VdPT1 Encoding a Neutral Trehalase of Verticillium dahliae Is Required for Growth and Virulence of the Pathogen
Abstract
1. Introduction
2. Results
2.1. Identification of Trehalase-Encoding Genes in V. dahliae
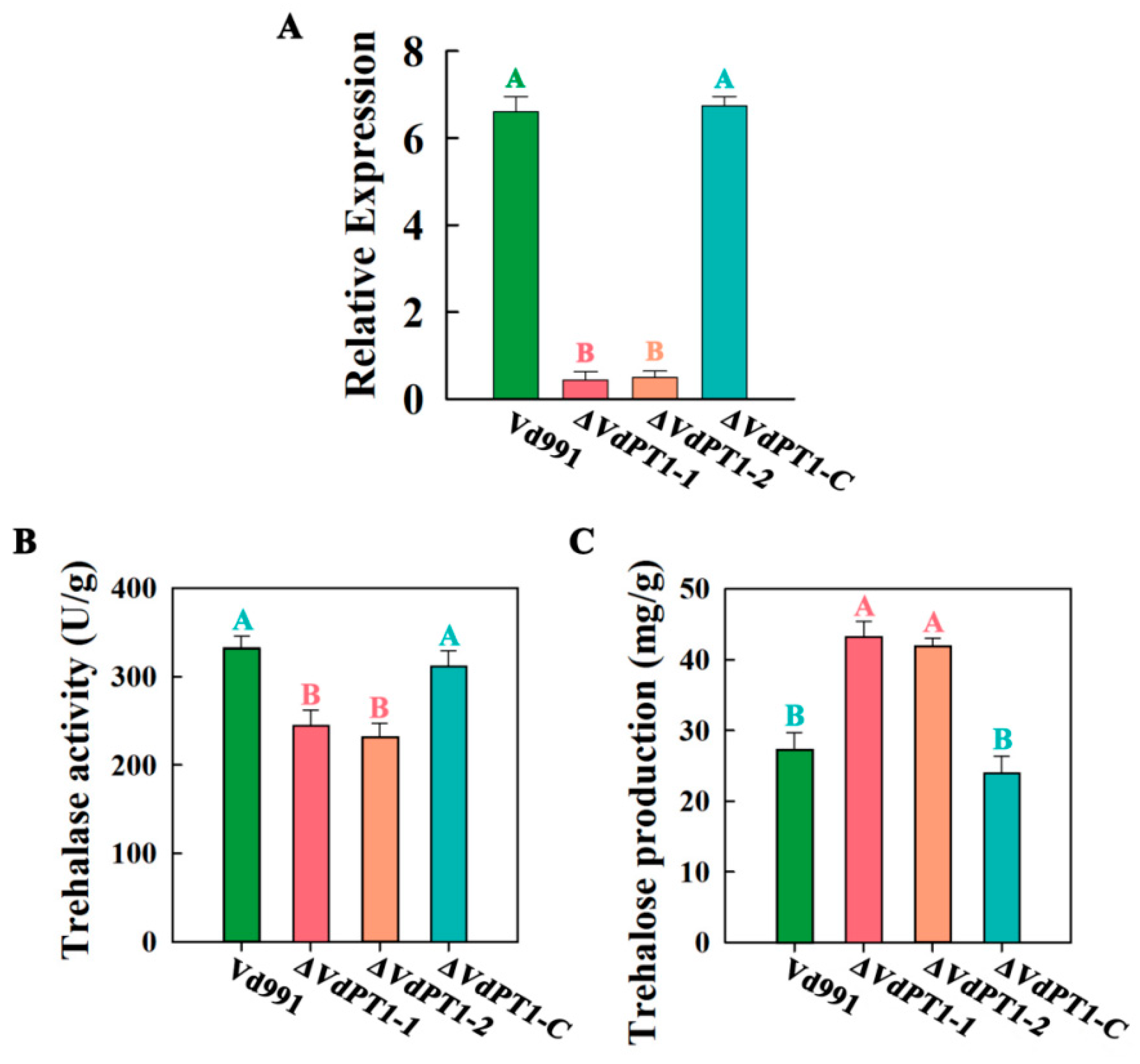
2.2. VdPT1 Deletion Mutants Had a Reduced Trehalase Activity and Increased Trehalose Content
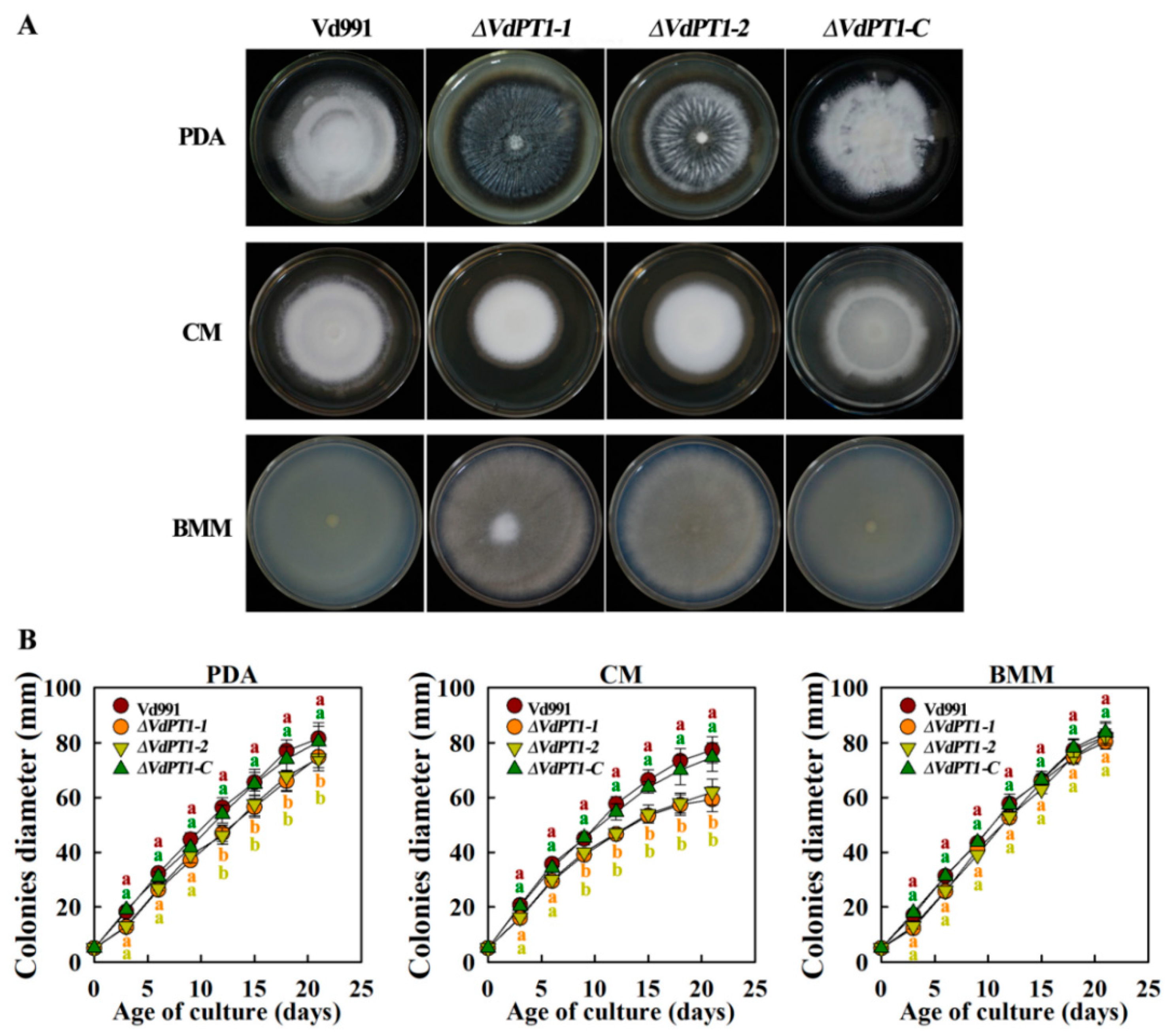
2.3. VdPT1 Deletion Mutants Had a Reduced Colony Growth Rate and Increased Melanin Production
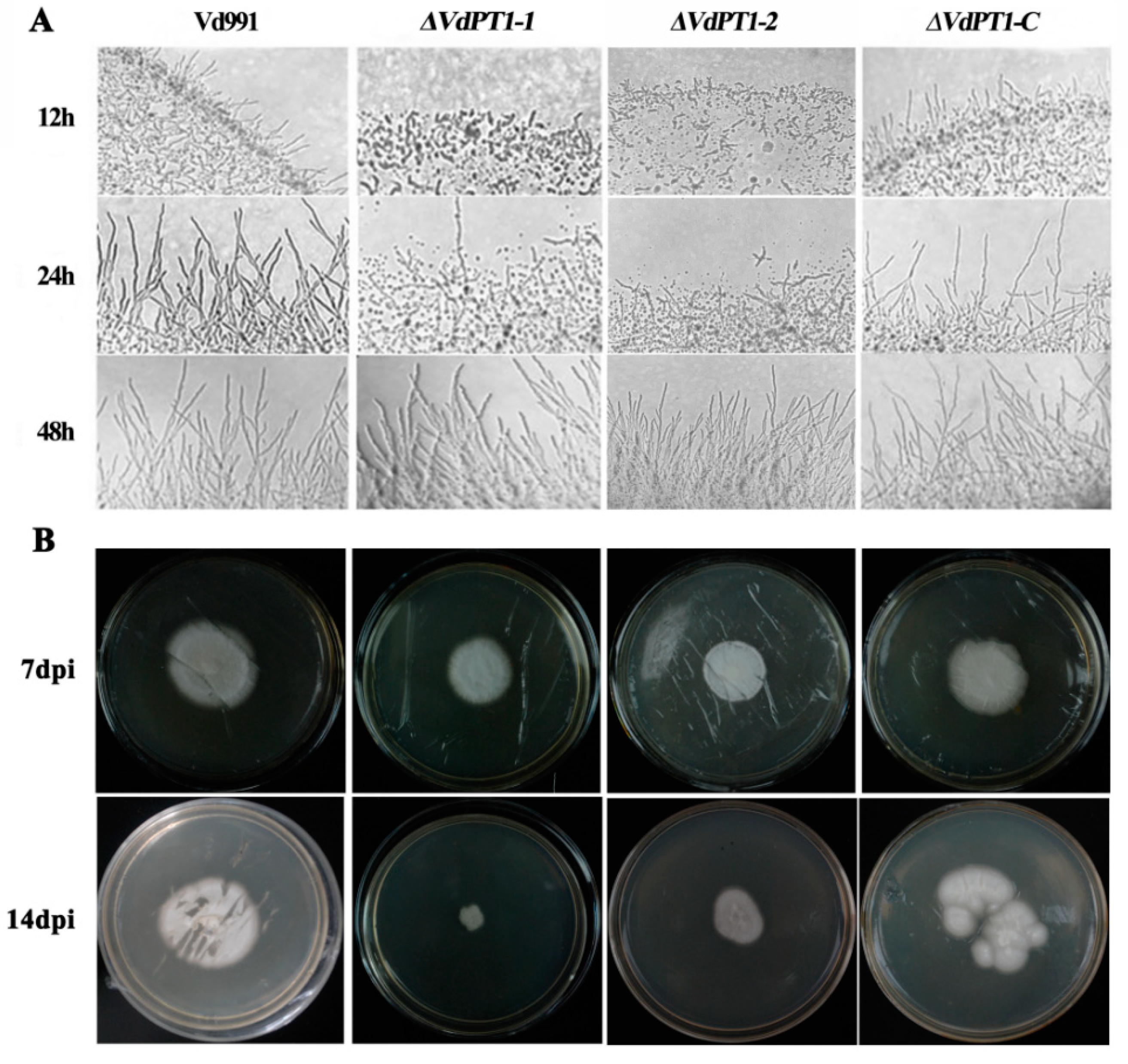
2.4. VdPT1 Deletion Led to a Delay in Mycelial Growth and Impaired Mycelial Penetration
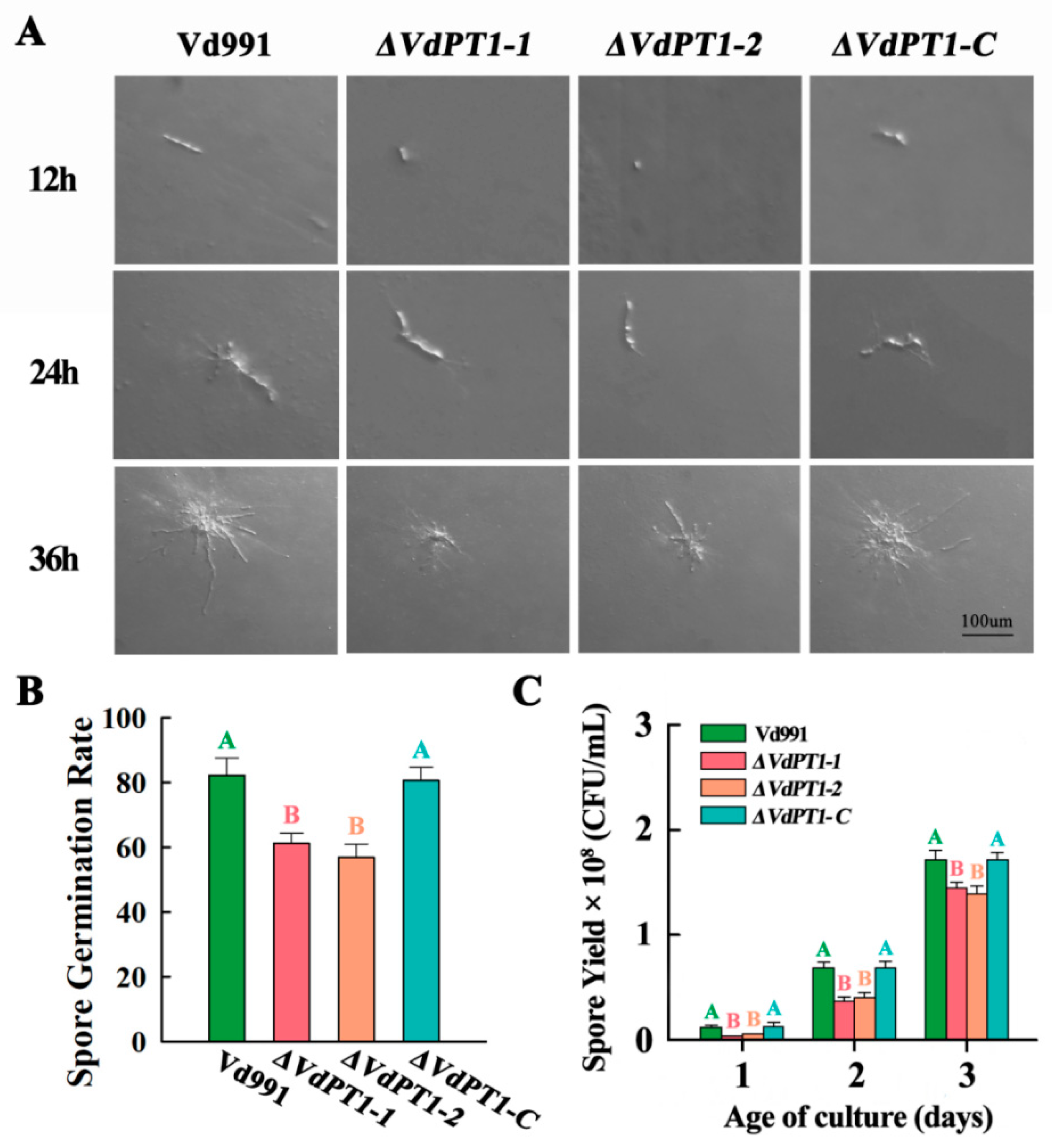
2.5. VdPT1 Deletion Resulted in a Delay of Conidial Germination and Reduced Conidial Production
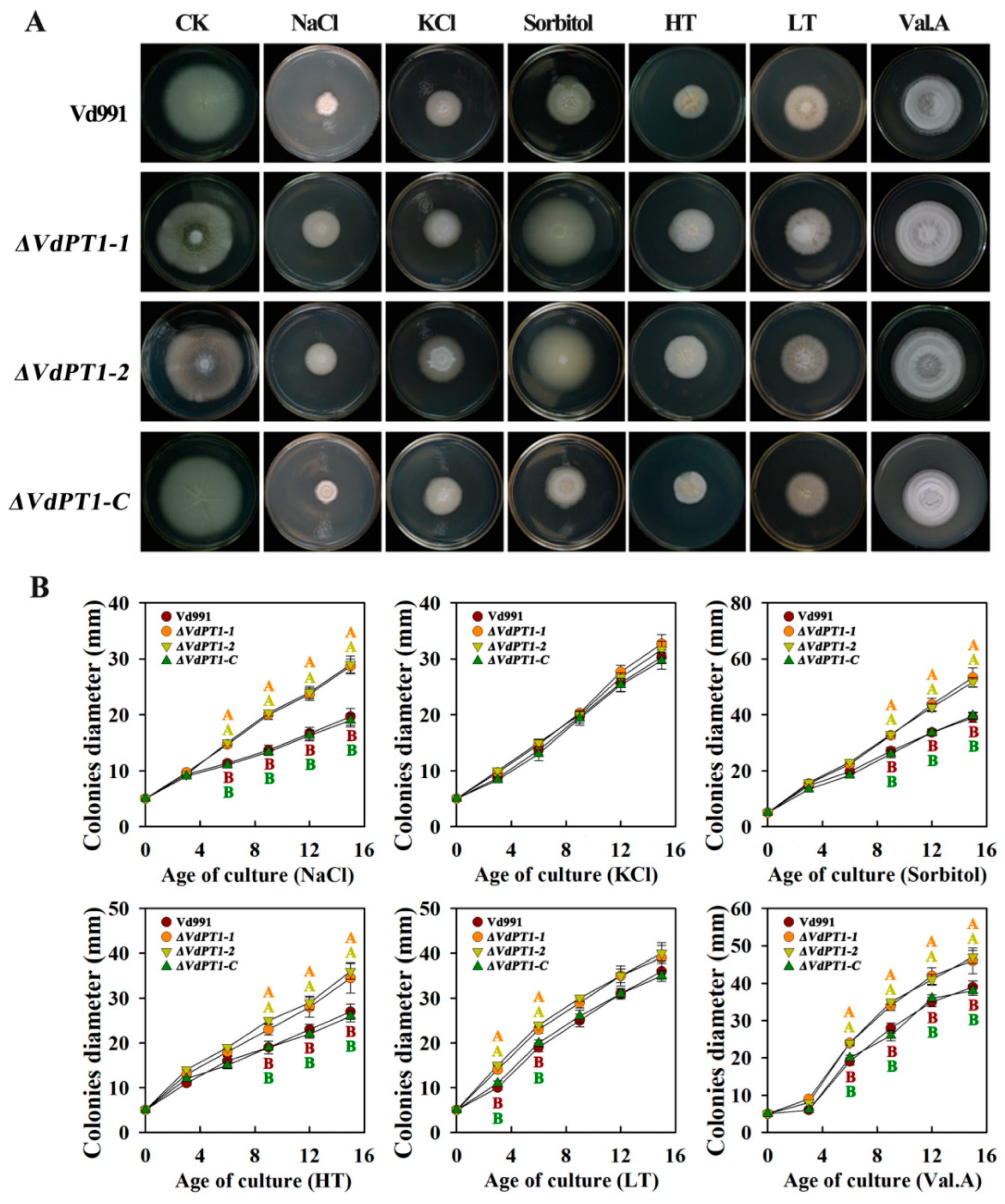
2.6. VdPT1 Deletion Increased Stress Resistance of V. dahliae
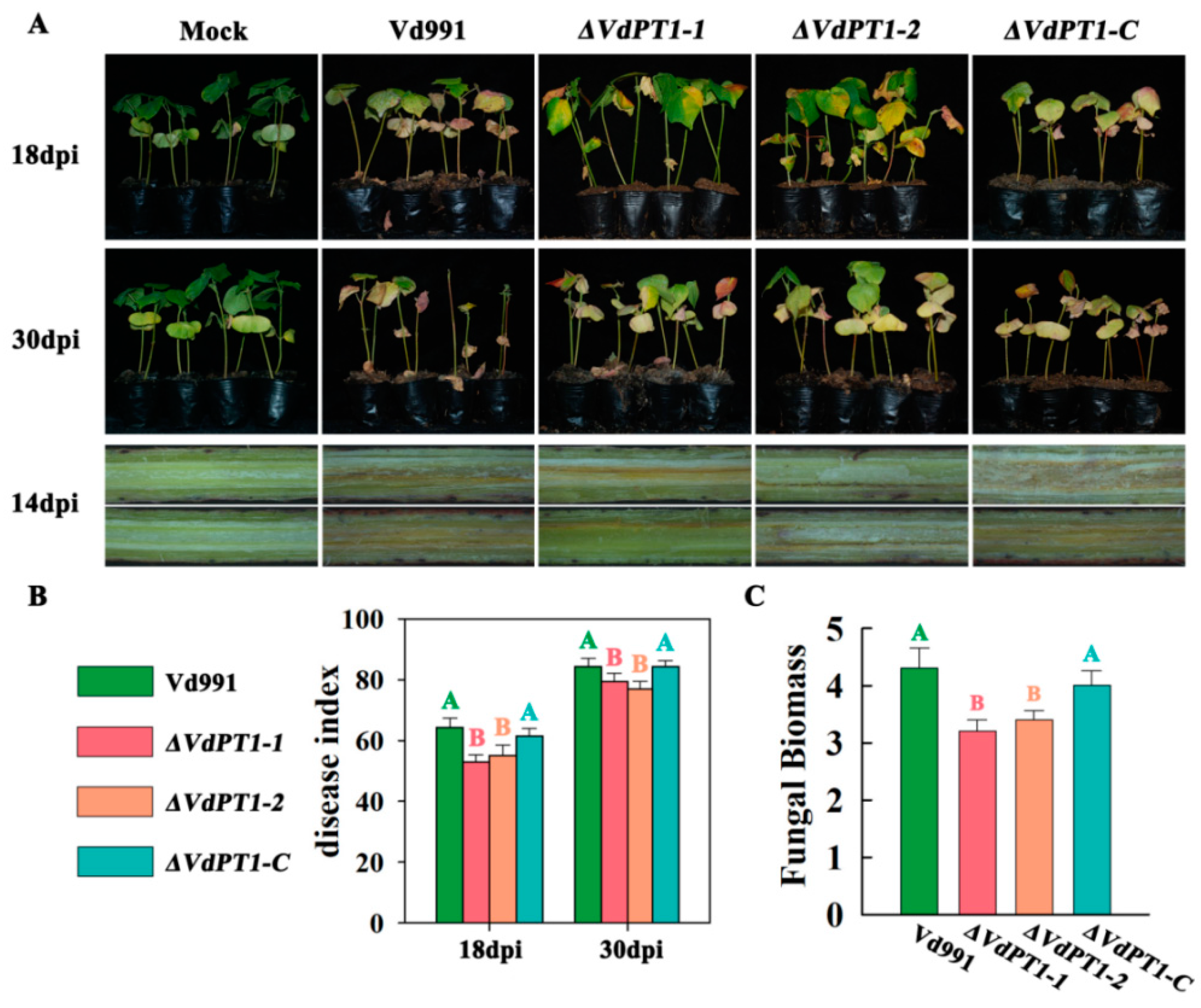
2.7. VdPT1 Deletion Reduced the Pathogenicity of V. dahliae
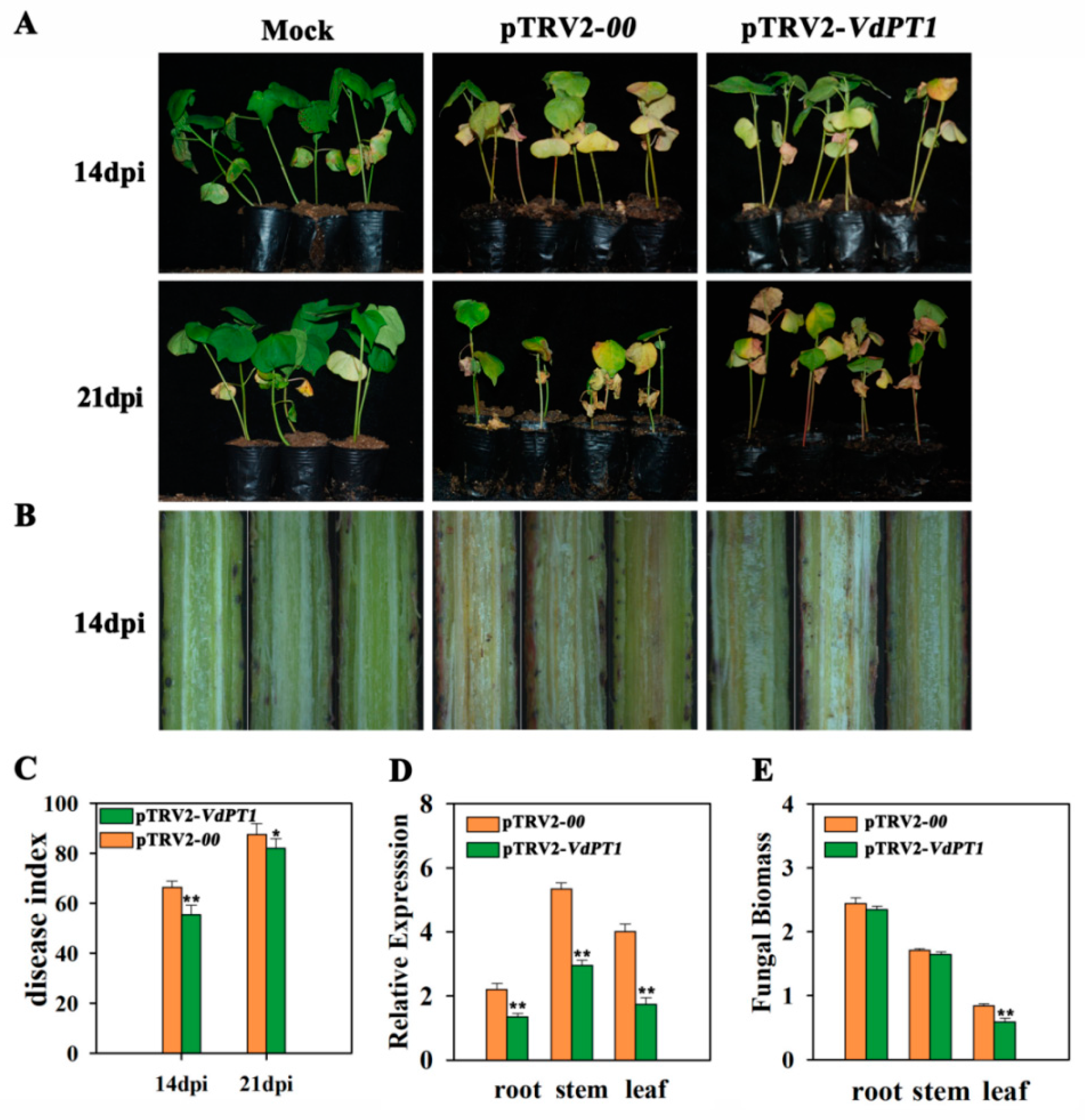
2.8. Host-Induced Silencing of VdPT1 Increased the Resistance of Cotton to V. dahliae
2.9. RNA-Seq Analysis
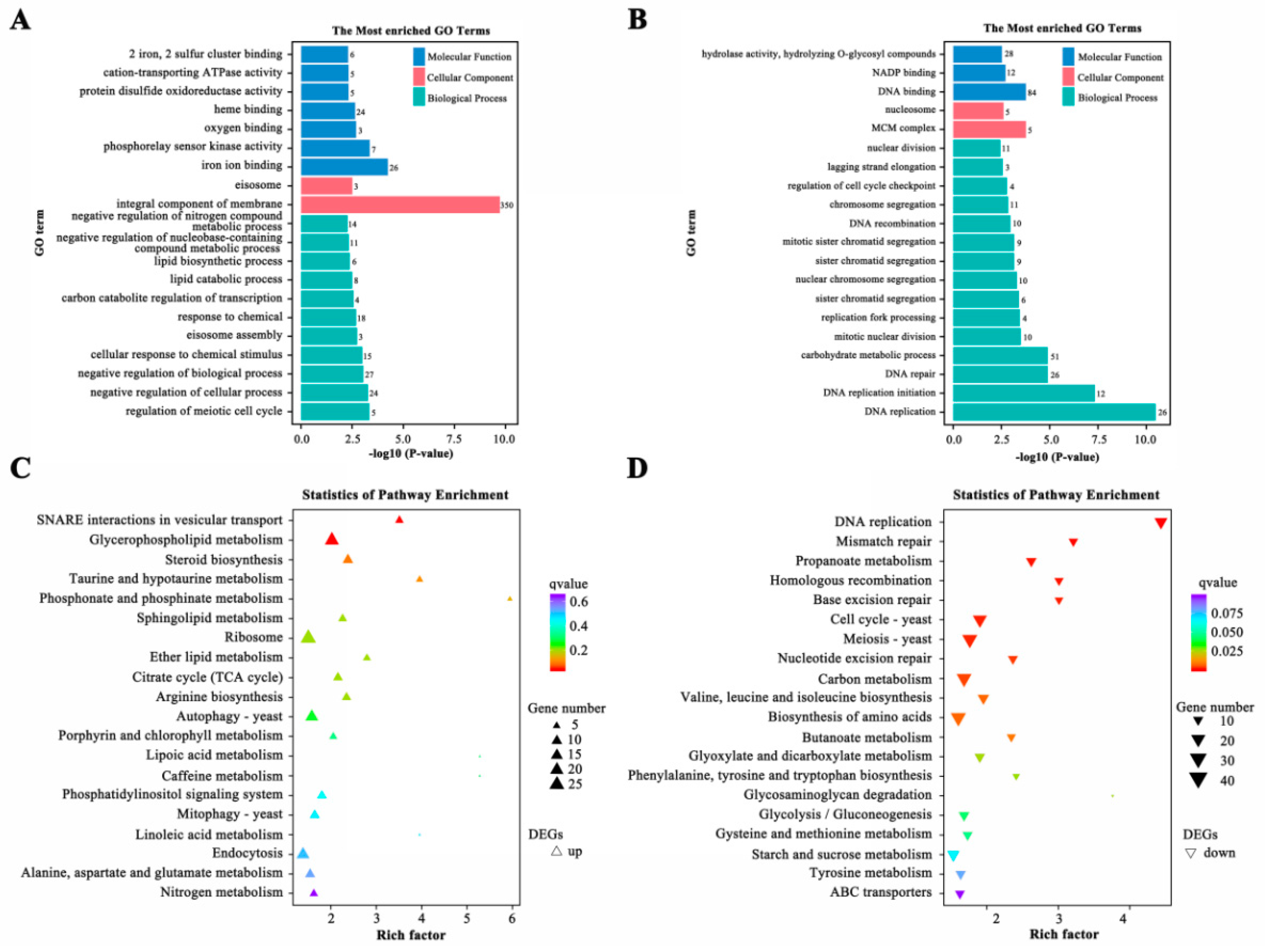
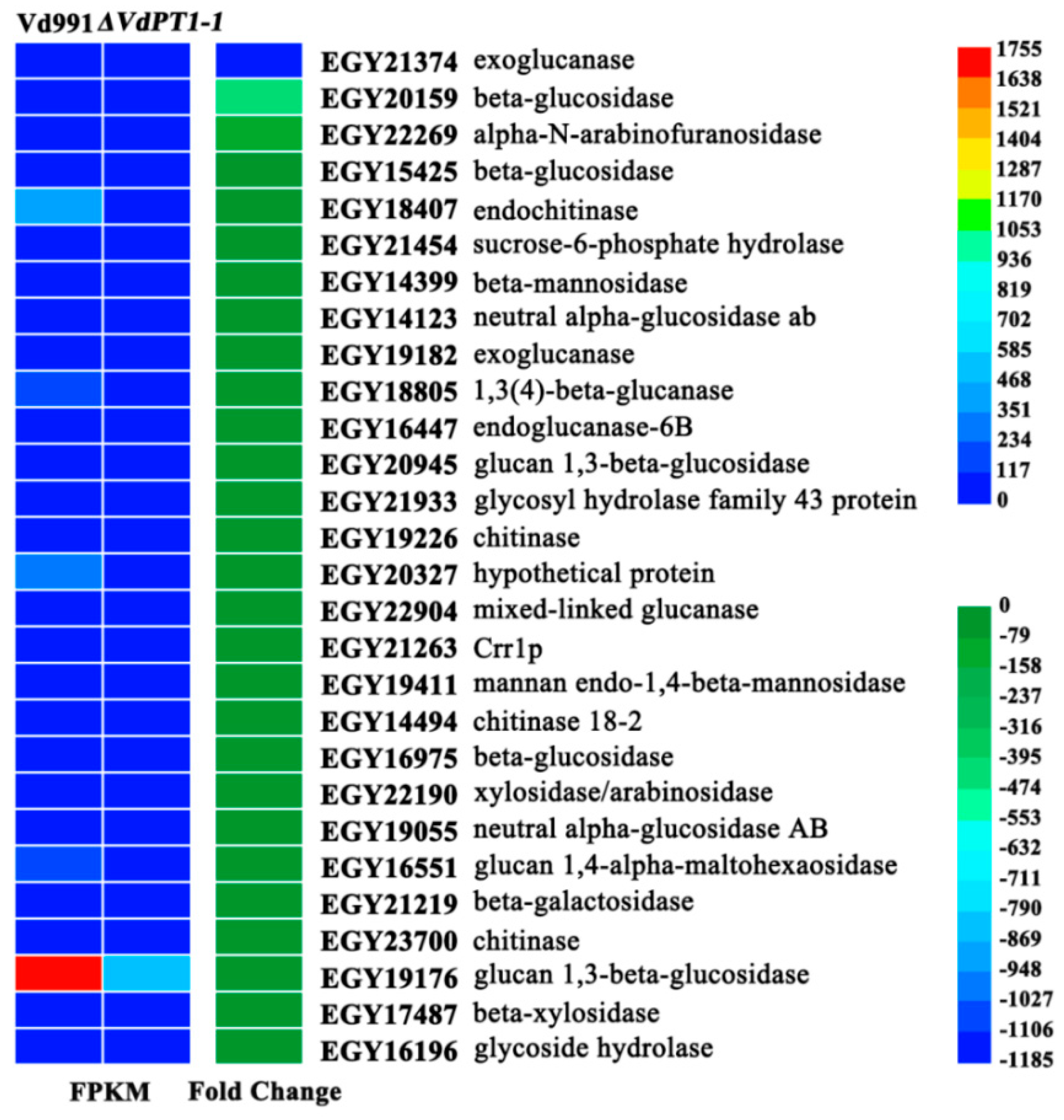
2.10. DEGs Related to Hydrolase Activity Hydrolyzing O-glycosyl Compounds
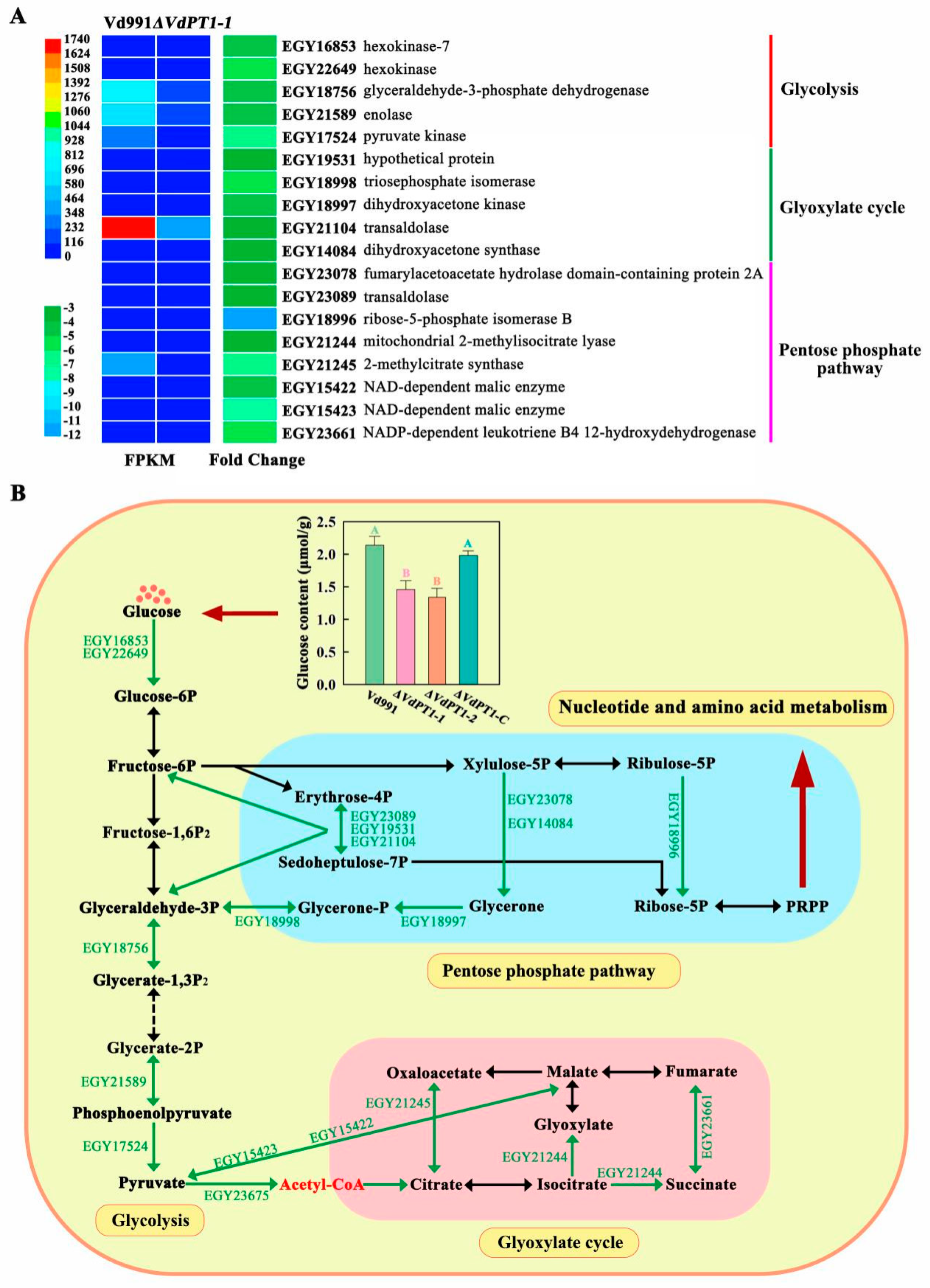
2.11. DEGs Related to Carbon Metabolism, DNA, and Amino Acids Biosynthesis
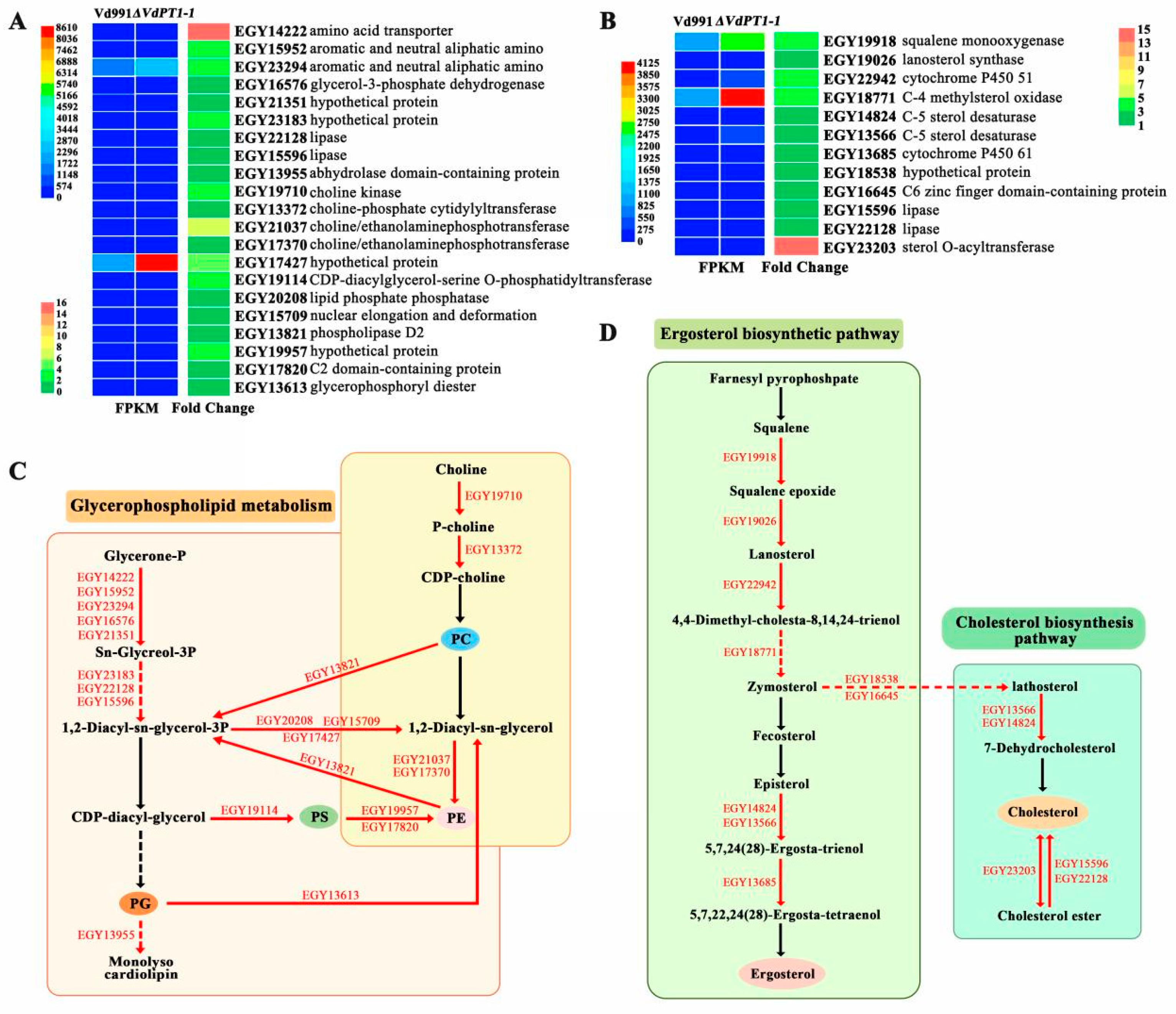
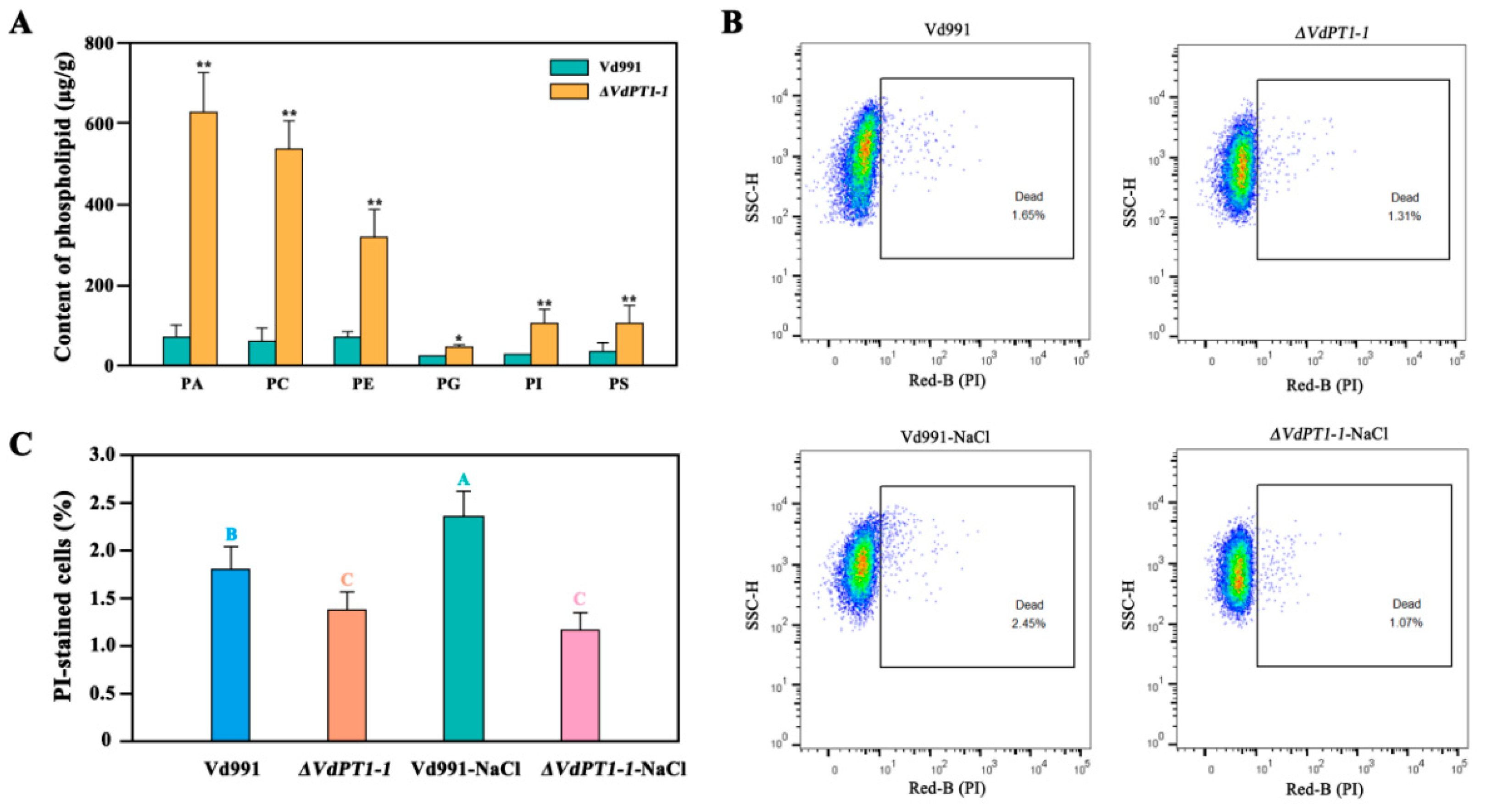
2.12. DEGs Related to Glycerophospholipid Metabolism and Steroid Biosynthesis
3. Discussion
3.1. VdPT1 Is a Neutral Trehalase and Is Required for Growth and Pathogenicity of V. dahliae
3.2. Down-Regulation of Cell Wall-Degrading Enzyme Genes Is Responsible for the Decreased Pathogenicity of V. dahliae
3.3. Down-Regulation of Carbon, DNA, and Amino Acid Metabolism-Related Genes Is Responsible for the Slow Growth of V. dahliae
3.4. Up-Regulation of Glycerophospholipid Metabolism-Related Genes Is Responsible for the Enhanced Stress Resistance of V. dahliae
4. Materials and Methods
4.1. Pathogen Strains and Plant Growth Conditions
4.2. DNA, RNA Extraction, and cDNA Synthesis
4.3. Bioinformatics Analysis
4.4. Generation and Confirmation of VdPT1 Deletion and Complementary Mutants
4.5. Fungal Growth Assays
4.6. Stress Treatments
4.7. Pathogenicity Assays
4.8. TRV (Tobacco Rattle Virus) Treatment
4.9. Determination of Trehalase Activity, Trehalose and Glucose Content
4.10. RNA-Sequencing (RNA-Seq)
4.11. Gene Expression Analysis
4.12. Cell Membrane Integrity Analysis
4.13. Lipidomics Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pegg, G.F. Effect of host substrate on germination and growth of verticillium albo-atrum and V. dahliae conidia and mycelia. Trans. Br. Mycol. Soc. 1978, 71, 483–489. [Google Scholar] [CrossRef]
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An Overview of the Molecular Genetics of Plant Resistance to the Verticillium Wilt Pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.J.; Chen, J.Y.; Zhang, D.D.; Li, N.Y.; Li, T.G.; Zhang, W.Q.; Wang, X.Y.; Short, D.P.G.; Li, L.; Guo, W.; et al. Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 protens in conjunction with carbohydrate-binding module 1. Environ. Microbiol. 2017, 19, 1914–1932. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wang, K.; Sun, L.; Xing, H.; Wang, S.; Li, L.; Chen, S.; Guo, H.S.; Zhang, J. The plant-specific transcription factors CBP60g and SARD1 are targeted by a Verticillium secretory protein VdSCP41 to modulate immunity. Elife 2018, 7, e34902. [Google Scholar] [CrossRef]
- Zhang, L.; Ni, H.; Du, X.; Wang, S.; Ma, X.W.; Nürnberger, T.; Guo, H.S.; Hua, C. The Verticillium-specific protein VdSCP7 localizes to the plant nucleus and modulates immunity to fungal infections. New Phytol. 2017, 215, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Li, Y.; Zeng, J.; Wang, G.; Deng, C.; Guo, W. Host-induced gene silencing of a regulator of G protein signalling gene (VdRGS1) confers resistance to Verticillium wilt in cotton. Plant Biotechnol. J. 2018, 16, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.B.; Krakowka, S.; Dexter, L.B.; Schmid, H.; Wolterbeek, A.P.; Waalkens-Berendsen, D.H.; Shigoyuki, A.; Kurimoto, M. Trehalose: A review of properties, history of use and human tolerance, and results of multiple safety studies. Food Chem. Toxicol. 2002, 40, 871–898. [Google Scholar] [CrossRef]
- Sussman, A.S.; Lingappa, B.T. Role of Trehalose in Ascospores of Neurospora Tetrasperma. Science 1959, 130, 1343. [Google Scholar] [CrossRef]
- Elbein, A.D. The metabolism of alpha, alpha-trehalose. Adv. Carbohydr. Chem. Biochem. 1974, 30, 227–256. [Google Scholar] [CrossRef]
- Shleeva, M.O.; Trutneva, K.A.; Demina, G.R.; Zinin, A.I.; Sorokoumova, G.M.; Laptinskaya, P.K.; Shumkova, E.S.; Kaprelyants, A.S. Free Trehalose Accumulation in Dormant Mycobacterium smegmatis Cells and Its Breakdown in Early Resuscitation Phase. Front. Microbiol. 2017, 8, 524. [Google Scholar] [CrossRef]
- Zeidler, S.; Hubloher, J.; Schabacker, K.; Lamosa, P.; Santos, H.; Müller, V. Trehalose, a temperature- and salt-induced solute with implications in pathobiology of Acinetobacter baumannii. Environ. Microbiol. 2017, 19, 5088–5099. [Google Scholar] [CrossRef] [PubMed]
- Plourde-Owobi, L.; Durner, S.; Goma, G.; François, J. Trehalose reserve in Saccharomyces cerevisiae: Phenomenon of transport, accumulation and role in cell viability. Int. J. Food Microbiol. 2000, 55, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Svanström, A.; Melin, P. Intracellular trehalase activity is required for development, germination and heat-stress resistance of Aspergillus niger conidia. Res. Microbiol. 2013, 164, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fresneda, R.; Martínez-Esparza, M.; Maicas, S.; Argüelles, J.C.; Valentín, E. In Candida parapsilosis the ATC1 gene encodes for an acid trehalase involved in trehalose hydrolysis, stress resistance and virulence. PLoS ONE. 2014, 9, e99113. [Google Scholar] [CrossRef] [PubMed]
- Zähringer, H.; Thevelein, J.M.; Nwaka, S. Induction of neutral trehalase Nth1 by heat and osmotic stress is controlled by STRE elements and Msn2/Msn4 transcription factors: Variations of PKA effect during stress and growth. Mol. Microbiol. 2000, 35, 397–406. [Google Scholar] [CrossRef]
- Andersson, U.; Levander, F.; Rådström, P. Trehalose-6-phosphate phosphorylase is part of a novel metabolic pathway for trehalose utilization in Lactococcus lactis. J. Biol. Chem. 2001, 276, 42707–42713. [Google Scholar] [CrossRef]
- Argüelles, J.C. Physiological roles of trehalose in bacteria and yeasts: A comparative analysis. Arch. Microbiol. 2000, 174, 217–224, Erratum in: Arch. Microbiol. 2000, 174, 456. [Google Scholar] [CrossRef]
- Parrou, J.L.; Jules, M.; Beltran, G.; François, J. Acid trehalase in yeasts and filamentous fungi: Localization, regulation and physiological function. FEMS Yeast Res. 2005, 5, 503–511. [Google Scholar] [CrossRef]
- Gao, H.; Gong, J.S.; Su, C.; Li, H.; Xu, Z.-H.; Shi, J.-S. Characterization, heterologous expression and engineering of trehalase for biotechnological applications. Syst. Microbiol. Biomanuf. 2022, 2, 445–460. [Google Scholar] [CrossRef]
- Jorge, J.A.; Polizeli, M.L.; Thevelein, J.M.; Terenzi, H.F. Trehalases and trehalose hydrolysis in fungi. FEMS Microbiol. Lett. 1997, 154, 165–171. [Google Scholar] [CrossRef][Green Version]
- Garre, E.; Matallana, E. The three trehalases Nth1p, Nth2p and Ath1p participate in the mobilization of intracellular trehalose required for recovery from saline stress in Saccharomyces cerevisiae. Microbiology 2009, 155 Pt 9, 3092–3099. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barraza, A.; Sánchez, F. Trehalases: A neglected carbon metabolism regulator? Plant Signal Behav. 2013, 8, e24778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fillinger, S.; Chaveroche, M.K.; van Dijck, P.; de Vries, R.; Ruijter, G.; Thevelein, J.; d’Enfert, C. Trehalose is required for the acquisition of tolerance to a variety of stresses in the filamentous fungus Aspergillus nidulans. Microbiology 2001, 147 Pt 7, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Conlin, L.K.; Nelson, H.C. The natural osmolyte trehalose is a positive regulator of the heat-induced activity of yeast heat shock transcription factor. Mol. Cell. Biol. 2007, 27, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.J.; Jenkinson, J.M.; Talbot, N.J. Trehalose synthesis and metabolism are required at different stages of plant infection by Magnaporthe grisea. EMBO J. 2003, 22, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Botts, M.R.; Huang, M.; Borchardt, R.K.; Hull, C.M. Developmental cell fate and virulence are linked to trehalose homeostasis in Cryptococcus neoformans. Eukaryot. Cell. 2014, 13, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Berndt, P.; Hahn, M. Trehalose metabolism is important for heat stress tolerance and spore germination of Botrytis cinerea. Microbiology 2006, 152 Pt 9, 2625–2634. [Google Scholar] [CrossRef]
- Danielson, N.D.; Collins, J.; Stothard, A.I.; Dong, Q.Q.; Kalera, K.; Woodruff, P.J.; DeBosch, B.J.; Britton, R.A.; Swarts, B.M. Degradation-resistant trehalose analogues block utilization of trehalose by hypervirulent Clostridioides difficile. Chem. Commun. (Camb.) 2019, 55, 5009–5012. [Google Scholar] [CrossRef]
- Matassini, C.; Parmeggiani, C.; Cardona, F. New Frontiers on Human Safe Insecticides and Fungicides: An Opinion on Trehalase Inhibitors. Molecules 2020, 25, 3013. [Google Scholar] [CrossRef]
- García, M.D.; Argüelles, J.C. Trehalase inhibition by validamycin A may be a promising target to design new fungicides and insecticides. Pest Manag. Sci. 2021, 77, 3832–3835. [Google Scholar] [CrossRef]
- Pedreño, Y.; González-Párraga, P.; Martínez-Esparza, M.; Sentandreu, R.; Valentín, E.; Argüelles, J.C. Disruption of the Candida albicans ATC1 gene encoding a cell-linked acid trehalase decreases hypha formation and infectivity without affecting resistance to oxidative stress. Microbiology 2007, 153 Pt 5, 1372–1381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, B.; Yang, M.; Shen, Q.; Xu, Y.; Wang, H.; Wang, S. Suppressing the activity of trehalase with validamycin disrupts the trehalose and chitin biosynthesis pathways in the rice brown planthopper, Nilaparvata lugens. Pestic. Biochem. Physiol. 2017, 137, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Adhav, A.S.; Kokane, S.R.; Joshi, R.S. Functional characterization of Helicoverpa armigera trehalase and investigation of physiological effects caused due to its inhibition by Validamycin A formulation. Int. J. Biol. Macromol. 2018, 112, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Shirouzu, K.; Nakashita, H.; Lee, H.Y.; Motoyama, T.; Yamaguchi, I.; Teraoka, T.; Arie, T. Foliar spray of validamycin a or validoxylamine a controls tomato fusarium wilt. Phytopathology 2005, 95, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, N.; Zhao, L.; Zhu, H.; Tang, C. Transcriptome analysis reveals the defense mechanism of cotton against Verticillium dahliae in the presence of the biocontrol fungus Chaetomium globosum CEF-082. BMC Plant Biol. 2020, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, V.; Vélëz, H.; Tzelepis, G. The Role of Glycoside Hydrolases in Phytopathogenic Fungi and Oomycetes Virulence. Int. J. Mol. Sci. 2021, 22, 9359. [Google Scholar] [CrossRef] [PubMed]
- Kondakova, T.; D’Heygère, F.; Feuilloley, M.J.; Orange, N.; Heipieper, H.J.; Duclairoir Poc, C. Glycerophospholipid synthesis and functions in Pseudomonas. Chem. Phys. Lipids 2015, 190, 27–42. [Google Scholar] [CrossRef]
- Zahumensky, J.; Malinsky, J. Role of MCC/Eisosome in Fungal Lipid Homeostasis. Biomolecules 2019, 9, 305. [Google Scholar] [CrossRef]
- Renne, M.F.; de Kroon, A.I.P.M. The role of phospholipid molecular species in determining the physical properties of yeast membranes. FEBS Lett. 2018, 592, 1330–1345. [Google Scholar] [CrossRef]
- Powers, M.J.; Trent, M.S. Intermembrane transport: Glycerophospholipid homeostasis of the Gram-negative cell envelope. Proc. Natl. Acad. Sci. USA 2019, 116, 17147–17155. [Google Scholar] [CrossRef]
- Dalebroux, Z.D. Cues from the Membrane: Bacterial Glycerophospholipids. J. Bacteriol. 2017, 199, e00136-17. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef] [PubMed]
- Thevelein, J.M. Regulation of trehalose mobilization in fungi. Microbiol. Rev. 1984, 48, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, G.; Suárez, R.; Nova-Franco, B. Trehalose metabolism: From osmoprotection to signaling. Int. J. Mol. Sci. 2009, 10, 3793–3810. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, P.; Halvorson, H.O.; Bulla, L.A., Jr.; St Julian, G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J. Bacteriol. 1972, 109, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Cantu, D.; Vicente, A.R.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L. Strangers in the matrix: Plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008, 13, 610–617. [Google Scholar] [CrossRef]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing: Wellingford, UK, 2002. [Google Scholar]
- Klosterman, S.J.; Subbarao, K.V.; Kang, S.; Veronese, P.; Gold, S.E.; Thomma, B.P.; Chen, Z.; Henrissat, B.; Lee, Y.H.; Park, J.; et al. Comparative genomics yields insights into niche adaptation of plant vascular wilt pathogens. PLoS Pathog. 2011, 7, e1002137. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xiao, H.L.; Gui, Y.J.; Zhang, D.D.; Li, L.; Bao, Y.M.; Dai, X.F. Characterization of the Verticillium dahliae Exoproteome Involves in Pathogenicity from Cotton-Containing Medium. Front. Microbiol. 2016, 7, 1709. [Google Scholar] [CrossRef]
- Gui, Y.J.; Zhang, W.Q.; Zhang, D.D.; Zhou, L.; Short, D.P.G.; Wang, J.; Ma, X.F.; Li, T.G.; Kong, Z.Q.; Wang, B.L.; et al. A Verticillium dahliae Extracellular Cutinase Modulates Plant Immune Responses. Mol. Plant Microbe Interact. 2018, 31, 260–273. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.Y.; Song, J.; Li, J.J.; Klosterman, S.J.; Li, R.; Kong, Z.Q.; Subbarao, K.V.; Dai, X.F.; Zhang, D.D. Cytotoxic function of xylanase VdXyn4 in the plant vascular wilt pathogen Verticillium dahliae. Plant Physiol. 2021, 187, 409–429. [Google Scholar] [CrossRef]
- Liu, S.Y.; Chen, J.Y.; Wang, J.L.; Li, L.; Xiao, H.L.; Adam, S.M.; Dai, X.F. Molecular characterization and functional analysis of a specific secreted protein from highly virulent defoliating Verticillium dahliae. Gene 2013, 529, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Tzima, A.K.; Paplomatas, E.J.; Rauyaree, P.; Ospina-Giraldo, M.D.; Kang, S. VdSNF1, the sucrose nonfermenting protein kinase gene of Verticillium dahliae, is required for virulence and expression of genes involved in cell-wall degradation. Mol. Plant Microbe Interact. 2011, 24, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Gui, Y.J.; Short, D.P.G.; Li, T.G.; Zhang, D.D.; Zhou, L.; Liu, C.; Bao, Y.M.; Subbarao, K.V.; Chen, J.Y.; et al. Verticillium dahliae transcription factor VdFTF1 regulates the expression of multiple secreted virulence factors and is required for full virulence in cotton. Mol. Plant Pathol. 2018, 19, 841–857. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, K.; Qin, Q.; Raimi, O.G.; Du, C.; Wang, B.; Ahamefule, C.S.; Kowalski, B.; Jin, C.; van Aalten, D.M.F.; et al. Phosphoglucose Isomerase Is Important for Aspergillus fumigatus Cell Wall Biogenesis. Mbio 2022, 13, e0142622. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, G.; Schoonbeek, H.; De Waard, M.A. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Qadri, H.; Qureshi, M.F.; Mir, M.A.; Shah, A.H. Glucose—The X factor for the survival of human fungal pathogens and disease progression in the host. Microbiol. Res. 2021, 247, 126725. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Peng, G.; Jin, K.; Liu, Y.; Xia, Y. Enhancing the utilization of host trehalose by fungal trehalase improves the virulence of fungal insecticide. Appl. Microbiol. Biotechnol. 2015, 99, 8611–8618. [Google Scholar] [CrossRef]
- Tang, M.; Waring, A.J.; Hong, M. Trehalose-protected lipid membranes for determining membrane protein structure and insertion. J. Magn. Reson. 2007, 184, 222–227. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Usov, A.I.; Mysyakina, I.S.; Kochkina, G.A. Trehalose: Chemical structure, biological functions, and practical application. Mikrobiologiia 2014, 83, 271–283. (In Russian) [Google Scholar] [CrossRef]
- Amen, T.; Kaganovich, D. Stress granules sense metabolic stress at the plasma membrane and potentiate recovery by storing active Pkc1. Sci. Signal. 2020, 13, eaaz6339. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.P.; Kado, T.; Prithviraj, M.; Siegrist, M.S.; Morita, Y.S. Inositol acylation of phosphatidylinositol mannosides: A rapid mass response to membrane fluidization in mycobacteria. J. Lipid Res. 2022, 63, 100262. [Google Scholar] [CrossRef] [PubMed]
- Ciumac, D.; Campbell, R.A.; Clifton, L.A.; Xu, H.; Fragneto, G.; Lu, J.R. Influence of Acyl Chain Saturation on the Membrane-Binding Activity of a Short Antimicrobial Peptide. ACS Omega 2017, 2, 7482–7492. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Boumann, H.A.; de Kroon, A.I. The contributions of biosynthesis and acyl chain remodelling to the molecular species profile of phosphatidylcholine in yeast. Biochem. Soc. Trans. 2005, 33 Pt 5, 1146–1149. [Google Scholar] [CrossRef] [PubMed]
- McGee, T.P.; Skinner, H.B.; Bankaitis, V.A. Functional redundancy of CDP-ethanolamine and CDP-choline pathway enzymes in phospholipid biosynthesis: Ethanolamine-dependent effects on steady-state membrane phospholipid composition in Saccharomyces cerevisiae. J. Bacteriol. 1994, 176, 6861–6868. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McMaster, C.R.; Bell, R.M. Phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. Regulatory insights from studies employing null and chimeric sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J. Biol. Chem. 1994, 269, 28010–28016. [Google Scholar] [CrossRef] [PubMed]
- Hjelmstad, R.H.; Morash, S.C.; McMaster, C.R.; Bell, R.M. Chimeric enzymes. Structure-function analysis of segments of sn-1,2-diacylglycerol choline- and ethanolaminephosphotransferases. J. Biol. Chem. 1994, 269, 20995–21002. [Google Scholar] [CrossRef]
- Chen, Y.; Li, B.; Cen, K.; Lu, Y.; Zhang, S.; Wang, C. Diverse effect of phosphatidylcholine biosynthetic genes on phospholipid homeostasis, cell autophagy and fungal developments in Metarhizium robertsii. Environ. Microbiol. 2018, 20, 293–304. [Google Scholar] [CrossRef]
- Liu, X.L.; Shiihara, M.; Taniwaki, N.; Shirasaka, N.; Atsumi, Y.; Shiojiri, M. 6-Phosphatidylserine: Biology, technologies, and applications. Polar Lipids 2015, 145–184. [Google Scholar] [CrossRef]
- Hartmann, A.; Gupta, N. Multiple Routes of Phosphatidylethanolamine Biogenesis Ensure Membrane Integrity of Toxoplasma Gondii. Ph.D. Thesis, der Humboldt-Universität zu Berlin, Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, S.; Xiong, D.; Tian, C. Genetic transformation, infection process and qPCR quantification of Verticillium dahliae on smoke-tree Cotinus coggygria. Australas. Plant Pathol. 2013, 42, 33–41. [Google Scholar] [CrossRef]
- Neumann, M.J.; Dobinson, K.F. Sequence tag analysis of gene expression during pathogenic growth and microsclerotia development in the vascular wilt pathogen Verticillium dahliae. Fungal Genet. Biol. 2003, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.P.; Sun, S.C.; Zhang, X.Y.; Li, Y.J.; Liu, F.; Zhu, Q.H.; Xue, F.; Sun, J. Corrigendum: GhWRKY70D13 Regulates Resistance to Verticillium dahliae in Cotton Through the Ethylene and Jasmonic Acid Signaling Pathways. Front. Plant Sci. 2020, 11, 1045, Erratum in: Front. Plant Sci. 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Ma, X.; Sun, T.; Zhu, Q.-H.; Feng, H.; Li, Y.; Liu, F.; Zhang, X.; Sun, J.; Li, Y. VdPT1 Encoding a Neutral Trehalase of Verticillium dahliae Is Required for Growth and Virulence of the Pathogen. Int. J. Mol. Sci. 2024, 25, 294. https://doi.org/10.3390/ijms25010294
Chen L, Ma X, Sun T, Zhu Q-H, Feng H, Li Y, Liu F, Zhang X, Sun J, Li Y. VdPT1 Encoding a Neutral Trehalase of Verticillium dahliae Is Required for Growth and Virulence of the Pathogen. International Journal of Molecular Sciences. 2024; 25(1):294. https://doi.org/10.3390/ijms25010294
Chicago/Turabian StyleChen, Lihua, Xiaohu Ma, Tiange Sun, Qian-Hao Zhu, Hongjie Feng, Yongtai Li, Feng Liu, Xinyu Zhang, Jie Sun, and Yanjun Li. 2024. "VdPT1 Encoding a Neutral Trehalase of Verticillium dahliae Is Required for Growth and Virulence of the Pathogen" International Journal of Molecular Sciences 25, no. 1: 294. https://doi.org/10.3390/ijms25010294
APA StyleChen, L., Ma, X., Sun, T., Zhu, Q.-H., Feng, H., Li, Y., Liu, F., Zhang, X., Sun, J., & Li, Y. (2024). VdPT1 Encoding a Neutral Trehalase of Verticillium dahliae Is Required for Growth and Virulence of the Pathogen. International Journal of Molecular Sciences, 25(1), 294. https://doi.org/10.3390/ijms25010294






