Abstract
The hybridization of inorganic and organic components is a promising strategy to build functional materials. Among several functions, luminescence is an important function which should be considered for practical usage. Inorganic–organic hybrid luminescent materials have been investigated as phosphors, sensors, and lasers. Organic luminescent centers such as dye molecules have often been hybridized with inorganic matrices. Polyoxometalate anions (POMs) are effective inorganic luminescent centers due to their luminescent properties and structural designability. However, most luminescent POM components are limited to lanthanide-based POMs. In this report, a photoluminescent inorganic–organic hybrid crystal based on a non-lanthanide POM was successfully synthesized as a single crystal. Anderson-type hexamolybdochromate ([CrMo6O18(OH)6]3−, CrMo6) anion exhibiting emission derived from Cr3+ was utilized with n-dodecylammonium ([C12H25NH3]+, C12NH3) surfactant cation to obtain a photoluminescent hybrid crystal. The grown single crystal of C12NH3-CrMo6 comprised a distinct layered structure consisting of inorganic CrMo6 layers and interdigitated C12NH3 layers. In the CrMo6 layers, the CrMo6 anions were associated with water molecules by hydrogen bonding to form a densely packed two-dimensional network. Steady-state and time-resolved photoluminescence spectroscopy revealed that the C12NH3-CrMo6 hybrid crystal exhibited characteristic emission from the CrMo6 anion. Preliminary lasing properties were also observed for C12NH3-CrMo6, which shows the possibility of using the C12NH3-CrMo6 hybrid crystal as an inorganic–organic hybrid laser.
1. Introduction
Synthetic methodology is crucial for the construction of functional compounds and/or materials. The hybridization of inorganic and organic components is a promising strategy to build functional materials [1,2,3]. Unprecedented functions can emerge from the combination of inorganic and organic components. Inorganic motifs can contribute to thermal stability and elemental variation, whereas organic motifs enable flexible molecular design. The synergy of inorganic and organic characteristics has been realized in conductive [4], adsorbent [5], and magnetic materials [6].
Luminescence is another important function which should be considered for several reasons. Inorganic–organic hybrid materials have been investigated as phosphors, sensors, and lasers [7,8,9,10]. Organic luminophores or dyes are often utilized in inorganic matrices [11]. Several inorganic–organic hybrids contain inorganic luminescent centers such as lanthanides, which behave as distinct luminescent centers exhibiting sharp spectra due to the transitions of inner-shell 4f electrons [12,13,14,15].
Polyoxometalate molecular clusters (POMs) are also effective inorganic luminescent centers due to their luminescent properties and structural designability [16,17,18]. Lanthanide-containing POMs [19,20,21] are typically employed, and several luminescent inorganic–organic hybrids have been reported [22,23]. Anderson-type POMs [24,25,26] are another class of luminescent POMs. Hexamolybdochromate [CrMo6O18(OH)6]3− (CrMo6, Figure 1a) anion comprises Cr3+ surrounded by six oxygen atoms exhibiting red emission similar to ruby lasers [27,28,29,30,31]. In addition, Anderson-type POMs possess planar molecular shapes leading to layered structures [32,33,34,35]. Layered packing of luminescent species gives rise to anisotropic emission, which is beneficial for lasing properties. However, Anderson-type POMs have rarely been employed as luminescent centers in inorganic–organic hybrid materials [30,31,35].
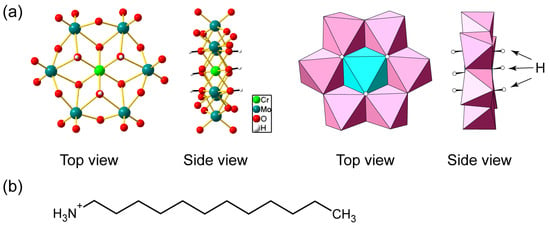
Figure 1.
(a) Molecular structure of hexamolybdochromate (CrMo6) anion. Mo: teal, Cr: light green, O: red, H: white in ball-and-stick representation, Mo: pink, and Cr: light blue in polyhedral representation; (b) molecular structure of n-dodecylammonium (C12NH3) cation.
In this report, we constructed an inorganic–organic hybrid with a luminescent Anderson-type CrMo6 anion and surfactant cation. The use of n-dodecylammonium ([C12H25NH3]+, C12NH3, Figure 1b) derived from aliphatic primary amines enabled the crystallization of the CrMo6-surfactant hybrid as single crystals. The C12NH3-CrMo6 hybrid crystal possessed a distinct layered structure due to the planar CrMo6 anion and the structure-directing C12NH3 surfactant. The emission properties, including the preliminary lasing behavior, were investigated.
2. Results
2.1. Synthesis of C12NH3-CrMo6 Hybrid Crystal
The C12NH3-CrMo6 hybrid crystal was obtained by an ion exchange reaction between the sodium salt of CrMo6 (Na3[CrMo6O18(OH)6]·8H2O, Na-CrMo6) and C12NH3 cations. The single crystals of C12NH3-CrMo6 were successfully grown from the synthetic filtrate after the removal of a precipitate of the C12NH3-CrMo6 hybrid crystal. The IR spectra (Figure 2) exhibited characteristic peaks of CrMo6 (400–1100 cm−1), which were assigned as terminal Mo–Ot bonds (950–890 cm−1) and bridging Mo–O–Mo bonds (700–400 cm−1) [36,37,38]. Characteristic peaks of C12NH3 were observed in the range of 2800–3000 cm−1, 2920 cm−1 for νas(−CH2−), and 2850 cm−1 for νs(−CH2−), respectively. The almost identical IR spectra of the precipitate (Figure 2b) and single crystal (Figure 2c) indicated that the precipitate and single crystal of the C12NH3-CrMo6 hybrid were the same in terms of their molecular structures.
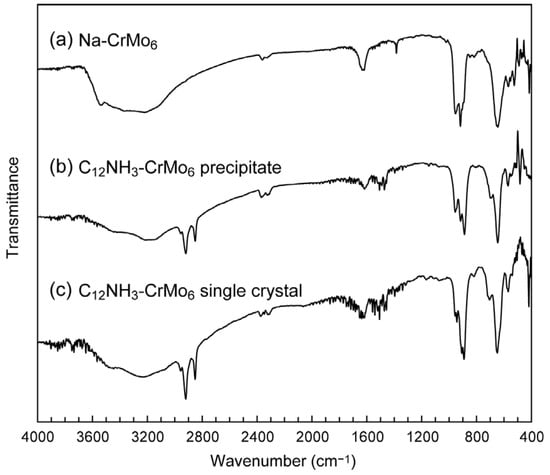
Figure 2.
IR spectra of C12NH3-CrMo6 hybrid crystal: (a) starting material of Na-CrMo6; (b) precipitate of C12NH3-CrMo6; (c) single crystal of C12NH3-CrMo6.
The precipitate and single crystal of the C12NH3-CrMo6 hybrid crystal exhibited slightly different XRD patterns (Figure 3a,b). Both XRD patterns showed strong peaks assignable to 001 and 002 reflections in the lower 2-theta range featuring layered materials. Estimated interlayer spacing was 24.5 Å for the C12NH3-CrMo6 precipitate and 26.6 Å for the C12NH3-CrMo6 single crystal, respectively. The XRD pattern of the C12NH3-CrMo6 single crystal was similar to that calculated from the single-crystal X-ray analysis data (Figure 3c). Subtle differences in the peak intensity and position of the patterns may be due to the difference in the measurement temperature (powder: room temperature, single crystal: 103 K), and to the preferred orientation derived from the predominant layered structure of C12NH3-CrMo6.
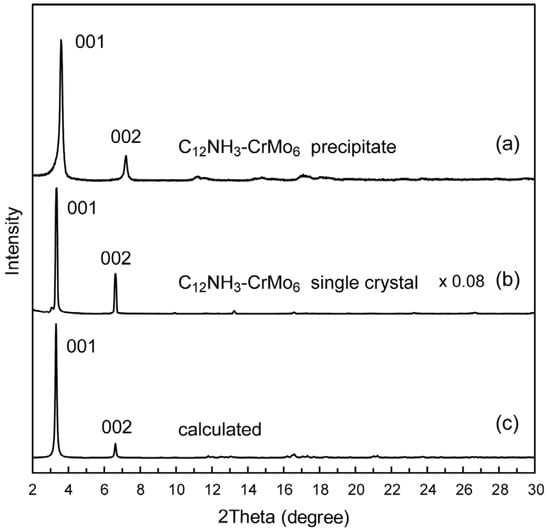
Figure 3.
Powder X-ray diffraction patterns of C12NH3-CrMo6 hybrid crystal: (a) precipitate of C12NH3-CrMo6; (b) single crystal of C12NH3-CrMo6; (c) calculated pattern of C12NH3-CrMo6 from the structure revealed by single-crystal X-ray diffraction.
2.2. Crystal Structure of C12NH3-CrMo6 Hybrid Crystal
The presence and molecular structure of the CrMo6 anion were revealed via X-ray structural analysis (Table 1, Figure 4). The asymmetric unit consisted of two half-anions of CrMo6 and three C12NH3 cations including water of crystallization (Figure 4a). This indicates that one CrMo6 anion (3− charge) was connected with the three C12NH3 cations due to charge compensation, which was verified by CHN elemental analysis. Four water molecules (O25, O26, O27, and O28) were assigned unambiguously. Some terminal atoms (C11 and C12) in the C12NH3 dodecyl chain were disordered, and a small electron density (2.62 eÅ−3) remained ca. 2.8 Å away from C12A in the main part of the disordered atoms (Supplementary Material, Figure S1). We assigned this small electron density to a disordered water molecule (O29B) in the minor disordered part together with C11B and C12B, which was refined to have a site occupancy of 0.32. Therefore, the C12NH3-CrMo6 single crystal contained 4.32 water molecules per CrMo6 anion, leading to the chemical formula of [C12H25NH3]3[CrMo6O18(OH)6]·4.32H2O. The six H atoms in the hydroxo group of CrMo6 were identified using the X-ray diffraction analysis and bond valence sum (BVS) calculations [39]. The BVS values of the protonated O atoms (O8, O11, O12, O16, O23, and O24) in CrMo6 were in the range of 1.19–1.28, whereas those for other O atoms were in the range of 1.65–1.86.

Table 1.
Crystallographic data.
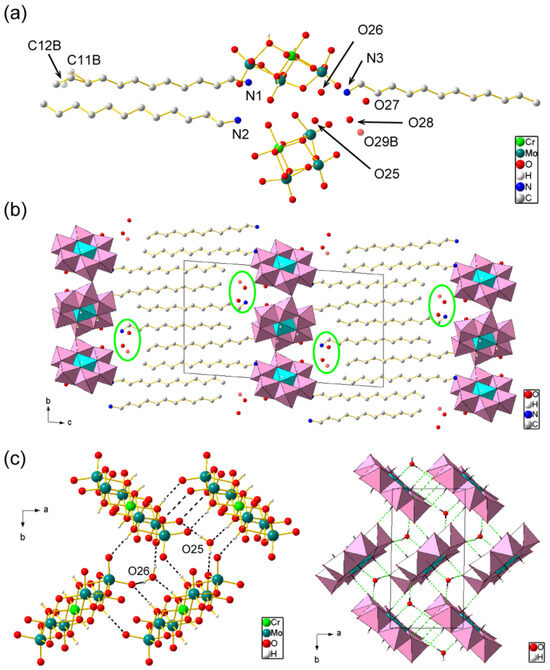
Figure 4.
Crystal structure of C12NH3-CrMo6 (Mo: teal, Cr: light green, C: gray, N: blue, O: red, and H: white). H atoms of C12NH3 cations and solvents were omitted for clarity: (a) asymmetric unit. Disordered atoms (C11B, C12B, and O29B) in the minor part are indicated in transparent color; (b) packing diagram along a-axis. CrMo6 anions are depicted in a polyhedral model (Mo: pink; Cr: light blue). Some solvents (O27, O28, and O29B) are highlighted by green circles; (c) molecular arrangement of the inorganic monolayer (ab plane) in ball-and-stick (left) and polyhedral (right) representations. Broken lines represent short contacts including O–H···O hydrogen bonding between the CrMo6 anions and solvents.
The crystal of C12NH3-CrMo6 was composed of alternating CrMo6 inorganic monolayers and C12NH3 organic bilayers parallel to the ab plane (Figure 4b), as typically observed for surfactant-POM hybrid single crystals [40]. The layered periodicity was 26.7 Å, which was consistent with the powder XRD pattern (Figure 3b). The aliphatic chains of C12NH3 were interdigitated in a straight line. Some solvent water molecules (O27, O28, and O29B) were located at the interface between the CrMo6 and C12NH3 layers (Figure 4b). On the other hand, two water molecules (O25 and O26) were located inside the inorganic CrMo6 monolayer. These CrMo6 anions and water molecules were densely packed to form a two-dimensional network via short contacts, including O–H⋯O hydrogen bonding (Figure 4c) [41]. The O⋯O distance ranged from 2.71 to 3.00 Å (mean value: 2.80 Å). The hydrophilic heads of C12NH3 did not penetrate the densely packed CrMo6-H2O monolayers, but were located at the dip between the CrMo6 anions [42] by forming N–H⋯O hydrogen bonds with distances of 2.71–2.84 Å (mean value: 2.78 Å) [41].
2.3. Photoluminescent Properties of C12NH3-CrMo6 Hybrid Crystal
As mentioned above, the CrMo6 anion exhibits a distinct red emission due to the presence of Cr3+ like ruby lasers [27,28,29,30,31]. The photoluminescent properties of C12NH3-CrMo6 were evaluated using steady-state and time-resolved spectroscopy. The diffuse reflectance spectra (Figure 5a) of both C12NH3-CrMo6 and Na-CrMo6 (starting material) showed broad peaks around 390 and 540 nm, corresponding to the transitions of the d3 electron in Cr3+ from the 4A2 ground state to the 4T1 and 4T2 excited states, respectively [27]. These electron transitions were also observed in the excitation spectra of C12NH3-CrMo6 and Na-CrMo6, as shown in Figure 5b. Characteristic emission derived from Cr3+ was observed around 680–710 nm (Figure 5b), which was investigated in detail using time-resolved spectroscopy.
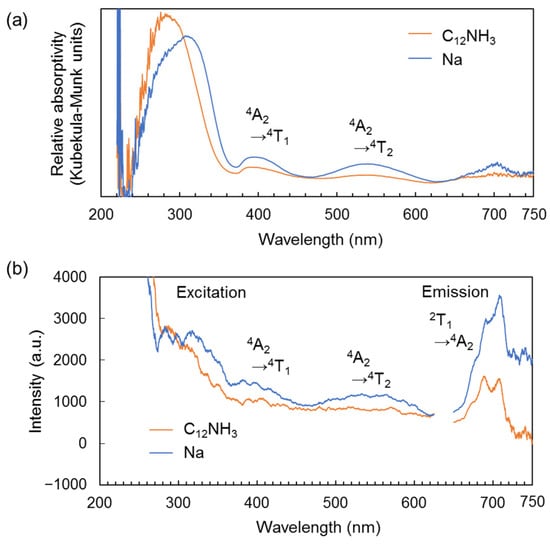
Figure 5.
Steady-state spectra of C12NH3-CrMo6 and Na-CrMo6 (starting material). The measurement temperature was 300 K: (a) diffuse reflectance spectra; (b) excitation and emission spectra. Excitation spectra were monitored for the emission at 705 nm. Emission spectra were measured with an excitation wavelength of 300 nm.
Figure 6a shows the emission spectra of C12NH3-CrMo6 and Na-CrMo6 obtained using a single pulse excitation. Emission peaks at around 682, 688, 701, and 707 nm were derived from the 2T1 → 4A2 transition known as “R-lines” [27,28,29,30,31]. The peaks at around 730 nm were assigned to the 2E → 4A2 transition. The spectral shapes of C12NH3-CrMo6 were almost identical to those of Na-CrMo6 irrespective of the measurement temperatures. On the other hand, the emission intensity of C12NH3-CrMo6 was lower than that of Na-CrMo6 (Figure 6a), and the emission of C12NH3-CrMo6 decayed faster than that of Na-CrMo6 at each temperature (Figure 6b). The estimated emission lifetimes were 26 μs (15 K) and 11 μs (300 K) for C12NH3-CrMo6 and 55 μs (15 K) and 24 μs (300 K) for Na-CrMo6.
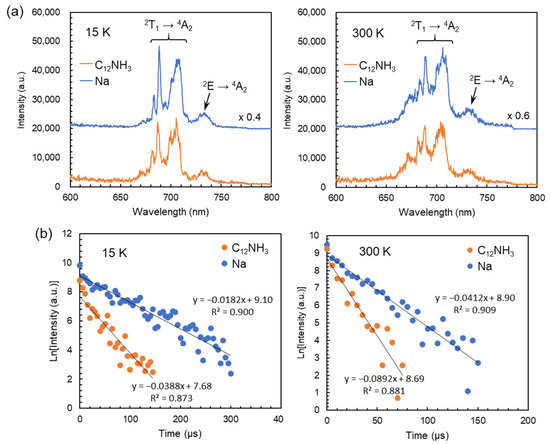
Figure 6.
Photoluminescence properties of C12NH3-CrMo6 and Na-CrMo6 (starting material) investigated using time-resolved spectroscopy. Each spectrum or decay profile was obtained using a single-pulse excitation with a wavelength of 266 nm: (a) emission spectra measured at 15 K (left) and 300 K (right) with an acquisition time of 0–50 μs; (b) emission decay profiles measured at 15 K (left) and 300 K (right) on the emission at 688 nm.
The title compound C12NH3-CrMo6 had a distinct layered structure together with characteristic emission as described above, which is associated with the plausible emergence of lasing properties. To evaluate the possibility of inorganic–organic hybrid laser materials, the emission intensity of C12NH3-CrMo6 was explored by changing the excitation laser power. Figure 7 shows the emission intensity-excitation laser power dependency at 15 and 300 K. The emission intensity increased linearly with an increase in the excitation laser power above the threshold value, which indicates the emergence of preliminary lasing properties [43,44,45,46]. The threshold values were 22.4 mJ cm−2 for 15 K and 26.7 mJ cm−2 for 300 K, respectively.
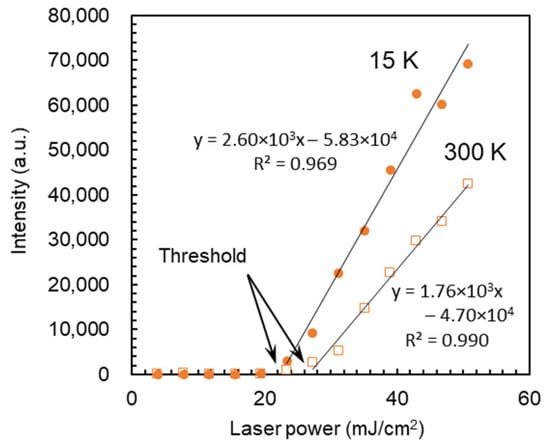
Figure 7.
Emission intensity-excitation laser power dependency of C12NH3-CrMo6 at 15 and 300 K. Each data point was obtained using a single-pulse excitation with a wavelength of 266 nm on the emission at 688 nm. Data acquisition time: 0–50 μs.
3. Discussion
We first synthesized a photoluminescent polyoxometalate-surfactant hybrid crystal by using an Anderson-type CrMo6 anion and C12NH3 surfactant cation. Using primary alkylammonium cations seems essentially effective for the crystallization of the CrMo6 anion with surfactant cations. The powder XRD patterns of the C12NH3-CrMo6 hybrid crystal (Figure 3) suggest that the precipitates and single crystals of the C12NH3-CrMo6 hybrid crystal had slightly different phases in their interlayer periodicities. The interlayer spacing was 24.5 Å for the precipitate and 26.6 Å for the single crystal. The precipitate and single crystal possessed distinct layered structures, both of which are believed to be essentially similar, except for the interlayer distances. The difference is derived from the desorption of water molecules (O27, O28, and O29B highlighted in Figure 4b) from the crystalline lattice, shrinking the layered distance to 24.5 Å of the precipitate from 26.6 Å of the single crystal.
Single-crystal X-ray diffraction analysis confirmed a distinct layered structure consisting of the CrMo6 inorganic layers and C12NH3 organic layers. Notably, the CrMo6 anions formed a two-dimensional infinite layer with the water molecules through O–H⋯O hydrogen bonding in the CrMo6 inorganic layers. The hydrophilic heads of the C12NH3 surfactant did not penetrate the inorganic CrMo6-H2O monolayers as observed in another POM crystal hybridized with C12NH3 [42]. This implies that a densely packed POM inorganic layer is easily formed by using a primary alkylammonium cation with a smaller hydrophobic head [40].
The photoluminescent properties of C12NH3-CrMo6 were investigated by steady-state and time-resolved spectroscopy, revealing the characteristic ruby-like emission derived from Cr3+ in the CrMo6 anion. As shown in Figure 6a, the emission spectra of C12NH3-CrMo6 were almost identical in shape to those of Na-CrMo6 irrespective of the measurement temperature. This suggests that the crystal fields of Cr3+ in C12NH3-CrMo6 and Na-CrMo6 were similar due to the structural rigidity of the CrMo6 anion. The spectral shape of the C12NH3-CrMo6 emission was similar to that of previous compounds containing the CrMo6 anion [29,30,31]. On the other hand, the emission lifetimes of C12NH3-CrMo6 (26 μs at 15 K and 11 μs at 300 K) were shorter than those (55 μs at 15 K and 24 μs at 300 K) of Na-CrMo6 and another inorganic–organic hybrid consisting of CrMo6 anions (240 μs) [30]. These results indicate that the emission efficiency of the CrMo6 anion depends on the countercation and that the C12NH3 cation reduces the emission efficiency. In C12NH3-CrMo6, nonradiative deactivation of the excitation energy (O → Mo ligand-to-metal charge transfer) occurs more easily than in Na-CrMo6 through the vibration states of the high-frequency C–H oscillators in C12NH3 [35].
The distinct layered structure of the C12NH3-CrMo6 hybrid crystal is beneficial for the emergence of lasing properties. Preliminary lasing behavior was observed by changing the excitation laser power, exhibiting the lasing threshold values of 22.4 mJ cm−2 at 15 K and 26.7 mJ cm−2 at 300 K, respectively (Figure 7). These threshold values were much larger than those of recent organic lasers (order of μJ cm−2) [44,45,46], indicating a lower lasing efficiency in C12NH3-CrMo6, probably due to the use of randomly oriented crystalline powder [47]. In the future, the fabrication of large single crystals [48,49] in higher yields or the construction of microcavity structures [47] will be a promising strategy to improve the lasing emission efficiency. The thermal gravimetric (TG) profiles (Figure S2) suggest that the hybrid crystal of C12NH3-CrMo6 started to decompose from ca. 423 K (150 °C) due to the removal of the C12NH3 cation. A temperature of 423 K (150 °C) may be rather low for the removal of the C12NH3 cation, and the details of this phenomenon are still unclear. Our previous results showed that three hybrid crystals consisting of decavanadate anions and double-headed primary ammonium cation started to decompose from ca. 473 K (200 °C) [50]. In these cases, the organic ammonium cation was divalent and more strongly associated with the polyoxometalate anion than the monovalent C12NH3 cation. Therefore, we speculate that the C12NH3-CrMo6 hybrid crystal could start to decompose due to the removal of the C12NH3 cation at ca. 423 K (150 °C). The thermal stability of the C12NH3-CrMo6 hybrid crystal may be improved by blending C12NH3-CrMo6 with other polymers or glassy matrices to dilute the C12NH3-CrMo6 component. Although a more sophisticated fabrication process should be applied to improve the lasing efficiency, the C12NH3-CrMo6 hybrid crystal has shown potential as another category of inorganic–organic hybrid laser materials.
4. Materials and Methods
4.1. Materials
Chemical reagents including n-dodecylammonium chloride (C12NH3-Cl) were obtained from commercial sources (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan, and Tokyo Chemical Industry Co., Ltd. (TCI), Tokyo, Japan) and used without further purification. The sodium salt of CrMo6 (Na3[CrMo6O18(OH)6]·8H2O, Na-CrMo6) was prepared according to the literature [26,27]: Na2MoO4·2H2O (36.3 g, 150 mmol) was dissolved in 75 mL of H2O, and the solution pH was adjusted to 4.4 with HNO3. An aqueous solution (10 mL) containing 10 g of Cr(NO3)3·9H2O (25.0 mmol) was added to a pH-adjusted solution, and the solution was sequentially heated at 353 K for 1 h. The resultant supernatant was obtained by filtration and kept at room temperature for a week to isolate pink-purple block crystals of Na-CrMo6 (7.0 g, yield: 23% based on Mo).
4.2. Synthesis of C12NH3-CrMo6 Hybrid Crystal
An ethanol solution (30 mL) of C12NH3-Cl (0.11 g, 0.50 mmol) was added to an aqueous solution (30 mL) of Na-CrMo6 (0.34 g, 0.28 mmol) with heating at 333 or 343 K, and stirred for 5 min. The resultant suspension was filtrated and dried in the air to obtain a pale pink precipitate of C12NH3-CrMo6 (0.21–0.25 g, yield 45–54%). Single crystals of C12NH3-CrMo6 were grown from the synthetic filtrate kept at 315 or 323 K (0.01 g, yield ca. 2%). Some water molecules were removed from the crystal lattice in an ambient atmosphere. Anal. Calcd for C36H94N3CrMo6O24: C, 26.81; H, 5.87; N, 2.61%. Found: C, 26.85; H, 5.75; N, 2.69%. IR (KBr disk): 956 (m), 917 (s), 888 (s), 806 (w), 693 (m), 646 (s), 570 (w), 551 (w), 518 (w), and 416 (w) cm−1.
4.3. Measurements
Infrared (IR) spectra were measured using an FT/IR-4200ST spectrometer (Jasco Corporation, Tokyo, Japan, KBr pellet method). Powder X-ray diffraction (XRD) patterns were recorded using a MiniFlex300 diffractometer (Rigaku Corporation, Tokyo, Japan, Cu Kα radiation, λ = 1.54056 Å) under an ambient atmosphere. CHN (carbon, hydrogen, and nitrogen) elemental analyses were performed using a 2400II elemental analyzer (PerkinElmer, Inc., Waltham, MA, USA). Thermal gravimetric (TG) analyses were carried out on a TG/DTA-6200 (Seiko Instruments, Chiba, Japan) at a heating rate of 10 °C min−1 in a nitrogen atmosphere.
Steady-state diffuse reflectance, excitation, and emission spectra were recorded at 300 K using an FP-6500 fluorescence spectrometer (Jasco Corporation, Tokyo, Japan) equipped with an Xe lamp. Time-resolved emission spectra were obtained at 15 and 300 K with an Ultra CFR 400 YAG:Nd3+ laser (Big Sky Laser Technologies, Inc., Bozeman, MT, USA, 266 nm fourth harmonics, pulse duration 10 ns with a repetition rate of 10 Hz) as an excitation source. A Spectra Pro 2300i (Princeton Instruments, Inc., Trenton, NJ, USA) was utilized as a spectrometer, and a PI-Max with an intensified CCD camera (Princeton Instruments, Inc., Trenton, NJ, USA) was used as a detector. Pelletized samples of the C12NH3-CrMo6 precipitate were used for the aforementioned photoluminescence measurements.
4.4. X-ray Crystallography
Single crystal X-ray diffraction was measured with an XtaLAB PRO P200 diffractometer (Rigaku Corporation, Tokyo, Japan) using graphite monochromated Mo Kα radiation (λ = 0.71073 Å). The acquisition and processing of diffraction images including absorption correction were performed using CrysAlisPro (Version 1.171.39.46) [51]. Crystal structures were solved using SHELXT (Version 2018/2) [52] and refined by the full-matrix least-squares using SHELXL (Version 2018/3) [53]. The diffraction data recorded at the 2D beamline of the Pohang Accelerator Laboratory (PAL) confirmed the same crystal structure. CCDC 2312544.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25010345/s1.
Author Contributions
Conceptualization, T.I; methodology, A.M., K.M. and T.S.; validation, T.K., Y.S. and T.I.; formal analysis, T.I., T.K., Y.S., H.I., Y.O. (Yoshiki Oda) and Y.O. (Yosuke Okamura); investigation, A.M., T.K., Y.S., K.M., T.S., N.M. and A.W.; resources, Y.S., Y.O. (Yoshiki Oda) and Y.O. (Yosuke Okamura); writing—original draft preparation, T.I.; writing—review and editing, T.I. and Y.S.; visualization, T.I., Y.S. and A.M.; funding acquisition, T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI (grant numbers JP26410245 and JP21K05232), and the JSPS Core-to-Core Program ((grant number A31-4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Further details of the crystal structure investigation (CCDC 2312544) can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 7 December 2023), or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033.
Acknowledgments
X-ray diffraction measurements with synchrotron radiation were performed at the Pohang Accelerator Laboratory (Beamline 2D, proposal No. 2019-3rd-2D-M003), a synchrotron radiation facility in Pohang, Republic of Korea, supported by Pohang University of Science and Technology (POSTECH).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sanchez, C.; Soler-Illia, G.J.A.A.; Ribot, F.; Lalot, T.; Mayer, C.R.; Cabuil, V. Designed hybrid organic-inorganic nanocomposites from functional nanobuilding blocks. Chem. Mater. 2001, 13, 3061–3083. [Google Scholar] [CrossRef]
- Coronado, E.; Gómez-García, C.J. Polyoxometalate-based molecular materials. Chem. Rev. 1998, 98, 273–296. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Inagaki, S. Self-organization of organosilica solids with molecular-scale and mesoscale periodicities. Chem. Mater. 2008, 20, 891–908. [Google Scholar] [CrossRef]
- Horike, S.; Umeyama, D.; Kitagawa, S. Ion conductivity and transport by porous coordination polymers and metal-organic frameworks. Acc. Chem. Res. 2013, 46, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Coronado, E. Molecular magnetism: From chemical design to spin control in molecules, materials and devices. Nature Rev. Mater. 2020, 5, 87–104. [Google Scholar] [CrossRef]
- Ogawa, M.; Kuroda, K. Photofunctions of intercalation compounds. Chem. Rev. 1995, 95, 399–438. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.-P. Optical properties of functional hybrid organic–inorganic nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Reisfeld, R.; Weiss, A.; Saraidarov, T.; Yariv, E.; Ishchenko, A.A. Solid-state lasers based on inorganic–organic hybrid materials obtained by combined sol–gel polymer technology. Polym. Adv. Technol. 2004, 15, 291–301. [Google Scholar] [CrossRef]
- Smith, M.D.; Connor, B.A.; Karunadasa, H.I. Tuning the luminescence of layered halide perovskites. Chem. Rev. 2019, 119, 3104–3139. [Google Scholar] [CrossRef]
- Lebeau, B.; Innocenzi, P. Hybrid materials for optics and photonics. Chem. Soc. Rev. 2011, 40, 886–906. [Google Scholar] [CrossRef] [PubMed]
- Carlos, L.D.; Ferreira, R.A.; de Zea Bermudez, V.; Julian-Lopez, B.; Escribano, P. Progress on lanthanide-based organic–inorganic hybrid phosphors. Chem. Soc. Rev. 2011, 40, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Wada, Y.; Yanagida, S. Strategies for the design of luminescent lanthanide(III) complexes and their photonic applications. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 183–202. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shang, J.; Xin, J.; Xie, B.; Li, Y.; Möhwald, H. Self-assemblies of luminescent rare earth compounds in capsules and multilayers. Adv. Colloid Interface Sci. 2014, 207, 361–375. [Google Scholar] [CrossRef]
- Misra, A.; Kozma, K.; Streb, C.; Nyman, M. Beyond charge balance: Counter-cations in polyoxometalate chemistry. Angew. Chem. Int. Ed. 2020, 59, 596–612. [Google Scholar] [CrossRef] [PubMed]
- Anyushin, A.V.; Kondinski, A.; Parac-Vogt, T.N. Hybrid polyoxometalates as post-functionalization platforms: From fundamentals to emerging applications. Chem. Soc. Rev. 2020, 49, 382–432. [Google Scholar] [CrossRef]
- Cameron, J.M.; Guillemot, G.; Galambos, T.; Amin, S.S.; Hampson, E.; Mall Haidaraly, K.; Newton, G.N.; Izzet, G. Supramolecular assemblies of organo-functionalised hybrid polyoxometalates: From functional building blocks to hierarchical nanomaterials. Chem. Soc. Rev. 2022, 51, 293–328. [Google Scholar] [CrossRef]
- Yamase, T. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–325. [Google Scholar] [CrossRef]
- Zhao, J.-W.; Li, Y.-Z.; Chen, L.-J.; Yang, G.-Y. Research progress on polyoxometalate-based transition-metal–rare-earth heterometallic derived materials: Synthetic strategies, structural overview and functional applications. Chem. Commun. 2016, 52, 4418–4445. [Google Scholar] [CrossRef]
- Boskovic, C. Rare Earth Polyoxometalates. Acc. Chem. Res. 2017, 50, 2205–2214. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wu, L. Polyoxometalate/polymer hybrid materials: Fabrication and properties. Polym. Int. 2009, 58, 1217–1225. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Lanthanopolyoxometalates: From the structure of polyanions to the design of functional materials. Polyhedron 2013, 52, 10–24. [Google Scholar] [CrossRef]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Perloff, A. The crystal structure of sodium hexamolybdochromate(III) octahydrate, Na3(CrMo6O24H6)·8H2O. Inorg. Chem. 1970, 9, 2228–2239. [Google Scholar] [CrossRef]
- Yamase, T.; Sugeta, M. Charge-transfer photoluminescence of polyoxo-tungstates and -molybdates. J. Chem. Soc. Dalton Trans. 1993, 759–765. [Google Scholar] [CrossRef]
- Yusov, A.B.; Yin, M.; Fedosseev, A.M.; Andreev, G.B.; Shirokova, I.B.; Krupa, J.-C. Luminescence study of new Ln(III) compounds formed with Anderson’s heteropolyanions Al(OH)6Mo6O183− and Cr(OH)6Mo6O183−. J. Alloys Compd. 2002, 344, 289–292. [Google Scholar] [CrossRef]
- Tewari, S.; Adnan, M.; Balendra; Kumar, V.; Jangra, G.; Prakash, G.V.; Ramanan, A. Photoluminescence properties of two closely related isostructural series based on Anderson-Evans cluster coordinated with lanthanides [Ln(H2O)7{X(OH)6Mo6O18}]·yH2O, X = Al, Cr. Front. Chem. 2019, 6, 631. [Google Scholar] [CrossRef]
- Kumar, D.; Ahmad, S.; Prakash, G.V.; Ramanujachary, K.V.; Ramanan, A. Photoluminescent chromium molybdate cluster coordinated with rare earth cations: Synthesis, structure, optical and magnetic properties. CrystEngComm 2014, 16, 7097–7105. [Google Scholar] [CrossRef]
- Li, X.-M.; Guo, Y.; Shi, T.; Chen, Y.-G. Crystal structures and photoluminescence of two inorganic–organic hybrids of Anderson anions. J. Clust. Sci. 2016, 27, 1913–1922. [Google Scholar] [CrossRef]
- An, H.; Xiao, D.; Wang, E.; Sun, C.; Xu, L. Organic–inorganic hybrids with three-dimensional supramolecular channels based on Anderson type polyoxoanions. J. Mol. Struct. 2005, 743, 117–123. [Google Scholar] [CrossRef]
- Dessapt, R.; Gabard, M.; Bujoli-Doeuff, M.; Deniard, P.; Jobic, S. Smart heterostructures for tailoring the optical properties of photochromic hybrid organic–inorganic polyoxometalates. Inorg. Chem. 2011, 50, 8790–8796. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.; Lin, H.; Chang, Z.; Liu, G.; Wang, X. A series of novel Anderson-type polyoxometalate-based MnII complexes constructed from pyridyl-derivatives: Assembly, structures, electrochemical and photocatalytic properties. CrystEngComm 2017, 19, 3167–3177. [Google Scholar] [CrossRef]
- Ito, T.; Yashiro, H.; Yamase, T. Regular two-dimensional molecular array of photoluminescent Anderson-type polyoxometalate constructed by Langmuir-Blodgett technique. Langmuir 2006, 22, 2806–2810. [Google Scholar] [CrossRef]
- Nomiya, K.; Takahashi, T.; Shirai, T.; Niwa, M. Anderson-type heteropolyanions of molybdenum(VI) and tungsten(VI). Polyhedron 1987, 6, 213–218. [Google Scholar] [CrossRef]
- Botto, I.L.; Garcia, A.C.; Thomas, H.J. Spectroscopical approach to some heteropolymolybdates with the anderson structure. J. Phys. Chem. Solids 1992, 53, 1075–1080. [Google Scholar] [CrossRef]
- Bridgeman, A.J. Computational study of solvent effects and the vibrational spectra of Anderson Polyoxometalates. Chem. Eur. J. 2006, 12, 2094–2102. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. Sect. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Ito, T. Inorganic–organic hybrid surfactant crystals: Structural aspects and functions. Crystals 2016, 6, 24. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999; pp. 12–16. [Google Scholar]
- Ito, T.; Ide, R.; Kosaka, K.; Hasegawa, S.; Mikurube, K.; Taira, M.; Naruke, H.; Koguchi, S. Polyoxomolybdate–surfactant layered crystals derived from long-tailed alkylamine and ionic liquid. Chem. Lett. 2013, 42, 1400–1402. [Google Scholar] [CrossRef]
- Svelto, O. Principles of Lasers, 5th ed.; Springer: New York, NY, USA, 2010; Chapter 7. [Google Scholar]
- Tessler, N.; Denton, G.J.; Friend, R.H. Lasing from conjugated-polymer microcavities. Nature 1996, 382, 695–697. [Google Scholar] [CrossRef]
- Samuel, I.D.W.; Turnbull, G.A. Organic semiconductor lasers. Chem. Rev. 2007, 107, 1272–1295. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, A.J.; Gather, M.C. Organic lasers: Recent developments on materials, device geometries, and fabrication techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Song, Q.; Nie, D.; Li, F.; Bian, Z.; Liu, L.; Xu, L.; Huang, C. Synthesis of amphiphilic dye-self-assembled mesostructured powder silica with enhanced emission for directional random laser. Chem. Mater. 2008, 20, 3814–3820. [Google Scholar] [CrossRef]
- Park, S.; Kwon, O.-H.; Kim, S.; Park, S.; Choi, M.-G.; Cha, M.; Park, S.Y.; Jang, D.-J. Imidazole-based excited-state intramolecular proton-transfer materials: synthesis and amplified spontaneous emission from a large single crystal. J. Am. Chem. Soc. 2005, 127, 10070–10074. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.; Tahi, M.; Muhammad, F.; Said, S.M.; Sabri, M.F.M.; Sarker, M.R.; Ali, S.H.M.; Wahab, F. Amplified spontaneous emission and optical gain in organic single crystal quinquethiophene. Crystals 2019, 9, 609. [Google Scholar] [CrossRef]
- Kiyota, Y.; Kojima, T.; Kawahara, R.; Taira, M.; Naruke, H.; Kawano, M.; Uchida, S.; Ito, T. Porous layered inorganic-organic hybrid frameworks constructed from polyoxovanadate and bolaamphiphiles. Cryst. Growth Des. 2021, 21, 7230–7239. [Google Scholar] [CrossRef]
- CrysAlisPro; Rigaku Oxford Diffraction: Tokyo, Japan, 2015.
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).