Anti-Algics in the Therapeutic Response of Breast and Urological Cancers
Abstract
:1. Introduction
2. Results
2.1. Las Present Selective Anti-Tumor Effects
2.2. LAs and Dtx Act Synergistically on Metastatic Prostate Cancer Cell Lines
2.3. Combined Therapies Decreased Cell Viability and Increased Cell Death by Necrosis
2.4. Combined Therapies Induce Alteration in Cell Migration
2.5. Clinical Outcomes of Castrate-Resistant Prostate Cancer Patients Treated with Opioids
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Metabolic Activity Assay and Selectivity Index Assessment
4.3. Combination Studies
4.4. Cell Viability and Cell Death Profile Analisys
4.5. Migration Assay
4.6. Observational Study
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Afsharimani, B.; Cabot, P.J.; Parat, M.-O. Morphine Use in Cancer Surgery. Front. Pharmacol. 2011, 2, 46. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Ji, M.; Wang, H.L.; Padhya, T.; Mcmillan, S.C. Cancer Pain and Quality of Life. J. Hosp. Palliat. Nurs. 2019, 21, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Matos, A.C.; Marques, I.A.; Pires, A.S.; Valentim, A.; Abrantes, A.M.; Botelho, M.F. The Potential Effect of Lidocaine, Ropivacaine, Levobupivacaine and Morphine on Breast Cancer Pre-Clinical Models: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 1894. [Google Scholar] [CrossRef] [PubMed]
- Kaye, A.D.; Patel, N.; Bueno, F.R.; Hymel, B.; Vadivelu, N.; Kodumudi, G.; Urman, R.D. Effect of Opiates, Anesthetic Techniques, and Other Perioperative Factors on Surgical Cancer Patients. Ochsner J. 2014, 14, 216–228. [Google Scholar] [PubMed]
- Al-Hashimi, M.; Scott, S.W.M.; Thompson, J.P.; Lambert, D.G. Opioids and Immune Modulation: More Questions than Answers. Br. J. Anaesth. 2013, 111, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Rangel, F.P.; Auler, J.O.C.; Carmona, M.J.C.; Cordeiro, M.D.; Nahas, W.C.; Coelho, R.F.; Simões, C.M. Opioids and Premature Biochemical Recurrence of Prostate Cancer: A Randomised Prospective Clinical Trial. Br. J. Anaesth. 2021, 126, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Barbieri, A.; Rea, D.; Palma, G.; Luciano, A.; Cuomo, A.; Arra, C.; Izzo, F. Morphine Promotes Tumor Angiogenesis and Increases Breast Cancer Progression. BioMed Res. Int. 2015, 2015, 161508. [Google Scholar] [CrossRef]
- Nguyen, J.; Luk, K.; Vang, D.; Soto, W.; Vincent, L.; Robiner, S.; Saavedra, R.; Li, Y.; Gupta, P.; Gupta, K. Morphine Stimulates Cancer Progression and Mast Cell Activation and Impairs Survival in Transgenic Mice with Breast Cancer. Br. J. Anaesth. 2014, 113 (Suppl. S1), i4–i13. [Google Scholar] [CrossRef]
- Ge, Z.H.; Wang, Z.X.; Yu, T.L.; Yang, N.; Sun, Y.; Hao, C.L.; Sun, L.X. Morphine Improved the Antitumor Effects on MCF-7 Cells in Combination with 5-Fluorouracil. Biomed. Pharmacother. 2014, 68, 299–305. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, Y.; Pan, R.; Ding, K.; Chen, R.; Meng, Q. Mechanisms of Cancer Inhibition by Local Anesthetics. Front. Pharmacol 2021, 12, 770694. [Google Scholar] [CrossRef] [PubMed]
- Biki, B.; Mascha, E.; Moriarty, D.C.; Fitzpatrick, J.M.; Sessler, D.I.; Buggy, D.J. Anesthetic Technique for Radical Prostatectomy Surgery Affects Cancer Recurrence a Retrospective Analysis. 2008. Available online: www.cancer.org/downloads/STT/CAFF2007PWSecured.pdf (accessed on 21 May 2022).
- Zhang, L.; Hu, R.; Cheng, Y.; Wu, X.; Xi, S.; Sun, Y.; Jiang, H. Lidocaine Inhibits the Proliferation of Lung Cancer by Regulating the Expression of GOLT1A. Cell Prolif. 2017, 50, e12364. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Dan, J.; Li, F.; Wang, L. Suppression of Mitochondrial Respiration with Local Anesthetic Ropivacaine Targets Breast Cancer Cells. J. Thorac. Dis. 2018, 10, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Vellky, J.E.; Ricke, W.A. Development and Prevalence of Castration-Resistant Prostate Cancer Subtypes. Neoplasia 2020, 22, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 1988. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. Effects of Local Anesthetics on Cancer Cells. Pharmacol. Ther. 2020, 212, 107558. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Piroli, A.; Marinangeli, F.; D’Angelo, M.; Benedetti, E.; Ippoliti, R.; Zis, P.; Varrassi, G.; Giordano, A.; Paladini, A.; et al. Local Anesthetics Counteract Cell Proliferation and Migration of Human Triple-Negative Breast Cancer and Melanoma Cells. J. Cell Physiol. 2019, 235, 3474–3484. [Google Scholar] [CrossRef]
- Jose, C.; Hebert-chatelain, E.; Dias, N.; Roche, E.; Obre, E.; Lacombe, D.; Reza, H.; Pourquier, P. Redox Biology Redox Mechanism of Levobupivacaine Cytostatic e Ff Ect on Human Prostate Cancer Cells. Redox. Biol. 2018, 18, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, L.; Zhao, H.; Wu, L.; Masters, J.; Han, C.; Hirota, K.; Ma, D. Both Bupivacaine and Levobupivacaine Inhibit Colon Cancer Cell Growth but Not Melanoma Cells In Vitro. J. Anesth. 2019, 33, 17–25. [Google Scholar] [CrossRef]
- Li, R.; Xiao, C.; Liu, H.; Huang, Y.; Dilger, J.P.; Lin, J. Effects of Local Anesthetics on Breast Cancer Cell Viability and Migration. BMC Cancer 2018, 18, 666. [Google Scholar] [CrossRef]
- Laniado, M.E.; Lalani, E.N.; Fraser, S.P.; Grimes, J.A.; Bhangal, G.; Djamgoz, M.B.A.; Abel, P.D. Expression and Functional Analysis of Voltage-Activated Na+ Channels in Human Prostate Cancer Cell Lines and Their Contribution to Invasion In Vitro. Am. J. Pathol. 1997, 150, 1213. [Google Scholar]
- Grimes, J.A.; Fraser, S.P.; Stephens, G.J.; Downing, J.E.G.; Laniado, M.E.; Foster, C.S.; Abel, P.D.; Djamgoz, M.B.A. Differential Expression of Voltage-Activated Na+ Currents in Two Prostatic Tumour Cell Lines: Contribution to Invasiveness In Vitro. FEBS Lett. 1995, 369, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B.A.; Fraser, S.P.; Brackenbury, W.J. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 2019, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Djamgoz, M.B. Biophysics of Cancer: Cellular Excitability (“CELEX”) Hypothesis of Metastasis. Clin. Exp. Oncol. 2014, S1, 005. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Canta, A.; Chiorazzi, A. Chemotherapy-Induced Peripheral Neuropathy: What Do We Know about Mechanisms? Neurosci. Lett. 2015, 596, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme. Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, J.; Han, X. Lidocaine Sensitizes the Cytotoxicity of Cisplatin in Breast Cancer Cells via Up-Regulation of RARβ2 and RASSF1A Demethylation. Int. J. Mol. Sci. 2014, 15, 23519–23536. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, Z.; Xu, H.; Ma, C. Lidocaine Alleviates Cytotoxicity-Resistance in Lung Cancer A549/DDP Cells via down-Regulation of MiR-21. Mol. Cell Biochem. 2019, 456, 63–72. [Google Scholar] [CrossRef]
- Chen, D.; Yan, Y.; Xie, J.; Pan, J.; Chen, Y.; Li, Q.; Yuan, Y.; Zeng, W.; Xing, W. Amide-Type Local Anesthetics May Suppress Tumor Cell Proliferation and Sensitize Human Hepatocellular Carcinoma Cells to Cisplatin via Upregulation of RASSF1A Expression and Demethylation. J. Cancer 2020, 11, 7312–7319. [Google Scholar] [CrossRef]
- Xing, W.; Chen, D.T.; Pan, J.H.; Chen, Y.H.; Yan, Y.; Li, Q.; Xue, R.F.; Yuan, Y.F.; Zeng, W.A. Lidocaine Induces Apoptosis and Suppresses Tumor Growth in Human Hepatocellular Carcinoma Cells In Vitro and in a Xenograft Model In Vivo. Anesthesiology 2017, 126, 868–881. [Google Scholar] [CrossRef]
- Zhang, X.; Pang, W.; Liu, H.; Wang, J. Lidocine Potentiates the Cytotoxicity of 5-Fluorouracil to Choriocarcinoma Cells by Downregulating ABC Transport Proteins Expression. J. Cell Biochem. 2019, 120, 16533–16542. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, J.; Liu, W.; Zhang, R.; Huang, S.; Xing, Y. Lidocaine Sensitizes the Cytotoxicity of 5-Fluorouacil in Melanoma Cells via Upregulation of MicroRNA-493. Pharmazie 2017, 72, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.; Gong, X.; Li, D.; Zhu, G.; Wang, L.; Li, F. Inhibition of Gastric Cancer by Local Anesthetic Bupivacaine through Multiple Mechanisms Independent of Sodium Channel Blockade. Biomed. Pharmacother. 2018, 103, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, L.; Li, M.; Yan, L.; Zhang, S.; Mi, Z.; Ren, L.; Xu, J. Lidocaine Enhances the Effects of Chemotherapeutic Drugs against Bladder Cancer. Sci. Rep. 2018, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, L.; Dan, J.; Zhu, Q. Differential Effects and Mechanisms of Local Anesthetics on Esophageal Carcinoma Cell Migration, Growth, Survival and Chemosensitivity. BMC Anesthesiol. 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.I.d.A.L. Local Anesthetics as a New Therapeutic Approach in Oral Squamous Cell Carcinoma—An In Vitro Study. Master’s Thesis, Universidade de Coimbra, Coimbra, Portugal, 2016. [Google Scholar]
- Zheng, Q.; Peng, X.; Zhang, Y. Cytotoxicity of Amide-Linked Local Anesthetics on Melanoma Cells via Inhibition of Ras and RhoA Signaling Independent of Sodium Channel Blockade. BMC Anesthesiol. 2020, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Liu, S.-T.; Huang, S.-M.; Wu, Z.-F. Apoptosis, Proliferation, and Autophagy Are Involved in Local Anesthetic-Induced Cytotoxicity of Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15455. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, J.; Wang, W.; Zhou, H.; Cai, S.; Zhu, L.; Qian, X.; Wang, J.; Lu, Z.; Huang, C. The Impact of Pain and Opioids Use on Survival in Cancer Patients: Results from a Population-Based Cohort Study and a Meta-Analysis. Medicine 2020, 99, e19306. [Google Scholar] [CrossRef]
- Zylla, D.; Gourley, B.L.; Vang, D.; Jackson, S.; Boatman, S.; Lindgren, B.; Kuskowski, M.A.; Le, C.; Gupta, K.; Gupta, P. Opioid Requirement, Opioid Receptor Expression, and Clinical Outcomes in Patients with Advanced Prostate Cancer. Cancer 2013, 119, 4103–4110. [Google Scholar] [CrossRef]
- Zylla, D.; Steele, G.; Gupta, P. A Systematic Review of the Impact of Pain on Overall Survival in Patients with Cancer. Support. Care Cancer 2017, 25, 1687–1698. [Google Scholar] [CrossRef]
- Liu, H. A Clinical Mini-Review: Clinical Use of Local Anesthetics in Cancer Surgeries. Gaz. Med. Sci. 2020, 1, 30–34. [Google Scholar] [CrossRef]
- Almeida-Ferreira, C.; Silva-Teixeira, R.; Laranjo, M.; Almeida, N.; Brites, G.; Dias-Ferreira, J.; Marques, I.; Neves, R.; Serambeque, B.; Teixo, R.; et al. Open-Air Cold Plasma Device Leads to Selective Tumor Cell Cytotoxicity. Appl. Sci. 2021, 11, 4171. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective Cytotoxic Activities of Two Novel Synthetic Drugs on Human Breast Carcinoma MCF-7 Cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Tavares-da-Silva, E.J.; Varela, C.L.; Pires, A.S.; Encarnação, J.C.; Abrantes, A.M.; Botelho, M.F.; Carvalho, R.A.; Proença, C.; Freitas, M.; Fernandes, E.; et al. Combined Dual Effect of Modulation of Human Neutrophils’ Oxidative Burst and Inhibition of Colon Cancer Cells Proliferation by Hydroxycinnamic Acid Derivatives. Bioorg. Med. Chem. 2016, 24, 3556–3564. [Google Scholar] [CrossRef]
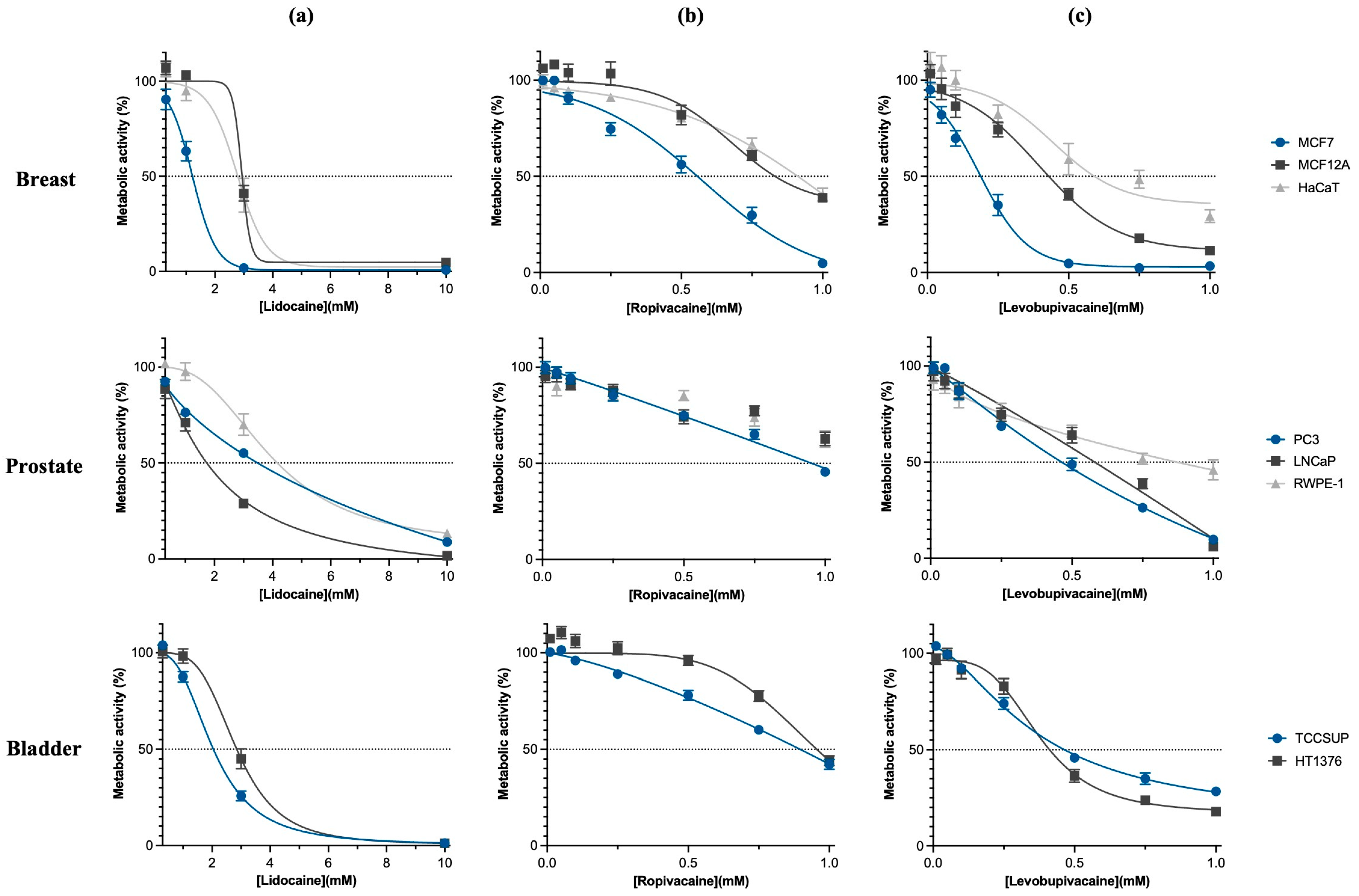

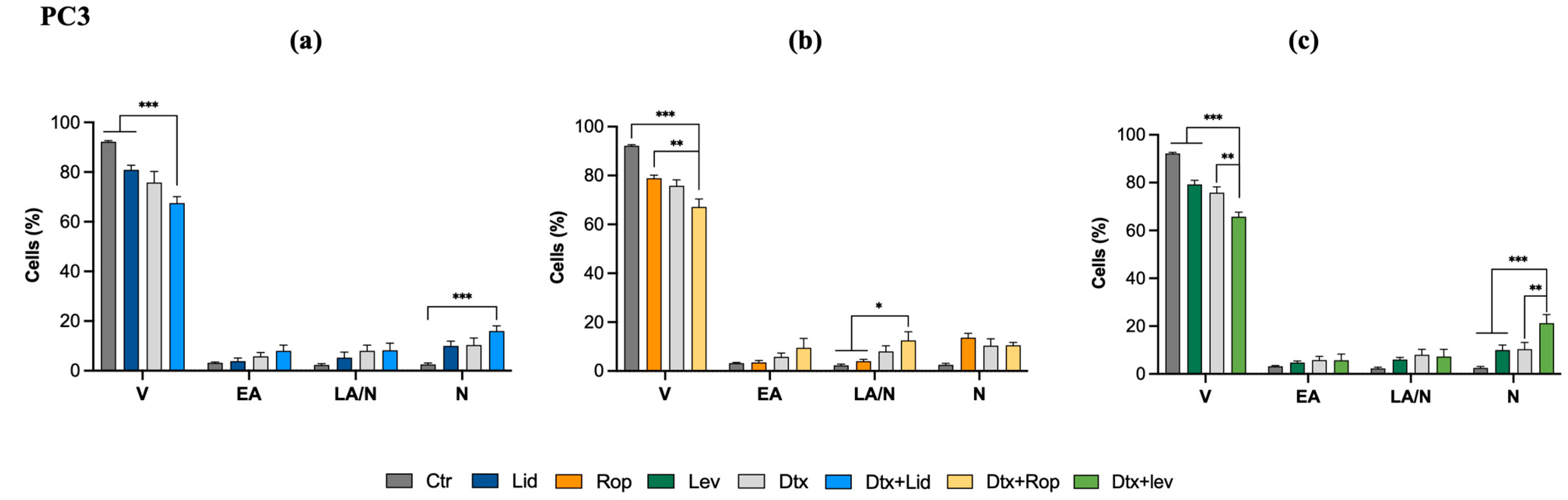
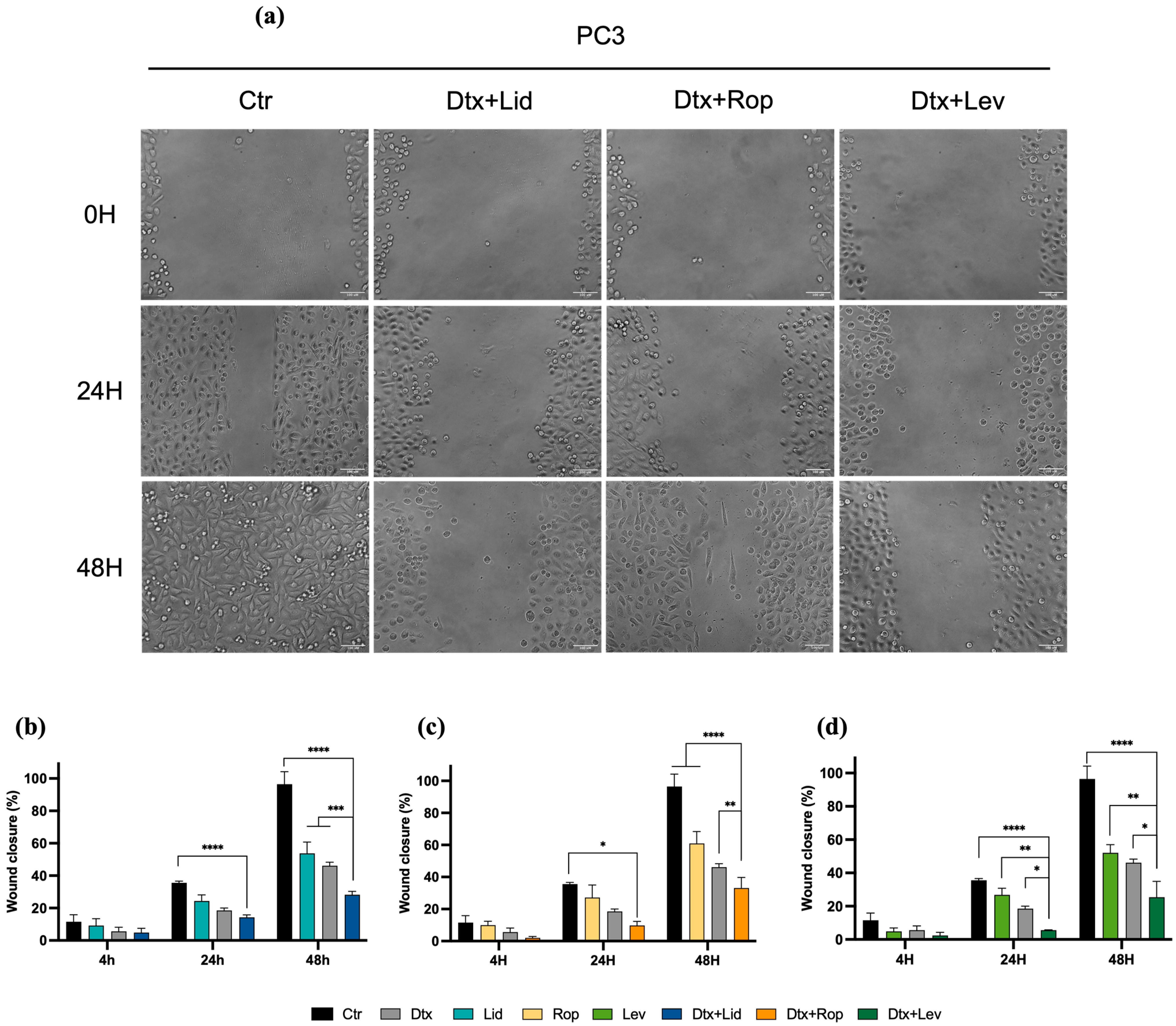
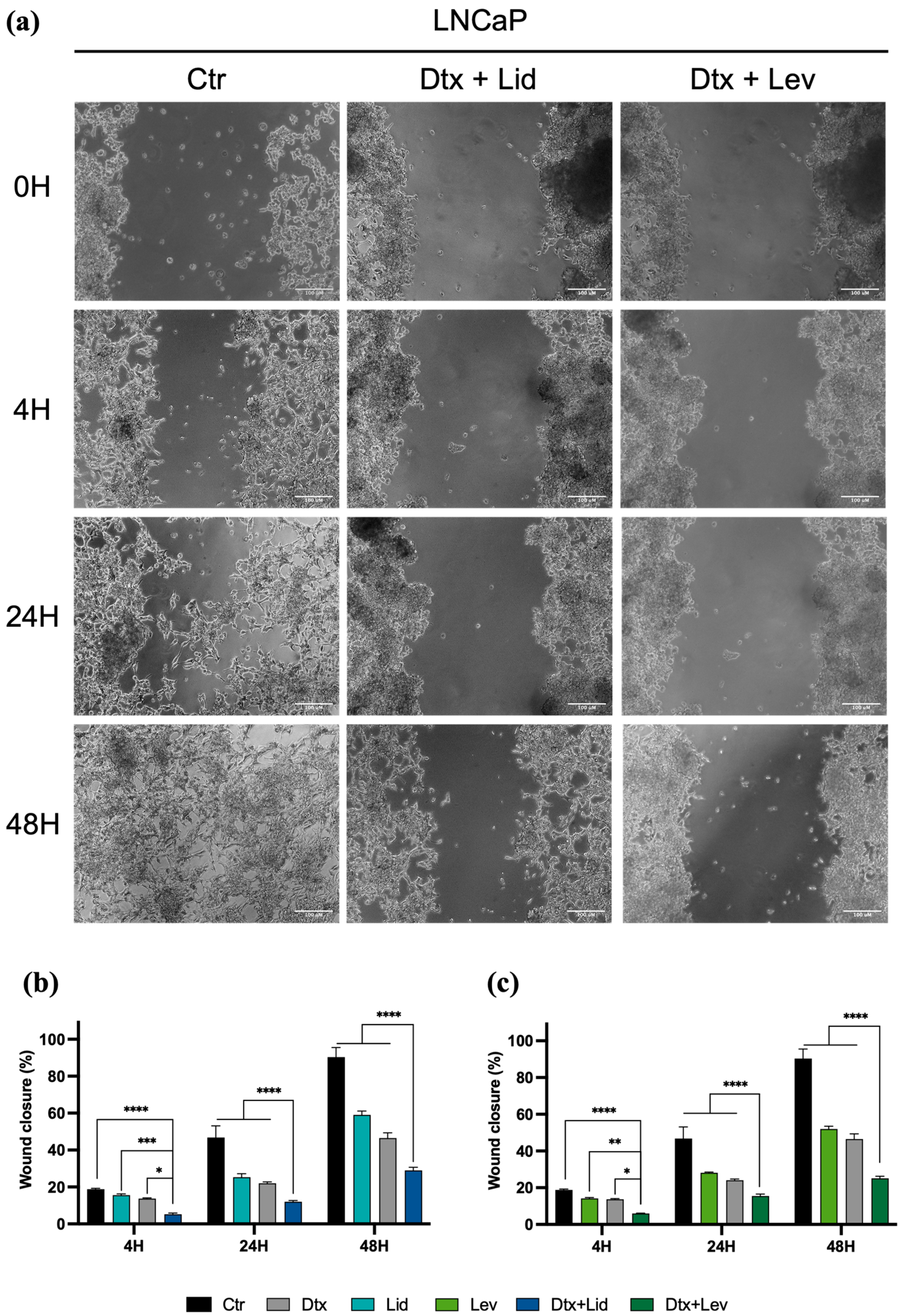
| Time (h) | Lidocaine | Ropivacaine | Levobupivacaine | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (mM) | R2 | 95% CoI (mM) | IC50 (mM) | R2 | 95% CoI (mM) | IC50 (mM) | R2 | 95% CoI (mM) | ||
| Human cancer cell lines | ||||||||||
| MCF7 | 24 | 1.87 | 0.94 | [1.67; 2.11] | 0.90 | 0.96 | [0.84; 0.96] | 0.35 | 0.88 | [0.31; 0.39] |
| 48 | 1.16 | 0.99 | [0.99; 1.34] | 0.55 | 0.97 | [0.47; 0.60] | 0.16 | 0.91 | [0.14; 0.19] | |
| 72 | 0.91 | 0.97 | [0.84; 0.98] | 0.42 | 0.84 | [0.37; 0.46] | 0.11 | 0.88 | [0.10; 0.13] | |
| LNCaP | 48 | 1.74 | 0.99 | [1.48; 2.06] | a | 0.56 | 0.95 | [0.51; 0.61] | ||
| PC3 | 48 | 3.17 | 0.98 | [2.90; 3.50] | 0.99 | 0.98 | [0.89; 1.15] | 0.40 | 0.97 | [0.37; 0.44] |
| HT1376 | 48 | 1.99 | 0.98 | [1.81; 2.18] | 0.86 | 0.99 | [0.81; 0.92] | 0.47 | 0.99 | [0.46; 0.54] |
| TCCSUP | 48 | 1.84 | 0.99 | [2.82; 2.85] | 0.99 | 0.99 | [0.94; 1.06] | 0.39 | 0.97 | [0.36; 0.42] |
| Human normal cell lines | ||||||||||
| RWPE-1 | 48 | 4.25 | 0.99 | [4.02; 4.72] | a | 0.86 | 0.99 | [0.76; 1.06] | ||
| MCF12A | 48 | 2.69 | 0.99 | [2.60; 2.99] | 0.86 | 0.99 | [0.82; 0.90] | 0.42 | 0.98 | [0.36; 0.45] |
| HaCaT | 48 | 2.55 | 0.99 | [2.21; 3.08] | 0.90 | 0.98 | [0.84; 0.98] | 0.64 | 0.99 | [0.54; 0.73] |
| Drug/Combination | PC3 | LNCaP | ||||||
|---|---|---|---|---|---|---|---|---|
| IC50 (nM) | R2 | 95% CoI (nM) | DRI | IC50 (nM) | R2 | 95% CoI (nM) | DRI | |
| Dtx | 82.79 | 0.96 | [66.84; 104.7] | - | 68.40 | 0.93 | [43.60; 95.43] | - |
| Dtx/Lid | 0.54 | 0.93 | [0.36; 0.73] | 153.31 | 3.59 | 0.93 | [1.52; 7.87] | 19.05 |
| Dtx/Rop | 8.50 | 0.86 | [5.64; 12.30] | 9.74 | - 1 | - | - | - |
| Dtx/Lev | 4.61 | 0.88 | [2.89; 7.01] | 17.96 | 0.003 | 0.94 | [0.0005; 0.01] | 22,800 |
| Variable | Total, N = 165 | Non-Opioid Group, N = 103 | Opioid Group, N = 62 | p-Value a |
|---|---|---|---|---|
| Age (Years; mean ± SD) | 74.4 ± 9.6 | 74.0 ± 9.8 | 74.9 ± 9.3 | NS |
| CHAARTED | <0.001 | |||
| Low volume | 69 (50.7%) | 51 (37.5%) | 18 (13.2%) | |
| High volume | 67 (49.3%) | 28 (20.6%) | 39 (28.7%) | |
| Missing | 29 (17.6%) | 24 (23.3%) | 5 (8.1%) | |
| LATITUDE | <0.001 | |||
| Low risk | 88 (67.7%) | 63 (48.5%) | 25 (19.2%) | |
| High risk | 42 (32.3%) | 14 (10.8%) | 28 (21.5%) | |
| Missing | 35 (21.2%) | 26 (25.2%) | 9 (14.5%) | |
| Stage | NS | |||
| MHSPC | 48 (29.3%) | 38 (23.2%) | 10 (6.1%) | |
| MCRPC | 116 (70.7%) | 64 (39.0%) | 52 (31.7%) | |
| Missing | 1 (0.6%) | 1 (1.0%) | 0 (0.0%) | |
| Histology | NS | |||
| ISUP1 | 14 (10.5%) | 10 (7.5%) | 4 (3.0%) | |
| ISUP2 | 27 (20.3%) | 19 (14.3%) | 8 (6.0%) | |
| ISUP3 | 48 (36.1%) | 33 (24.8%) | 15 (11.3%) | |
| ISUP4 | 14 (10.6%) | 7 (5.3%) | 7 (5.3%) | |
| ISUP5 | 30 (22.5%) | 14 (10.5%) | 16 (12.0%) | |
| Missing | 32 (19.4%) | 20 (19.4%) | 12 (19.4%) | |
| Local Treatment | NS | |||
| No | 77 (57.5%) | 43 (32.1%) | 34 (25.4%) | |
| Yes | 57 (42.5%) | 40 (29.8%) | 17 (12.7%) | |
| Missing | 31 (18.8%) | 20 (19.4%) | 11 (17.7%) | |
| Therapeutic Line | ||||
| First | 86 (57.3%) | 69 (46.0%) | 17 (11.3%) | <0.001 |
| Second | 41 (27.3%) | 16 (10.7%) | 25 (16.7%) | |
| Third | 23 (15.3%) | 6 (4.0%) | 17 (11.3%) | |
| Missing | 15 (9.1%) | 12 (11.7%) | 3 (4.8%) | |
| Death | <0.001 | |||
| No | 129 (78.2%) | 98 (59.4%) | 31 (18.8%) | |
| Yes | 36 (21.8%) | 5 (3.0%) | 31 (18.8%) | |
| Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Univariate Analysis | Multivariate Model, n = 181 | |||||
|---|---|---|---|---|---|---|
| Independent Variables | HR | CI (95%) | p-Value | HR | CI (95%) | p-Value |
| Age, n = 209 | 0.999 | [0.983, 1.017] | 0.938 | |||
| CHAARTED, n = 189 | ||||||
| Low-volume, n = 83 | 1 | -- | -- | 1 | -- | -- |
| High-volume, n = 106 | 2.518 | [1.687, 3.756] | <0.001 | 1.936 | [1.174, 3.195] | 0.010 |
| LATITUDE, n = 184 | ||||||
| Low-risk, n = 114 | 1 | -- | -- | 1 | -- | -- |
| High-risk, n = 70 | 1.691 | [1.175, 2.435] | 0.004 | 0.891 | [0.544, 1.460] | 0.647 |
| Stage, n = 209 | ||||||
| CPHSM, n = 50 | 1 | -- | -- | |||
| CPRCM, n = 159 | 1.455 | [0.831, 2.546] | 0.189 | |||
| Histology, n = 180 | ||||||
| ISUP1, n = 16 | 1 | -- | -- | |||
| ISUP2, n = 31 | 0.848 | [0.361, 1.992] | 0.706 | |||
| ISUP3, n = 69 | 0.741 | [0.356, 1.541] | 0.422 | |||
| ISUP4, n = 21 | 0.835 | [0.360, 1.939] | 0.675 | |||
| ISUP5, n = 43 | 1.005 | [0.500, 2.020] | 0.988 | |||
| Local treatment, n = 188 | ||||||
| No, n = 113 | 1 | -- | -- | |||
| Yes, n = 75 | 0.809 | [0.557, 1.175] | 0.266 | |||
| Opioid, n = 209 | ||||||
| No, n = 108 | 1 | -- | -- | 1 | -- | -- |
| Yes, n = 101 | 3.069 | [2.034, 4.632] | <0.001 | 2.399 | [1.467, 3.925] | <0.001 |
| Concordance = 0.705 (very good) | ||||||
| Drug | Plasmatic Concentration (μM) | Local Infiltration Concentration (μM) |
|---|---|---|
| Lidocaine | 10 | 17,500 (0.5%) |
| Ropivacaine | 3.5 | 7288 (0.2%) |
| Levobupivacaine | 2.5 | 8667 (0.25%) |
| Cell Line | PC3 | LNCaP | ||
|---|---|---|---|---|
| Condition | [LA] (mM) | [Dtx] (nM) | [LA] (mM) | [Dtx] (nM) |
| Control | 0 | 0 | 0 | 0 |
| Lid | 3.17 | 0 | 1.74 | 0 |
| Rop | 0.99 | 0 | a | a |
| Lev | 0.40 | 0 | 0.56 | 0 |
| Dtx | 0 | 50 | 0 | 10 |
| Dtx/Lid | 3.17 | 50 | 1.74 | 10 |
| Dtx/Rop | 0.99 | 50 | a | a |
| Dtx/Lev | 0.40 | 50 | 0.56 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, A.C.; Lorigo, J.; Marques, I.A.; Abrantes, A.M.; Jóia-Gomes, M.; Sa-Couto, P.; Gonçalves, A.C.; Valentim, A.; Tavares-Silva, E.; Figueiredo, A.; et al. Anti-Algics in the Therapeutic Response of Breast and Urological Cancers. Int. J. Mol. Sci. 2024, 25, 468. https://doi.org/10.3390/ijms25010468
Matos AC, Lorigo J, Marques IA, Abrantes AM, Jóia-Gomes M, Sa-Couto P, Gonçalves AC, Valentim A, Tavares-Silva E, Figueiredo A, et al. Anti-Algics in the Therapeutic Response of Breast and Urological Cancers. International Journal of Molecular Sciences. 2024; 25(1):468. https://doi.org/10.3390/ijms25010468
Chicago/Turabian StyleMatos, Ana Catarina, João Lorigo, Inês Alexandra Marques, Ana Margarida Abrantes, Matilde Jóia-Gomes, Pedro Sa-Couto, Ana Cristina Gonçalves, Ana Valentim, Edgar Tavares-Silva, Arnaldo Figueiredo, and et al. 2024. "Anti-Algics in the Therapeutic Response of Breast and Urological Cancers" International Journal of Molecular Sciences 25, no. 1: 468. https://doi.org/10.3390/ijms25010468
APA StyleMatos, A. C., Lorigo, J., Marques, I. A., Abrantes, A. M., Jóia-Gomes, M., Sa-Couto, P., Gonçalves, A. C., Valentim, A., Tavares-Silva, E., Figueiredo, A., Pires, A. S., & Botelho, M. F. (2024). Anti-Algics in the Therapeutic Response of Breast and Urological Cancers. International Journal of Molecular Sciences, 25(1), 468. https://doi.org/10.3390/ijms25010468











