Genome-Wide Identification and Expression Analysis of Calmodulin and Calmodulin-like Genes, Revealing CaM3 and CML13 Participating in Drought Stress in Phoebe bournei
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization of CaM/CML Genes in P. bournei
2.2. Phylogenetic Analysis of CaM/CML Genes in P. bournei
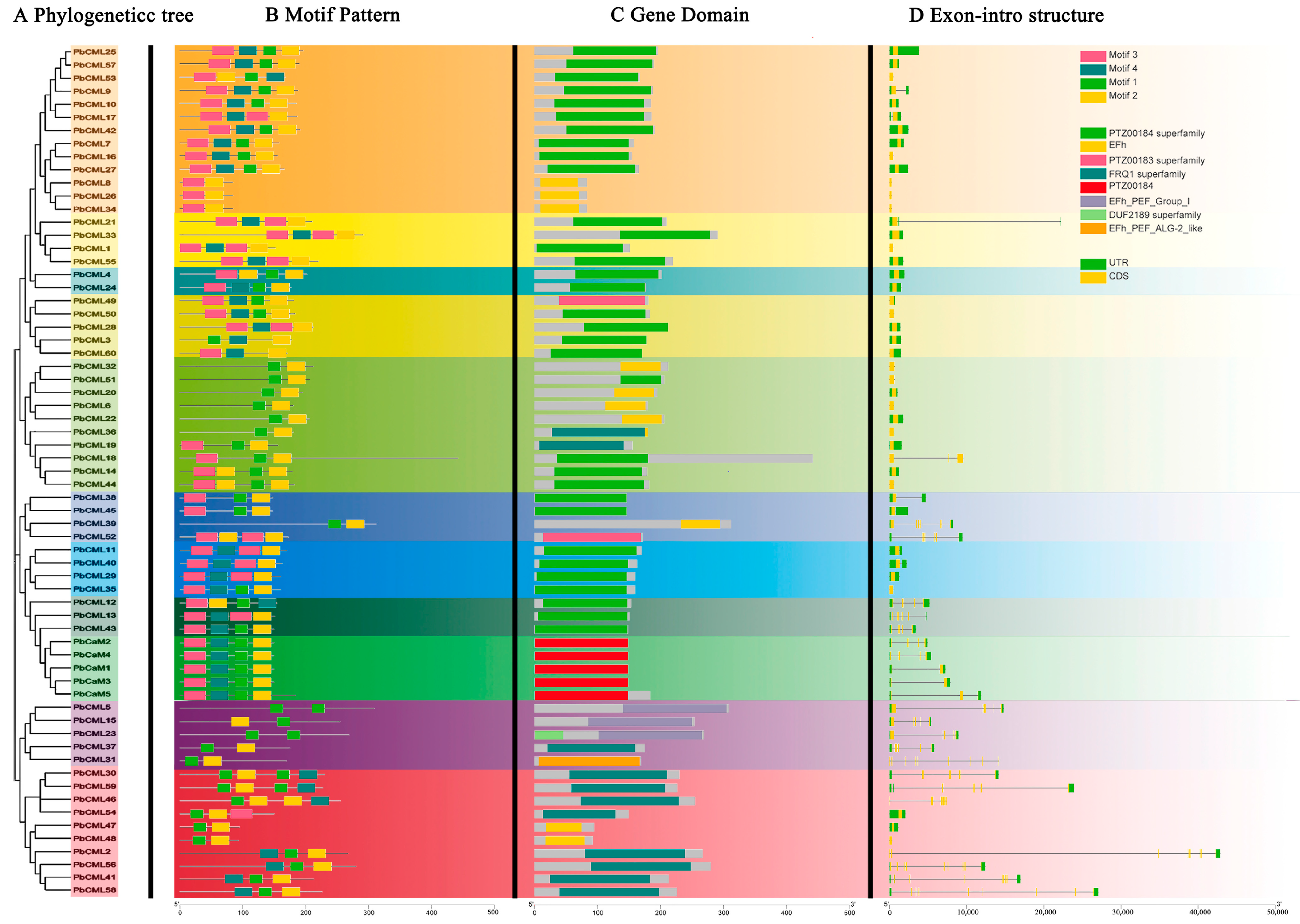
2.3. Gene Structure and Conserved Motifs Analysis of PbCaMs/CMLs
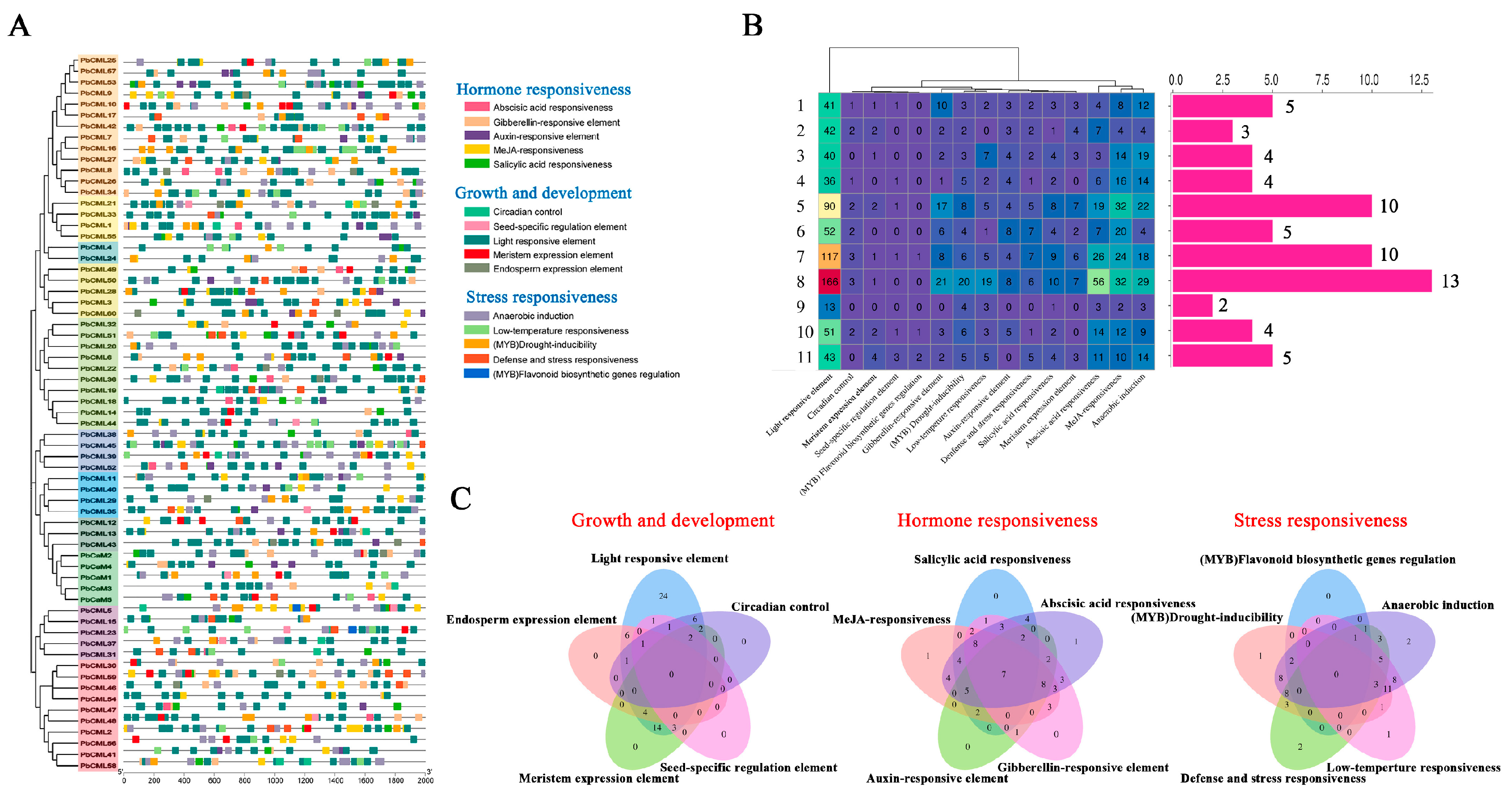
2.4. Cis-Regulatory Elements Analysis in the Promoters of PbCaM/CML Genes
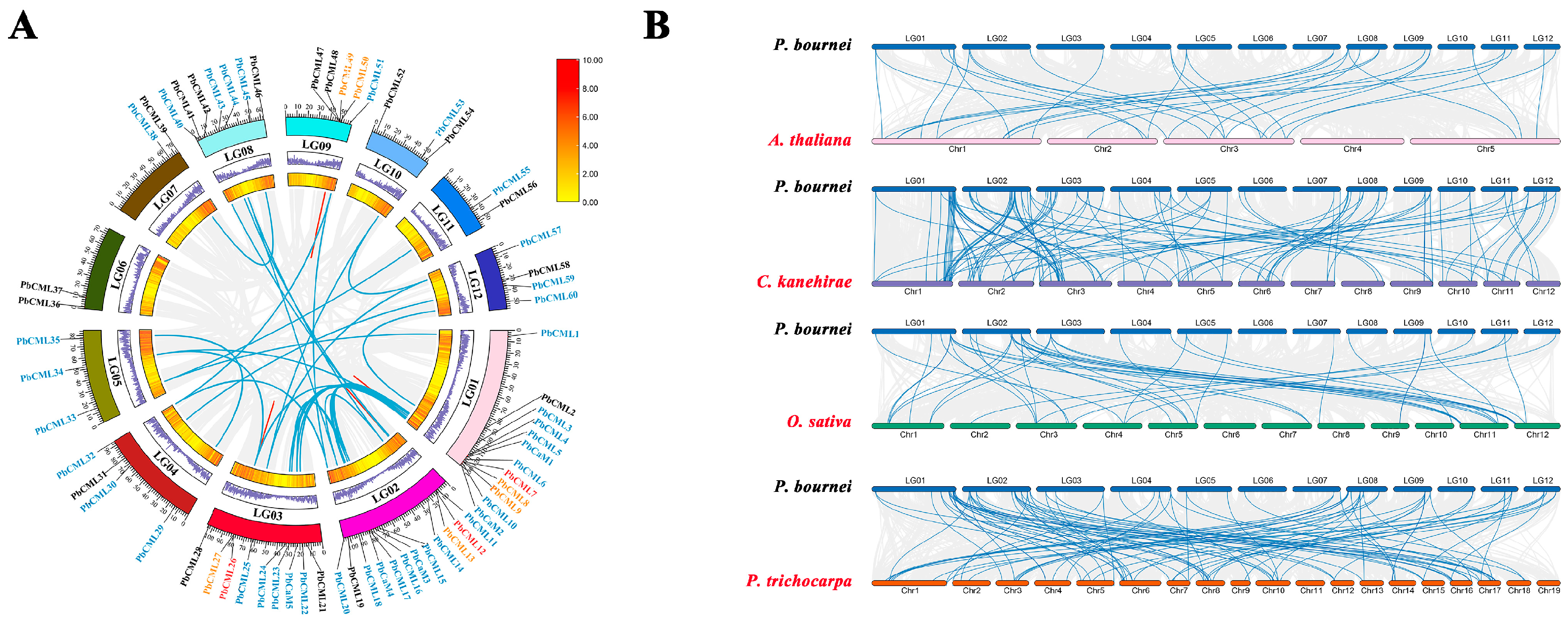
2.5. Chromosome Locations and Synteny Analysis of PbCaM/CML Genes
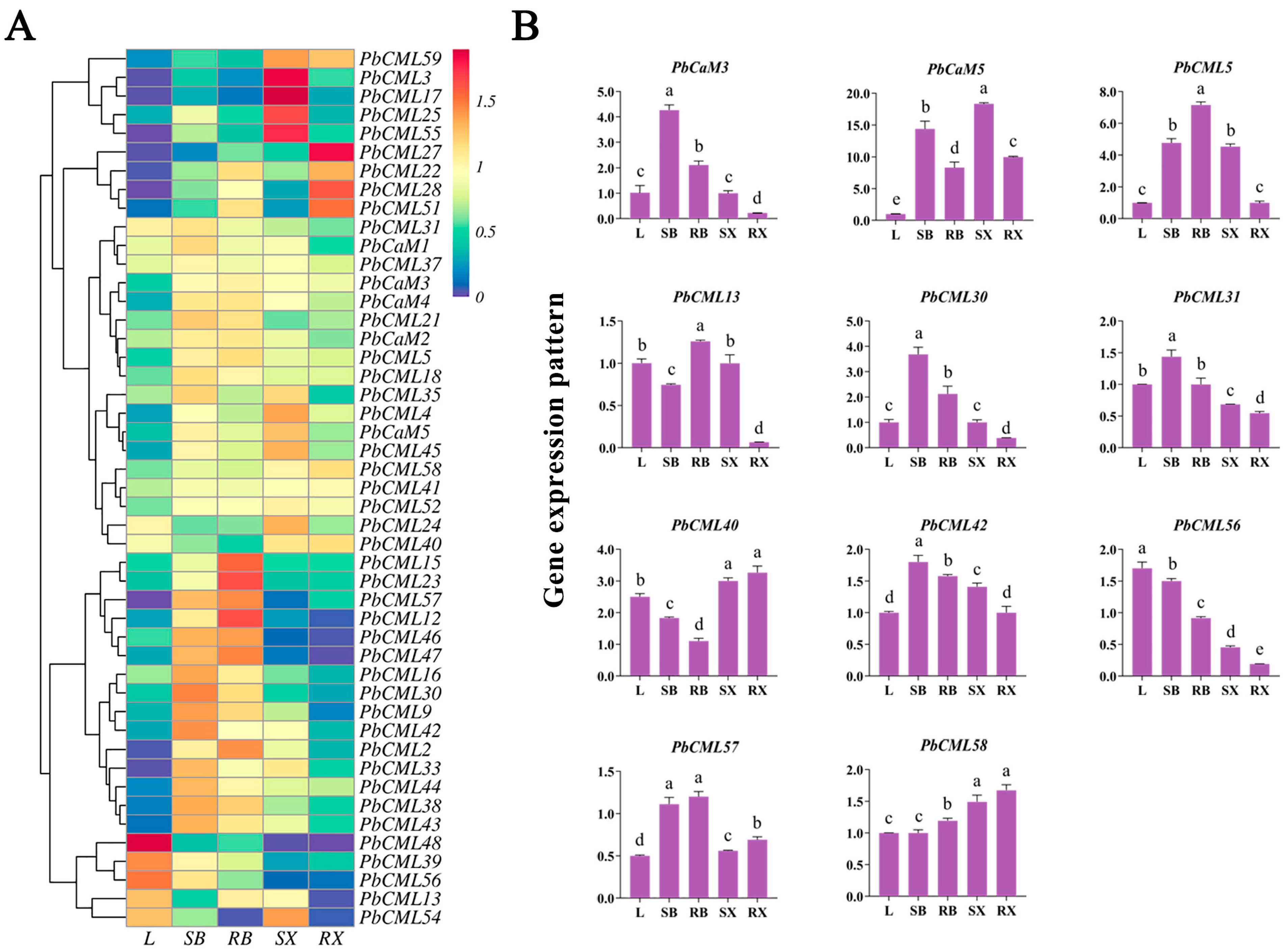
2.6. Expression Patterns of PbCaMs/CMLs in Different Tissues
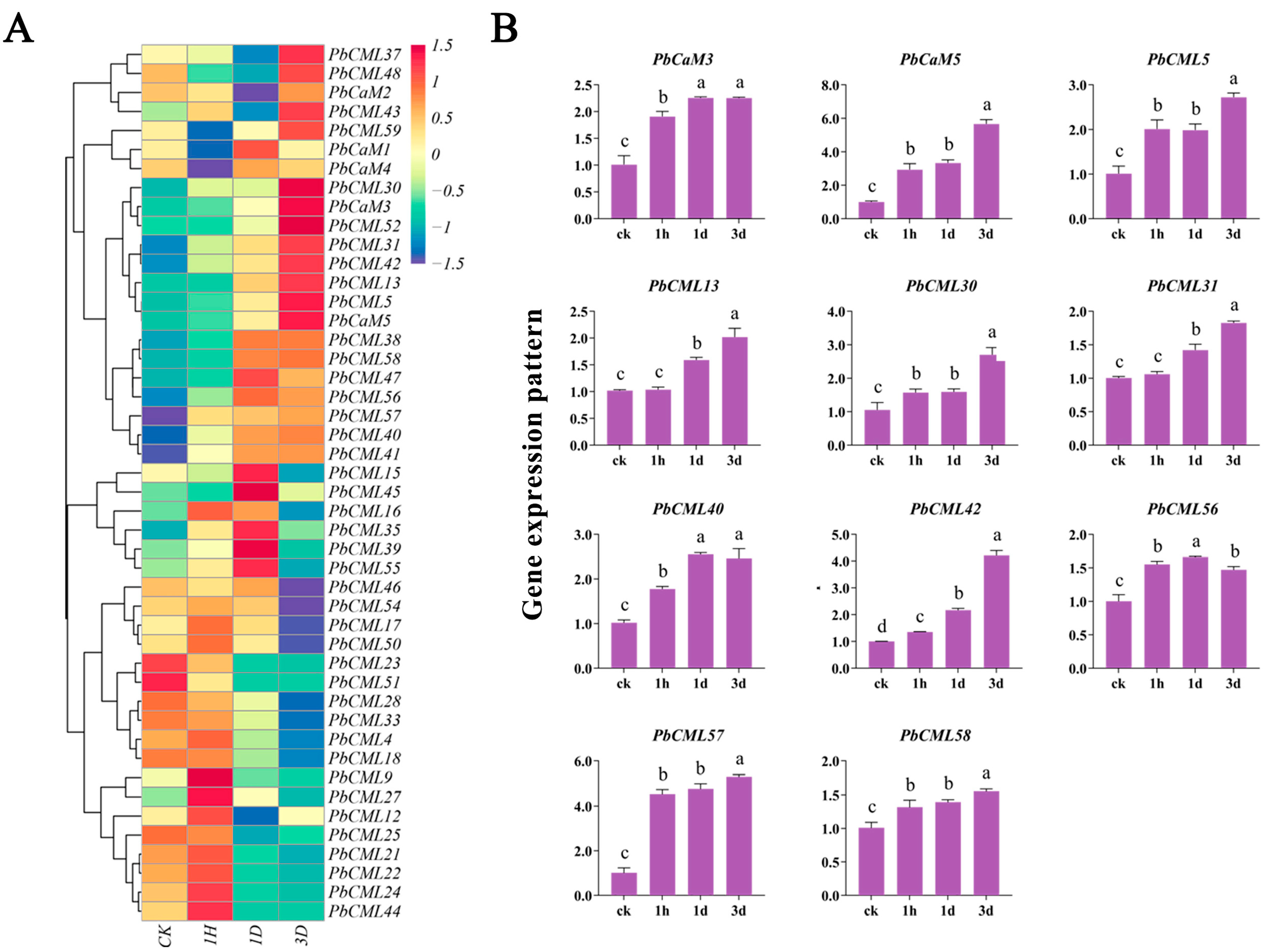
2.7. Expression Patterns of PbCaM/CML Genes in Response to Drought Stress
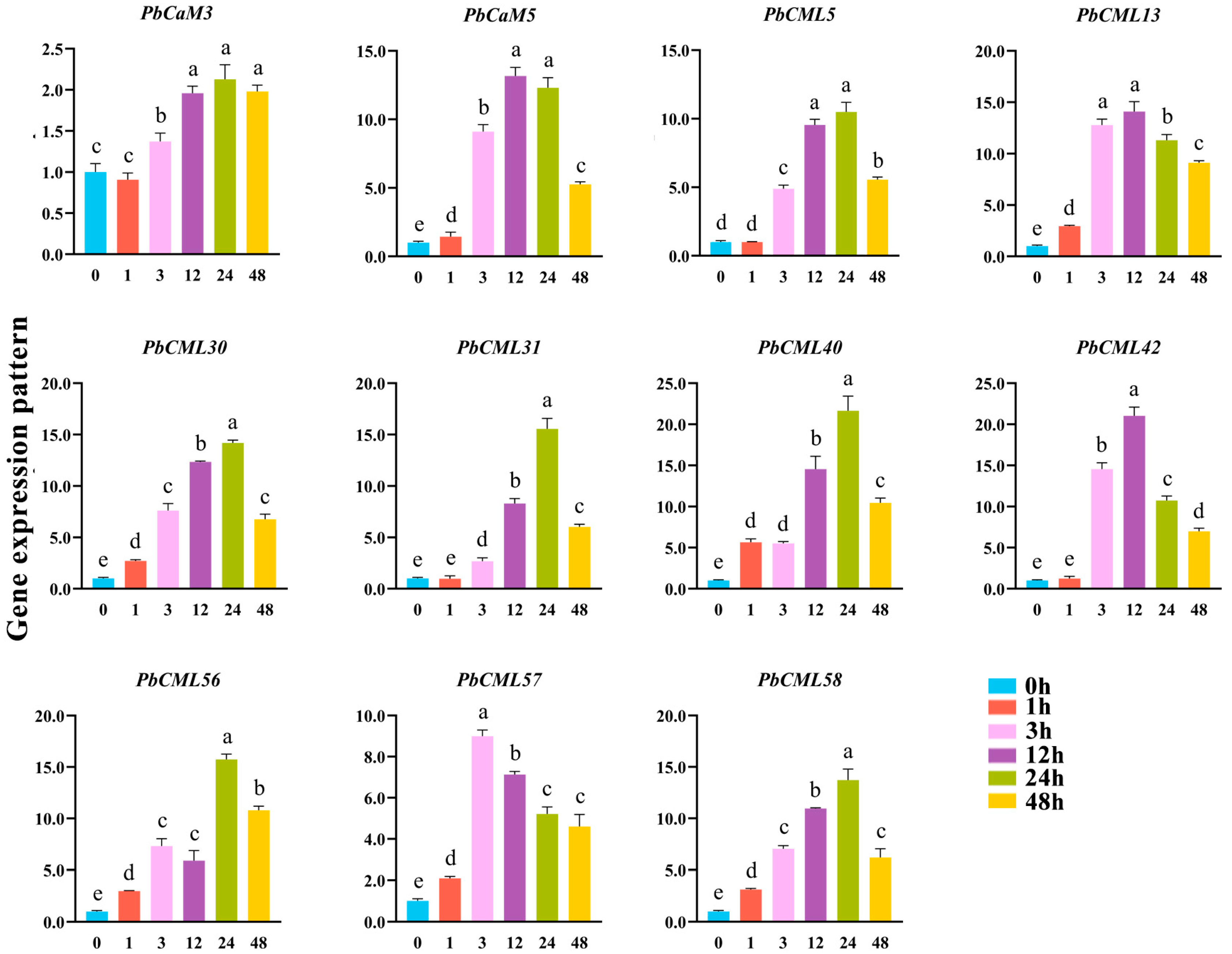
2.8. Expression Patterns of PbCaM/CML Genes in Response to ABA Treatment
2.9. Expression Patterns of PbCaM/CML Genes in Response to MeJA Treatment
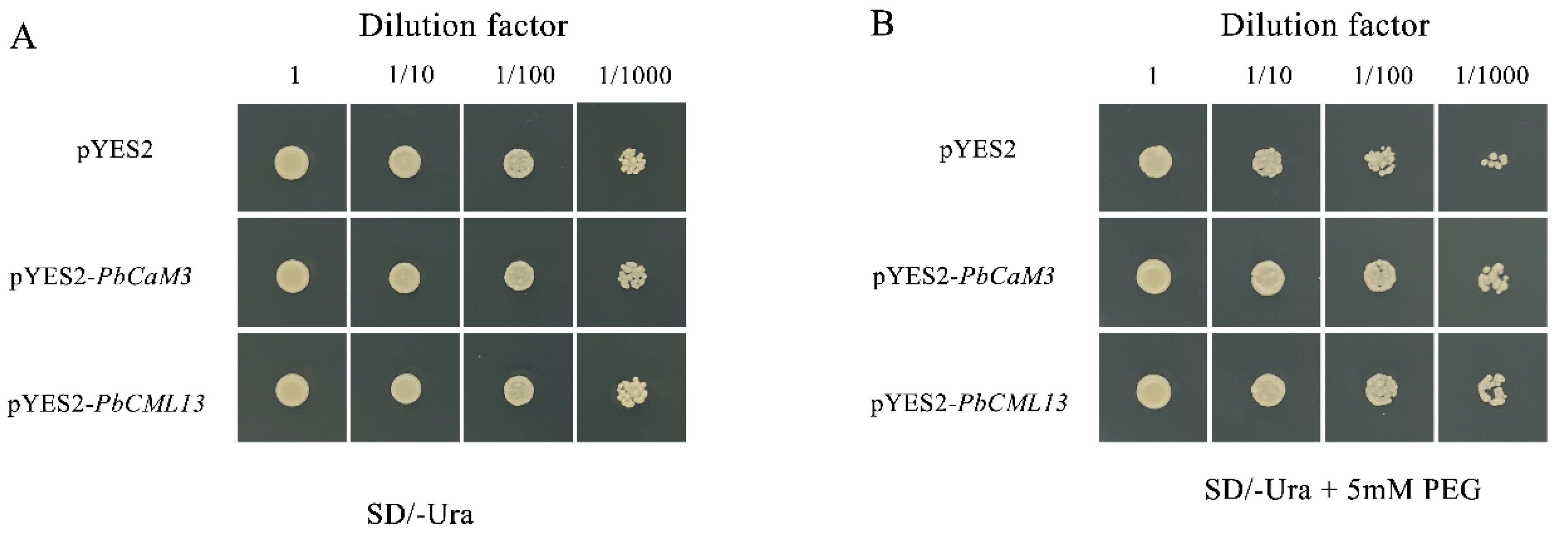
2.10. PbCaM3/PbCML13 Increased the Drought Tolerance of Yeast Cells
3. Discussion
4. Materials and Methods
4.1. Identification and Analysis of the CaM/CML Gene Family in P. bournei
4.2. Sequence Alignment and Phylogenetic Analysis of PbCaM/CML Proteins
4.3. Gene Structure and Conserved Motifs Analysis of PbCaMs/CMLs
4.4. Analysis of Cis-Acting Elements in the Promoters of PbCaMs/CMLs
4.5. Chromosomal Locations and Synteny Analysis of PbCaMs/CMLs
4.6. Expression Patterns of PbCaMs/CMLs in Different Tissues
4.7. Plant Materials and Treatments
4.8. RNA Isolation and Gene Expression Analysis
4.9. Molecular Cloning of PbCaM3/PbCML13
4.10. Analysis of Drought Tolerance in PbCaM3/CML13 Transgenic Yeasts
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathur, P.; Roy, S. Insights into the plant responses to drought and decoding the potential of root associated microbiome for inducing drought tolerance. Physiol. Plant. 2021, 172, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-F.; Wang, J.-D.; Xiong, M.; Wei, K.; Zhou, P.; Huang, L.-C.; Zhang, C.-Q.; Fan, X.-L.; Liu, Q.-Q. iTRAQ-based analysis of proteins co-regulated by brassinosteroids and gibberellins in rice embryos during seed germination. Int. J. Mol. Sci. 2018, 19, 3460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Huang, G.-T.; Ma, S.-L.; Bai, L.-P.; Zhang, L.; Ma, H.; Jia, P.; Liu, J.; Zhong, M.; Guo, Z.-F. Signal transduction during cold, salt, and drought stresses in plants. Mol. Biol. Rep. 2012, 39, 969–987. [Google Scholar] [CrossRef]
- La Verde, V.; Dominici, P.; Astegno, A. Towards understanding plant calcium signaling through calmodulin-like proteins: A biochemical and structural perspective. Int. J. Mol. Sci. 2018, 19, 1331. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kudla, J. Calcium decoding mechanisms in plants. Biochimie 2011, 93, 2054–2059. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef]
- Nehra, A.; Kundu, P.; Ahlawat, K.; Chhikara, A.; Agarwala, N.; Tuteja, N.; Gill, S.S.; Gill, R. Comprehensive genomic insight deciphers significance of EF-hand gene family in foxtail millet [Setaria italica (L.) P. Beauv.]. S. Afr. J. Bot. 2022, 148, 652–665. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnóczky, G. Intracellular Ca2+ sensing: Its role in calcium homeostasis and signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhang, M.; Zhang, L. Genome-wide identification and expression analysis of calmodulin-like (CML) genes in Chinese cabbage (Brassica rapa L. ssp. pekinensis). BMC Genom. 2017, 18, 842. [Google Scholar] [CrossRef] [PubMed]
- McCormack, E.; Tsai, Y.-C.; Braam, J. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci. 2005, 10, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, L.; Li, J.; Yang, W.; Ci, J.; Ren, X.; Wang, W.; Wang, Y.; Jiang, L.; Yang, W. Identification and expression analysis revealed drought stress-responsive calmodulin and calmodulin-like genes in maize. J. Plant Interact. 2022, 17, 450–461. [Google Scholar] [CrossRef]
- Munir, S.; Khan, M.R.G.; Song, J.; Munir, S.; Zhang, Y.; Ye, Z.; Wang, T. Genome-wide identification, characterization and expression analysis of calmodulin-like (CML) proteins in tomato (Solanum lycopersicum). Plant Physiol. Biochem. 2016, 102, 167–179. [Google Scholar] [CrossRef]
- Li, Q.; Gao, L.; Yu, F.; Lü, S.; Yang, P. Evolution and diversification of CaM/CML gene family in green plants. Plant Physiol. Biochem. 2023, 202, 107922. [Google Scholar] [CrossRef]
- He, X.; Liu, W.; Li, W.; Liu, Y.; Wang, W.; Xie, P.; Kang, Y.; Liao, L.; Qian, L.; Liu, Z.; et al. Genome-wide identification and expression analysis of CaM/CML genes in Brassica napus under abiotic stress. J. Plant Physiol. 2020, 255, 153251. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, T.; Liang, L.; Li, L.; Liu, L. Genome-wide analysis of CaM/CML gene family in two orchidaceae species. For. Res. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Xue, N.; Sun, M.; Gai, Z.; Bai, M.; Sun, J.; Sai, S.; Zhang, L. Genome-wide identification and expression analysis of calmodulin (CaM) and calmodulin-like (CML) genes in the brown algae Saccharina japonica. Plants 2023, 12, 1934. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.-S.; Wang, M.; Qiao, Z.; Bao, C.-C.; Zhang, W. Arabidopsis thaliana calmodulin-like protein CML24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic Ca2+ concentration. Plant Mol. Biol. 2014, 86, 225–236. [Google Scholar] [CrossRef]
- Tang, M.; Xu, C.; Cao, H.; Shi, Y.; Chen, J.; Chai, Y.; Li, Z. Tomato calmodulin-like protein SlCML37 is a calcium (Ca2+) sensor that interacts with proteasome maturation factor SlUMP1 and plays a role in tomato fruit chilling stress tolerance. J. Plant Physiol. 2021, 258–259, 153373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, T.; Liu, M.; Sun, W.; Zhang, W.-H. Calmodulin-like gene MtCML40 is involved in salt tolerance by regulating MtHKTs transporters in Medicago truncatula. Environ. Exp. Bot. 2019, 157, 79–90. [Google Scholar] [CrossRef]
- Yin, X.; Huang, L.; Wang, M.; Cui, Y.; Xia, X. OsDSR-1, a calmodulin-like gene, improves drought tolerance through scavenging of reactive oxygen species in rice (Oryza sativa L.). Mol. Breed. 2017, 37, 75. [Google Scholar] [CrossRef]
- Sun, Y.; Oh, D.-H.; Duan, L.; Ramachandran, P.; Ramirez, A.; Bartlett, A.; Tran, K.-N.; Wang, G.; Dassanayake, M.; Dinneny, J.R. Divergence in the ABA gene regulatory network underlies differential growth control. Nat. Plants 2022, 8, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.P.; Aldon, D. Mutations in AtCML9, a calmodulin-like protein from Arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008, 56, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, J.; Han, S.; Chong, S.L.; Meng, G.; Song, M.; Wang, Y.; Zhou, S.; Liu, C.; Lou, L.; et al. The chromosome-scale genome of Phoebe bournei reveals contrasting fates of terpene synthase (TPS)-a and TPS-b subfamilies. Plant Commun. 2022, 3, 100410. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Fu, N.; Song, M.; Han, X.; Yang, Q.; Zhang, Y.; Tong, Z.; Zhang, J. Cyanidin-3-O-glucoside contributes to leaf color change by regulating two bHLH transcription factors in Phoebe bournei. Int. J. Mol. Sci. 2023, 24, 3829. [Google Scholar] [CrossRef]
- Ge, Y.; He, X.; Wang, J.; Jiang, B.; Ye, R.; Lin, X. Physiological and biochemical responses of Phoebe bournei seedlings to water stress and recovery. Acta Physiol. Plant. 2014, 36, 1241–1250. [Google Scholar] [CrossRef]
- Liao, W.; Tang, X.; Li, J.; Zheng, Q.; Wang, T.; Cheng, S.; Chen, S.; Cao, S.; Cao, G. Genome wide investigation of Hsf gene family in Phoebe bournei: Identification, evolution, and expression after abiotic stresses. J. For. Res. 2023, 35, 11. [Google Scholar] [CrossRef]
- Cai, K.; Kuang, L.; Yue, W.; Xie, S.; Xia, X.; Zhang, G.; Wang, J. Calmodulin and calmodulin-like gene family in barley: Identification, characterization and expression analyses. Front. Plant Sci. 2022, 13, 964888. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Zhang, X.; Pi, E.; Zhu, Y. Analysis of EF-hand proteins in soybean genome suggests their potential roles in environmental and nutritional stress signaling. Front. Plant Sci. 2017, 8, 877. [Google Scholar] [CrossRef] [PubMed]
- McAinsh, M.R.; Pittman, J.K. Shaping the calcium signature. New Phytol. 2009, 181, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Bergey, D.R.; Kandel, R.; Tyree, B.K.; Dutt, M.; Dhekney, S.A. The role of calmodulin and related proteins in plant cell function: An ever-thickening plot. Springer Sci. Rev. 2014, 2, 145–159. [Google Scholar] [CrossRef]
- Sarwat, M.; Ahmad, P.; Nabi, G.; Hu, X. Ca2+ signals: The versatile decoders of environmental cues. Crit. Rev. Biotechnol. 2013, 33, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.-P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Boonburapong, B.; Buaboocha, T. Genome-wide identification and analyses of the rice calmodulin and related potential calcium sensor proteins. BMC Plant Biol. 2007, 7, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, D.; Yang, Q. Expression patterns of NbrgsCaM family genes in Nicotiana benthamiana and their potential roles in development and stress responses. Sci. Rep. 2020, 10, 9652. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Liu, L.; Su, Y.; Li, Y.; Jia, W.; Jiao, B.; Wang, J.; Yang, F.; Dong, F.; et al. Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in wheat (Triticum aestivum L.). Plant Signal. Behav. 2022, 17, 2013646. [Google Scholar] [CrossRef]
- Vandelle, E.; Vannozzi, A.; Wong, D.; Danzi, D.; Digby, A.-M.; Dal Santo, S.; Astegno, A. Identification, characterization, and expression analysis of calmodulin and calmodulin-like genes in grapevine (Vitis vinifera) reveal likely roles in stress responses. Plant Physiol. Biochem. 2018, 129, 221–237. [Google Scholar] [CrossRef]
- Li, C.; Meng, D.; Zhang, J.; Cheng, L. Genome-wide identification and expression analysis of calmodulin and calmodulin-like genes in apple (Malus × domestica). Plant Physiol. Biochem. 2019, 139, 600–612. [Google Scholar] [CrossRef]
- Gao, L.; Damaris, R.N.; Yu, F.; Yang, P. Genome-wide identification and expression analysis of CaM/CML gene family in sacred lotus (Nelumbo nucifera). Plant Mol. Biol. Rep. 2022, 40, 418–432. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Kumar, P.; Bae, H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Arab, M.; Najafi Zarrini, H.; Nematzadeh, G.; Heidari, P.; Hashemipetroudi, S.H.; Kuhlmann, M. Comprehensive analysis of calcium sensor families, CBL and CIPK, in Aeluropus littoralis and their expression profile in response to salinity. Genes 2023, 14, 753. [Google Scholar] [CrossRef] [PubMed]
- Yaghobi, M.; Heidari, P. Genome-wide analysis of aquaporin gene family in Triticum turgidum and its expression profile in response to salt stress. Genes 2023, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.; Liu, T.; Xiang, L.; Zhou, B.-F. Genome-wide identification and expression analysis of calmodulin-like gene family in Paspalums vaginatium revealed their role in response to salt and cold stress. Curr. Issues Mol. Biol. 2023, 45, 1693–1711. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Dutta, S.; Pal, A.; Sengupta, M.; Chattopadhyay, S. Calmodulin 7: Recent insights into emerging roles in plant development and stress. Plant Mol. Biol. 2021, 107, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Midhat, U.; Ting, M.K.Y.; Teresinski, H.J.; Snedden, W.A. The calmodulin-like protein, CML39, is involved in regulating seed development, germination, and fruit development in Arabidopsis. Plant Mol. Biol. 2018, 96, 375–392. [Google Scholar] [CrossRef]
- Shi, J.; Du, X. Identification, characterization and expression analysis of calmodulin and calmodulin-like proteins in Solanum pennellii. Sci. Rep. 2020, 10, 7474. [Google Scholar] [CrossRef]
- Yin, X.M.; Huang, L.F.; Zhang, X.; Wang, M.L.; Xu, G.Y.; Xia, X.J. OsCML4 improves drought tolerance through scavenging of reactive oxygen species in rice. J. Plant Biol. 2015, 58, 68–73. [Google Scholar] [CrossRef]
- Aslam, M.M.; Waseem, M.; Jakada, B.H.; Okal, E.J.; Lei, Z.; Saqib, H.S.A.; Yuan, W.; Xu, W.; Zhang, Q. Mechanisms of abscisic acid-mediated drought stress responses in plants. Int. J. Mol. Sci. 2022, 23, 1084. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, Y.; Zhang, X.; Lang, D.; Zhang, X. The roles of methyl jasmonate to stress in plants. Funct. Plant Biol. 2019, 46, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Hou, J.; Iqbal, M.Z.; Zhang, Y.; Cheng, B.; Feng, H.; Li, Z.; Liu, L.; Zhou, J.; Feng, G.; et al. Overexpression of the white clover TrSAMDC1 gene enhanced salt and drought resistance in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 165, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Berardini, T.Z.; Reiser, L.; Li, D.; Mezheritsky, Y.; Muller, R.; Strait, E.; Huala, E. The Arabidopsis information resource: Making and mining the “gold standard” annotated reference plant genome. Genesis 2015, 53, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Liang, J.; Fang, Y.; An, C.; Yao, Y.; Wang, X.; Zhang, W.; Liu, R.; Wang, L.; Aslam, M.; Cheng, Y.; et al. Genome-wide identification and expression analysis of the bHLH gene family in passion fruit (Passiflora edulis) and its response to abiotic stress. Int. J. Biol. Macromol. 2023, 225, 389–403. [Google Scholar] [CrossRef]
- Song, M.; Wang, L.; Zhang, Y.; Wang, Q.; Han, X.; Yang, Q.; Zhang, J.; Tong, Z. Temporospatial pattern of flavonoid metabolites and potential regulatory pathway of PbMYB211-coordinated kaempferol-3-O-rhamnoside biosynthesis in Phoebe bournei. Plant Physiol. Biochem. 2023, 202, 107913. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, N.; Wang, L.; Han, X.; Yang, Q.; Zhang, Y.; Tong, Z.; Zhang, J. Genome-Wide Identification and Expression Analysis of Calmodulin and Calmodulin-like Genes, Revealing CaM3 and CML13 Participating in Drought Stress in Phoebe bournei. Int. J. Mol. Sci. 2024, 25, 545. https://doi.org/10.3390/ijms25010545
Fu N, Wang L, Han X, Yang Q, Zhang Y, Tong Z, Zhang J. Genome-Wide Identification and Expression Analysis of Calmodulin and Calmodulin-like Genes, Revealing CaM3 and CML13 Participating in Drought Stress in Phoebe bournei. International Journal of Molecular Sciences. 2024; 25(1):545. https://doi.org/10.3390/ijms25010545
Chicago/Turabian StyleFu, Ningning, Li Wang, Xiao Han, Qi Yang, Yuting Zhang, Zaikang Tong, and Junhong Zhang. 2024. "Genome-Wide Identification and Expression Analysis of Calmodulin and Calmodulin-like Genes, Revealing CaM3 and CML13 Participating in Drought Stress in Phoebe bournei" International Journal of Molecular Sciences 25, no. 1: 545. https://doi.org/10.3390/ijms25010545
APA StyleFu, N., Wang, L., Han, X., Yang, Q., Zhang, Y., Tong, Z., & Zhang, J. (2024). Genome-Wide Identification and Expression Analysis of Calmodulin and Calmodulin-like Genes, Revealing CaM3 and CML13 Participating in Drought Stress in Phoebe bournei. International Journal of Molecular Sciences, 25(1), 545. https://doi.org/10.3390/ijms25010545






