Proof-of-Concept Study on the Use of Tangerine-Derived Nanovesicles as siRNA Delivery Vehicles toward Colorectal Cancer Cell Line SW480
Abstract
:1. Introduction
2. Results
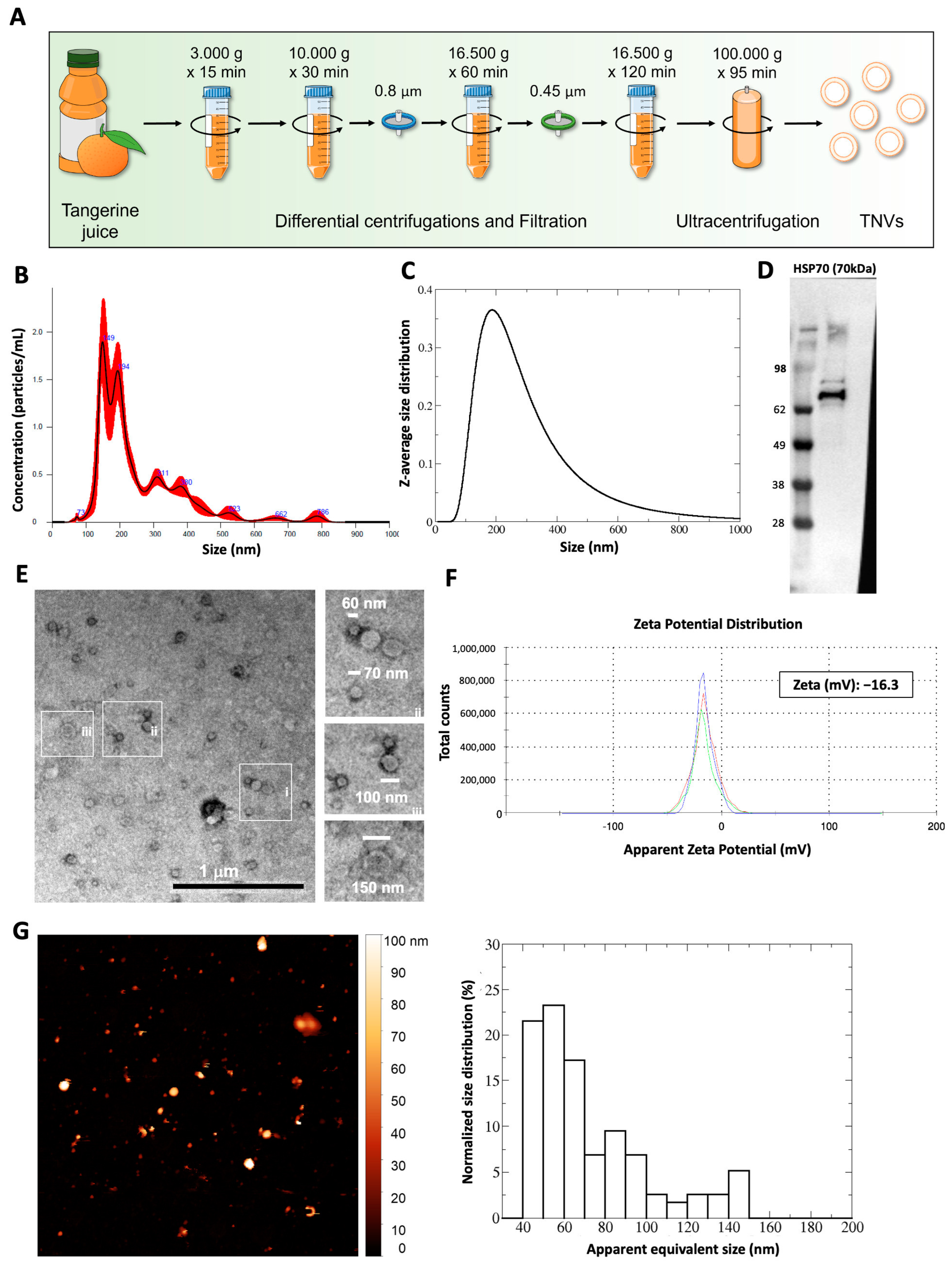
2.1. Isolation and Characterization of Tangerine-Derived Nanovesicles (TNVs)
2.2. TNVs Can Be Loaded with siRNA through Electroporation
2.3. TNVs Delivered DDHD1-siRNA in Target Cells and Affected DDHD1 Expression
3. Discussion
4. Materials and Methods
4.1. Tangerine Nanovesicles Isolation
4.2. Nanoparticle Tracking Analysis
4.3. Dynamic Light Scattering
4.4. Cell Culture
4.5. Western Blotting
4.6. Transmission Electron Microscopy
4.7. Zeta Potential
4.8. Atomic Force Microscopy
4.9. Proteomics
4.9.1. Sample Preparation
4.9.2. LC-MS/MS
4.10. Reversed-Phase HPLC/MS
4.11. Cell Viability Assays
4.12. TNV Internalization in Human Cell Lines
4.13. siRNA Labeling
4.14. TNVs Electroporation
4.15. RNAse Treatment
4.16. Confocal Microscopy
4.17. Cell Transfection
4.18. RNA Isolation and Real-Time PCR
4.19. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younis, M.A.; Tawfeek, H.M.; Abdellatif, A.A.H.; Abdel-Aleem, J.A.; Harashima, H. Clinical translation of nanomedicines: Challenges, opportunities, and keys. Adv. Drug Deliv. Rev. 2022, 181, 114083. [Google Scholar] [CrossRef] [PubMed]
- Schirrmacher, V. From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- van der Meel, R.; Fens, M.H.; Vader, P.; van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control Release 2014, 195, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for rna therapeutics. Nat. Rev. Genet 2022, 23, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(r)—The first fda-approved nano-drug: Lessons learned. J. Control Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal formulations in clinical use: An updated review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.; et al. Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from hek293t cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (misev2018): A position statement of the international society for extracellular vesicles and update of the misev2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. Rna delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekstrom, K.; Bossios, A.; Sjostrand, M.; Lee, J.J.; Lotvall, J.O. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Bonsergent, E.; Grisard, E.; Buchrieser, J.; Schwartz, O.; Thery, C.; Lavieu, G. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat. Commun. 2021, 12, 1864. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of exosome-encapsulated paclitaxel to overcome mdr in cancer cells. Nanomed. Nanotechnol. Biol. Med. 2016, 2, 655–664. [Google Scholar] [CrossRef]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and update on methods for cargo loading into extracellular vesicles. Processes 2021, 9, 356. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of sirna to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Faruqu, F.N.; Xu, L.; Al-Jamal, K.T. Preparation of exosomes for sirna delivery to cancer cells. J. Vis. Exp. 2018, 142, e58814. [Google Scholar]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced sirna precipitation obscures the efficiency of sirna loading into extracellular vesicles. J. Control Release 2013, 172, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Urzi, O.; Gasparro, R.; Ganji, N.R.; Alessandro, R.; Raimondo, S. Plant-rna in extracellular vesicles: The secret of cross-kingdom communication. Membranes 2022, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Karamanidou, T.; Tsouknidas, A. Plant-derived extracellular vesicles as therapeutic nanocarriers. Int. J. Mol. Sci. 2021, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The emerging role of plant-derived exosomes-like nanoparticles in immune regulation and periodontitis treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Gao, J.; He, Y.; Jiang, L. Plant extracellular vesicles. Protoplasma 2020, 257, 3–12. [Google Scholar] [CrossRef]
- Raimondo, S.; Naselli, F.; Fontana, S.; Monteleone, F.; Lo Dico, A.; Saieva, L.; Zito, G.; Flugy, A.; Manno, M.; Di Bella, M.A.; et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress cml xenograft growth by inducing trail-mediated cell death. Oncotarget 2015, 6, 19514–19527. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Yin, H.; Bennett, C.; Zhang, H.G.; Guo, P. Arrowtail rna for ligand display on ginger exosome-like nanovesicles to systemic deliver sirna for cancer suppression. Sci. Rep. 2018, 8, 14644. [Google Scholar] [CrossRef]
- Raimondo, S.; Cristaldi, M.; Fontana, S.; Saieva, L.; Monteleone, F.; Calabrese, G.; Giavaresi, G.; Parenti, R.; Alessandro, R. The phospholipase ddhd1 as a new target in colorectal cancer therapy. J. Exp. Clin. Cancer Res. 2018, 37, 82. [Google Scholar] [CrossRef]
- Pinedo, M.; de la Canal, L.; de Marcos Lousa, C. A call for rigor and standardization in plant extracellular vesicle research. J. Extracell. Vesicles 2021, 10, e12048. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, Y.; Li, S.; Ye, X.; Jiang, Y.; Tang, L.; Wang, J. Proteomics analysis of exosomes from patients with active tuberculosis reveals infection profiles and potential biomarkers. Front. Microbiol. 2021, 12, 800807. [Google Scholar] [CrossRef]
- Christensen, J.; El-Gebali, S.; Natoli, M.; Sengstag, T.; Delorenzi, M.; Bentz, S.; Bouzourene, H.; Rumbo, M.; Felsani, A.; Siissalo, S.; et al. Defining new criteria for selection of cell-based intestinal models using publicly available databases. BMC Genom. 2012, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.G. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- Zhuang, X.; Teng, Y.; Samykutty, A.; Mu, J.; Deng, Z.; Zhang, L.; Cao, P.; Rong, Y.; Yan, J.; Miller, D.; et al. Grapefruit-derived nanovectors delivering therapeutic mir17 through an intranasal route inhibit brain tumor progression. Mol. Ther. 2016, 24, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.G. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Mu, J.; Hu, X.; Samykutty, A.; Zhuang, X.; Deng, Z.; Zhang, L.; Cao, P.; Yan, J.; Miller, D.; et al. Grapefruit-derived nanovectors deliver mir-18a for treatment of liver metastasis of colon cancer by induction of m1 macrophages. Oncotarget 2016, 7, 25683–25697. [Google Scholar] [CrossRef]
- Xiao, Q.; Zhao, W.; Wu, C.; Wang, X.; Chen, J.; Shi, X.; Sha, S.; Li, J.; Liang, X.; Yang, Y.; et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv. Sci. 2022, 9, e2105274. [Google Scholar] [CrossRef]
- Garaeva, L.; Kamyshinsky, R.; Kil, Y.; Varfolomeeva, E.; Verlov, N.; Komarova, E.; Garmay, Y.; Landa, S.; Burdakov, V.; Myasnikov, A.; et al. Delivery of functional exogenous proteins by plant-derived vesicles to human cells in vitro. Sci. Rep. 2021, 11, 6489. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef]
- Arafa, E.A.; Shurrab, N.T.; Buabeid, M.A. Therapeutic implications of a polymethoxylated flavone, tangeretin, in the management of cancer via modulation of different molecular pathways. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 4709818. [Google Scholar] [CrossRef]
- Mdkhana, B.; Zaher, D.M.; Abdin, S.M.; Omar, H.A. Tangeretin boosts the anticancer activity of metformin in breast cancer cells via curbing the energy production. Phytomedicine 2021, 83, 153470. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Fattah, M.M.; Mohamed, W.R.; Hassanein, E.H.M.; Arab, H.A.; Arafa, E.A. Role of nf-kappab/icam-1, jak/stat-3, and apoptosis signaling in the anticancer effect of tangeretin against urethane-induced lung cancer in balb/c mice. Life Sci. 2023, 325, 121749. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Amin, M.; Aktar, S.; Ali, M.C.; Rahim, Z.B.; Hossain, M.A.; Al Mamun, A.; Amin, M.N.; et al. Chemotherapeutic potential of hesperetin for cancer treatment, with mechanistic insights: A comprehensive review. Heliyon 2022, 8, e08815. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin relieves cisplatin-induced acute kidney injury by mitigating oxidative stress, inflammation and apoptosis. Chem. Biol. Interact 2019, 308, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.E.; Ebrahim, H.Y.; Mohyeldin, M.M.; Siddique, A.B.; Kamal, A.M.; Haggag, E.G.; El Sayed, K.A. Rutin as a novel c-met inhibitory lead for the control of triple negative breast malignancies. Nutr. Cancer 2017, 69, 1256–1271. [Google Scholar] [CrossRef]
- Kim, K.; Yoo, H.J.; Jung, J.H.; Lee, R.; Hyun, J.K.; Park, J.H.; Na, D.; Yeon, J.H. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020, 11, 22. [Google Scholar] [CrossRef]
- Demirgan, R.; Karagöz, A.; Pekmez, M.; Önay-Uçar, E.; Artun, F.T.; Gürer, Ç.U.; Mat, A. In vitro anticancer activity and cytotoxicity of some papaver alkaloids on cancer and normal cell lines. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 22–26. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, H.; Shi, W.; Chen, L.; Chen, T.; Chen, G.; Wang, W.; Lan, J.; Huang, Z.; Zhang, J.; et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J. Nanobiotechnol. 2021, 19, 439. [Google Scholar] [CrossRef]
- Umezu, T.; Takanashi, M.; Murakami, Y.; Ohno, S.I.; Kanekura, K.; Sudo, K.; Nagamine, K.; Takeuchi, S.; Ochiya, T.; Kuroda, M. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Mol. Ther. Methods Clin. Dev. 2021, 21, 199–208. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Han, M.K.; Collins, J.F.; Merlin, D. Oral administration of ginger-derived nanolipids loaded with sirna as a novel approach for efficient sirna drug delivery to treat ulcerative colitis. Nanomedicine 2017, 12, 1927–1943. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Luo, Q.; Chen, X.; Chen, F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J. Nanobiotechnol. 2021, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Woith, E.; Guerriero, G.; Hausman, J.F.; Renaut, J.; Leclercq, C.C.; Weise, C.; Legay, S.; Weng, A.; Melzig, M.F. Plant extracellular vesicles and nanovesicles: Focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int. J. Mol. Sci. 2021, 22, 3719. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved loading of plasma-derived extracellular vesicles to encapsulate antitumor mirnas. Mol. Ther. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lennaard, A.J.; Mamand, D.R.; Wiklander, R.J.; El Andaloussi, S.; Wiklander, O.P.B. Optimised electroporation for loading of extracellular vesicles with doxorubicin. Pharmaceutics 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed]
- Naseri, Z.; Oskuee, R.K.; Jaafari, M.R.; Forouzandeh Moghadam, M. Exosome-mediated delivery of functionally active mirna-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int. J. Nanomed. 2018, 13, 7727–7747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yang, Y.; Tao, R.; Yao, C.; Zhou, Z.; Zhang, Y. Exosomes loaded with mir-665 inhibit the progression of osteosarcoma in vivo and in vitro. Am. J. Transl. Res. 2022, 14, 7012–7026. [Google Scholar]
- Rong, Y.; Wang, Z.; Tang, P.; Wang, J.; Ji, C.; Chang, J.; Zhu, Y.; Ye, W.; Bai, J.; Liu, W.; et al. Engineered extracellular vesicles for delivery of sirna promoting targeted repair of traumatic spinal cord injury. Bioact. Mater. 2023, 23, 328–342. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Ali, D.J.; Tian, T.; Xu, H.; Si, K.; Sun, B.; Chen, B.; Xiao, Z. Engineered exosomes for targeted co-delivery of mir-21 inhibitor and chemotherapeutics to reverse drug resistance in colon cancer. J. Nanobiotechnol. 2020, 18, 10. [Google Scholar] [CrossRef]
- Dorfmüller, T. B. J. Berne and R. Pecora: Dynamic light scattering, john wiley and sons ltd., baffins lane 1976, 376 seiten, preis: £ 14,—. Berichte Bunsenges. Phys. Chem. 1977, 81, 101. [Google Scholar] [CrossRef]
- Adamo, G.; Fierli, D.; Romancino, D.P.; Picciotto, S.; Barone, M.E.; Aranyos, A.; Bozic, D.; Morsbach, S.; Raccosta, S.; Stanly, C.; et al. Nanoalgosomes: Introducing extracellular vesicles produced by microalgae. J. Extracell. Vesicles 2021, 10, e12081. [Google Scholar] [CrossRef] [PubMed]
- Paterna, A.; Rao, E.; Adamo, G.; Raccosta, S.; Picciotto, S.; Romancino, D.; Noto, R.; Touzet, N.; Bongiovanni, A.; Manno, M. Isolation of extracellular vesicles from microalgae: A renewable and scalable bioprocess. Front. Bioeng. Biotechnol. 2022, 10, 836747. [Google Scholar] [CrossRef] [PubMed]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
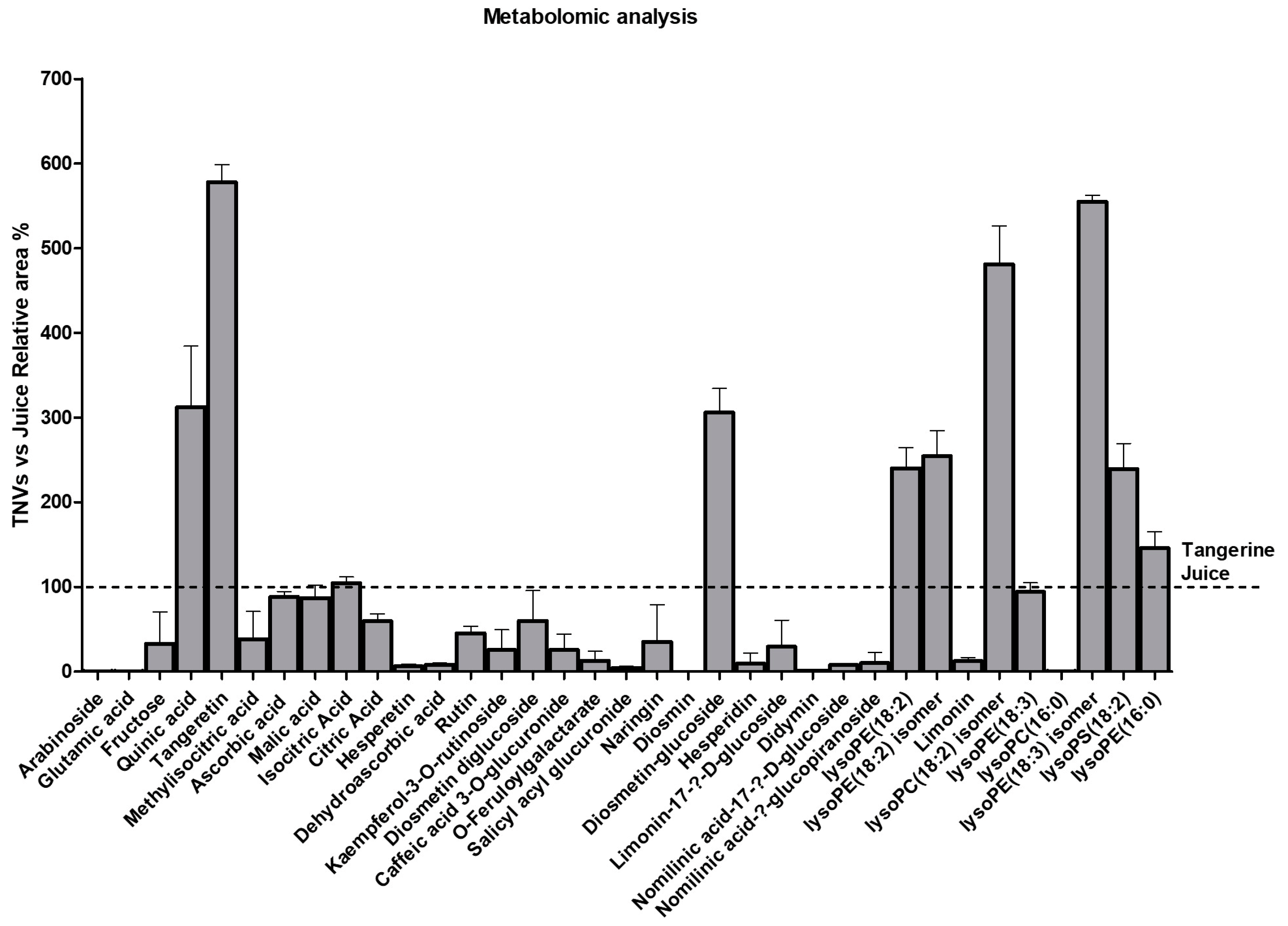


| Compound | Molecular Formula | Chemical Class | ESI- [M-H]- (m/z) (Teor.) | ESI- [M-H]- (m/z) (Exp.) | Rt (min) | |
|---|---|---|---|---|---|---|
| 1 | Arabinoside | C10H15N5O5 | Carbohydrate | 284.10 | 284.0970 | 3.33 |
| 2 | Glutamic acid | C5H9NO4 | Amino acid | 146.0459 | 146.0459 | 3.42 |
| 3 | Fructose | C6H12O6 | Carbohydrate | 179.0561 | 179.0575 | 3.45 |
| 4 | Quinic acid | C7H12O6 | Organic acid | 191.0561 | 191.0551 | 3.77 |
| 5 | Tangeretin | C20H20O7 | Methoxylated flavonoid | 371.1136 | 371.1153 | 3.89 |
| 6 | Methylisocitric acid | C7H10O7 | Organic acid | 205.0354 | 205.0343 | 4.08 |
| 7 | Ascorbic acid | C6H8O6 | Organic acid | 175.0248 | 175.0246 | 4.19 |
| 8 | Malic acid | C4H6O5 | Organic acid | 133.0142 | 133.0143 | 4.29 |
| 9 | Isocitric acid | C6H8O7 | Organic acid | 191.0197 | 191.0198 | 5.02 |
| 10 | Citric acid | C6H8O7 | Organic acid | 191.0197 | 191.0192 | 5.26 |
| 11 | Hesperetin | C16H14O6 | Methoxylated flavanone | 301.0714 | 301.0671 | 6.58 |
| 12 | Dehydroascorbic acid | C6H6O6 | Organic acid | 173.0092 | 173.0086 | 7.10 |
| 13 | Rutin | C27H30O16 | Non-methoxylated flavonol glycoside | 609.1461 | 609.1404 | 7.57 |
| 14 | Kaempferol-3-O-rutinoside | C27H30O15 | Non-methoxylated flavonol glycoside | 593.1512 | 593.1461 | 7.67 |
| 15 | Diosmetin diglucoside | C28H32O16 | Methoxylated flavone glycoside | 623.1618 | 623.1580 | 7.74 |
| 16 | Caffeic acid 3-O-glucuronide | C15H16O10 | Cinnamic acid derivative | 355.0671 | 355.0645 | 7.95 |
| 17 | O-feruloylgalactarate | C16H18O11 | Cinnamic acid derivative | 385.0776 | 385.0752 | 8.00 |
| 18 | Salicyl acyl glucuronide | C13H14O9 | Salicylate | 313.0565 | 313.0548 | 8.08 |
| 19 | Naringin | C27H32O14 | Non-methoxylated flavanone glycoside | 579.1719 | 579.1658 | 8.19 |
| 20 | Diosmin | C28H32O15 | Methoxylated flavonoid glycoside | 607.1668 | 607.1636 | 8.21 |
| 21 | Diosmetin glucoside | C22H22O11 | Methoxylated flavone glycoside | 461.1089 | 461.1079 | 8.25 |
| 22 | Hesperidin | C28H34O15 | Methoxylated flavonoid glycoside | 609.1825 | 609.1781 | 8.27 |
| 23 | Limonin-17-β-D-glucoside | C32H42O14 | Limonoid | 649.2502 | 649.2449 | 8.52 |
| 24 | Didymin | C28H34O14 | Methoxylated flavonoid glycoside | 593.1876 | 593.185 | 8.65 |
| 25 | Nomilinic acid-17-β-D-glucoside | C34H48O16 | Limonoid | 711.2870 | 711.2806 | 8.75 |
| 26 | Nomilinic acid-β-glucopiranoside | C34H46O15 | Limonoid | 693.2764 | 693.2693 | 8.88 |
| 27 | lysoPE(18:2) | C23H44NO7P | Lysophospholipid | 476.2783 | 476.2738 | 9.50 |
| 28 | lysoPE(18:2) isomer | C23H44NO7P | Lysophospholipid | 476.2783 | 476.2752 | 10.24 |
| 29 | Limonin | C26H30O8 | Limonoid | 515.1923 | 515.1863 (M+FA-H) | 10.45 |
| 30 | lysoPC(18:2) isomer | C26H50NO7P | Lysophospholipid | 564.3307 | 564.3317 (M+FA-H) | 10.70 |
| 31 | lysoPE(18:3) | C23H42NO7P | Lysophospholipid | 474.2626 | 474.2592 | 13.15 |
| 32 | lysoPC(16:0) | C24H50NO7P | Lysophospholipid | 540.3307 | 540.3284 (M+FA-H) | 13.19 |
| 33 | lysoPE(18:3) isomer | C23H42NO7P | Lysophospholipid | 474.2626 | 474.2675 | 13.59 |
| 34 | lysoPS(18:2) | C24H44NO9P | Lysophospholipid | 520.2681 | 520.2585 | 13.85 |
| 35 | lysoPE(16:0) | C21H44NO7P | Lysophospholipid | 452.2783 | 452.2747 | 13.91 |
| Gene | Forward 5′ → 3′ | Reverse 5′ → 3′ |
|---|---|---|
| ACT | TCCCTTGCCATCCTAAAAAGCCACCC | CTGGGCCATTCTTCCTTAGAGAGAAG |
| DDHD1 | TTTCTCAACCCAGCTAAAGAACCTA | TGATCCAACTCCAATGCAGAAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabienezhad Ganji, N.; Urzì, O.; Tinnirello, V.; Costanzo, E.; Polito, G.; Palumbo Piccionello, A.; Manno, M.; Raccosta, S.; Gallo, A.; Lo Pinto, M.; et al. Proof-of-Concept Study on the Use of Tangerine-Derived Nanovesicles as siRNA Delivery Vehicles toward Colorectal Cancer Cell Line SW480. Int. J. Mol. Sci. 2024, 25, 546. https://doi.org/10.3390/ijms25010546
Rabienezhad Ganji N, Urzì O, Tinnirello V, Costanzo E, Polito G, Palumbo Piccionello A, Manno M, Raccosta S, Gallo A, Lo Pinto M, et al. Proof-of-Concept Study on the Use of Tangerine-Derived Nanovesicles as siRNA Delivery Vehicles toward Colorectal Cancer Cell Line SW480. International Journal of Molecular Sciences. 2024; 25(1):546. https://doi.org/10.3390/ijms25010546
Chicago/Turabian StyleRabienezhad Ganji, Nima, Ornella Urzì, Vincenza Tinnirello, Elisa Costanzo, Giulia Polito, Antonio Palumbo Piccionello, Mauro Manno, Samuele Raccosta, Alessia Gallo, Margot Lo Pinto, and et al. 2024. "Proof-of-Concept Study on the Use of Tangerine-Derived Nanovesicles as siRNA Delivery Vehicles toward Colorectal Cancer Cell Line SW480" International Journal of Molecular Sciences 25, no. 1: 546. https://doi.org/10.3390/ijms25010546
APA StyleRabienezhad Ganji, N., Urzì, O., Tinnirello, V., Costanzo, E., Polito, G., Palumbo Piccionello, A., Manno, M., Raccosta, S., Gallo, A., Lo Pinto, M., Calligaris, M., Scilabra, S. D., Di Bella, M. A., Conigliaro, A., Fontana, S., Raimondo, S., & Alessandro, R. (2024). Proof-of-Concept Study on the Use of Tangerine-Derived Nanovesicles as siRNA Delivery Vehicles toward Colorectal Cancer Cell Line SW480. International Journal of Molecular Sciences, 25(1), 546. https://doi.org/10.3390/ijms25010546













