Abstract
Atherosclerosis is a chronic inflammatory disease driven by lipid accumulation in the arteries, leading to narrowing and thrombosis that causes mortality. Emerging evidence has confirmed that atherosclerosis affects younger people and is involved in the majority of deaths worldwide. EVs are associated with critical steps in atherosclerosis, cholesterol metabolism, immune response, endothelial dysfunction, vascular inflammation, and remodeling. Endothelial cell-derived EVs can interact with platelets and monocytes, thereby influencing endothelial dysfunction, atherosclerotic plaque destabilization, and the formation of thrombus. EVs are potential diagnostic and prognostic biomarkers in atherosclerosis (AS) and cardiovascular disease (CVD). Importantly, EVs derived from stem/progenitor cells are essential mediators of cardiogenesis and cardioprotection and may be used in regenerative medicine and tissue engineering.
1. Introduction
Atherosclerosis is a chronic inflammatory disease driven by lipid accumulation in the arteries, leading to narrowing and thrombosis that causes mortality [1]. Emerging evidence has confirmed that AS affects younger people and is involved in the majority of deaths worldwide [2,3]. Vascular inflammation contributes to atherogenesis by activating molecular and cellular pathways and plays a key role in atherosclerosis plaques, which are unstable and can rupture, leading to stroke or myocardial infarction (MI) [4]. The molecular mechanisms associated with thrombotic complications of atherosclerosis have evolved far beyond the ‘vulnerable plaque’ concept [5]. This advance in knowledge has opened a path to efficient therapeutic interventions promising an improvement in the prevention and treatment of CVD [6]. However, the modulation of inflammatory components in atherosclerosis by gene therapy and targeting the effects on plaques remain as difficult challenges [7]. Extracellular vesicles (EVs) are lipid bilayer-enclosed structures secreted by endothelial cells, immune cells, and other cardiovascular tissues [8]. They are important mediators of intercellular and extracellular communications because they carry protein, DNA, mRNA, miRNA, non-coding RNA, lipids, and other bioactive molecules and deliver this genetic information to target cells [9,10]. miRNAs play a pivotal role in cardiogenesis, heart regeneration, and the pathophysiology of CVD [11]. miRNA transfer is important in cardiovascular systems and diseases because it modulates atherosclerosis [12]. The activation of cellular changes and the modulation of disease phenotypes are caused by EV miRNA transfer [13]. EVs can modulate phenotypes and cellular functions by regulating epigenetic alteration, transcription, and translation [14,15]. They have potential as biomarkers in diagnosis and prognosis as well as monitoring therapy in individuals with CVD and as new therapeutic targets and/or drug delivery vehicles [16,17,18].
1.1. Minimal Information for Studies of EVs (MISEV)
The International Society for Extracellular Vesicles (ISEV) has developed MISEV standardization in the EV research community [19,20]. In 2014, the MISEV2014 was provided, covering EV separation and isolation, characterization, and functional studies, and it was updated in 2018 (MISEV2018). The updated MISEV2018 offers vital suggestions, emphasizing precise terminology, characterization, and transparent reporting. In brief, MISEV18 describes “extracellular vesicle” as the preferred term and states that subtypes should be defined by their biochemical and physical characteristics and/or sources and conditions. Another conclusion is that diverse EV separation techniques require detailed reporting for replicability. Due to the development of EV characterization possibilities, protein and lipid markers seem to be crucial for demonstrating generic EV structure, adapted according to cellular origin and claim specificity. The authors also underline that demonstrating EV-associated functions demands rigorous evidence, emphasizing independence from cell–cell contact and differentiation from soluble factors. It is worth mentioning that the ISEV endorses the EV-TRACK knowledgebase, aligning with the MISEV for enhanced rigor [21,22].
1.2. Extracellular Vesicle (EV) Structure and Functions
EVs include exosomes, apoptotic bodies (ABs), and microvesicles (MVs), which play a crucial role in physiology and pathology [23]. They can be released by most cell types and detected in most body fluids, like plasma, urine, and saliva [24]. EVs have immunoregulatory functions in innate and adaptive immunity, including inflammation, antigen presentation, and the development and activation of B and T cells [25,26]. EVs have natural stability in circulation, biocompatibility, low toxicity, and low immunogenicity [27]. EVs contain tetraspanins (CD9, CD63, and CD81), membrane transport and fusion proteins (GTPases, annexins, and flotillin), heat shock proteins (Hsp60, Hsp70, and Hsp90), proteins involved in multivesicular body biogenesis (Alix andTSG101), cytoskeletal proteins (actin, cofilin, and tubulin), adhesion molecules (integrin-α and -β and P-selectin), glycoproteins (β-galactosidase, O-linked glycans, and N-linked glycans), growth factors and cytokines (TNF-α, TGF-β, TNF-related apoptosis-inducing ligand (TRAIL), and other signaling receptors (Fas ligand (FasL), TNF receptor, and transferrin receptor (TfR)) [28] (Figure 1).
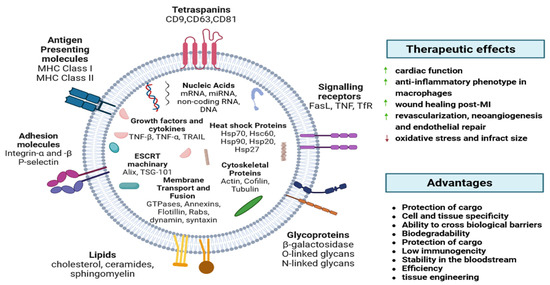
Figure 1.
EVs’ exterior and interior components. Major histocompatibility complex (MHC), heat shock proteins (Hsp), tumor susceptibility gene (TSG), tumor necrosis factor (TNF), TNF-related apoptosis-inducing ligand (TRAIL), Fas ligand (FasL), transferrin receptor (TfR) [28].
2. EVs in Atherosclerosis and Cardiovascular Diseases
Atherosclerosis is an inflammatory process of the small- and medium-sized arteries and is primarily responsible for most CVD. AS is characterized by the accumulation of lipids due to impaired cholesterol metabolism, fibrous elements, and vascular calcification (VC) in the arteries. This process occurs due to inflammation, the oxidation of LDL particles, endothelial dysfunction, foam cell formation, proliferation, and the migration of vascular smooth muscle cells (VSMCs) [29]. The preceding vascular modifications are also affected by extracellular matrix molecules, soluble factors, and interactions between endothelial cells (EC) and EVs [30]. EVs are associated with critical steps in atherosclerosis, cholesterol metabolism, immune response endothelial dysfunction, and vascular inflammation and remodeling [31,32]. Recent studies have focused on EVs’ contents and biological functions, particularly their potential effects on atherosclerosis and the associated cholesterol metabolism [31].
Endothelial- and platelet-derived EVs have been shown to be independent biomarkers for coronary artery disease (CAD) [33,34,35,36]. Patients with endothelial dysfunction or atherosclerosis have demonstrated increased levels of EVs levels, which suggests that they are potential disease markers [37,38,39]. Moreover, it has also been proven that EC-derived EVs can affect the initial steps of atherosclerosis by aggravating endothelial barrier dysfunction, which leads to the transfer of Src kinase and the subsequent destabilization of tight junction proteins [36,40]. Platelet-derived EVs interact with subendothelial elements, so they participate in the phenotypic modulation of immune and vascular cells [41]. Regarding macrophages, studies have shown that platelet EVs can reduce macrophage reactivity by altering their differentiation into the M2 phenotype [42,43]. Additionally, these EVs hinder the production of nitric oxide (NO) in ECs, which worsens endothelial function [44]. Oxidized lipoproteins can modify the cargo of EVs produced by ECs. This alteration promotes the inclusion of proinflammatory substances, triggering the expression of adhesion molecules like intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) [45,46,47,48] (Figure 2).
EVs can interact with platelets and monocytes, thereby influencing endothelial dysfunction, atherosclerotic plaque destabilization, and the formation of thrombous [49,50,51,52,53]. Plaque ruptures are mainly caused by fibrous cap weakening and the destabilization of atherosclerotic plaque caps by circulating EVs [54]. The breakdown of the extracellular matrix and the destabilization of plaques have been attributed to MMPs which are carried by EVs as their cargo [55,56]. EC-derived EVs have been shown to carry MMP-2, MMP-9, MMP-10, and MMP-14 on their external surface [57,58,59]. The cell source of EVs can affect their proteolytic cargo and fibrous cap integrity. For example, microvesicles isolated from atherosclerotic lesions express the metalloprotease TNF-α-converting enzyme (TACE/ADAM-17) [60,61]. Investigations have also detected the existence of EVs within calcified plaques and demonstrated their participation in the associated biological processes [62,63,64,65]. Endothelial EVs have been documented to exhibit elevated levels of calcium and bone morphogenetic protein 2 (BMP-2), leading to calcification and the induction of an osteogenic phenotype in smooth muscle cells (SMCs) [63].
Endothelial dysfunction, the initial step of atherogenesis, triggers the release of EVs, reflected in exosomal proteins and RNAs [66,67]. Notably, human atherosclerotic plaques have been observed to contain EVs originating from various cell types: leukocytes, macrophages, erythrocytes, lymphocytes, SMCs, and platelets [37,49,50,51,68,69]. Endothelial dysfunction, a stand-alone predictor of vascular disease, was evaluated in patients with stable CAD through quantitative measurements of CD31+/Annexin V+. It was shown that individuals with elevated levels of EVs developed significant cardiovascular or cerebral events afterward [70]. Endothelial cell-derived EVs may regulate the VSMC phenotype via cargos [71,72]. It was confirmed that miR-143/145-containing EVs derived from KLF2-expressing ECs reduced atherosclerotic lesions in ApoE−/− mice [73]. By transferring miR10a, exosomes derived from ECs can modulate monocyte activation [74]. They can also reduce hypertension by regulating the VSMC phenotype via the ACE2/NF-κB/Ang II pathway [75]. Non-coding RNAs (ncRNAs), including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), which are encapsulated in EVs, are involved in AS and VC [76,77]. ncRNAs shuttled via EVs may control post-hypoxia tissue survival and remodeling by regulating cell injury and inflammatory responses in MI and stroke [78,79]. Cardiomyocytes increase the secretion of EVs during stress, such as hypoxia, inflammation, or injury [80]. Cardiac-derived EVs are rich in microRNAs and are key to controlling cellular processes. Importantly, EVs secreted by cardiac progenitor cells (CPCs) and cardiosphere-derived cells are essential mediators of cardiogenesis and cardioprotection. Therefore, EVs from stem cells could exert a therapeutic effect on the damaged heart [81].
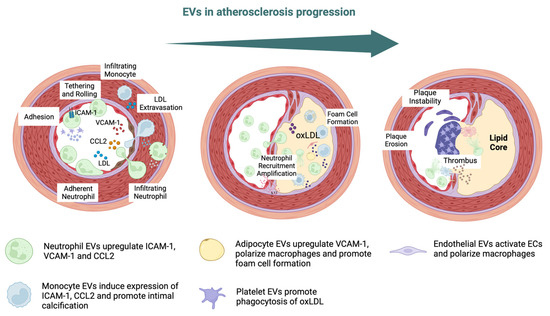
Figure 2.
EVs in atherosclerosis progression. Intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), chemokine C–C motif chemokine ligand 2 (CCL2), low-density lipoprotein (LDL), oxidized LDL (oxLDL) [48,82].
3. EVs as Biomarkers and Therapeutic Targets in AS and CVD
EVs have potential as prognostic and diagnostic biomarkers of atherosclerosis initiation, progression, and complications [83]. EVs shield their molecular cargo from degradation, contributing to the progression of plaques and making them an excellent therapeutic target [84]. EVs’ presence in early and advanced plaques indicates their involvement in both the initial and later stages of plaque formation in humans, providing a chance to use them as progression markers [49,50,85]. Evaluating vascular well-being through EVs may offer valuable insights for preventing atherosclerosis in individuals who show no symptoms but have early signs of the disease [86,87]. For instance, EVs originating from ECs reflect endothelial impairment and indicate potential cardiovascular results [35,70,88] (Figure 3).
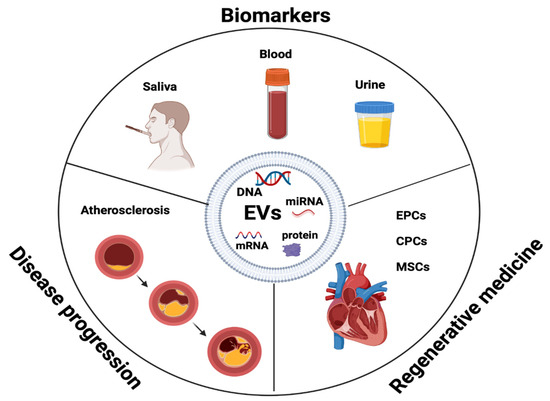
Figure 3.
EVs as biomarkers in disease progression and regenerative medicine. Endothelial progenitor cells (EPCs), cardiac progenitor cells (CPCs), mesenchymal stem cells (MSCs).
Increased levels of EVs are linked to risk factors like smoking, diabetes, and high cholesterol [89]. Exosomes are valuable prognostic and diagnostic biomarkers in CVD [90]. Elevated levels of specific categories of procoagulant EVs have been observed in individuals with acute coronary syndrome (ACS) compared with healthy individuals, and this phenomenon is linked to plaque rupture [34,91,92,93,94,95,96,97,98,99,100]. EV levels in the blood, urine, and saliva have been linked to clinical risk in patients with stable CVD [33]. Table 1 presents EVs as potential markers in cardiovascular diseases [34,35,70,94,100,101,102,103,104,105,106,107,108,109,110,111,112].

Table 1.
EVs as markers for diagnosing and predicting the progression of atherosclerosis.
According to the revascularization success rate achieved in the culprit artery through PCI, there is an overall reduction in the levels of circulating EVs 72 h after a STEMI (ST elevation myocardial infarction). According to the successful rate of PCI revascularization in the culprit artery, there is a global decrease in circulating EVs 72 h after STEMI [86,92].
4. miRNA as a Biomarker in AS and CVD
miRNA is a class of small regulatory RNAs that regulate protein expression in recipient cells in a paracrine fashion. A promising biomarker for AS diagnosis, related to the occurrence of adverse cardiovascular events, is miR-18a-5p [114]. Also, serum miR-211-5p and miR-675-3p are new diagnostic biomarkers that are associated with the poor prognosis of AS [115]. miR-637 showed important predictive value for the occurrence of future cardiovascular events [116]. Circulating miRNAs, such as miR-17, miR-17-5p, miR-29b, miR-30, miR-92a, miR-126, miR-143, miR-145, miR-146a, miR-212, miR-218, miR-221, miR-222, and miR- 361-5p, are promising biomarkers for the clinical diagnosis of atherosclerosis [117]. Furthermore, miR-122-5p and miR-223-3p might be markers of plaque instability [118].
Importantly, exosomal miRNAs play an important role in the pathophysiology of CAD and acute myocardial infarction (AMI). miR-942-5p, miR-149-5p, and miR-32-5p may serve as potential diagnostic biomarkers for stable coronary artery disease (SCAD) [119]. miR-1915-3p, miR-4,507, and miR-3,656 were significantly less expressed in AMI samples than in SCAD [120]. Exosomal miR-152-5p and miR-3681-5p may serve as potential biomarkers for ST-segment elevation MI [121]. Also, MV miR-126 and miR-199a expression can predict the occurrence of cardiovascular (CV) events in patients with SCAD [110] Figure 4.
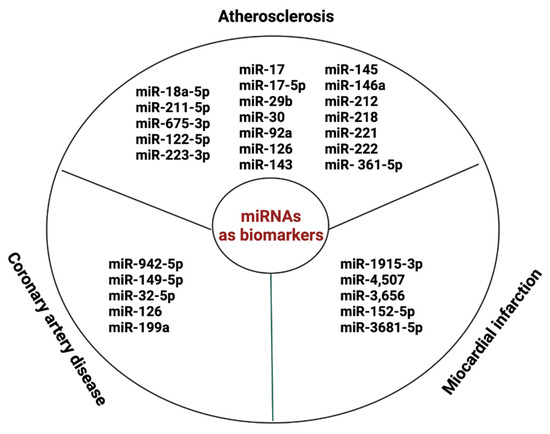
Figure 4.
miRNAs as biomarkers in AS and CVD.
On the other hand, EV microRNAs are key players in cardiac regeneration and have cardioprotective and regenerative properties for cardiac and non-cardiac cells, stem cells, and progenitor cells [80,122]. It was found that exosomal microRNAs may deliver signals to mediate cardiac repair after MI [123]. It was shown that miR-22 derived from bone marrow mesenchymal stem cell-derived EVs (MSC-EVs) may reduce fibrosis and decrease apoptosis in mouse models of MI [124]. miR-19a and miR-221 from GATA-4 overexpressing MSCs also showed cardioprotective effects [125]. Cardiac progenitor cell-derived EVs (CPC-derived EVs) enriched in miR-210 and miR-132 may prevent stress-induced apoptosis in HL1 cardiomyocytes [126]. By restoring the miR-21/PDCD4 pathway, CPC-derived EVs inhibited oxidative stress-related apoptosis in H9c2 cells [127]. miR-210 and miR-132 play an important role in improving angiogenesis and cardiac function in MI models [128,129].
5. EVs in Tissue Regeneration and Repair in AS and CVD
Cell therapy holds great promise for treating diseases that are irreversible or incurable at the moment [130]. This is due to the fact that stem cells exhibit multilineage differentiation and self-renewal; moreover, they possess several immunomodulatory functions and promote tissue regeneration [131]. The success of regenerative medicine is based on two goals: one includes the direct restoration of the damaged tissue by cell transplantation; the other one is to repair the tissue via neurotrophic or immunomodulatory factors produced by cells or gap junction formation [132,133]. In many studies, the regenerative potential of stem cells has been seen in EV release [130,134].
It has been confirmed that EVs serve as efficient carriers of molecular cargo and are prime candidates for tissue engineering and regenerative medicine because of their favorable biological properties, including biocompatibility and stability [135,136]. EVs may modulate the activity of the immune system, playing an important role in regulating inflammation [25]. They exhibit a broad spectrum of immunomodulatory and pro-regenerative activity. By regulating cell proliferation, they are crucial for local tissue repair [137].
Via EV stimulation, many regenerative process-linked signaling pathways have been identified, including Wnt/β-catenin; PI3K/Akt; Notch; TGF-β/Smad; Hedgehog; CaMKII; and Efna3, AMPK and JAK2/STAT3/NF-κB [138,139,140,141] (Figure 5). For example, in MI, EVs from human umbilical cord MSCs were shown to stimulate angiogenesis through the microRNA-423-5p/EFNA3 pathway [142]. Other findings have suggested that EVs derived from human bone marrow MSCs effectively enhance cutaneous wound healing by inhibiting the TGF-β/Smad signaling pathway. Notably, this study revealed that the EVs induced a significant downregulation of TGF-β1, Smad2, Smad3, and Smad4 expression while concurrently upregulating TGF-β3 and Smad7 expression in the TGF-β/Smad signaling pathway [143]. Moreover, the pivotal role of the Wnt/β-catenin pathway as a key regulator of proangiogenic effects in EVs derived from tubular epithelial cells (TEC) was determined [141]. To develop an effective strategy for promoting vascularized bone regeneration, the impact on angiogenesis was also studied in EVs from hypoxia-induced MSCs. These EVs were observed to significantly enhance the proliferation, migration, and angiogenesis of human umbilical vein endothelial cells (HUVECs). The study revealed that HIF-1α induced the upregulation of miR-210-3p, which subsequently inhibited EFNA3 expression, leading to an enhancement in phosphorylation in the PI3K/AKT pathway [144]. Recent reports highlighted human placenta-derived MSC EVs as a promising therapeutic approach for nerve injury-induced neuropathic pain. The study reported that miR-26a-5p in EVs plays a regulatory role in the Wnt5a/Ryk/CaMKII/NFAT pathway. This regulation partially contributes to analgesic effects through anti-neuroinflammation [145]. Another study showed that EVs derived from MSCs effectively reduced apoptosis and alleviated tissue damage induced by middle cerebral artery occlusion (MCAO) in rats. This protective mechanism was shown to be likely mediated through the regulation of the AMPK pathway and the JAK2/STAT3/NF-κB signaling pathways. The findings highlight the neuroprotective potential of EVs in the context of MCAO, suggesting a promising therapeutic opportunity for ischemic stroke treatment [140] (Figure 5).
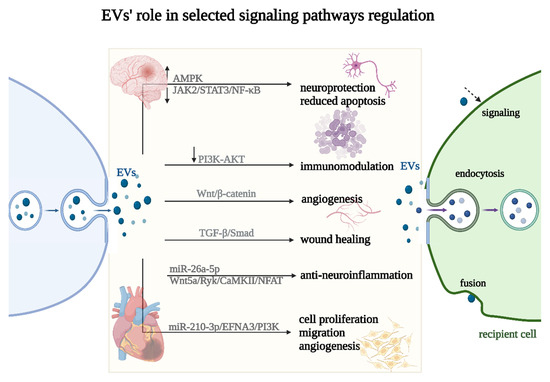
Figure 5.
Selected signaling pathways’ regulation (↑—upregulation, ↓—downregulation) by EVs. EVs can impact the recipient cell phenotype using three different uptake mechanisms: endocytosis, fusion, or signaling (marked with a dashed arrow).
EV phenotype can vary depending on the stem cell source. For example, EVs derived from endothelial progenitor cells (EPCs) may be used in regenerative medicine, especially in cardiac repair, because of their rich anti-inflammatory properties, while the EVs isolated from cardiomyocytes or cardiosphere-derived cells were proposed to be applied mainly due to their pro-proliferative effects [146]
EPCs seem to promote wound healing via angiogenesis and collagen synthesis stimulation [147]. EPCs are characterized by strong growth potential and the ability to differentiate into endothelial cells [148,149]. It was shown that EPC-derived exosomes presented a cardioprotective effect in angiotensin II-induced hypertrophy and apoptosis [150]. Moreover, they can control angiogenesis by promoting endothelial cell survival, proliferation, and organization [151].
The enhancement of angiogenesis was also reported to be a property of cardiomyocyte EVs after oxidative and metabolic stress [11]. Under physiological conditions, the released EVs regulated cell survival and growth. Other candidates for myocardial disease treatment, cardiac progenitor cells (CPCs), seem to be very attractive because of their origin and the fact that they contribute to maintaining the population of cardiac myocytes and coronary vessels under normal conditions [152,153]. Their protective effect can be achieved both by their further differentiation and paracrine abilities [154]. CPCs, however, can hamper myocardial regeneration due to the limited proliferative potential [155]. By contrast, exosomes derived from cardiospheres (CDCs) stimulate cardiomyocytes’ proliferative, angiogenic, and anti-apoptotic potential. CDCs are clusters composed of cardiomyocytes, cardiac stem cells, CPCs, endothelial cells, and smooth muscle cells [156]. It was observed recently that CDCs’ EVs seem to reduce the inflammatory response by suppressing monocytes’ expression of CCR2 in patients with acute MI, offering a new prospect for existing clinical therapies for elevated pro-inflammatory responses following this incident [157].
The beneficial therapeutic impacts of MSCs are largely associated with their exosomal paracrine functions [158]. Mesenchymal stem cells (MSCs) are multipotent stem cells with self-renewal abilities, and they induce tissue regeneration by their direct recruitment into injured tissues, including the heart [159,160]. MSCs may be found in many tissue sources, including the bone marrow, adipose tissue (AD-MSCs) and umbilical cord blood, Wharton’s jelly (WJ-MSCs), and even the brain, placenta, liver, kidney, lungs, and thymus [161]. Due to the great access to many MSC sources, these cells have attracted the attention of researchers and are commonly used in experiments. Recently, it was shown that MSCs can release several EVs whose activity in regenerative processes is similar to that of the MSCs [162]. Moreover, it was confirmed that MSC-EVs are cardioprotective [163]. EVs from these cells are beneficial in various ways—for example, their anti-inflammatory, anti-apoptotic, and anti-remodeling actions have regenerative and neovascular properties [164]. MSC-EVs were also reported to decrease oxidative stress [165]. MSCs, through their advantageous properties, such as accelerating wound closure, improving re-epithelialization, elevating angiogenesis, suppressing inflammation, and modulating extracellular matrix (ECM) remodeling, can accelerate wound repair [166]. MSC-derived EVs can shuttle bioactive molecules, causing the exchange of genetic information and reprogramming of the recipient cells. Importantly, EVs could mediate and induce cell differentiation/self-renewal [167].
The most attractive MSCs regarding cardiovascular diseases seem to be adipose tissue-derived mesenchymal stem cells (AD-MSCs). AD-MSCs are relatively easy to isolate and culture and are characterized by a high proliferation rate and multilineage differentiation [168,169]. Despite their relatively low survival rate in a cardiac niche, their paracrine potential is known to be the most promising one nowadays [146,168,170]. Luo et al. reported that AD-MSC-derived exosomes can protect myocardial cells from apoptosis, inflammation, and fibrosis, and they can promote angiogenesis, thereby preventing myocardial damage [171].
MSC-derived EVs may be used as an alternative MSC-based therapy in regenerative medicine and tissue engineering [172,173]. They pave the way to an effective, cost-efficient, and safe therapeutic option in cell-free regenerative medicine, presumably offering a viable alternative to MSCs. Therefore, understanding their functional mechanisms is essential to fully address the regenerative potential of MSC-derived EVs in therapeutic applications.
6. Conclusions and Future Directions
It has been confirmed that EVs are implicated in key aspects of atherosclerosis progression by regulating pathophysiologic pathways like inflammation, angiogenesis, and senescence [174,175]. They play an important role in physiological and pathological conditions [176]. EVs have potential as biomarkers in the diagnosis, prognosis, and monitoring of atherosclerosis in patients and as new therapeutic targets and/or drug delivery vehicles [17]. It is worth mentioning that altered levels of EVs have been evidenced in patients suffering from atrial fibrillation [177]. They may be clinically useful as therapeutic targets in cardiovascular diseases. A novel therapeutic approach for atherosclerosis prevention and treatment is the precise targeting of genes involved in lipoprotein metabolism. Therefore, the roles of EVs-derived RNAs as therapeutic products or theranostic markers demonstrate unequivocally encouraging benefits. The homeostatic functions of EVs may facilitate the development of new regenerative therapies. Importantly, EVs obtained from various stem/progenitor cells are safer and more effective in repairing damaged tissues [178]. They play an important role in intracardiac communication and present huge potential in regenerative medicine [179]. EPCs represent more biocompatible, less immunogenic populations and have potential as a cell-free therapy for CVD [180]. Also, tissue engineering and targeted therapies by using MSCs are capable of reducing inflammation and increasing regenerative potential. The MISEV has established essential principles while fostering flexibility, collaboration, and continuous improvement in EV research. By collectively advancing isolation, detection, and standardization, the full potential of EVs can be unlocked, enhancing our understanding of their roles in health and disease. However, it should be emphasized that despite the success in engineering EVs toward clinical applications, the use of EVs is still hampered by challenges encountered in EV isolation and detection and the lack of standardization methods.
Author Contributions
Conceptualization, W.O.; writing—original draft preparation, W.O., K.S. and K.R.; writing—review and editing, W.O., K.S. and K.R.; visualization, W.O., K.S. and K.R.; supervision, W.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Botts, S.R.; Fish, J.E.; Howe, K.L. Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights into Pathogenesis and Treatment. Front. Pharmacol. 2021, 12, 787541. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Spacek, M.; Zemanek, D.; Hutyra, M.; Sluka, M.; Taborsky, M. Vulnerable atherosclerotic plaque—A review of current concepts and advanced imaging. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub. 2018, 162, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.J.; Sheats, J.L.; King, A.C. The Use of Behavior Change Techniques and Theory in Technologies for Cardiovascular Disease Prevention and Treatment in Adults: A Comprehensive Review. Prog. Cardiovasc. Dis. 2016, 58, 605–612. [Google Scholar] [CrossRef]
- Bugger, H.; Zirlik, A. Anti-inflammatory Strategies in Atherosclerosis. Hamostaseologie 2021, 41, 433–442. [Google Scholar] [CrossRef]
- Jia, G.; Sowers, J.R. Targeting endothelial exosomes for the prevention of cardiovascular disease. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165833. [Google Scholar] [CrossRef]
- Peng, M.; Liu, X.; Xu, G. Extracellular Vesicles as Messengers in Atherosclerosis. J. Cardiovasc. Transl. Res. 2020, 13, 121–130. [Google Scholar] [CrossRef]
- Sluijter, J.P.; Verhage, V.; Deddens, J.C.; van den Akker, F.; Doevendans, P.A. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kaminska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Thavarajah, T.; Gu, W.; Cai, J.; Xu, Q. Impact of miRNA in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, e159–e170. [Google Scholar] [CrossRef]
- Das, S.; Halushka, M.K. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc. Pathol. 2015, 24, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Gaceb, A.; Martinez, M.C.; Andriantsitohaina, R. Extracellular vesicles: New players in cardiovascular diseases. Int. J. Biochem. Cell Biol. 2014, 50, 24–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tesovnik, T.; Jenko Bizjan, B.; Sket, R.; Debeljak, M.; Battelino, T.; Kovac, J. Technological Approaches in the Analysis of Extracellular Vesicle Nucleotide Sequences. Front. Bioeng. Biotechnol. 2021, 9, 787551. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Boulanger, C.M.; Aikawa, E.; Badimon, L.; Barile, L.; Binder, C.J.; Brisson, A.; Buzas, E.; Emanueli, C.; Jansen, F.; et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies: From exosomes to microvesicles. Cardiovasc. Res. 2023, 119, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Buntsma, N.; van der Pol, E.; Nieuwland, R.; Gasecka, A. Extracellular Vesicles in Coronary Artery Disease. Adv. Exp. Med. Biol. 2023, 1418, 81–103. [Google Scholar] [CrossRef]
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.W. Extracellular vesicles in cardiovascular disease. Adv. Clin. Chem. 2021, 103, 47–95. [Google Scholar] [CrossRef]
- Witwer, K.W.; Goberdhan, D.C.; O’Driscoll, L.; Thery, C.; Welsh, J.A.; Blenkiron, C.; Buzas, E.I.; Di Vizio, D.; Erdbrugger, U.; Falcon-Perez, J.M.; et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef]
- Royo, F.; Thery, C.; Falcon-Perez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Consortium, E.-T.; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, O.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Loyer, X.; Boulanger, C.M. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur. J. Pharmacol. 2015, 763 Pt A, 90–103. [Google Scholar] [CrossRef]
- Loyer, X.; Vion, A.C.; Tedgui, A.; Boulanger, C.M. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ. Res. 2014, 114, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2023, 23, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Peng, Y.; Feng, Y.; Xu, Z.; Feng, P.; Cao, J.; Chen, Y.; Chen, X.; Cao, X.; Yang, Y.; et al. Immune Cell-Derived Extracellular Vesicles—New Strategies in Cancer Immunotherapy. Front. Immunol. 2021, 12, 771551. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, D.S.; Badawi, M.; Phelps, M.A.; Schmittgen, T.D. Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading. Pharm. Res. 2017, 34, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun. Signal 2021, 19, 47. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Schiano, C.; Balbi, C.; de Nigris, F.; Napoli, C. Basic Pathogenic Mechanisms and Epigenetic Players Promoted by Extracellular Vesicles in Vascular Damage. Int. J. Mol. Sci. 2023, 24, 7509. [Google Scholar] [CrossRef]
- Deng, W.; Tang, T.; Hou, Y.; Zeng, Q.; Wang, Y.; Fan, W.; Qu, S. Extracellular vesicles in atherosclerosis. Clin. Chim. Acta 2019, 495, 109–117. [Google Scholar] [CrossRef]
- Lu, M.; Yuan, S.; Li, S.; Li, L.; Liu, M.; Wan, S. The Exosome-Derived Biomarker in Atherosclerosis and Its Clinical Application. J. Cardiovasc. Transl. Res. 2019, 12, 68–74. [Google Scholar] [CrossRef]
- Nozaki, T.; Sugiyama, S.; Koga, H.; Sugamura, K.; Ohba, K.; Matsuzawa, Y.; Sumida, H.; Matsui, K.; Jinnouchi, H.; Ogawa, H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J. Am. Coll. Cardiol. 2009, 54, 601–608. [Google Scholar] [CrossRef]
- Bernal-Mizrachi, L.; Jy, W.; Jimenez, J.J.; Pastor, J.; Mauro, L.M.; Horstman, L.L.; de Marchena, E.; Ahn, Y.S. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am. Heart J. 2003, 145, 962–970. [Google Scholar] [CrossRef]
- Werner, N.; Wassmann, S.; Ahlers, P.; Kosiol, S.; Nickenig, G. Circulating CD31+/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 112–116. [Google Scholar] [CrossRef]
- Tash, J.S.; Roby, K.F.; Gupta, V.; Holets, L. Long-term spaceflight impacts male reproductive health. In Fundamental Space Biology Division; NASA: Washington, DC, USA, 2012. [Google Scholar]
- Chironi, G.; Simon, A.; Hugel, B.; Del Pino, M.; Gariepy, J.; Freyssinet, J.M.; Tedgui, A. Circulating leukocyte-derived microparticles predict subclinical atherosclerosis burden in asymptomatic subjects. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2775–2780. [Google Scholar] [CrossRef]
- Heiss, C.; Amabile, N.; Lee, A.C.; Real, W.M.; Schick, S.F.; Lao, D.; Wong, M.L.; Jahn, S.; Angeli, F.S.; Minasi, P.; et al. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: Sustained vascular injury and blunted nitric oxide production. J. Am. Coll. Cardiol. 2008, 51, 1760–1771. [Google Scholar] [CrossRef]
- Dignat-George, F.; Boulanger, C.M. The many faces of endothelial microparticles. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef]
- Chatterjee, V.; Yang, X.; Ma, Y.; Cha, B.; Meegan, J.E.; Wu, M.; Yuan, S.Y. Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis. Cardiovasc. Res. 2020, 116, 1525–1538. [Google Scholar] [CrossRef]
- Merten, M.; Pakala, R.; Thiagarajan, P.; Benedict, C.R. Platelet microparticles promote platelet interaction with subendothelial matrix in a glycoprotein IIb/IIIa-dependent mechanism. Circulation 1999, 99, 2577–2582. [Google Scholar] [CrossRef]
- Sadallah, S.; Eken, C.; Martin, P.J.; Schifferli, J.A. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J. Immunol. 2011, 186, 6543–6552. [Google Scholar] [CrossRef] [PubMed]
- Vasina, E.M.; Cauwenberghs, S.; Feijge, M.A.; Heemskerk, J.W.; Weber, C.; Koenen, R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011, 2, e211. [Google Scholar] [CrossRef]
- Brodsky, S.V.; Zhang, F.; Nasjletti, A.; Goligorsky, M.S. Endothelium-derived microparticles impair endothelial function in vitro. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1910–H1915. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Vales, A.; Mitulovic, G.; Blumer, M.; Schmid, R.; Witztum, J.L.; Binder, B.R.; Leitinger, N. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 101–107. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, C.; Xiao, J.; Li, D.; Sun, Z.; Li, M. Endothelial extracellular vesicles modulate the macrophage phenotype: Potential implications in atherosclerosis. Scand. J. Immunol. 2018, 87, e12648. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Yang, X.; Franklin, B.S.; Hoelscher, M.; Schmitz, T.; Bedorf, J.; Nickenig, G.; Werner, N. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc. Res. 2013, 98, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Akhmerov, A.; Parimon, T. Extracellular Vesicles, Inflammation, and Cardiovascular Disease. Cells 2022, 11, 2229. [Google Scholar] [CrossRef] [PubMed]
- Leroyer, A.S.; Isobe, H.; Leseche, G.; Castier, Y.; Wassef, M.; Mallat, Z.; Binder, B.R.; Tedgui, A.; Boulanger, C.M. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J. Am. Coll. Cardiol. 2007, 49, 772–777. [Google Scholar] [CrossRef]
- Perrotta, I.; Aquila, S. Exosomes in human atherosclerosis: An ultrastructural analysis study. Ultrastruct. Pathol. 2016, 40, 101–106. [Google Scholar] [CrossRef]
- Mayr, M.; Grainger, D.; Mayr, U.; Leroyer, A.S.; Leseche, G.; Sidibe, A.; Herbin, O.; Yin, X.; Gomes, A.; Madhu, B.; et al. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ. Cardiovasc. Genet. 2009, 2, 379–388. [Google Scholar] [CrossRef]
- Heloire, F.; Weill, B.; Weber, S.; Batteux, F. Aggregates of endothelial microparticles and platelets circulate in peripheral blood. Variations during stable coronary disease and acute myocardial infarction. Thromb. Res. 2003, 110, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Chirinos, J.A.; Heresi, G.A.; Velasquez, H.; Jy, W.; Jimenez, J.J.; Ahn, E.; Horstman, L.L.; Soriano, A.O.; Zambrano, J.P.; Ahn, Y.S. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J. Am. Coll. Cardiol. 2005, 45, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Boulanger, C.M.; Loyer, X.; Rautou, P.E.; Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Shah, N.; Zanetti, B.R.; Maugeri, M.; Silvestre, R.N.; Fatima, F.; Neder, L.; Valadi, H. Extracellular Vesicles and Matrix Remodeling Enzymes: The Emerging Roles in Extracellular Matrix Remodeling, Progression of Diseases and Tissue Repair. Cells 2018, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Olejarz, W.; Lacheta, D.; Kubiak-Tomaszewska, G. Matrix Metalloproteinases as Biomarkers of Atherosclerotic Plaque Instability. Int. J. Mol. Sci. 2020, 21, 3946. [Google Scholar] [CrossRef] [PubMed]
- Lozito, T.P.; Tuan, R.S. Endothelial cell microparticles act as centers of matrix metalloproteinsase-2 (MMP-2) activation and vascular matrix remodeling. J. Cell Physiol. 2012, 227, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Lizarrondo, S.; Roncal, C.; Calvayrac, O.; Rodriguez, C.; Varo, N.; Purroy, A.; Lorente, L.; Rodriguez, J.A.; Doeuvre, L.; Hervas-Stubbs, S.; et al. Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Taraboletti, G.; D’Ascenzo, S.; Borsotti, P.; Giavazzi, R.; Pavan, A.; Dolo, V. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am. J. Pathol. 2002, 160, 673–680. [Google Scholar] [CrossRef]
- Canault, M.; Leroyer, A.S.; Peiretti, F.; Leseche, G.; Tedgui, A.; Bonardo, B.; Alessi, M.C.; Boulanger, C.M.; Nalbone, G. Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-α converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am. J. Pathol. 2007, 171, 1713–1723. [Google Scholar] [CrossRef]
- Folkesson, M.; Li, C.; Frebelius, S.; Swedenborg, J.; Wagsater, D.; Williams, K.J.; Eriksson, P.; Roy, J.; Liu, M.L. Proteolytically active ADAM10 and ADAM17 carried on membrane microvesicles in human abdominal aortic aneurysms. Thromb. Haemost. 2015, 114, 1165–1174. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Chatrou, M.L.; Drozdov, I.; Zheng, Y.; Davidson, S.M.; Soong, D.; Furmanik, M.; Sanchis, P.; De Rosales, R.T.; Alvarez-Hernandez, D.; et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 2015, 116, 1312–1323. [Google Scholar] [CrossRef] [PubMed]
- Buendia, P.; Montes de Oca, A.; Madueno, J.A.; Merino, A.; Martin-Malo, A.; Aljama, P.; Ramirez, R.; Rodriguez, M.; Carracedo, J. Endothelial microparticles mediate inflammation-induced vascular calcification. FASEB J. 2015, 29, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, A.N.; Davies, J.D.; Reynolds, J.L.; McNair, R.; Jones, G.T.; Sidibe, A.; Schurgers, L.J.; Skepper, J.N.; Proudfoot, D.; Mayr, M.; et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ. Res. 2011, 109, e1–e12. [Google Scholar] [CrossRef] [PubMed]
- New, S.E.; Goettsch, C.; Aikawa, M.; Marchini, J.F.; Shibasaki, M.; Yabusaki, K.; Libby, P.; Shanahan, C.M.; Croce, K.; Aikawa, E. Macrophage-derived matrix vesicles: An alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ. Res. 2013, 113, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Verma, S. Evolving role of microparticles in the pathophysiology of endothelial dysfunction. Clin. Chem. 2013, 59, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Nikdoust, F.; Pazoki, M.; Mohammadtaghizadeh, M.; Aghaali, M.K.; Amrovani, M. Exosomes: Potential Player in Endothelial Dysfunction in Cardiovascular Disease. Cardiovasc. Toxicol. 2022, 22, 225–235. [Google Scholar] [CrossRef]
- Dickhout, A.; Koenen, R.R. Extracellular Vesicles as Biomarkers in Cardiovascular Disease; Chances and Risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Crespo, J.; Sionis, A.; Alonso, R.; Mata, P.; Badimon, L. Liquid Biopsy of Extracellular Microvesicles Predicts Future Major Ischemic Events in Genetically Characterized Familial Hypercholesterolemia Patients. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1172–1181. [Google Scholar] [CrossRef]
- Sinning, J.M.; Losch, J.; Walenta, K.; Bohm, M.; Nickenig, G.; Werner, N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. Eur. Heart J. 2011, 32, 2034–2041. [Google Scholar] [CrossRef]
- Togliatto, G.; Dentelli, P.; Rosso, A.; Lombardo, G.; Gili, M.; Gallo, S.; Gai, C.; Solini, A.; Camussi, G.; Brizzi, M.F. PDGF-BB Carried by Endothelial Cell-Derived Extracellular Vesicles Reduces Vascular Smooth Muscle Cell Apoptosis in Diabetes. Diabetes 2018, 67, 704–716. [Google Scholar] [CrossRef]
- Li, B.; Zang, G.; Zhong, W.; Chen, R.; Zhang, Y.; Yang, P.; Yan, J. Activation of CD137 signaling promotes neointimal formation by attenuating TET2 and transferrring from endothelial cell-derived exosomes to vascular smooth muscle cells. Biomed. Pharmacother. 2020, 121, 109593. [Google Scholar] [CrossRef] [PubMed]
- Hergenreider, E.; Heydt, S.; Treguer, K.; Boettger, T.; Horrevoets, A.J.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Njock, M.S.; Cheng, H.S.; Dang, L.T.; Nazari-Jahantigh, M.; Lau, A.C.; Boudreau, E.; Roufaiel, M.; Cybulsky, M.I.; Schober, A.; Fish, J.E. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015, 125, 3202–3212. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Cheng, C.; Liu, S. Angiotensin-converting enzyme 2 augments the effects of endothelial progenitor cells-exosomes on vascular smooth muscle cell phenotype transition. Cell Tissue Res. 2020, 382, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Duan, Y.; Liu, C.; Huang, H.; Xiao, X.; He, Z. Extracellular vesicles in atherosclerosis and vascular calcification: The versatile non-coding RNAs from endothelial cells and vascular smooth muscle cells. Front. Med. 2023, 10, 1193660. [Google Scholar] [CrossRef]
- Jiapaer, Z.; Li, C.; Yang, X.; Sun, L.; Chatterjee, E.; Zhang, L.; Lei, J.; Li, G. Extracellular Non-Coding RNAs in Cardiovascular Diseases. Pharmaceutics 2023, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Hermann, D.M.; Xin, W.; Bahr, M.; Giebel, B.; Doeppner, T.R. Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke. Theranostics 2022, 12, 5776–5802. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Liu, Y.; Wei, W.; Yang, X.; Wang, Z.; Xin, W. Extracellular Vesicles as Delivery Shippers for Noncoding RNA-Based Modulation of Angiogenesis: Insights from Ischemic Stroke and Cancer. Small 2023, 19, e2205739. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac Extracellular Vesicles in Normal and Infarcted Heart. Int. J. Mol. Sci. 2016, 17, 63. [Google Scholar] [CrossRef]
- Rezaie, J.; Rahbarghazi, R.; Pezeshki, M.; Mazhar, M.; Yekani, F.; Khaksar, M.; Shokrollahi, E.; Amini, H.; Hashemzadeh, S.; Sokullu, S.E.; et al. Cardioprotective role of extracellular vesicles: A highlight on exosome beneficial effects in cardiovascular diseases. J. Cell Physiol. 2019, 234, 21732–21745. [Google Scholar] [CrossRef]
- Irwandi, R.A.; Chiesa, S.T.; Hajishengallis, G.; Papayannopoulos, V.; Deanfield, J.E.; D’Aiuto, F. The Roles of Neutrophils Linking Periodontitis and Atherosclerotic Cardiovascular Diseases. Front. Immunol. 2022, 13, 915081. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Guo, M.K.; Abdul Samad, M.; Howe, K.L. Extracellular vesicles as biomarkers and modulators of atherosclerosis pathogenesis. Front. Cardiovasc. Med. 2023, 10, 1202187. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, J.P.G.; Davidson, S.M.; Boulanger, C.M.; Buzas, E.I.; de Kleijn, D.P.V.; Engel, F.B.; Giricz, Z.; Hausenloy, D.J.; Kishore, R.; Lecour, S.; et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 2018, 114, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Bobryshev, Y.V.; Killingsworth, M.C.; Orekhov, A.N. Increased shedding of microvesicles from intimal smooth muscle cells in athero-prone areas of the human aorta: Implications for understanding of the predisease stage. Pathobiology 2013, 80, 24–31. [Google Scholar] [CrossRef]
- Sionis, A.; Suades, R.; Sans-Rosello, J.; Sanchez-Martinez, M.; Crespo, J.; Padro, T.; Cubedo, J.; Ferrero-Gregori, A.; Vila-Perales, M.; Duran-Cambra, A.; et al. Circulating microparticles are associated with clinical severity of persistent ST-segment elevation myocardial infarction complicated with cardiogenic shock. Int. J. Cardiol. 2018, 258, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Landers-Ramos, R.Q.; Addison, O.A.; Beamer, B.; Katzel, L.I.; Blumenthal, J.B.; Robinson, S.; Hagberg, J.M.; Prior, S.J. Circulating microparticle concentrations across acute and chronic cardiovascular disease conditions. Physiol. Rep. 2020, 8, e14534. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, J.M.; Moon, H.J.; Bahn, J.J.; Im, W.S.; Sunwoo, J.; Moon, J.; Kim, M.; et al. Circulating CD62E+ microparticles and cardiovascular outcomes. PLoS ONE 2012, 7, e35713. [Google Scholar] [CrossRef] [PubMed]
- Amabile, N.; Rautou, P.E.; Tedgui, A.; Boulanger, C.M. Microparticles: Key protagonists in cardiovascular disorders. Semin. Thromb. Hemost. 2010, 36, 907–916. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.W.; Zheng, L.; Wang, Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell Biol. 2017, 36, 202–211. [Google Scholar] [CrossRef]
- Montoro-Garcia, S.; Shantsila, E.; Tapp, L.D.; Lopez-Cuenca, A.; Romero, A.I.; Hernandez-Romero, D.; Orenes-Pinero, E.; Manzano-Fernandez, S.; Valdes, M.; Marin, F.; et al. Small-size circulating microparticles in acute coronary syndromes: Relevance to fibrinolytic status, reparative markers and outcomes. Atherosclerosis 2013, 227, 313–322. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Crespo, J.; Ramaiola, I.; Martin-Yuste, V.; Sabate, M.; Sans-Rosello, J.; Sionis, A.; Badimon, L. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int. J. Cardiol. 2016, 202, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Morel, O.; Hugel, B.; Jesel, L.; Lanza, F.; Douchet, M.P.; Zupan, M.; Chauvin, M.; Cazenave, J.P.; Freyssinet, J.M.; Toti, F. Sustained elevated amounts of circulating procoagulant membrane microparticles and soluble GPV after acute myocardial infarction in diabetes mellitus. Thromb. Haemost. 2004, 91, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M.; Tedgui, A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, P.M.; Biro, E.; Ko, Y.; de Winter, R.J.; Hack, C.E.; Sturk, A.; Nieuwland, R. P-selectin- and CD63-exposing platelet microparticles reflect platelet activation in peripheral arterial disease and myocardial infarction. Clin. Chem. 2006, 52, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Stepien, E.; Stankiewicz, E.; Zalewski, J.; Godlewski, J.; Zmudka, K.; Wybranska, I. Number of microparticles generated during acute myocardial infarction and stable angina correlates with platelet activation. Arch. Med. Res. 2012, 43, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulos, G.; Oudatzis, G.; Paterakis, G.; Synetos, A.; Tampaki, E.; Bouras, G.; Hahalis, G.; Alexopoulos, D.; Tousoulis, D.; Cleman, M.W.; et al. Red blood cell and platelet microparticles in myocardial infarction patients treated with primary angioplasty. Int. J. Cardiol. 2014, 176, 145–150. [Google Scholar] [CrossRef]
- Biasucci, L.M.; Porto, I.; Di Vito, L.; De Maria, G.L.; Leone, A.M.; Tinelli, G.; Tritarelli, A.; Di Rocco, G.; Snider, F.; Capogrossi, M.C.; et al. Differences in microparticle release in patients with acute coronary syndrome and stable angina. Circ. J. 2012, 76, 2174–2182. [Google Scholar] [CrossRef]
- Min, P.K.; Kim, J.Y.; Chung, K.H.; Lee, B.K.; Cho, M.; Lee, D.L.; Hong, S.Y.; Choi, E.Y.; Yoon, Y.W.; Hong, B.K.; et al. Local increase in microparticles from the aspirate of culprit coronary arteries in patients with ST-segment elevation myocardial infarction. Atherosclerosis 2013, 227, 323–328. [Google Scholar] [CrossRef]
- Porto, I.; Biasucci, L.M.; De Maria, G.L.; Leone, A.M.; Niccoli, G.; Burzotta, F.; Trani, C.; Tritarelli, A.; Vergallo, R.; Liuzzo, G.; et al. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur. Heart J. 2012, 33, 2928–2938. [Google Scholar] [CrossRef]
- Zacharia, E.; Antonopoulos, A.S.; Oikonomou, E.; Papageorgiou, N.; Pallantza, Z.; Miliou, A.; Mystakidi, V.C.; Simantiris, S.; Kriebardis, A.; Orologas, N.; et al. Plasma signature of apoptotic microvesicles is associated with endothelial dysfunction and plaque rupture in acute coronary syndromes. J. Mol. Cell Cardiol. 2020, 138, 110–114. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L.; Li, Y.; Yin, Z.; Xu, Z.; Wang, C. Quantification of endothelial microparticles on modified cytometric bead assay and prognosis in chest pain patients. Circ. J. 2014, 78, 206–214. [Google Scholar] [CrossRef]
- Vagida, M.S.; Arakelyan, A.; Lebedeva, A.M.; Grivel, J.C.; Shpektor, A.V.; Vasilieva, E.Y.; Margolis, L.B. Analysis of Extracellular Vesicles Using Magnetic Nanoparticles in Blood of Patients with Acute Coronary Syndrome. Biochemistry 2016, 81, 382–391. [Google Scholar] [CrossRef]
- Cheng, G.; Shan, X.F.; Wang, X.L.; Dong, W.W.; Li, Z.; Liu, X.H.; Zhang, W.; Xing, K.; Chang, F.J. Endothelial damage effects of circulating microparticles from patients with stable angina are reduced by aspirin through ERK/p38 MAPKs pathways. Cardiovasc. Ther. 2017, 35, e12273. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, L.; Jiang, M.; Jia, R.; Zhang, X.; Quan, Q.; Du, G.; Shen, D.; Zhao, X.; Sun, W.; et al. Circulating microparticles in patients with coronary heart disease and its correlation with interleukin-6 and C-reactive protein. Mol. Biol. Rep. 2013, 40, 6437–6442. [Google Scholar] [CrossRef]
- Wang, B.; Li, T.; Han, X.; Li, Y.; Cheng, W.; Wang, L.; Lu, Z.; Yang, J.; Zhao, M. The Level of Circulating Microparticles in Patients with Coronary Heart Disease: A Systematic Review and Meta-Analysis. J. Cardiovasc. Transl. Res. 2020, 13, 702–712. [Google Scholar] [CrossRef]
- Marei, I.; Chidiac, O.; Thomas, B.; Pasquier, J.; Dargham, S.; Robay, A.; Vakayil, M.; Jameesh, M.; Triggle, C.; Rafii, A.; et al. Angiogenic content of microparticles in patients with diabetes and coronary artery disease predicts networks of endothelial dysfunction. Cardiovasc. Diabetol. 2022, 21, 17. [Google Scholar] [CrossRef]
- Sansone, R.; Baaken, M.; Horn, P.; Schuler, D.; Westenfeld, R.; Amabile, N.; Kelm, M.; Heiss, C. Endothelial microparticles and vascular parameters in subjects with and without arterial hypertension and coronary artery disease. Data Brief. 2018, 19, 495–500. [Google Scholar] [CrossRef]
- Wekesa, A.L.; Cross, K.S.; O’Donovan, O.; Dowdall, J.F.; O’Brien, O.; Doyle, M.; Byrne, L.; Phelan, J.P.; Ross, M.D.; Landers, R.; et al. Predicting carotid artery disease and plaque instability from cell-derived microparticles. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 489–495. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Alonso, R.; Mata, P.; Badimon, L. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb. Haemost. 2015, 114, 1310–1321. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Alonso, R.; Lopez-Miranda, J.; Mata, P.; Badimon, L. Circulating CD45+/CD3+ lymphocyte-derived microparticles map lipid-rich atherosclerotic plaques in familial hypercholesterolaemia patients. Thromb. Haemost. 2014, 111, 111–121. [Google Scholar] [CrossRef]
- Suades, R.; Greco, M.F.; Padro, T.; Badimon, L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells 2022, 11, 1845. [Google Scholar] [CrossRef]
- Teng, P.; Liu, Y.; Zhang, M.; Ji, W. Diagnostic and Prognostic Significance of serum miR-18a-5p in Patients with Atherosclerosis. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211050642. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Xia, Y. The expression of miR-211-5p in atherosclerosis and its influence on diagnosis and prognosis. BMC Cardiovasc. Disord. 2021, 21, 371. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, L.; Liu, H.; Sun, B.; Zhao, X. Diagnostic value of miR-637 in patients with atherosclerosis and its predictive significance for the future cardiovascular events. Vascular 2021, 29, 704–710. [Google Scholar] [CrossRef]
- Sharma, A.R.; Sharma, G.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. Circulating miRNA in Atherosclerosis: A Clinical Biomarker and Early Diagnostic Tool. Curr. Mol. Med. 2022, 22, 250–262. [Google Scholar] [CrossRef]
- Singh, S.; de Ronde, M.W.J.; Kok, M.G.M.; Beijk, M.A.; De Winter, R.J.; van der Wal, A.C.; Sondermeijer, B.M.; Meijers, J.C.M.; Creemers, E.E.; Pinto-Sietsma, S.J. MiR-223-3p and miR-122-5p as circulating biomarkers for plaque instability. Open Heart 2020, 7, e001223. [Google Scholar] [CrossRef]
- Zhang, P.; Liang, T.; Chen, Y.; Wang, X.; Wu, T.; Xie, Z.; Luo, J.; Yu, Y.; Yu, H. Circulating Exosomal miRNAs as Novel Biomarkers for Stable Coronary Artery Disease. Biomed. Res. Int. 2020, 2020, 3593962. [Google Scholar] [CrossRef]
- Su, J.; Li, J.; Yu, Q.; Wang, J.; Li, X.; Yang, J.; Xu, J.; Liu, Y.; Xu, Z.; Ji, L.; et al. Exosomal miRNAs as potential biomarkers for acute myocardial infarction. IUBMB Life 2020, 72, 384–400. [Google Scholar] [CrossRef]
- Chen, X.; Huang, F.; Liu, Y.; Liu, S.; Tan, G. Exosomal miR-152-5p and miR-3681-5p function as potential biomarkers for ST-segment elevation myocardial infarction. Clinics 2022, 77, 100038. [Google Scholar] [CrossRef]
- Zamani, P.; Fereydouni, N.; Butler, A.E.; Navashenaq, J.G.; Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 2019, 29, 313–323. [Google Scholar] [CrossRef]
- Yuan, M.J.; Maghsoudi, T.; Wang, T. Exosomes Mediate the Intercellular Communication after Myocardial Infarction. Int. J. Med. Sci. 2016, 13, 113–116. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef]
- Barile, L.; Cervio, E.; Lionetti, V.; Milano, G.; Ciullo, A.; Biemmi, V.; Bolis, S.; Altomare, C.; Matteucci, M.; Di Silvestre, D.; et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: Role of pregnancy-associated plasma protein-A. Cardiovasc. Res. 2018, 114, 992–1005. [Google Scholar] [CrossRef]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef]
- Hu, S.; Huang, M.; Li, Z.; Jia, F.; Ghosh, Z.; Lijkwan, M.A.; Fasanaro, P.; Sun, N.; Wang, X.; Martelli, F.; et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010, 122 (Suppl. S11), S124–S131. [Google Scholar] [CrossRef]
- Katare, R.; Riu, F.; Mitchell, K.; Gubernator, M.; Campagnolo, P.; Cui, Y.; Fortunato, O.; Avolio, E.; Cesselli, D.; Beltrami, A.P.; et al. Transplantation of human pericyte progenitor cells improves the repair of infarcted heart through activation of an angiogenic program involving micro-RNA-132. Circ. Res. 2011, 109, 894–906. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef]
- Jin, Y.; Li, S.; Yu, Q.; Chen, T.; Liu, D. Application of stem cells in regeneration medicine. MedComm 2023, 4, e291. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Jadczyk, T.; Pedziwiatr, D.; Wojakowski, W. New advances in stem cell research: Practical implications for regenerative medicine. Pol. Arch. Med. Wewn. 2014, 124, 417–426. [Google Scholar] [CrossRef]
- Teng, Y.D. Functional multipotency of stem cells: Biological traits gleaned from neural progeny studies. Semin. Cell Dev. Biol. 2019, 95, 74–83. [Google Scholar] [CrossRef]
- Fuloria, S.; Subramaniyan, V.; Dahiya, R.; Dahiya, S.; Sudhakar, K.; Kumari, U.; Sathasivam, K.; Meenakshi, D.U.; Wu, Y.S.; Sekar, M.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Regenerative Potential and Challenges. Biology 2021, 10, 172. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Mossell, J.; Suo, Z. Extracellular vesicles: Emerging frontiers in wound healing. Med. Res. Rev. 2022, 42, 2102–2125. [Google Scholar] [CrossRef]
- Hade, M.D.; Suire, C.N.; Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells 2021, 10, 1959. [Google Scholar] [CrossRef]
- De Jong, O.G.; Van Balkom, B.W.; Schiffelers, R.M.; Bouten, C.V.; Verhaar, M.C. Extracellular vesicles: Potential roles in regenerative medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef]
- Adamo, A.; Brandi, J.; Caligola, S.; Delfino, P.; Bazzoni, R.; Carusone, R.; Cecconi, D.; Giugno, R.; Manfredi, M.; Robotti, E.; et al. Extracellular Vesicles Mediate Mesenchymal Stromal Cell-Dependent Regulation of B Cell PI3K-AKT Signaling Pathway and Actin Cytoskeleton. Front. Immunol. 2019, 10, 446. [Google Scholar] [CrossRef]
- Maggio, S.; Ceccaroli, P.; Polidori, E.; Cioccoloni, A.; Stocchi, V.; Guescini, M. Signal Exchange through Extracellular Vesicles in Neuromuscular Junction Establishment and Maintenance: From Physiology to Pathology. Int. J. Mol. Sci. 2019, 20, 2804. [Google Scholar] [CrossRef]
- Han, M.; Cao, Y.; Xue, H.; Chu, X.; Li, T.; Xin, D.; Yuan, L.; Ke, H.; Li, G.; Wang, Z. Neuroprotective Effect of Mesenchymal Stromal Cell-Derived Extracellular Vesicles Against Cerebral Ischemia-Reperfusion-Induced Neural Functional Injury: A Pivotal Role for AMPK and JAK2/STAT3/NF-κB Signaling Pathway Modulation. Drug Des. Dev. Ther. 2020, 14, 2865–2876. [Google Scholar] [CrossRef]
- Lombardo, G.; Gili, M.; Grange, C.; Cavallari, C.; Dentelli, P.; Togliatto, G.; Taverna, D.; Camussi, G.; Brizzi, M.F. IL-3R-α blockade inhibits tumor endothelial cell-derived extracellular vesicle (EV)-mediated vessel formation by targeting the β-catenin pathway. Oncogene 2018, 37, 1175–1191. [Google Scholar] [CrossRef]
- Gao, T.; Fan, H.; Wang, J.; Wang, R. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells stimulate angiogenesis in myocardial infarction via the microRNA-423-5p/EFNA3 axis. Postep. Kardiol. Interwencyjnej 2022, 18, 373–391. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Sun, J. Human bone marrow mesenchymal stem cell-derived exosomes stimulate cutaneous wound healing mediates through TGF-β/Smad signaling pathway. Stem Cell Res. Ther. 2020, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, J.; Zeng, F.; Wang, P.; Guo, X.; Wang, H.; Qin, Z.; Tao, T. Human PMSCs-derived small extracellular vesicles alleviate neuropathic pain through miR-26a-5p/Wnt5a in SNI mice model. J. Neuroinflamm. 2022, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, K.; Inouye, K.; Takhmazyan, T.; Bonavida, V.; Yang, J.W.; de Barros, N.R.; Thankam, F.G. Engineered Vesicles and Hydrogel Technologies for Myocardial Regeneration. Gels 2023, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Zhao, C.; Liu, H.; Fan, Y. Endothelial Progenitor Cell-Derived Extracellular Vesicles: A Novel Candidate for Regenerative Medicine and Disease Treatment. Adv. Healthc. Mater. 2020, 9, e2000255. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Valgimigli, M.; Giannico, M.B.; Zaccone, V.; Perfetti, M.; D’Amario, D.; Rebuzzi, A.G.; Crea, F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J. 2009, 30, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, R.; Jiang, L.; Xu, Q.; Guo, X. Endothelial repair by stem and progenitor cells. J. Mol. Cell Cardiol. 2022, 163, 133–146. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, W.; Chen, J.; Ma, R.; Xiao, X.; Ma, X.; Yao, Z.; Chen, Y. EPC-derived microvesicles protect cardiomyocytes from Ang II-induced hypertrophy and apoptosis. PLoS ONE 2014, 9, e85396. [Google Scholar] [CrossRef]
- Deregibus, M.C.; Cantaluppi, V.; Calogero, R.; Lo Iacono, M.; Tetta, C.; Biancone, L.; Bruno, S.; Bussolati, B.; Camussi, G. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007, 110, 2440–2448. [Google Scholar] [CrossRef]
- Bearzi, C.; Rota, M.; Hosoda, T.; Tillmanns, J.; Nascimbene, A.; De Angelis, A.; Yasuzawa-Amano, S.; Trofimova, I.; Siggins, R.W.; Lecapitaine, N.; et al. Human cardiac stem cells. Proc. Natl. Acad. Sci. USA 2007, 104, 14068–14073. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Rokosh, G.; Sanganalmath, S.K.; Yuan, F.; Sato, H.; Mu, J.; Dai, S.; Li, C.; Chen, N.; Peng, Y.; et al. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation 2010, 121, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Honda, A.; Nagai, T.; Fukushima, N.; Iwanaga, K.; Tokunaga, M.; Shimizu, T.; Okano, T.; Kasanuki, H.; Hagiwara, N.; et al. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J. Clin. Investig. 2009, 119, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Maring, J.A.; Beez, C.M.; Falk, V.; Seifert, M.; Stamm, C. Myocardial Regeneration via Progenitor Cell-Derived Exosomes. Stem Cells Int. 2017, 2017, 7849851. [Google Scholar] [CrossRef] [PubMed]
- Lazar, E.; Benedek, T.; Korodi, S.; Rat, N.; Lo, J.; Benedek, I. Stem cell-derived exosomes—An emerging tool for myocardial regeneration. World J. Stem Cells 2018, 10, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Tarvirdizadeh, T.; Pandey, R.; Mentkowski, K.I.; Schiffmacher, P.; Eagler, L.; Reynolds, J.; Lang, J.K. Abstract 15771: Cardiosphere-Derived Cell Extracellular Vesicles (CDC-EVs) Alter C-C Chemokine Receptor Type 2 (CCR2) Mediated Monocyte Pro-Inflammatory Signaling Following Cardiac Injury. Circulation 2023, 148 (Suppl. S1), A15771. [Google Scholar] [CrossRef]
- Khayambashi, P.; Iyer, J.; Pillai, S.; Upadhyay, A.; Zhang, Y.; Tran, S.D. Hydrogel Encapsulation of Mesenchymal Stem Cells and Their Derived Exosomes for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 684. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 6480. [Google Scholar] [CrossRef]
- Zhang, B.; Tian, X.; Hao, J.; Xu, G.; Zhang, W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant. 2020, 29, 963689720908500. [Google Scholar] [CrossRef]
- Lelek, J.; Zuba-Surma, E.K. Perspectives for Future Use of Extracellular Vesicles from Umbilical Cord- and Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells in Regenerative Therapies-Synthetic Review. Int. J. Mol. Sci. 2020, 21, 799. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.; Al-Massri, K. New Approaches for Enhancement of the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cardiovascular Diseases. Tissue Eng. Regen. Med. 2022, 19, 1129–1146. [Google Scholar] [CrossRef] [PubMed]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Saadh, M.J.; Ramirez-Coronel, A.A.; Saini, R.S.; Arias-Gonzales, J.L.; Amin, A.H.; Gavilan, J.C.O.; Sarbu, I. Advances in mesenchymal stem/stromal cell-based therapy and their extracellular vesicles for skin wound healing. Hum. Cell 2023, 36, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Nooshabadi, V.T.; Mardpour, S.; Yousefi-Ahmadipour, A.; Allahverdi, A.; Izadpanah, M.; Daneshimehr, F.; Ai, J.; Banafshe, H.R.; Ebrahimi-Barough, S. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J. Cell Biochem. 2018, 119, 8048–8073. [Google Scholar] [CrossRef] [PubMed]
- Bruun, K.; Schermer, E.; Sivendra, A.; Valaik, E.; Wise, R.B.; Said, R.; Bracht, J.R. Therapeutic applications of adipose-derived stem cells in cardiovascular disease. Am. J. Stem Cells 2018, 7, 94–103. [Google Scholar] [PubMed]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Ma, T.; Sun, J.; Zhao, Z.; Lei, W.; Chen, Y.; Wang, X.; Yang, J.; Shen, Z. A brief review: Adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res. Ther. 2017, 8, 124. [Google Scholar] [CrossRef]
- Luo, Q.; Guo, D.; Liu, G.; Chen, G.; Hang, M.; Jin, M. Exosomes from MiR-126-Overexpressing Adscs Are Therapeutic in Relieving Acute Myocardial Ischaemic Injury. Cell Physiol. Biochem. 2017, 44, 2105–2116. [Google Scholar] [CrossRef]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Tsiapalis, D.; O’Driscoll, L. Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells 2020, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Coly, P.M.; Boulanger, C.M. Role of extracellular vesicles in atherosclerosis: An update. J. Leukoc. Biol. 2022, 111, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Konkoth, A.; Saraswat, R.; Dubrou, C.; Sabatier, F.; Leroyer, A.S.; Lacroix, R.; Duchez, A.C.; Dignat-George, F. Multifaceted role of extracellular vesicles in atherosclerosis. Atherosclerosis 2021, 319, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Procyk, G.; Bilicki, D.; Balsam, P.; Lodzinski, P.; Grabowski, M.; Gasecka, A. Extracellular Vesicles in Atrial Fibrillation-State of the Art. Int. J. Mol. Sci. 2022, 23, 7591. [Google Scholar] [CrossRef] [PubMed]
- Manchon, E.; Hirt, N.; Bouaziz, J.D.; Jabrane-Ferrat, N.; Al-Daccak, R. Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases. Int. J. Mol. Sci. 2021, 22, 3130. [Google Scholar] [CrossRef] [PubMed]
- Bei, Y.; Das, S.; Rodosthenous, R.S.; Holvoet, P.; Vanhaverbeke, M.; Monteiro, M.C.; Monteiro, V.V.S.; Radosinska, J.; Bartekova, M.; Jansen, F.; et al. Extracellular Vesicles in Cardiovascular Theranostics. Theranostics 2017, 7, 4168–4182. [Google Scholar] [CrossRef]
- Terriaca, S.; Fiorelli, E.; Scioli, M.G.; Fabbri, G.; Storti, G.; Cervelli, V.; Orlandi, A. Endothelial Progenitor Cell-Derived Extracellular Vesicles: Potential Therapeutic Application in Tissue Repair and Regeneration. Int. J. Mol. Sci. 2021, 22, 6375. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).