MitoNEET Provides Cardioprotection via Reducing Oxidative Damage and Conserving Mitochondrial Function
Abstract
1. Introduction
1.1. Cardiovascular Diseases
1.2. Metabolic Diseases
1.3. MitoNEET as a Potential Therapeutic Target
2. Mitochondrial Homeostasis and mitoNEET
2.1. MitoNEET, Mitochondria, and Metabolic Diseases
2.2. MitoNEET Regulates Mitochondrial Morphology
2.3. Mitochondrial Health and MitoNEET
3. Iron Metabolism and MitoNEET
3.1. The Role of Dysregulated Iron Metabolism in Cardiovascular and Metabolic Diseases
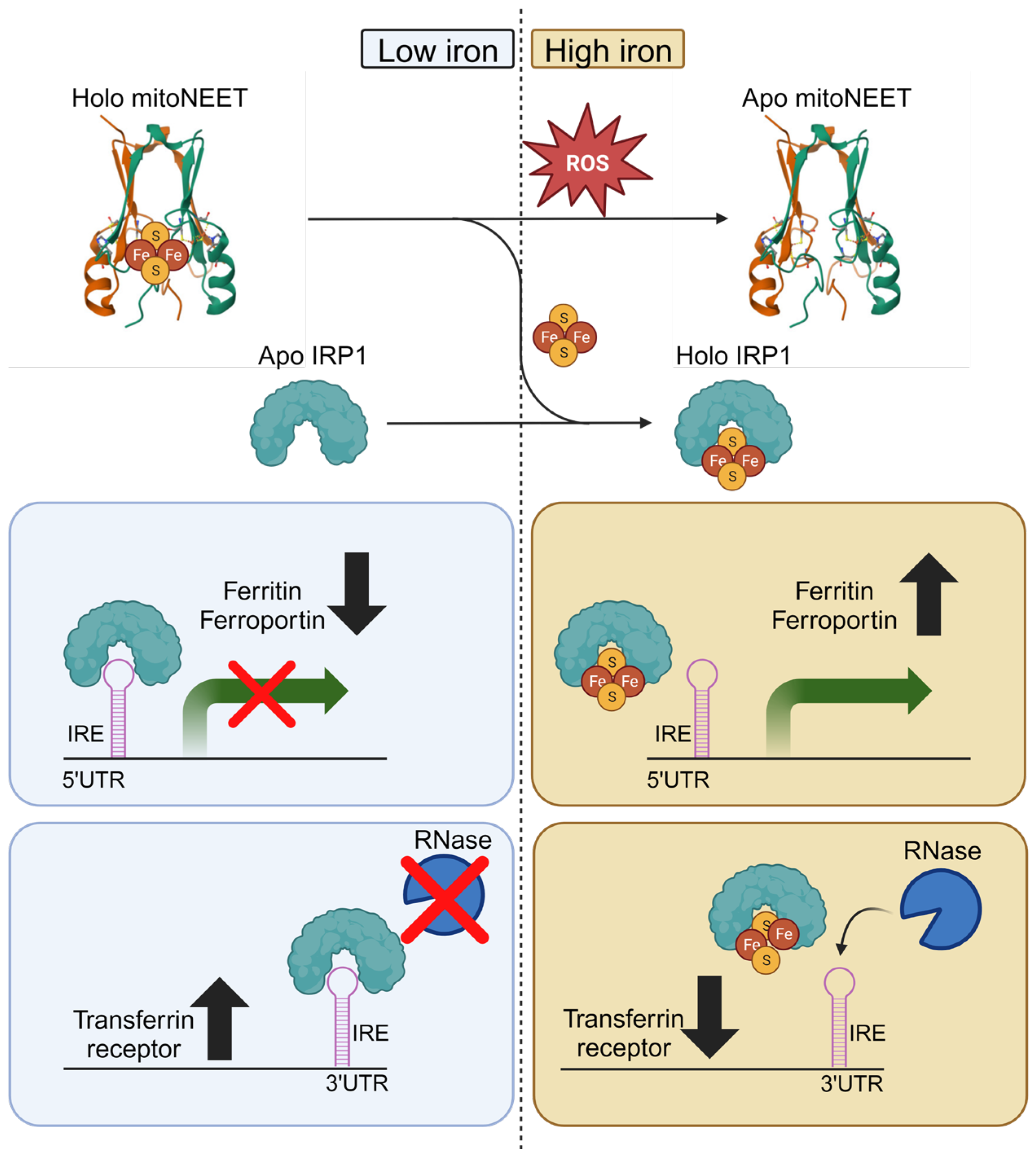
3.2. MitoNEET and Iron Metabolism
3.3. MitoNEET Regulates Mitochondrial Iron
4. MitoNEET and Redox Homeostasis
4.1. Limitations in Using Antioxidants as Therapeutics
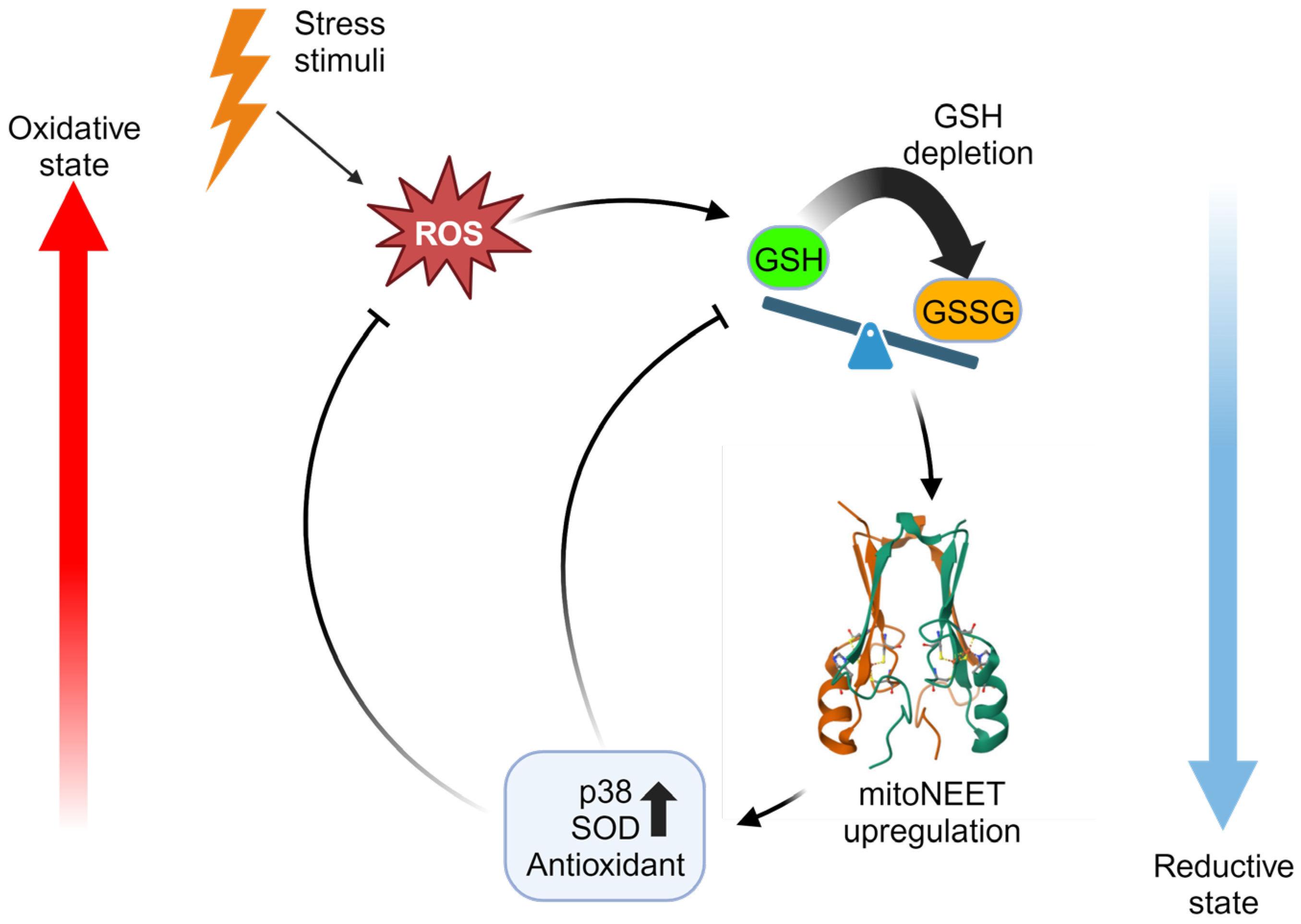
4.2. MitoNEET Iron-Sulfur Cluster Status Acts as a Redox Sensor
4.3. MitoNEET Mitigates Oxidative Stress
5. Cardioprotective Potential of MitoNEET via Mitigating Cell Death
5.1. Implications of Cell Death on Cardiovascular Diseases
5.2. MitoNEET Regulates Apoptosis in Cardiomyocytes and Cardiomyoblasts
5.3. MitoNEET Reduces Cell Death via Supression of Ferroptosis
5.4. Physiological Applications of MitoNEET in Cardiovascular Disease
6. Metabolic Effects of MitoNEET in Cardioprotection
6.1. MitoNEET Preserves Metabolic Health in Obesity
6.2. MitoNEET as a Therapeutic Target in Metabolic Dysfunction
6.3. MitoNEET in Regulating Crosstalk between Adipose and Cardiac Tissue
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Ding, W.; Ji, X.; Ao, X.; Liu, Y.; Yu, W.; Wang, J. Oxidative Stress in Cell Death and Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9030563. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef] [PubMed]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Chong, B.; Kong, G.; Shankar, K.; Chew, H.J.; Lin, C.; Goh, R.; Chin, Y.H.; Tan, D.J.H.; Chan, K.E.; Lim, W.H.; et al. The global syndemic of metabolic diseases in the young adult population: A consortium of trends and projections from the Global Burden of Disease 2000–2019. Metabolism 2023, 141, 155402. [Google Scholar] [CrossRef]
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C.; Cosials, J.B.; Reyero, J.B.; Martínez, J.D.; Diego, P.G.; Uche, A.M.G.; et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef]
- Zhu, L.; Spence, C.; Yang, W.J.; Ma, G.X. The IDF Definition Is Better Suited for Screening Metabolic Syndrome and Estimating Risks of Diabetes in Asian American Adults: Evidence from NHANES 2011–2016. J. Clin. Med. 2020, 9, 3871. [Google Scholar] [CrossRef]
- MacRae, C.A.; Roden, D.M.; Loscalzo, J. The Future of Cardiovascular Therapeutics. Circulation 2016, 133, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Hartley, R.C. Mitochondria as a therapeutic target for common pathologies. Nat. Rev. Drug Discov. 2018, 17, 865–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Y.; Ren, H.-H.; Wang, D.; Chen, Y.; Qu, C.-J.; Pan, Z.-H.; Liu, X.-N.; Hao, W.-J.; Xu, W.-J.; Wang, K.-J.; et al. Isoliquiritigenin Induces Mitochondrial Dysfunction and Apoptosis by Inhibiting mitoNEET in a Reactive Oxygen Species-Dependent Manner in A375 Human Melanoma Cells. Oxidative Med. Cell. Longev. 2019, 2019, 9817576. [Google Scholar] [CrossRef] [PubMed]
- Inupakutika, M.A.; Sengupta, S.; Nechushtai, R.; Jennings, P.A.; Onuchic, J.N.; Azad, R.K.; Padilla, P.; Mittler, R. Phylogenetic analysis of eukaryotic NEET proteins uncovers a link between a key gene duplication event and the evolution of vertebrates. Sci. Rep. 2017, 7, srep42571. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Holland, W.L.; Sun, K.; Park, J.; Spurgin, S.B.; Lin, Y.; Askew, G.R.; Simcox, J.A.; McClain, D.A.; Li, C.; et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 2012, 18, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Hu, M.; Qiu, Y.; Zhang, L.; Yan, Y.; Feng, Y.; Feng, C.; Hou, X.; Wang, Z.; Zhang, D.; et al. Mitoglitazone ameliorates renal ischemia/reperfusion injury by inhibiting ferroptosis via targeting mitoNEET. Toxicol. Appl. Pharmacol. 2023, 465, 116440. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.; Sung, H.K.; Lam, N.H.; You, S.; Cho, S.; Ahmed, S.M.; Abdul-Sater, A.A.; Sweeney, G. Role of Mitochondrial Iron Overload in Mediating Cell Death in H9c2 Cells. Cells 2022, 12, 118. [Google Scholar] [CrossRef]
- Tam, E.; Sung, H.K.; Sweeney, G. MitoNEET prevents iron overload-induced insulin resistance in H9c2 cells through regulation of mitochondrial iron. J. Cell. Physiol. 2023, 238, 1867–1875. [Google Scholar] [CrossRef]
- Yonutas, H.M.; Hubbard, W.B.; Pandya, J.D.; Vekaria, H.J.; Geldenhuys, W.J.; Sullivan, P.G. Bioenergetic restoration and neuroprotection after therapeutic targeting of mitoNEET: New mechanism of pioglitazone following traumatic brain injury. Exp. Neurol. 2020, 327, 113243. [Google Scholar] [CrossRef]
- Colca, J.R.; McDonald, W.G.; Waldon, D.J.; Leone, J.W.; Lull, J.M.; Bannow, C.A.; Lund, E.T.; Mathews, W.R. Identification of a novel mitochondrial protein (“mitoNEET”) cross-linked specifically by a thiazolidinedione photoprobe. Am. J. Physiol. Metab. 2004, 286, E252–E260. [Google Scholar] [CrossRef]
- Furihata, T.; Takada, S.; Kakutani, N.; Maekawa, S.; Tsuda, M.; Matsumoto, J.; Mizushima, W.; Fukushima, A.; Yokota, T.; Enzan, N.; et al. Cardiac-specific loss of mitoNEET expression is linked with age-related heart failure. Commun. Biol. 2021, 4, 138. [Google Scholar] [CrossRef] [PubMed]
- Geldenhuys, W.J.; Benkovic, S.A.; Lin, L.; Yonutas, H.M.; Crish, S.D.; Sullivan, P.G.; Darvesh, A.S.; Brown, C.M.; Richardson, J.R. MitoNEET (CISD1) Knockout Mice Show Signs of Striatal Mitochondrial Dysfunction and a Parkinson’s Disease Phenotype. ACS Chem. Neurosci. 2017, 8, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Habener, A.; Chowdhury, A.; Echtermeyer, F.; Lichtinghagen, R.; Theilmeier, G.; Herzog, C. MitoNEET Protects HL-1 Cardiomyocytes from Oxidative Stress Mediated Apoptosis in an In Vitro Model of Hypoxia and Reoxygenation. PLoS ONE 2016, 11, e0156054. [Google Scholar] [CrossRef] [PubMed]
- Saralkar, P.; Arsiwala, T.; Geldenhuys, W.J. Nanoparticle formulation and in vitro efficacy testing of the mitoNEET ligand NL-1 for drug delivery in a brain endothelial model of ischemic reperfusion-injury. Int. J. Pharm. 2020, 578, 119090. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Mughal, A.; Kumar, D.; Vikram, A. Effects of Thiazolidinediones on metabolism and cancer: Relative influence of PPARγ and IGF-1 signaling. Eur. J. Pharmacol. 2015, 768, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Mitochondrial dysfunction in diabetes and the regulatory roles of antidiabetic agents on the mitochondrial function. J. Cell. Physiol. 2019, 234, 8402–8410. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Park, J.; Scherer, P.E. MitoNEET-mediated effects on browning of white adipose tissue. Nat. Commun. 2014, 5, 3962. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.; Lee, S.-J.; Chung, S.W. Inhibition of mitoNEET induces Pink1-Parkin-mediated mitophagy. BMB Rep. 2022, 55, 354–359. [Google Scholar] [CrossRef]
- Wiley, S.E.; Murphy, A.N.; Ross, S.A.; van der Geer, P.; Dixon, J.E. MitoNEET is an iron-containing outer mitochondrial membrane protein that regulates oxidative capacity. Proc. Natl. Acad. Sci. USA 2007, 104, 5318–5323. [Google Scholar] [CrossRef]
- Sergi, D.; Naumovski, N.N.; Heilbronn, L.H.K.; Abeywardena, M.; O’callaghan, N.; Lionetti, L.; Luscombe-Marsh, N.L.-M. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Front. Physiol. 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Weng, S.-W.; Chang, Y.-H.; Su, Y.-J.; Chang, C.-M.; Tsai, C.-J.; Shen, F.-C.; Chuang, J.-H.; Lin, T.-K.; Liou, C.-W.; et al. The Causal Role of Mitochondrial Dynamics in Regulating Insulin Resistance in Diabetes: Link through Mitochondrial Reactive Oxygen Species. Oxidative Med. Cell. Longev. 2018, 2018, 7514383. [Google Scholar] [CrossRef] [PubMed]
- Vernay, A.; Marchetti, A.; Sabra, A.; Jauslin, T.N.; Rosselin, M.; Scherer, P.E.; Demaurex, N.; Orci, L.; Cosson, P. MitoNEET-dependent formation of intermitochondrial junctions. Proc. Natl. Acad. Sci. USA 2017, 114, 8277–8282. [Google Scholar] [CrossRef]
- Saralkar, P.; Mdzinarishvili, A.; Arsiwala, T.A.; Lee, Y.-K.; Sullivan, P.G.; Pinti, M.V.; Hollander, J.M.; Kelley, E.E.; Ren, X.; Hu, H.; et al. The Mitochondrial mitoNEET Ligand NL-1 Is Protective in a Murine Model of Transient Cerebral Ischemic Stroke. Pharm. Res. 2021, 38, 803–817. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Jiang, X.; Yang, J.; Zhang, J.; Yang, F.; Cao, H. Mitophagy: A potential therapeutic target for insulin resistance. Front. Physiol. 2022, 13, 957968. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef] [PubMed]
- Ayer, A.; Fazakerley, D.J.; James, D.E.; Stocker, R. The role of mitochondrial reactive oxygen species in insulin resistance. Free. Radic. Biol. Med. 2022, 179, 339–362. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Chang, H.-C.; Shapiro, J.S.; Ardehali, H. Getting to the “Heart” of Cardiac Disease by Decreasing Mitochondrial Iron. Circ. Res. 2016, 119, 1164–1166. [Google Scholar] [CrossRef]
- Chang, H.; Wu, R.; Shang, M.; Sato, T.; Chen, C.; Shapiro, J.S.; Liu, T.; Thakur, A.; Sawicki, K.T.; Prasad, S.V.; et al. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol. Med. 2016, 8, 247–267. [Google Scholar] [CrossRef]
- Duan, G.; Li, J.; Duan, Y.; Zheng, C.; Guo, Q.; Li, F.; Zheng, J.; Yu, J.; Zhang, P.; Wan, M.; et al. Mitochondrial Iron Metabolism: The Crucial Actors in Diseases. Molecules 2022, 28, 29. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Cilibrizzi, A.; Pourzand, C.; Abbate, V.; Reelfs, O.; Versari, L.; Floresta, G.; Hider, R. The synthesis and properties of mitochondrial targeted iron chelators. BioMetals 2022, 36, 321–337. [Google Scholar] [CrossRef] [PubMed]
- Danielpur, L.; Sohn, Y.-S.; Karmi, O.; Fogel, C.; Zinger, A.; Abu-Libdeh, A.; Israeli, T.; Riahi, Y.; Pappo, O.; Birk, R.; et al. GLP-1-RA Corrects Mitochondrial Labile Iron Accumulation and Improves β-Cell Function in Type 2 Wolfram Syndrome. J. Clin. Endocrinol. Metab. 2016, 101, 3592–3599. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Pantopoulos, K. The IRP/IRE system in vivo: Insights from mouse models. Front. Pharmacol. 2014, 5, 176. [Google Scholar] [CrossRef] [PubMed]
- Ferecatu, I.; Gonçalves, S.; Golinelli-Cohen, M.-P.; Clémancey, M.; Martelli, A.; Riquier, S.; Guittet, E.; Latour, J.-M.; Puccio, H.; Drapier, J.-C.; et al. The Diabetes Drug Target MitoNEET Governs a Novel Trafficking Pathway to Rebuild an Fe-S Cluster into Cytosolic Aconitase/Iron Regulatory Protein 1. J. Biol. Chem. 2014, 289, 28070–28086. [Google Scholar] [CrossRef] [PubMed]
- Mons, C.; Botzanowski, T.; Nikolaev, A.; Hellwig, P.; Cianférani, S.; Lescop, E.; Bouton, C.; Golinelli-Cohen, M.-P. The H2O2-Resistant Fe–S Redox Switch MitoNEET Acts as a pH Sensor to Repair Stress-Damaged Fe–S Protein. Biochemistry 2018, 57, 5616–5628. [Google Scholar] [CrossRef]
- Batista-Jorge, G.; Barcala-Jorge, A.; Silveira, M.; Lelis, D.; Andrade, J.; de Paula, A.; Guimarães, A.; Santos, S. Oral resveratrol supplementation improves Metabolic Syndrome features in obese patients submitted to a lifestyle-changing program. Life Sci. 2020, 256, 117962. [Google Scholar] [CrossRef]
- Mirmiran, P.; Hosseini-Esfahani, F.; Esfandiar, Z.; Hosseinpour-Niazi, S.; Azizi, F. Associations between dietary antioxidant intakes and cardiovascular disease. Sci. Rep. 2022, 12, 1504. [Google Scholar] [CrossRef]
- Cunnington, C.; Van Assche, T.; Shirodaria, C.; Kylintireas, I.; Lindsay, A.C.; Lee, J.M.; Antoniades, C.; Margaritis, M.; Lee, R.; Cerrato, R.; et al. Systemic and vascular oxidation limits the efficacy of oral tetrahydrobiopterin treatment in patients with coronary artery disease. Circulation 2012, 125, 1356–1366. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef] [PubMed]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Charron, M.J.; Williams, L.; Seki, Y.; Du, X.Q.; Chaurasia, B.; Saghatelian, A.; Summers, S.A.; Katz, E.B.; Vuguin, P.M.; Reznik, S.E. Antioxidant Effects of N-Acetylcysteine Prevent Programmed Metabolic Disease in Mice. Diabetes 2020, 69, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs 2018, 18, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Barteková, M.; Adameová, A.; Görbe, A.; Ferenczyová, K.; Pecháňová, O.; Lazou, A.; Dhalla, N.S.; Ferdinandy, P.; Giricz, Z. Natural and synthetic antioxidants targeting cardiac oxidative stress and redox signaling in cardiometabolic diseases. Free Radic. Biol. Med. 2021, 169, 446–477. [Google Scholar] [CrossRef] [PubMed]
- Yarema, M.; Chopra, P.; Sivilotti, M.L.A.; Johnson, D.; Nettel-Aguirre, A.; Bailey, B.; Victorino, C.; Gosselin, S.; Purssell, R.; Thompson, M.; et al. Anaphylactoid Reactions to Intravenous N-Acetylcysteine during Treatment for Acetaminophen Poisoning. J. Med. Toxicol. 2018, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Wiley, S.E.; Paddock, M.L.; Abresch, E.C.; Gross, L.; van der Geer, P.; Nechushtai, R.; Murphy, A.N.; Jennings, P.A.; Dixon, J.E. The outer mitochondrial membrane protein mitoNEET contains a novel redox-active 2Fe-2S cluster. J. Biol. Chem. 2007, 282, 23745–23749. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.W.; Zuris, J.A.; Paddock, M.L.; Jennings, P.A.; Elliott, S.J. Redox characterization of the FeS protein MitoNEET and impact of thiazolidinedione drug binding. Biochemistry 2009, 48, 10193–10195. [Google Scholar] [CrossRef]
- Landry, A.P.; Ding, H. Redox control of human mitochondrial outer membrane protein MitoNEET [2Fe-2S] clusters by biological thiols and hydrogen peroxide. J. Biol. Chem. 2014, 289, 4307–4315. [Google Scholar] [CrossRef]
- Logan, S.J.; Yin, L.; Geldenhuys, W.J.; Enrick, M.K.; Stevanov, K.M.; Carroll, R.T.; Ohanyan, V.A.; Kolz, C.L.; Chilian, W.M. Novel thiazolidinedione mitoNEET ligand-1 acutely improves cardiac stem cell survival under oxidative stress. Basic Res. Cardiol. 2015, 110, 19. [Google Scholar] [CrossRef]
- Boos, J.R.; Jandrain, H.N.; Hagiuda, E.; Taguchi, A.T.; Hasegawa, K.; Fedun, B.L.; Taylor, S.J.; Elad, S.M.; Faber, S.E.; Kumasaka, T.; et al. Structure and biological evaluation of Caenorhabditis elegans CISD-1/mitoNEET, a KLP-17 tail domain homologue, supports attenuation of paraquat-induced oxidative stress through a p38 MAPK-mediated antioxidant defense response. Adv. Redox Res. 2022, 6, 100048. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Cui, L.; Chen, R.; Liang, S.; Wang, C.; Wu, P. TT01001 attenuates oxidative stress and neuronal apoptosis by preventing mitoNEET-mediated mitochondrial dysfunction after subarachnoid hemorrhage in rats. NeuroReport 2020, 31, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamamoto, M.; Amikura, K.; Kato, K.; Serizawa, T.; Serizawa, K.; Akazawa, D.; Aoki, T.; Kawai, K.; Ogasawara, E.; et al. A novel MitoNEET ligand, TT01001, improves diabetes and ameliorates mitochondrial function in db/db mice. Experiment 2014, 352, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Li, X.; Zhang, X.; Kang, R.; Tang, D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem. Biophys. Res. Commun. 2016, 478, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Moe, G.W.; Marín-García, J. Role of cell death in the progression of heart failure. Heart Fail. Rev. 2016, 21, 157–167. [Google Scholar] [CrossRef]
- Dhani, S.; Zhao, Y.; Zhivotovsky, B. A long way to go: Caspase inhibitors in clinical use. Cell Death Dis. 2021, 12, 949. [Google Scholar] [CrossRef]
- Stockwell, B.R. Ferroptosis turns 10: Emerging mechanisms, physiological functions, and therapeutic applications. Cell 2022, 185, 2401–2421. [Google Scholar] [CrossRef]
- Nair, N.; Gongora, E. Stem cell therapy in heart failure: Where do we stand today? Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165489. [Google Scholar] [CrossRef]
- Butrous, H.; Hummel, S.L. Heart Failure in Older Adults. Can. J. Cardiol. 2016, 32, 1140–1147. [Google Scholar] [CrossRef]
- Guerra, F.; Brambatti, M.; Matassini, M.V.; Capucci, A. Current Therapeutic Options for Heart Failure in Elderly Patients. BioMed Res. Int. 2017, 2017, 1483873. [Google Scholar] [CrossRef]
- Wang, J.-S.; Xia, P.-F.; Ma, M.-N.; Li, Y.; Geng, T.-T.; Zhang, Y.-B.; Tu, Z.-Z.; Jiang, L.; Zhou, L.-R.; Zhang, B.-F.; et al. Trends in the Prevalence of Metabolically Healthy Obesity Among US Adults, 1999–2018. JAMA Netw. Open 2023, 6, e232145. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.E.; Crail, J.P.; Laffoon, M.M.; Fernandez, W.G.; Menze, M.A.; Konkle, M.E. Identification of disulfide bond formation between MitoNEET and glutamate dehydrogenase 1. Biochemistry 2013, 52, 8969–8971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilson, D.F.; Cember, A.T.J.; Matschinsky, F.M. Glutamate dehydrogenase: Role in regulating metabolism and insulin release in pancreatic β-cells. J. Appl. Physiol. 2018, 125, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Jahng, J.W.S.; Song, E.; Sweeney, G. Crosstalk between the heart and peripheral organs in heart failure. Exp. Mol. Med. 2016, 48, e217. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.-Y.; Qu, S.-L.; Xiong, W.-H.; Rom, O.; Chang, L.; Jiang, Z.-S. Perivascular adipose tissue (PVAT) in atherosclerosis: A double-edged sword. Cardiovasc. Diabetol. 2018, 17, 134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, H.; Garcia-Barrio, M.; Guo, Y.; Zhang, J.; Chen, Y.E.; Chang, L. RNA sequencing reveals perivascular adipose tissue plasticity in response to angiotensin II. Pharmacol. Res. 2022, 178, 106183. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, X.; Garcia-Barrio, M.T.; Zhang, J.; Lin, J.; Chen, Y.E.; Jiang, Z.; Chang, L. MitoNEET in Perivascular Adipose Tissue Blunts Atherosclerosis under Mild Cold Condition in Mice. Front. Physiol. 2017, 8, 1032. [Google Scholar] [CrossRef]
- Chang, L.; Zhao, X.; Garcia-Barrio, M.; Zhang, J.; Chen, Y.E. MitoNEET in Perivascular Adipose Tissue Prevents Arterial Stiffness in Aging Mice. Cardiovasc. Drugs Ther. 2018, 32, 531–539. [Google Scholar] [CrossRef]
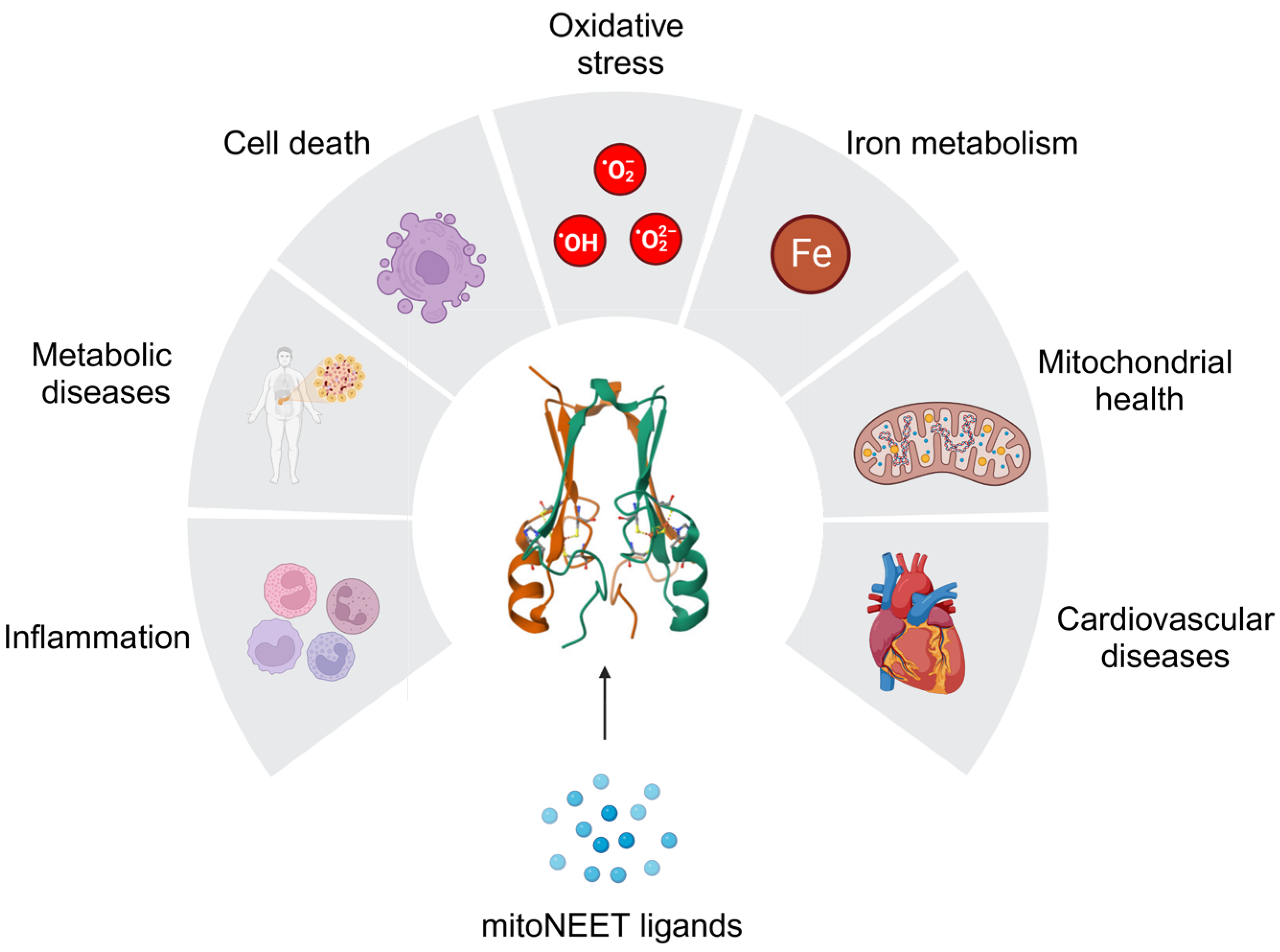

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tam, E.; Sweeney, G. MitoNEET Provides Cardioprotection via Reducing Oxidative Damage and Conserving Mitochondrial Function. Int. J. Mol. Sci. 2024, 25, 480. https://doi.org/10.3390/ijms25010480
Tam E, Sweeney G. MitoNEET Provides Cardioprotection via Reducing Oxidative Damage and Conserving Mitochondrial Function. International Journal of Molecular Sciences. 2024; 25(1):480. https://doi.org/10.3390/ijms25010480
Chicago/Turabian StyleTam, Eddie, and Gary Sweeney. 2024. "MitoNEET Provides Cardioprotection via Reducing Oxidative Damage and Conserving Mitochondrial Function" International Journal of Molecular Sciences 25, no. 1: 480. https://doi.org/10.3390/ijms25010480
APA StyleTam, E., & Sweeney, G. (2024). MitoNEET Provides Cardioprotection via Reducing Oxidative Damage and Conserving Mitochondrial Function. International Journal of Molecular Sciences, 25(1), 480. https://doi.org/10.3390/ijms25010480




