Current and Emerging Therapeutic Targets for the Treatment of Cholangiocarcinoma: An Updated Review
Abstract
:1. Introduction
1.1. Cholangiocarcinoma Epidemiology
1.2. Standard Treatment
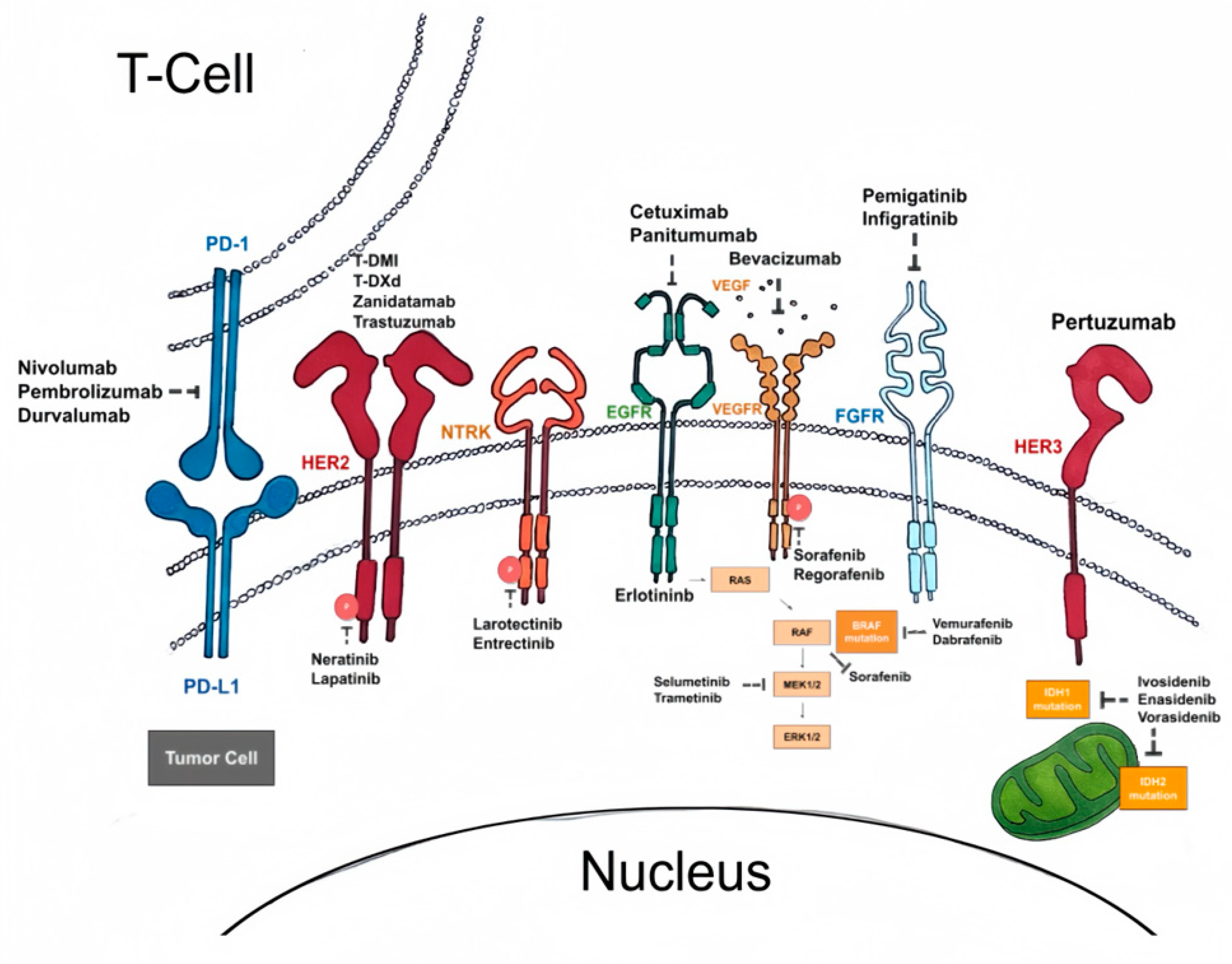
2. Molecular Targets
2.1. Overview
2.2. BRAF V600E
2.3. EGFR
2.4. FGFR
2.5. IDH1/IDH2
2.6. Her-2/NEU/ERBB2/ERBB3
2.7. Immune Checkpoint Inhibitors
2.8. NTRK
2.9. VEGF
2.10. RET
2.11. Future Therpatuic Targets
2.12. Antibody–Drug Conjugates
2.13. Adoptive Cellular Therapies
2.14. Cancer Vaccines
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.; Capanu, M.; Abou-Alfa, G.; Huitzil, D.; Jarnagin, W.; Fong, Y.; D’Angelica, M.; DeMatteo, R.; Blumgart, L.; O’Reilly, E. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J. Surg. Oncol. 2008, 98, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Nagino, M.; Ebata, T.; Yokoyama, Y.; Igami, T.; Sugawara, G.; Takahashi, Y.; Nimura, Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: A single-center 34-year review of 574 consecutive resections. Ann. Surg. 2013, 258, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Amano, H.; Yasuda, H.; Nimura, Y.; Matsushiro, T.; Nakayama, T. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer 2002, 95, 1685–1695. [Google Scholar] [PubMed]
- Horgan, A.M.; Amir, E.; Walter, T.; Knox, J.J. Adjuvant therapy in the treatment of biliary tract cancer: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, K.; Simmons, G.; Manas, D.; Malik, H.; Hamady, Z.Z. Systemic adjuvant chemotherapy for cholangiocarcinoma surgery: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46 Pt A, 684–693. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Lee, K.-H.; Lee, D.-W.; Yoon, J.; Kim, T.-Y.; Bang, J.-H.; Nam, A.-R.; Oh, K.-S.; Kim, J.-M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Farshidfar, F.; Zheng, S.; Gingras, M.-C.; Newton, Y.; Shih, J.; Robertson, A.G.; Hinoue, T.; Hoadley, K.A.; Gibb, E.A.; Roszik, J.; et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell. Rep. 2017, 18, 2780–2794. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Jain, P.; El-Refai, S.M.; Azad, N.S.; Zabransky, D.J.; Baretti, M.; Shroff, R.T.; Kelley, R.K.; El-Khouiery, A.B.; Hockenberry, A.J.; et al. Clinical, Genomic, and Transcriptomic Data Profiling of Biliary Tract Cancer Reveals Subtype-Specific Immune Signatures. JCO Precis. Oncol. 2022, 6, e2100510. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; Wainberg, Z.A. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Adashek, J.J.; Menta, A.K.; Reddy, N.K.; Desai, A.P.; Roszik, J.; Subbiah, V. Tissue-Agnostic Activity of BRAF plus MEK Inhibitor in BRAF V600-Mutant Tumors. Mol. Cancer Ther. 2022, 21, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Wang, G.; Pei, J.; Zhang, J.; Wang, J.; Ouyang, L.; Wang, Y.; Li, W. Emerging strategies to overcome resistance to third-generation EGFR inhibitors. J. Hematol Oncol. 2022, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Mykulowycz, K.; Uehara, T.; Teitelbaum, U.R.; Damjanov, N.; Giantonio, B.J.; Carberry, M.; Wissel, P.; Jacobs-Small, M.; O’Dwyer, P.J.; et al. A phase II trial of gemcitabine, irinotecan and panitumumab in advanced cholangiocarcinoma. Ann. Oncol. 2013, 24, 3061–3065. [Google Scholar] [CrossRef] [PubMed]
- Philip, P.A.; Mahoney, M.R.; Allmer, C.; Thomas, J.; Pitot, H.C.; Kim, G.; Donehower, R.C.; Fitch, T.; Picus, J.; Erlichman, C. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J. Clin. Oncol. 2005, 23, 6657–6663. [Google Scholar] [CrossRef] [PubMed]
- Malka, D.; Cervera, P.; Foulon, S.; Trarbach, T.; de la Fouchardière, C.; Boucher, E.; Fartoux, L.; Faivre, S.; Blanc, J.-F.; Viret, F.; et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): A randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014, 15, 819–828. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Ross, J.S.; Wang, K.; Gay, L.; Al-Rohil, R.; Rand, J.V.; Jones, D.M.; Lee, H.J.; Sheehan, C.E.; Otto, G.A.; Palmer, G.; et al. New routes to targeted therapy of intrahepatic cholangiocarcinomas revealed by next-generation sequencing. Oncologist 2014, 19, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.P.; Fritcher, E.G.B.; Pestova, E.; Schulz, J.; Sitailo, L.A.; Vasmatzis, G.; Murphy, S.J.; McWilliams, R.R.; Hart, S.N.; Halling, K.C.; et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum. Pathol. 2014, 45, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Churi, C.R.; Shroff, R.; Wang, Y.; Rashid, A.; Kang, H.C.; Weatherly, J.; Zuo, M.; Zinner, R.; Hong, D.; Meric-Bernstam, F.; et al. Mutation profiling in cholangiocarcinoma: Prognostic and therapeutic implications. PLoS ONE 2014, 9, e115383. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Bekaii-Saab, T.S.; Valle, J.W.; Van Cutsem, E.; Rimassa, L.; Furuse, J.; Ioka, T.; Melisi, D.; Macarulla, T.; Bridgewater, J.; Wasan, H.; et al. FIGHT-302: First-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020, 16, 2385–2399. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Weiss, K.H.; Waldschmidt, D.-T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.; et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: Mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol. Hepatol. 2021, 6, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Boscoe, A.N.; Rolland, C.; Kelley, R.K. Frequency and prognostic significance of isocitrate dehydrogenase 1 mutations in cholangiocarcinoma: A systematic literature review. J. Gastrointest Oncol. 2019, 10, 751–765. [Google Scholar] [CrossRef]
- de Botton, S.; Fenaux, P.; Yee, K.; Récher, C.; Wei, A.H.; Montesinos, P.; Cortes, J. Olutasidenib (FT-2102) induces durable complete remissions in patients with relapsed or refractory IDH1-mutated AML. Blood Adv. 2023, 7, 3117–3127. [Google Scholar] [CrossRef]
- Wang, Y.; Wild, A.T.; Turcan, S.; Wu, W.H.; Sigel, C.; Klimstra, D.S.; Ma, X.; Gong, Y.; Holland, E.C.; Huse, J.T.; et al. Targeting therapeutic vulnerabilities with PARP inhibition and radiation in IDH-mutant gliomas and cholangiocarcinomas. Sci. Adv. 2020, 6, eaaz3221. [Google Scholar] [CrossRef]
- Galogre, M.; Rodin, D.; Pyatnitskiy, M.; Mackelprang, M.; Koman, I. A review of HER2 overexpression and somatic mutations in cancers. Crit. Rev. Oncol. Hematol. 2023, 186, 103997. [Google Scholar] [CrossRef] [PubMed]
- Roa, I.; de Toro, G.; Schalper, K.; de Aretxabala, X.; Churi, C.; Javle, M. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients With Advanced Gallbladder Cancer. Gastrointest Cancer Res. 2014, 7, 42–48. [Google Scholar] [PubMed]
- Kim, H.J.; Yoo, T.W.; Park, D.I.; Park, J.H.; Cho, Y.K.; Sohn, C.I.; Sohn, J.H. Gene amplification and protein overexpression of HER-2/neu in human extrahepatic cholangiocarcinoma as detected by chromogenic in situ hybridization and immunohistochemistry: Its prognostic implication in node-positive patients. Ann. Oncol. 2007, 18, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Galdy, S.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Cella, C.A.; Fazio, N.; Valle, J.W. HER2/HER3 pathway in biliary tract malignancies; systematic review and meta-analysis: A potential therapeutic target? Cancer Metastasis Rev. 2017, 36, 141–157. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Raufi, A.G.; Sadeghi, S.; Chen, K.; Iuga, A.; Sun, Y.; Ahmed, F.; Bates, S.; Manji, G.A. Prolonged Response to HER2-Directed Therapy in Three Patients with HER2-Amplified Metastatic Carcinoma of the Biliary System: Case Study and Review of the Literature. Oncologist 2021, 26, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Borad, M.J.; Azad, N.S.; Kurzrock, R.; Abou-Alfa, G.K.; George, B.; Hainsworth, J.; Meric-Bernstam, F.; Swanton, C.; Sweeney, C.J.; et al. Pertuzumab and trastuzumab for HER2-positive, metastatic biliary tract cancer (MyPathway): A multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2021, 22, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.-Y.; Hanna, D.L.; Kang, Y.-K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell. Int. 2020, 20, 16. [Google Scholar] [CrossRef]
- Williams, A.S.; Huang, W.-Y. The analysis of microsatellite instability in extracolonic gastrointestinal malignancy. Pathology 2013, 45, 540–552. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H., Jr.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fidalgo, J.A.; Martinelli, E. Lenvatinib plus pembrolizumab a new effective combination of targeted agents. ESMO Open 2023, 8, 101157. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.-M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients With Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Manea, C.A.; Badiu, D.C.; Ploscaru, I.C.; Zgura, A.; Bacinschi, X.; Smarandache, C.G.; Serban, D.; Popescu, C.G.; Grigorean, V.T.; Botnarciuc, V. A review of NTRK fusions in cancer. Ann. Med. Surg. 2022, 79, 103893. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Hyman, D.M. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Moehler, M.; Maderer, A.; Schimanski, C.; Kanzler, S.; Denzer, U.; Kolligs, F.; Ebert, M.; Distelrath, A.; Geissler, M.; Trojan, J.; et al. Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: A double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur. J. Cancer 2014, 50, 3125–3135. [Google Scholar] [CrossRef]
- Demols, A.; Borbath, I.; Eynde, M.V.D.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.-C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: REACHIN, a randomized, double-blind, phase II trial. Ann. Oncol. 2020, 31, 1169–1177. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef]
- Farha, N.; Dima, D.; Ullah, F.; Kamath, S. Precision Oncology Targets in Biliary Tract Cancer. Cancers 2023, 15, 2105. [Google Scholar] [CrossRef] [PubMed]
- Thein, K.Z.; Velcheti, V.; Mooers, B.H.; Wu, J.; Subbiah, V. Precision therapy for RET-altered cancers with RET inhibitors. Trends Cancer 2021, 7, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Drilon, A. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Kuwatani, M.; Sakamoto, N. Promising Highly Targeted Therapies for Cholangiocarcinoma: A Review and Future Perspectives. Cancers 2023, 15, 3686. [Google Scholar] [CrossRef] [PubMed]
- Yokota, K.; Serada, S.; Tsujii, S.; Toya, K.; Takahashi, T.; Matsunaga, T.; Fujimoto, M.; Uemura, S.; Namikawa, T.; Murakami, I.; et al. Anti-Glypican-1 Antibody-drug Conjugate as Potential Therapy Against Tumor Cells and Tumor Vasculature for Glypican-1-Positive Cholangiocarcinoma. Mol. Cancer Ther. 2021, 20, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, X.; Shimura, T.; Huang, A.C.; Kong, N.; Dai, Y.; Fang, J.; Guo, P.; Ying, J.-E. Development of potent antibody drug conjugates against ICAM1(+) cancer cells in preclinical models of cholangiocarcinoma. NPJ Precis Oncol. 2023, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Danese, E.; Ruzzenente, A.; Montagnana, M.; Lievens, P.M.-J. Current and future roles of mucins in cholangiocarcinoma-recent evidences for a possible interplay with bile acids. Ann. Transl. Med. 2018, 6, 333. [Google Scholar] [CrossRef] [PubMed]
- Supimon, K.; Sangsuwannukul, T.; Sujjitjoon, J.; Phanthaphol, N.; Chieochansin, T.; Poungvarin, N.; Wongkham, S.; Junking, M.; Yenchitsomanus, P.-T. Anti-mucin 1 chimeric antigen receptor T cells for adoptive T cell therapy of cholangiocarcinoma. Sci. Rep. 2021, 11, 6276. [Google Scholar] [CrossRef]
- Feng, K.-C.; Guo, Y.-L.; Liu, Y.; Dai, H.-R.; Wang, Y.; Lv, H.-Y.; Huang, J.-H.; Yang, Q.-M.; Han, W.-D. Cocktail treatment with EGFR-specific and CD133-specific chimeric antigen receptor-modified T cells in a patient with advanced cholangiocarcinoma. J. Hematol. Oncol. 2017, 10, 4. [Google Scholar] [CrossRef]
- Huang, X.; Tang, T.; Zhang, G.; Liang, T. Identification of tumor antigens and immune subtypes of cholangiocarcinoma for mRNA vaccine development. Mol. Cancer 2021, 20, 50. [Google Scholar] [CrossRef]
| NTC Number | Phase | Disease | Intervention | Outcome | Status |
|---|---|---|---|---|---|
| NCT05876754 | IIIb | Unresectable and/or metastatic CCA | ivosidenib (IDH1) | AEs, SAEs, QT prolonging events, ECOG, lab abnormalities, changes from baseline of labs and vitals | Enrolling |
| NCT03603834 | II | Resectable CCA | neoadjuvant mFOLFOXIRI | ORR | Enrolling |
| NCT04989218 | I and II | Resectable and high-risk feature ICC | durvalumab/MEDI4736 (anti-PD-L1), tremelimumab (CTLA-4), GemCis | ORR | Enrolling |
| NCT05348811 | II | Unresectable and/or metastatic ICC | GEMOX hepatic arterial infusion chemotherapy (HAIC), donafenib (small molecule multikinase inhibitor), sintilimab (anti-PD-1) | ORR and hORR | Enrolling |
| NCT03820310 | II | Treated with radical resection completely ICC | Autologous Tcm cellular immunotherapy and traditional therapy | PFS, 2-year survival | Enrolling |
| NCT02773485 | III | Unresectable and nonmetastatic CCA | High-dose radiation with systemic themotherapy | OS | Enrolling |
| NCT03656536 | III | Unresectable and/or metastatic CCA | pemigatinib (FGFR-inhibitor) vs. GemCis | PFS | Enrolling |
| NCT05655949 | II | Unresectable and/or metastatic IHC | Y-90 SIRT with durvalumab (anti-PD-L1), GemCis | PFS, grade 3 or higher AE | Enrolling |
| NCT05532059 | II | Unresectable and/or metastatic CCA | lenvatinib (VEGFR1-3), tislelizumab (anti-PD-1), GemCis | ORR | Enrolling |
| NCT05400902 | II | Unresectable ICC with at least one assessable intrahepatic lesion | FOLFOX6 hepatic arterial infusion chemotherapy, sintilimab (anti-PD-1), bevacizumab (VEGF-i) | ORR | Enrolling |
| NCT05290116 | II | Unresectable ICC with at least one assessable intrahepatic lesion | FOLFOX6 hepatic arterial infusion chemotherapy, tislelizumab (anti-PD-1), apatinib (TKI) | ORR | Enrolling |
| NCT05251662 | II | Unresectable CCA | sintilimab (anti-PD-1), GEMOX, IBI305 (bevacizumab biosimilar) | ORR | Enrolling |
| NCT05430698 | II | Resectable perihilar CCA with positive metastatic LNs | Adjuvant PD-1 antibody, GEMOX | 12 mo relapse-free survival rate | Enrolling |
| NCT04295317 | II | Resectable ICC | camrelizumab (anti-PD-1) and capecitabine | Recurrence-free survival | Enrolling |
| NCT05823311 | III | Unresectable and/or metastatic CCA | lenvatinib (VEGFR1-3), tislelizumab (anti-PD-1), GemCis | ORR | Enrolling |
| NCT04954781 | II | Unresectable or metastatic CCA | Transcatheter arterial chemoembolization (TACE) tislelizumab (anti-PD-1) | ORR | Enrolling |
| NCT05010668 | II | Unresectable and/or metastatic ICC | Cryoablation, sintilimab (anti-PD-1), lenvatinib (VEGFR1-3) | ORR | Enrolling |
| NCT04299581 | II | Unresectable and/or metastatic ICC | Cryoablation and Camrelizumab (Anti-PD-1) | ORR | Enrolling |
| NCT04891289 | II | Unresectable and liver metastasis ICC | Gemcitabine, oxaliplatin, floxuridine, and dexamethasone pump | PFS | Enrolling |
| NCT03633773 | I and II | Nonsurgical candidate, ICC | MUC-1 CART cell immunotherapy | DCR | Enrolling |
| NCT05557578 | II | Resectable ICC and high-risk recurrence | Neoadjuvant tislelizumab (anti-PD-1) and GEMOX | ORR, R0 resection rate | Enrolling |
| NCT04961970 | III | Unresectable or local metastatic ICC | FOLFOX hepatic arterial infusion chemotherapy vs. GemCis | OS | Enrolling |
| NCT04782804 | I and II | Post R0 resection ICC | Adjuvant tislelizumab (anti-PD-1) and capecitabine | RFS | Enrolling |
| NCT05174650 | II | Non-resectable ICC and FGFR2+ | atezolizumab (anti-PD-L1) and derazantinib (FGFR1–3 kinase inhibitor) | ORR | Enrolling |
| NCT04454905 | II | Unresectable and/or metastatic ICC | camrelizumab (anti-PD-1) and apatinib (TKI) | PFS | Enrolling |
| NCT04238637 | II | Locally advanced OR limited metasized intrahepatic BTC | Y-90 SIRT with durvalumab (anti-PD-L1) or tremelimumab (CTLA-4i) | ORR | Enrolling |
| NCT05010681 | II | Unresectable and/or metastatic ICC or HCC | sintilimab (anti-PD-1), lenvatinib (VEGFR1-3) | ORR | Enrolling |
| NCT05678270 | II | Unresectable, recurrent or metastatic ICC and FGFR2+ | gunagratinib (FGFR-inhibitor) | ORR | Enrolling |
| NCT05921760 | I, II | Unresectable and/or metastatic CCA, IDH1+ | ivosidenib, nivolumab (anti-PD1), ipilimumab (CTLA-4i) | Dose-limiting toxicities (DLT), recommended combination dose (RDC), ORR, AEs, SAEs | Enrolling |
| NCT04353375 | II | Unresectable and/or metastatic ICC, FGFR2+ | HMPL-453 tartrate (FGFR1, FGFR2, FGFR3 antagonist) | ORR | Enrolling |
| NCT05835245 | II | Unresectable and/or metastatic ICC | Cryoablation with sintilimab (anti-PD-1), lenvatinib (VEGFR1-3) | ORR | Enrolling |
| NCT05978609 | II | Unresectable and/or metastatic CCA | Candonilimab (PD-1/CTLA-4), GemCis | ORR | Enrolling |
| NCT04951141 | I | Unresectable liver tumor, GPC3+ | anti-GPC3 CAR-T cells | AEs | Enrolling |
| NCT04708067 | I | Unresectable and/or metastatic ICC | bintrafusp alfa and hypofractionated radiation therapy | AEs | Enrolling |
| NCT05672537 | II | Resectable ICC | Neoadjuvant durvalumab (anti-PD-L1), surgery, GemCis | RFS | Enrolling |
| NCT05239169 | II | Unresectable and/or metastatic BTC | durvalumab (anti-PD-L1), tremelimumab (CTLA-4), capecitabine | RFS | Enrolling |
| NCT05223816 | IIa/IIb | Unresectable and/or metastatic ICC | VG-161 (IL-12, IL-15, PD-L1), nivolumab (anti-PD1) | OS, ORR, PFS | Enrolling |
| NCT05220722 | I, II | Unresectable and/or metastatic ICC | SD-101 (TLR 9 agonist), pembrolizumab (anti-PD-1), nivolumab (anti-PD1), ipilimumab (CTLA-4i) | Safety, maximum tolerable dose (MTD), ORR | Enrolling |
| NCT06037980 | II, III | Resectable BTC at high risk for recurrence | GemCis, nabpaclitaxel vs. upfront surgery | PFS | Enrolling |
| NCT03779035 | III | BTC after curative resection | Adjuvant GemCis vs. capecitabine | DFS | Enrolling |
| NCT04634058 | II | Unresectable and metastatic ICC | PD-L1 and CTLA-4 | ORR | Enrolling |
| NCT03364530 | II | Nonmetastatic unresectable ICC | Hepatic intra-aterial Gemcitabine-Oxaliplatin | ORR | Enrolling |
| NCT04298021 | II | Unresectable and/or recurrent BTC | AZD6738 (ATR Kinase-i), durvalumab (anti-PD-L1), olaparib (PARP-i) | DCR | Enrolling |
| NCT04298008 | II | Unresectable and/or recurrent BTC | AZD6738 (ATR Kinase-i) and durvalumab (anti-PD-L1) | DCR | Enrolling |
| NCT05727176 | II | Unresectable and/or metastatic CCA, FGFR2+ | Futibatinib (FGFR1–4 inhibitor) | ORR | Enrolling |
| NCT06081322 | I | Advanced cholangiocarcinoma | 177Lu-EB-FAPI PRRT (FAP inhibitor molecule) | Hematoxicity, ORR, hepatotoxicity, renal toxicity | Enrolling |
| NCT04413734 | II | Unresectable and/or metastatic BTC | triprilumab (anti-PD-1), GemCis | PFS | Enrolling |
| NCT04251715 | IId | Unresectable ICC | mFOLFIRINOX, hepatic arterial infusion of floxuridine, dexamethasone, and systemic mFOLFIRI | Incidence of abnormal liver function, DCR | Enrolling |
| NCT05568680 | I | Recurrent or relapsed advanced mesothelial CCA | SynKIR-110 (anti-mesothelin) | Safety and feasibility | Enrolling |
| NCT04565275 | I, II | Unresectable and/or metastatic CCA, FGFR+ | ICP-192 (pan-FGFR inhibitor) | AEs, MTD, OBD, RP2D, ORR | Enrolling |
| NCT03991832 | II | Adenocarcinoma BTC, IDH+ | olaparib (PARP-i), durvalumab (anti-PD-L1) | ORR, DCR | Enrolling |
| NCT04264260 | II | ICC | colchicine | OS | Enrolling |
| NCT04669496 | II, III | Resectable ICC and high-risk recurrence | neoadjuvant toripalimab (anti-PD-1), lenvatinib (VEGF1-3), GEMOX | Event free survival | Enrolling |
| NCT05286814 | II | Unresectable and/or metastatic CCA | M9241 (IL-12 heterodimers) hepatic artery infusion pump and systemic chemotherapy, dexamethasone | ORR | Enrolling |
| NCT03801083 | II | Unresectable, recurrent, or metastatic BTC | Autologous tumor-infiltrating lymphocytes (TIL) | ORR | Enrolling |
| NCT05242822 | I | Advanced stage CCA, FGFR2+ and/or FGFR3+ | KIN-3248 (small molecule pan-FGFRi) | DLT, AEs, ORR, DCR, duration of response, PFS | Enrolling |
| NCT05724563 | II | Unresectable and/or metastatic BTC | domvanalimab (anti-TIGIT), zimberelimab (anti-PD-1) | ORR | Enrolling |
| NCT05913661 | II | Unresectable and/or metastatic ICC | pemigatinib (FGFR-inhibitor) and anti-PD-1 | ORR | Enrolling |
| NCT04645160 | I, II | Unresectable BTC | tivozanib (VEGF-i) | ORR, RP2D | Enrolling |
| NCT04068194 | I, II | Unresectable and/or metastatic ICC | peposertib (DNA-PK-i), avelumab (anti-PD-L1), RT | MTD, ORR | Enrolling |
| NCT05565794 | II | curatively treatable localized ICC, FGFR2+ | pemigatinib (FGFR-inhibitor) | ORR | Enrolling |
| NCT04526106 | I, II | Unresectable and/or metastatic CCA | RLY-4008 (FGFR2-i) | MTD, AEs, SAEs, ORR | Enrolling |
| NCT05620498 | II | Potentially resectable ICC and GBC | tislelizumab (anti-PD-1), lenvatinib lenvatinib (VEGFR1-3), GEMOX | ORR | Enrolling |
| NCT05506943 | II, III | Unresectable, metastatic, or recurrent BTC | CTX-009 (Delta-like ligand 4/Notch-1 (DLL4) and VEGF-A inhibitor) | BOR | Enrolling |
| NCT03942328 | I, II | Unresectable ICC | intra-tumoral injection of autologous dendritic cells and prevnar vaccine after EBRT, atezolizumab (anti-PD-L1), bevacizumab (VEGF-i) | incidence of significant toxicity, PFS | Enrolling |
| NCT05564403 | II | Unresectable or recurrent BTC, RAS/RAF/MEK/ERK+ | binimetinib (MEK-i), FOLFOX | OS | Enrolling |
| NCT05211323 | II | Unresectable or metastatic cHCC-CC | atezolizumab (anti-PD-L1), bevacizumab (VEGF-i), GemCis | PFS | Enrolling |
| NCT03212274 | II | BTC, IDH1/2+ | olaparib (PARP-i), | ORR | Enrolling |
| NCT05805956 | I, II | Unresectable and/or metastatic BTC, HER2+ | IMM2902 (anti-CD47/anti-HER2 bispecific antibody) | MTD, RP2D, AEs, ORR, DOR, DCR, PFS | Enrolling |
| NCT05411133 | I | Unresectable and/or metastatic BTC, CDH17+ | ARB202 (CDH17-CD3 bispecific T-cell engager antibody) | SAEs | Enrolling |
| NCT04801095 | I | Unresectable and/or metastatic BTC | WM-S1-030 (inhibitor for mtRTK) | DLT, AEs, SAEs | Enrolling |
| NCT04430738 | I, II | Unresectable and/or metastatic BTC | tucatinib (HER2 tyrosine kinase inhibitor), trastazumab (anti-EGFR), oxaliplatin-based chemotherapy, pembrolizumab (anti-PD-1) | DLT; AEs; incidence of lab abnormalities, DLTs, dose alterations | Enrolling |
| NCT05285358 | I | BTC with peritoneal metastasis | pressurized intraperitoneal aerosolized nab-paclitaxel, GemCis | AEs | Enrolling |
| NCT06010862 | I | Unresectable and/or metastatic BTC, CEA+ | CEA-targeted CAR-T | AEs, MTD | Enrolling |
| NCT05185947 | II | BTC with peritoneal metastasis | intravenous and intraperitoneal paclitaxel, nilotinib (transduction inhibitor of BCR-ABL, c-kit and PDGF) | OS, PFS, safety, tolerability, QOL, ORR | Enrolling |
| NCT05991518 | I, II | Unresectable and/or metastatic BTC, HER2+ | IAH0968 (afucosylated anti-EGFR2), GemCIs | AE, SAEs, DLT, ORR | Enrolling |
| NCT06126406 | I | Unresectable and/or metastatic BTC, CEA+ | CEA-targeted CAR-T | AEs, MTD | Enrolling |
| NCT03907852 | I, II | BTC, MSLN+ | Gavocabtagene Autoleucel (anti-Mesothelin), fludarabine, cyclophosphamide, nivolumab (anti-PD1), ipilimumab (CTLA-4i) | RP2D, ORR, DCR | Enrolling |
| NCT06043466 | I | Unresectable and/or metastatic BTC, CEA+ | CEA-targeted CAR-T | DLT, MTD | Enrolling |
| NCT05277766 | I | BTC with peritoneal metastasis | intraperitoneal aerosolized nano liposomal irinotecan | MTD | Enrolling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadfield, M.J.; DeCarli, K.; Bash, K.; Sun, G.; Almhanna, K. Current and Emerging Therapeutic Targets for the Treatment of Cholangiocarcinoma: An Updated Review. Int. J. Mol. Sci. 2024, 25, 543. https://doi.org/10.3390/ijms25010543
Hadfield MJ, DeCarli K, Bash K, Sun G, Almhanna K. Current and Emerging Therapeutic Targets for the Treatment of Cholangiocarcinoma: An Updated Review. International Journal of Molecular Sciences. 2024; 25(1):543. https://doi.org/10.3390/ijms25010543
Chicago/Turabian StyleHadfield, Matthew J., Kathryn DeCarli, Kinan Bash, Grace Sun, and Khaldoun Almhanna. 2024. "Current and Emerging Therapeutic Targets for the Treatment of Cholangiocarcinoma: An Updated Review" International Journal of Molecular Sciences 25, no. 1: 543. https://doi.org/10.3390/ijms25010543
APA StyleHadfield, M. J., DeCarli, K., Bash, K., Sun, G., & Almhanna, K. (2024). Current and Emerging Therapeutic Targets for the Treatment of Cholangiocarcinoma: An Updated Review. International Journal of Molecular Sciences, 25(1), 543. https://doi.org/10.3390/ijms25010543





