- Yu, M.; Ren, Q.; Yu, S.Y. Role of Nephrin Phosphorylation Inducted by Dexamethasone and Angiotensin II in Podocytes. Mol. Biol. Rep. 2014, 41, 3591–3595. [Google Scholar] [CrossRef]
- Jiang, L.; Hindmarch, C.C.T.; Rogers, M.; Campbell, C.; Waterfall, C.; Coghill, J.; Mathieson, P.W.; Welsh, G.I. RNA Sequencing Analysis of Human Podocytes Reveals Glucocorticoid Regulated Gene Networks Targeting Non-Immune Pathways. Sci. Rep. 2016, 6, 35671. [Google Scholar] [CrossRef]
- Yu, S.; Yu, L. Dexamethasone Resisted Podocyte Injury via Stabilizing TRPC6 Expression and Distribution. Evid.-Based Complement. Altern. Med. 2012, 2012, 652059. [Google Scholar] [CrossRef]
- Liu, H.; Gao, X.; Xu, H.; Feng, C.; Kuang, X.; Li, Z.; Zha, X. α-Actinin-4 Is Involved in the Process by which Dexamethasone Protects Actin Cytoskeleton Stabilization from Adriamycin-Induced Podocyte Injury: Dexamethasone and Cytoskeleton. Nephrology 2012, 17, 669–675. [Google Scholar] [CrossRef]
- McCaffrey, J.C.; Webb, N.J.; Poolman, T.M.; Fresquet, M.; Moxey, C.; Zeef, L.A.H.; Donaldson, I.J.; Ray, D.W.; Lennon, R. Glucocorticoid Therapy Regulates Podocyte Motility by Inhibition of Rac1. Sci. Rep. 2017, 7, 6725. [Google Scholar] [CrossRef]
- Hirakawa, M.; Tsuruya, K.; Yotsueda, H.; Tokumoto, M.; Ikeda, H.; Katafuchi, R.; Fujimi, S.; Hirakata, H.; Iida, M. Expression of Synaptopodin and GLEPP1 as Markers of Steroid Responsiveness in Primary Focal Segmental Glomerulosclerosis. Life Sci. 2006, 79, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Chanley, M.A.; Westbrook, D.; Nie, X.; Kitao, T.; Guess, A.J.; Benndorf, R.; Hidalgo, G.; Smoyer, W.E. Pioglitazone Enhances the Beneficial Effects of Glucocorticoids in Experimental Nephrotic Syndrome. Sci. Rep. 2016, 6, 24392. [Google Scholar] [CrossRef] [PubMed]
- Lewko, B.; Waszkiewicz, A.; Maryn, A.; Gołos, M.; Latawiec, E.; Daca, A.; Witkowski, J.M.; Angielski, S.; Stępiński, J. Dexamethasone-Dependent Modulation of Cyclic GMP Synthesis in Podocytes. Mol. Cell Biochem. 2015, 409, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Mallipattu, S.K.; Guo, Y.; Revelo, M.P.; Roa-Peña, L.; Miller, T.; Ling, J.; Shankland, S.J.; Bialkowska, A.B.; Ly, V.; Estrada, C.; et al. Krüppel–Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers. J. Am. Soc. Nephrol. 2017, 28, 166–184. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pace, J.; Li, Z.; Ma’ayan, A.; Wang, Z.; Revelo, M.P.; Chen, E.; Gu, X.; Attalah, A.; Yang, Y.; et al. Podocyte-Specific Induction of Krüppel-Like Factor 15 Restores Differentiation Markers and Attenuates Kidney Injury in Proteinuric Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 2529–2545. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Zhang, H.; Ke, G.; Zhang, L.; Lian, Z.; Chen, X.; Zhao, X.; Chen, Y.; Li, R.; Ma, J.; et al. The Krüppel-like Factor 15-NFATc1 Axis Ameliorates Podocyte Injury: A Novel Rationale for Using Glucocorticoids in Proteinuria Diseases. Clin. Sci. 2020, 134, 1305–1318. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Bharati, J.; Nada, R.; Minz, R.; Kohli, H.S. Rituximab in Maintaining Remission in Adults with Podocytopathy. Nephrology 2020, 25, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Fenoglio, R.; Baldovino, S.; Sciascia, S.; De Simone, E.; Del Vecchio, G.; Ferro, M.; Quattrocchio, G.; Naretto, C.; Roccatello, D. Efficacy of Low or Standard Rituximab-Based Protocols and Comparison to Ponticelli’s Regimen in Membranous Nephropathy. J. Nephrol. 2021, 34, 565–571. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ikezumi, Y.; Saitoh, A. Rituximab Protects Podocytes and Exerts Anti-Proteinuric Effects in Rat Adriamycin-Induced Nephropathy Independent of B-Lymphocytes: Rituximab Ameliorates Proteinuria in Rat ADN. Nephrology 2017, 22, 49–57. [Google Scholar] [CrossRef]
- Jeruschke, S.; Alex, D.; Hoyer, P.F.; Weber, S. Protective Effects of Rituximab on Puromycin-Induced Apoptosis, Loss of Adhesion and Cytoskeletal Alterations in Human Podocytes. Sci. Rep. 2022, 12, 12297. [Google Scholar] [CrossRef]
- Perosa, F.; Favoino, E.; Caragnano, M.A.; Dammacco, F. Generation of Biologically Active Linear and Cyclic Peptides Has Revealed a Unique Fine Specificity of Rituximab and Its Possible Cross-Reactivity with Acid Sphingomyelinase-like Phosphodiesterase 3b Precursor. Blood 2006, 107, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Fornoni, A.; Sageshima, J.; Wei, C.; Merscher-Gomez, S.; Aguillon-Prada, R.; Jauregui, A.N.; Li, J.; Mattiazzi, A.; Ciancio, G.; Chen, L.; et al. Rituximab Targets Podocytes in Recurrent Focal Segmental Glomerulosclerosis. Sci. Transl. Med. 2011, 3, 85ra46. [Google Scholar] [CrossRef] [PubMed]
- Pescovitz, M.D.; Book, B.K.; Sidner, R.A. Resolution of Recurrent Focal Segmental Glomerulosclerosis Proteinuria after Rituximab Treatment. N. Engl. J. Med. 2006, 354, 1961–1963. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Faguer, S.; Esposito, L.; Guitard, J.; Nogier, M.B.; Durand, D.; Rostaing, L. Treatment of Focal Segmental Glomerular Sclerosis with Rituximab: 2 Case Reports. Clin. Nephrol. 2007, 67, 250–254. [Google Scholar] [CrossRef]
- Strologo, L.D.; Guzzo, I.; Laurenzi, C.; Vivarelli, M.; Parodi, A.; Barbano, G.; Camilla, R.; Scozzola, F.; Amore, A.; Ginevri, F.; et al. Use of Rituximab in Focal Glomerulosclerosis Relapses after Renal Transplantation. Transplantation 2009, 88, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Takasu, J.; Nihei, H.; Yonekura, T.; Aoki, Y.; Kawamura, T.; Mizuiri, S.; Aikawa, A. Protocol Biopsies for Focal Segmental Glomerulosclerosis Treated with Plasma Exchange and Rituximab in a Renal Transplant Patient: Plasma Exchange and Rituximab for FSGS. Clin. Transplant. 2010, 24, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.; Reggiani, F.; Moroni, G. Old and New Calcineurin Inhibitors in Lupus Nephritis. J. Clin. Med. 2021, 10, 4832. [Google Scholar] [CrossRef]
- Clipstone, N.A.; Crabtree, G.R. Identification of Calcineurin as a Key Signalling Enzyme in T-Lymphocyte Activation. Nature 1992, 357, 695–697. [Google Scholar] [CrossRef]
- Descazeaud, V.; Mestre, E.; Marquet, P.; Essig, M. Calcineurin Regulation of Cytoskeleton Organization: A New Paradigm to Analyse the Effects of Calcineurin Inhibitors on the Kidney. J. Cell. Mol. Med. 2012, 16, 218–227. [Google Scholar] [CrossRef]
- Mundel, P.; Reiser, J. Proteinuria: An Enzymatic Disease of the Podocyte? Kidney Int. 2010, 77, 571–580. [Google Scholar] [CrossRef]
- Faul, C.; Donnelly, M.; Merscher-Gomez, S.; Chang, Y.H.; Franz, S.; Delfgaauw, J.; Chang, J.-M.; Choi, H.Y.; Campbell, K.N.; Kim, K.; et al. The Actin Cytoskeleton of Kidney Podocytes Is a Direct Target of the Antiproteinuric Effect of Cyclosporine A. Nat. Med. 2008, 14, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Liu, Q.; Zheng, Z.; Fan, J.; Peng, W.; Kong, Q.; He, H.; Yang, S.; Chen, W.; Tang, X.; et al. Tacrolimus Protects Podocytes from Injury in Lupus Nephritis Partly by Stabilizing the Cytoskeleton and Inhibiting Podocyte Apoptosis. PLoS ONE 2015, 10, e0132724. [Google Scholar] [CrossRef]
- Sever, S.; Schiffer, M. Actin Dynamics at Focal Adhesions: A Common Endpoint and Putative Therapeutic Target for Proteinuric Kidney Diseases. Kidney Int. 2018, 93, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Sloan, A.J.; Hoenderop, J.G.J.; Flesche, J.; Van Goor, H.; Kistler, A.D.; Bakker, M.; Bindels, R.J.M.; De Boer, R.A.; Möller, C.C.; et al. Angiotensin II Contributes to Podocyte Injury by Increasing TRPC6 Expression via an NFAT-Mediated Positive Feedback Signaling Pathway. Am. J. Pathol. 2011, 179, 1719–1732. [Google Scholar] [CrossRef] [PubMed]
- Schlöndorff, J.; Del Camino, D.; Carrasquillo, R.; Lacey, V.; Pollak, M.R. TRPC6 Mutations Associated with Focal Segmental Glomerulosclerosis Cause Constitutive Activation of NFAT-Dependent Transcription. Am. J. Physiol.-Cell Physiol. 2009, 296, C558–C569. [Google Scholar] [CrossRef] [PubMed]
- Shengyou, Y.; Li, Y.; Zhihong, H.; Yuanyuan, M. Influence of Tacrolimus on Podocyte Injury Inducted by Angiotensin II. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Liu, Y.; Li, X.; Thilo, F.; Tepel, M. Insulin Increases Expression of TRPC6 Channels in Podocytes by a Calcineurin-Dependent Pathway. Cell Physiol. Biochem. 2016, 38, 659–669. [Google Scholar] [CrossRef]
- Li, X.; Ding, F.; Wang, S.; Li, B.; Ding, J. Cyclosporine A Protects Podocytes by Regulating WAVE1 Phosphorylation. Sci. Rep. 2015, 5, 17694. [Google Scholar] [CrossRef]
- Shen, X.; Weng, C.; Wang, Y.; Wang, C.; Feng, S.; Li, X.; Li, H.; Jiang, H.; Wang, H.; Chen, J. Lipopolysaccharide-Induced Podocyte Injury Is Regulated by Calcineurin/NFAT and TLR4/MyD88/NF-κB Signaling Pathways through Angiopoietin-like Protein 4. Genes Dis. 2022, 9, 443–455. [Google Scholar] [CrossRef]
- Li, J.-S.; Chen, X.; Peng, L.; Wei, S.-Y.; Zhao, S.-L.; Diao, T.-T.; He, Y.-X.; Liu, F.; Wei, Q.-J.; Zhang, Q.-F.; et al. Angiopoietin-Like-4, a Potential Target of Tacrolimus, Predicts Earlier Podocyte Injury in Minimal Change Disease. PLoS ONE 2015, 10, e0137049. [Google Scholar] [CrossRef]
- Yasuda, H.; Fukusumi, Y.; Ivanov, V.; Zhang, Y.; Kawachi, H. Tacrolimus Ameliorates Podocyte Injury by Restoring FK506 Binding Protein 12 (FKBP12) at Actin Cytoskeleton. FASEB J. 2021, 35, e21983. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-M.; Wang, J.; Xu, X.-X.; Li, Y.-Y.; Wu, Y.-G. FK506 Reduces Albuminuria through Improving Podocyte Nephrin and Podocin Expression in Diabetic Rats. Inflamm. Res. 2016, 65, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, S.; Yu, L.; Ge, L.; Zhang, Y.; Hao, Z.; Liu, G. Effects of Tacrolimus on Autophagy Protein LC3 in Puromycin-Damaged Mouse Podocytes. J. Int. Med. Res. 2020, 48, 300060520971422. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Dong, W.; Chen, Y.; Tang, T.; Zhao, X.; Zhang, L.; Liang, X. Effect of Cyclosporine A on Focal Segmental Glomerulosclerosis Caused by MYO1E Mutation in a Chinese Adult Patient: A Case Report. Medicine 2023, 102, e32683. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, I.; Terai, C.; Yabe, H.; Sugawara, H. Tacrolimus Induction Therapy for Nephrotic Syndrome Caused by Minimal Mesangial Lupus Nephritis with Lupus Podocytopathy: A Case-Based Review. Am. J. Case Rep. 2022, 23, e937201. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Sharpe, A.H. T-Cell Stimulation: An Abundance of B7s. Nat. Med. 1999, 5, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- Reiser, J.; Von Gersdorff, G.; Loos, M.; Oh, J.; Asanuma, K.; Giardino, L.; Rastaldi, M.P.; Calvaresi, N.; Watanabe, H.; Schwarz, K.; et al. Induction of B7-1 in Podocytes Is Associated with Nephrotic Syndrome. J. Clin. Investig. 2004, 113, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Fornoni, A.; Weins, A.; Hakroush, S.; Maiguel, D.; Sageshima, J.; Chen, L.; Ciancio, G.; Faridi, M.H.; Behr, D.; et al. Abatacept in B7-1–Positive Proteinuric Kidney Disease. N. Engl. J. Med. 2013, 369, 2416–2423. [Google Scholar] [CrossRef]
- Genovese, M.C.; Becker, J.-C.; Schiff, M.; Luggen, M.; Sherrer, Y.; Kremer, J.; Birbara, C.; Box, J.; Natarajan, K.; Nuamah, I.; et al. Abatacept for Rheumatoid Arthritis Refractory to Tumor Necrosis Factor α Inhibition. N. Engl. J. Med. 2005, 353, 1114–1123. [Google Scholar] [CrossRef]
- Ruperto, N.; Lovell, D.J.; Quartier, P.; Paz, E.; Rubio-Pérez, N.; Silva, C.A.; Abud-Mendoza, C.; Burgos-Vargas, R.; Gerloni, V.; Melo-Gomes, J.A.; et al. Abatacept in Children with Juvenile Idiopathic Arthritis: A Randomised, Double-Blind, Placebo-Controlled Withdrawal Trial. Lancet 2008, 372, 383–391. [Google Scholar] [CrossRef]
- Delville, M.; Baye, E.; Durrbach, A.; Audard, V.; Kofman, T.; Braun, L.; Olagne, J.; Nguyen, C.; Deschênes, G.; Moulin, B.; et al. B7–1 Blockade Does Not Improve Post–Transplant Nephrotic Syndrome Caused by Recurrent FSGS. J. Am. Soc. Nephrol. 2016, 27, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Isom, R.; Shoor, S.; Higgins, J.; Cara-Fuentes, G.; Johnson, R.J. Abatacept in Steroid-Dependent Minimal Change Disease and CD80-Uria. Kidney Int. Rep. 2019, 4, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Mühlbacher, T.; Amann, K.; Mahling, M.; Nadalin, S.; Heyne, N.; Guthoff, M. Successful Long-Term Management of Recurrent Focal Segmental Glomerulosclerosis after Kidney Transplantation with Costimulation Blockade. Clin. Kidney J. 2021, 14, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Burke, G.W.; Chandar, J.; Sageshima, J.; Ortigosa-Goggins, M.; Amarapurkar, P.; Mitrofanova, A.; Defreitas, M.J.; Katsoufis, C.P.; Seeherunvong, W.; Centeno, A.; et al. Benefit of B7-1 Staining and Abatacept for Treatment-Resistant Post-Transplant Focal Segmental Glomerulosclerosis in a Predominantly Pediatric Cohort: Time for a Reappraisal. Pediatr. Nephrol. 2023, 38, 145–159. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02592798?cond=NCT02592798&draw=2&rank=1 (accessed on 8 July 2023).
- Fiorina, P.; Vergani, A.; Bassi, R.; Niewczas, M.A.; Altintas, M.M.; Pezzolesi, M.G.; D’Addio, F.; Chin, M.; Tezza, S.; Ben Nasr, M.; et al. Role of Podocyte B7-1 in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2014, 25, 1415–1429. [Google Scholar] [CrossRef]
- Gagliardini, E.; Novelli, R.; Corna, D.; Zoja, C.; Ruggiero, B.; Benigni, A.; Remuzzi, G. B7–1 Is not Induced in Podocytes of Human and Experimental Diabetic Nephropathy. J. Am. Soc. Nephrol. 2016, 27, 999–1005. [Google Scholar] [CrossRef][Green Version]
- Herrera, M.; Söderberg, M.; Sabirsh, A.; Valastro, B.; Mölne, J.; Santamaria, B.; Valverde, A.M.; Guionaud, S.; Heasman, S.; Bigley, A.; et al. Inhibition of T-Cell Activation by the CTLA4-Fc Abatacept Is Sufficient to Ameliorate Proteinuric Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2017, 312, F748–F759. [Google Scholar] [CrossRef]
- Norlin, J.; Nielsen Fink, L.; Helding Kvist, P.; Douglas Galsgaard, E.; Coppieters, K. Abatacept Treatment Does Not Preserve Renal Function in the Streptozocin-Induced Model of Diabetic Nephropathy. PLoS ONE 2016, 11, e0152315. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, Y.; Wu, L.; Chen, S.; Han, F. Efficacy and Safety of Biologic Agents for Lupus Nephritis: A Systematic Review and Meta-Analysis. J. Clin. Rheumatol. 2023, 29, 95–100. [Google Scholar] [CrossRef]
- Furie, R.; Nicholls, K.; Cheng, T.; Houssiau, F.; Burgos-Vargas, R.; Chen, S.; Hillson, J.L.; Meadows-Shropshire, S.; Kinaszczuk, M.; Merrill, J.T. Efficacy and Safety of Abatacept in Lupus Nephritis: A Twelve-Month, Randomized, Double-Blind Study. Arthritis Rheumatol. 2014, 66, 379–389. [Google Scholar] [CrossRef]
- The ACCESS Trial Group. Treatment of Lupus Nephritis with Abatacept: The Abatacept and Cyclophosphamide Combination Efficacy and Safety Study: Abatacept in Lupus Nephritis. Arthritis Rheumatol. 2014, 66, 3096–3104. [Google Scholar] [CrossRef]
- Zhang, R.; Lee, D.M.; Jimah, J.R.; Gerassimov, N.; Yang, C.; Kim, S.; Luvsanjav, D.; Winkelman, J.; Mettlen, M.; Abrams, M.E.; et al. Dynamin Regulates the Dynamics and Mechanical Strength of the Actin Cytoskeleton as a Multifilament Actin-Bundling Protein. Nat. Cell Biol. 2020, 22, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Chang, J.; Shchedrina, V.A.; Pham, V.A.; Hartwig, J.H.; Suphamungmee, W.; Lehman, W.; Hyman, B.T.; Bacskai, B.J.; Sever, S. Regulation of Dynamin Oligomerization in Cells: The Role of Dynamin-Actin Interactions and Its GTPase Activity. Traffic 2014, 15, 819–838. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lee, H.W.; Garborcauskas, G.; Reiser, J.; Gupta, V.; Sever, S. Dynamin Autonomously Regulates Podocyte Focal Adhesion Maturation. J. Am. Soc. Nephrol. 2017, 28, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Gu, C.; Collins, A.; Mettlen, M.; Samelko, B.; Altintas, M.M.; Sudhini, Y.R.; Wang, X.; Bouley, R.; Brown, D.; et al. Simultaneous Stabilization of Actin Cytoskeleton in Multiple Nephron-Specific Cells Protects the Kidney from Diverse Injury. Nat. Commun. 2022, 13, 2422. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Kume, S.; Yasuda-Yamahara, M.; Yamahara, K.; Takeda, N.; Chin-Kanasaki, M.; Araki, H.; Sekine, O.; Yokoi, H.; Mukoyama, M.; et al. O-Linked β-N-Acetylglucosamine Modification of Proteins Is Essential for Foot Process Maturation and Survival in Podocytes. Nephrol. Dial. Transplant. 2017, 32, 1477–1487. [Google Scholar] [CrossRef]
- Müller-Deile, J.; Teng, B.; Schenk, H.; Haller, H.; Reiser, J.; Sever, S.; Schiffer, M. Drugs Targeting Dynamin Can Restore Cytoskeleton and Focal Contact Alterations of Urinary Podocytes Derived from Patients with Nephrotic Syndrome. Ann. Transl. Med. 2016, 4, 439. [Google Scholar] [CrossRef]
- Kushiyama, T.; Oda, T.; Yamamoto, K.; Higashi, K.; Watanabe, A.; Takechi, H.; Uchida, T.; Oshima, N.; Sakurai, Y.; Miura, S.; et al. Protective Effects of Rho Kinase Inhibitor Fasudil on Rats with Chronic Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2013, 304, F1325–F1334. [Google Scholar] [CrossRef]
- Shibata, S.; Nagase, M.; Fujita, T. Fluvastatin Ameliorates Podocyte Injury in Proteinuric Rats via Modulation of Excessive Rho Signaling. J. Am. Soc. Nephrol. 2006, 17, 754–764. [Google Scholar] [CrossRef]
- Tian, F.; Huang, S.; Xu, W.; Xie, G.; Gan, Y.; Huang, F.; Fan, Y.; Bao, J. Fasudil Compensates Podocyte Injury via CaMK4/Rho GTPases Signal and Actin Cytoskeleton-Dependent Activation of YAP in MRL/Lpr Mice. Int. Immunopharmacol. 2023, 119, 110199. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Su, K.; Zha, D.; Liang, W.; Hillebrands, J.; van Goor, H.; Ding, G. Angiotensin II Induces Reorganization of the Actin Cytoskeleton and Myosin Light-Chain Phosphorylation in Podocytes through Rho/ROCK-Signaling Pathway. Ren. Fail. 2016, 38, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-Y.; Li, C.-Y.; Chen, J.; Pan, L.; Saito, S.; Terashita, T.; Saito, K.; Miyawaki, K.; Shigemoto, K.; Mominoki, K.; et al. Rho-ROCK Signal Pathway Regulates Microtubule-Based Process Formation of Cultured Podocytes—Inhibition of ROCK Promoted Process Elongation. Nephron Exp. Nephrol. 2004, 97, e49–e61. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Fukusumi, Y.; Yamazaki, M.; Kayaba, M.; Kitazawa, Y.; Tomita, M.; Kawachi, H. Angiotensin II Type 1 Receptor Blockade Ameliorates Proteinuria in Puromycin Aminonucleoside Nephropathy by Inhibiting the Reduction of NEPH1 and Nephrin. J. Nephrol. 2014, 27, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, W.; Zhang, Y.; Cheng, Y.; Xu, Z. Effect of Angiotensin II Type 1 Receptor Blocker on 12-Lipoxygenase Activity and Slit Diaphragm Protein Expression in Type 2 Diabetic Rat Glomeruli. J. Nephrol. 2016, 29, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Eckel, J.; Lavin, P.J.; Finch, E.A.; Mukerji, N.; Burch, J.; Gbadegesin, R.; Wu, G.; Bowling, B.; Byrd, A.; Hall, G.; et al. TRPC6 Enhances Angiotensin II-Induced Albuminuria. J. Am. Soc. Nephrol. 2011, 22, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Ilatovskaya, D.V.; Palygin, O.; Chubinskiy-Nadezhdin, V.; Negulyaev, Y.A.; Ma, R.; Birnbaumer, L.; Staruschenko, A. Angiotensin II Has Acute Effects on TRPC6 Channels in Podocytes of Freshly Isolated Glomeruli. Kidney Int. 2014, 86, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Jeruschke, S.; Büscher, A.K.; Oh, J.; Saleem, M.A.; Hoyer, P.F.; Weber, S.; Nalbant, P. Protective Effects of the mTOR Inhibitor Everolimus on Cytoskeletal Injury in Human Podocytes Are Mediated by RhoA Signaling. PLoS ONE 2013, 8, e55980. [Google Scholar] [CrossRef] [PubMed]
- Jeruschke, S.; Jeruschke, K.; DiStasio, A.; Karaterzi, S.; Büscher, A.K.; Nalbant, P.; Klein-Hitpass, L.; Hoyer, P.F.; Weiss, J.; Stottmann, R.W.; et al. Everolimus Stabilizes Podocyte Microtubules via Enhancing TUBB2B and DCDC2 Expression. PLoS ONE 2015, 10, e0137043. [Google Scholar] [CrossRef]
- Bergwall, L.; Wallentin, H.; Elvin, J.; Liu, P.; Boi, R.; Sihlbom, C.; Hayes, K.; Wright, D.; Haraldsson, B.; Nyström, J.; et al. Amplification of the Melanocortin-1 Receptor in Nephrotic Syndrome Identifies a Target for Podocyte Cytoskeleton Stabilization. Sci. Rep. 2018, 8, 15731. [Google Scholar] [CrossRef]
- Chen, B.; Alam, Z.; Ge, Y.; Dworkin, L.; Gong, R. Pharmacological Melanocortin 5 Receptor Activation Attenuates Glomerular Injury and Proteinuria in Rats With Puromycin Aminonucleoside Nephrosis. Front. Physiol. 2022, 13, 887641. [Google Scholar] [CrossRef]
- Elvin, J.; Buvall, L.; Lindskog Jonsson, A.; Granqvist, A.; Lassén, E.; Bergwall, L.; Nyström, J.; Haraldsson, B. Melanocortin 1 Receptor Agonist Protects Podocytes through Catalase and RhoA Activation. Am. J. Physiol.-Ren. Physiol. 2016, 310, F846–F856. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, P.; Chang, M.; Chen, B.; Ge, Y.; Malhotra, D.K.; Dworkin, L.D.; Gong, R. Melanocortin Therapy Ameliorates Podocytopathy and Proteinuria in Experimental Focal Segmental Glomerulosclerosis Involving a Podocyte Specific Non-MC1R-Mediated Melanocortinergic Signaling. Clin. Sci. 2020, 134, 695–710. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.-C.; Miyata, K.N.; Chang, S.-Y.; Zhao, X.-P.; Lo, C.-S.; El-Mortada, M.-A.; Peng, J.; Chenier, I.; Yamashita, M.; Ingelfinger, J.R.; et al. Angiotensin II Type-2-Receptor Stimulation Ameliorates Focal and Segmental Glomerulosclerosis in Mice. Clin. Sci. 2022, 136, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, X.; Zhai, X.; Wang, G.; Qin, W.; Cheng, Z.; Chen, Z. CDDO-Me Ameliorates Podocyte Injury through Anti-Oxidative Stress and Regulation of Actin Cytoskeleton in Adriamycin Nephropathy. Biomed. Pharmacother. 2023, 167, 115617. [Google Scholar] [CrossRef] [PubMed]
- Benetti, A.; Martins, F.L.; Sene, L.B.; Shimizu, M.H.M.; Seguro, A.C.; Luchi, W.M.; Girardi, A.C.C. Urinary DPP4 Correlates with Renal Dysfunction, and DPP4 Inhibition Protects against the Reduction in Megalin and Podocin Expression in Experimental CKD. Am. J. Physiol.-Ren. Physiol. 2021, 320, F285–F296. [Google Scholar] [CrossRef] [PubMed]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin Reduces TRPC6 Expression through AMPK Activation and Modulates Cytoskeleton Dynamics in Podocytes under Diabetic Conditions. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165610. [Google Scholar] [CrossRef] [PubMed]
- Suk Kang, J.; Son, S.S.; Lee, J.-H.; Lee, S.W.; Jeong, A.R.; Lee, E.S.; Cha, S.-K.; Chung, C.H.; Lee, E.Y. Protective Effects of Klotho on Palmitate-Induced Podocyte Injury in Diabetic Nephropathy. PLoS ONE 2021, 16, e0250666. [Google Scholar] [CrossRef]
- Yao, X.; Guo, H.; Sun, M.; Meng, S.; Zhu, B.; Fang, J.; Huang, J.; Wang, H.; Xing, L. Klotho Ameliorates Podocyte Injury through Targeting TRPC6 Channel in Diabetic Nephropathy. J. Diabetes Res. 2022, 2022, 1329380. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Shan, Z.; Mi, W.; Zhao, Y.; Li, M.; Wang, B.; Zheng, X.; Feng, W. Catalpol Ameliorates Podocyte Injury by Stabilizing Cytoskeleton and Enhancing Autophagy in Diabetic Nephropathy. Front. Pharmacol. 2019, 10, 1477. [Google Scholar] [CrossRef]
- Liu, J.; Sun, M.; Xia, Y.; Cui, X.; Jiang, J. Phloretin Ameliorates Diabetic Nephropathy by Inhibiting Nephrin and Podocin Reduction through a Non-Hypoglycemic Effect. Food Funct. 2022, 13, 6613–6622. [Google Scholar] [CrossRef]
- Wei, L.; Yong, J.; Zhang, X.; Ling, C.; Wu, Y.; Xu, Z.; Zhang, H.; Cao, X.; Sheng, L.; Zhang, Q.; et al. Shenqi Granule Upregulates CD2AP and α-Actinin4 and Activates Autophagy through Regulation of mTOR/ULK1 Pathway in MPC5 Cells. J. Ethnopharmacol. 2023, 303, 115942. [Google Scholar] [CrossRef] [PubMed]
- Merchant, M.L.; Rood, I.M.; Deegens, J.K.J.; Klein, J.B. Isolation and Characterization of Urinary Extracellular Vesicles: Implications for Biomarker Discovery. Nat. Rev. Nephrol. 2017, 13, 731–749. [Google Scholar] [CrossRef] [PubMed]
- Thongboonkerd, V. Roles for Exosome in Various Kidney Diseases and Disorders. Front. Pharmacol. 2020, 10, 1655. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).