Transcriptomic and Metabolomic Profiling Reveals the Variations in Carbohydrate Metabolism between Two Blueberry Cultivars
Abstract
1. Introduction
2. Results
2.1. Fruit Appearance, Antioxidant System, Bioactive Compounds and Flavor Indicators
2.2. Transcriptomic Analysis
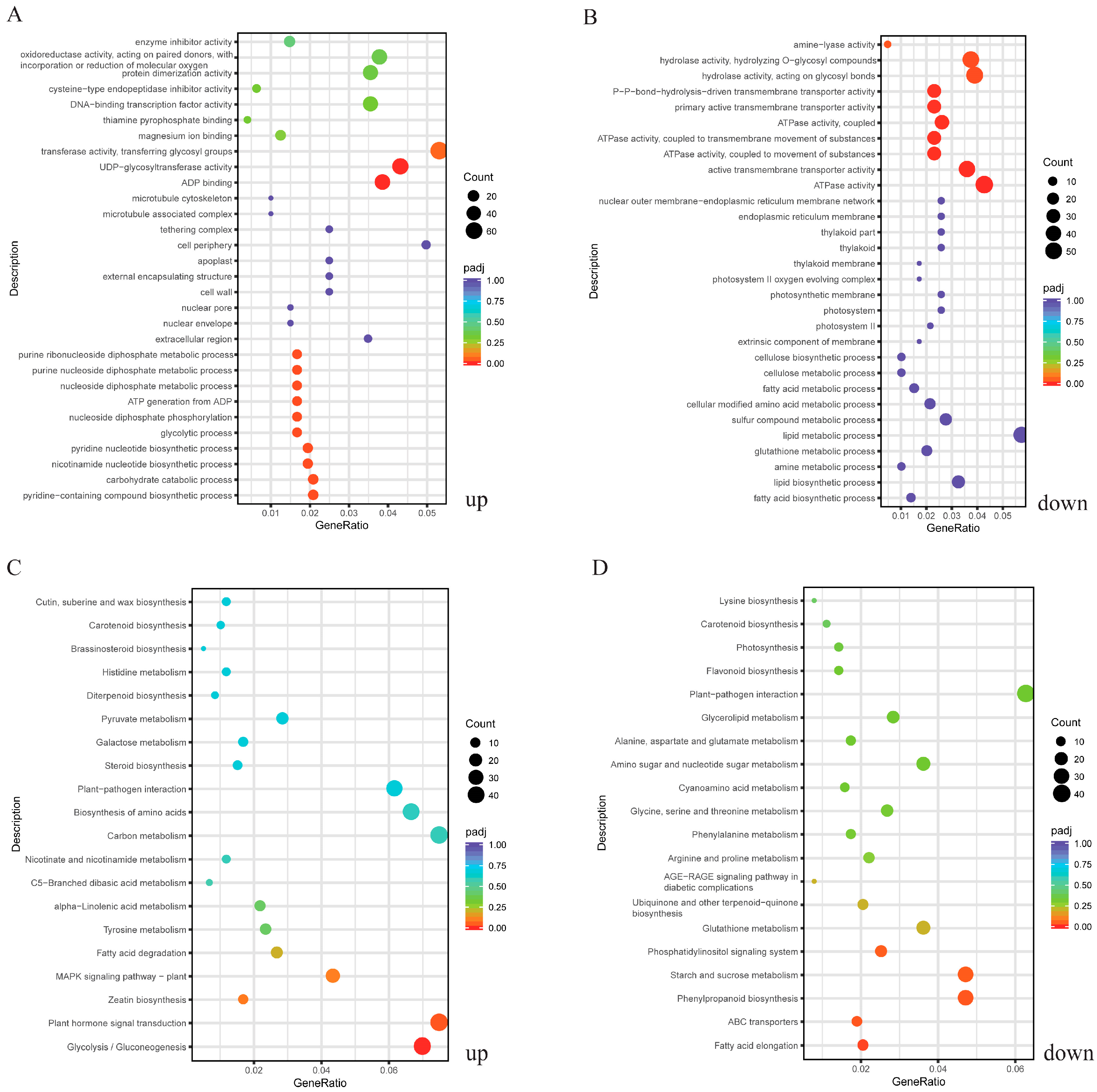
2.3. GO and KEGG Enrichment Analysis
2.4. DEGs in Phenylpropanoid Biosynthesis and Plant Hormone Signal Transduction Pathways
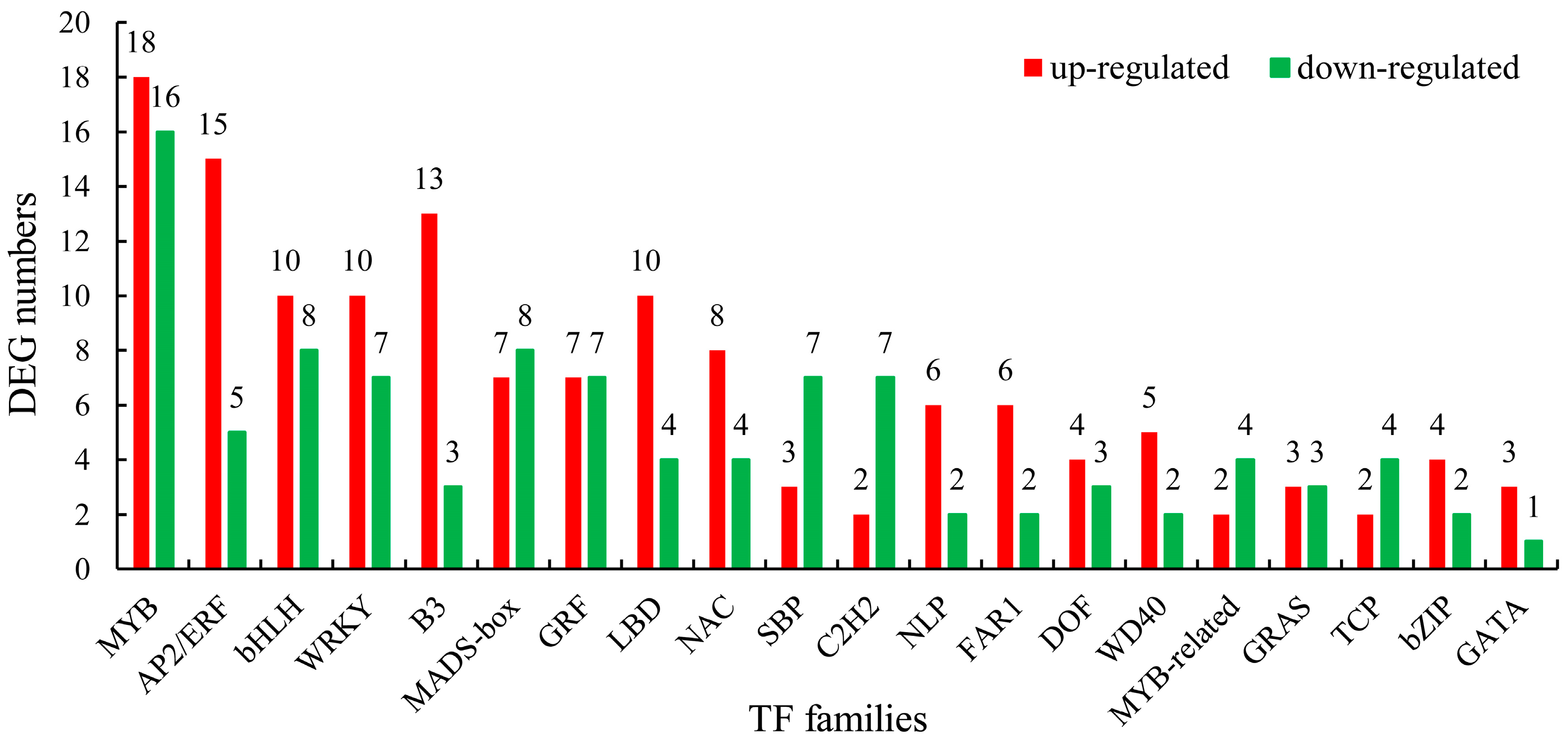
2.5. Transcription Factors (TFs)
2.6. Metabolic Profiling
2.7. Metabolite Multivariate Statistical Analysis
2.8. DAM Screening and Analysis
2.9. KEGG Enrichment Analysis of DAMs
2.10. Transcriptomics and Metabolomics Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Fruit Appearance Indicators and Firmness
4.3. Antioxidant-Related Parameters
4.4. Fruit-Quality-Related Parameters
4.5. Transcriptomic Analysis
4.6. Metabolite Extraction and Isolation
4.7. Metabolomic Data Processing and Analysis
4.8. Association Analysis of Transcriptomic and Metabolomic Data
4.9. qRT-PCR Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry phenolic antioxidants—Implications for human health? Front. Pharmacol. 2018, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Forney, C.F.; Qiu, S.; Jordan, M.A.; McCarthy, D.; Fillmore, S. Comparison of volatile compounds contributing to flavor of wild lowbush (Vaccinium augustifolium) and cultivated highbush (Vaccinium corymbosum) blueberry fruit using gas chromatography-olfactometry. Foods 2022, 11, 2516. [Google Scholar] [CrossRef] [PubMed]
- Su, M.-S.; Silva, J.L. Antioxidant activity, anthocyanins, and phenolics of rabbiteye blueberry (Vaccinium ashei) by-products as affected by fermentation. Food Chem. 2006, 97, 447–451. [Google Scholar] [CrossRef]
- Montecchiarini, M.L.; Silva-Sanzana, C.; Valderramo, L.; Alemano, S.; Gollán, A.; Rivadeneira, M.F.; Bello, F.; Vázquez, D.; Blanco-Herrera, F.; Podestá, F.E.; et al. Biochemical differences in the skin of two blueberries (Vaccinium corymbosum) varieties with contrasting firmness: Implication of ions, metabolites and cell wall related proteins in two developmental stages. Plant Physiol. Bioch. 2021, 162, 483–495. [Google Scholar] [CrossRef]
- Yang, H.Y.; Wu, Y.Q.; Duan, Y.K.; Zhang, C.H.; Huang, Z.J.; Wu, W.L.; Lyu, L.F.; Li, W.L. Metabolomics combined with physiological and transcriptomic analyses reveal regulatory features associated with blueberry growth in different soilless substrates. Sci. Hortic. 2022, 302, 111145. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, E.; Fu, Y.; Yuan, F.; Zhang, T.; Peng, S. High temperatures during flowering reduce fruit set in rabbiteye blueberry. J. Am. Soc. Hortic. Sci. 2019, 144, 339–351. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Sater, H.; Ferrão, L.F.V.; Olmstead, J.; Munoz, P.R.; Bai, J.; Hopf, A.; Plotto, A. Exploring environmental and storage factors affecting sensory, physical and chemical attributes of six southern highbush blueberry cultivars. Sci. Hortic. 2021, 289, 110468. [Google Scholar] [CrossRef]
- Liu, B.H.; Wang, K.F.; Shu, X.G.; Liang, J.; Fan, X.L.; Sun, L. Changes in fruit firmness, quality traits and cell wall constituents of two highbush blueberries (Vaccinium corymbosum L.) during postharvest cold storage. Sci. Hortic. 2019, 246, 557–562. [Google Scholar] [CrossRef]
- Yang, H.; Duan, Y.; Wei, Z.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Integrated physiological and metabolomic analyses reveal the differences in the fruit quality of the blueberry cultivated in three soilless substrates. Foods 2022, 11, 3965. [Google Scholar] [CrossRef] [PubMed]
- Montero, T.M.; MollÁ, E.M.; Esteban, R.M.; López-Andréu, F.J. Quality attributes of strawberry during ripening. Sci. Hortic. 1996, 65, 239–250. [Google Scholar] [CrossRef]
- Qin, G.; Zhu, Z.; Wang, W.; Cai, J.; Chen, Y.; Li, L.; Tian, S. A tomato vacuolar invertase inhibitor mediates sucrose metabolism and influences fruit ripening. Plant Physiol. 2016, 172, 1596–1611. [Google Scholar] [CrossRef] [PubMed]
- Ai, T.N.; Naing, A.H.; Arun, M.; Lim, S.-H.; Kil Kim, C. Sucrose-induced anthocyanin accumulation in vegetative tissue of Petunia plants requires anthocyanin regulatory transcription factors. Plant Sci. 2016, 252, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Wei, Z.W.; Duan, Y.K.; Wu, Y.Q.; Zhang, C.H.; Wu, W.L.; Lyu, L.F.; Li, W.L. Transcriptomic and metabolomic investigation of the adaptation mechanisms of blueberries to nitrogen deficiency stress. Sci. Hortic. 2023, 321, 112376. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, J.-Y.; Li, J.; Zhang, H.; Li, Y.; Farooq, S.; Bacha, S.A.S.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, H.C.; Gao, G.; Yang, F.; Guan, J.Y.; Qi, H.Y. CmMYB44 might interact with CmAPS2-2 to regulate starch metabolism in oriental melon fruit. Plant Physiol. Biochem. 2023, 196, 361–369. [Google Scholar] [CrossRef]
- Basson, C.E.; Groenewald, J.-H.; Kossmann, J.; Cronjé, C.; Bauer, R. Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: Invertase is the main sucrose hydrolysing enzyme. Food Chem. 2010, 121, 1156–1162. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, C.Y.; Dong, W.C.; Jiang, Q.; Wang, D.L.; Li, S.J.; Chen, M.; Liu, C.R.; Sun, C.D.; Chen, K.S. Transcriptome and metabolome analyses of sugar and organic acid metabolism in Ponkan (Citrus reticulata) fruit during fruit maturation. Gene 2015, 554, 64–74. [Google Scholar] [CrossRef]
- Wang, J.; Du, J.J.; Mu, X.P.; Wang, P.F. Cloning and characterization of the Cerasus Humilis sucrose phosphate synthase gene (ChSPS1). PLoS ONE 2017, 12, e0186650. [Google Scholar] [CrossRef]
- Aslam, M.M.; Deng, L.; Wang, X.B.; Wang, Y.; Pan, L.; Liu, H.; Niu, L.; Lu, Z.H.; Cui, G.C.; Zeng, W.F.; et al. Expression patterns of genes involved in sugar metabolism and accumulation during peach fruit development and ripening. Sci. Hortic. 2019, 257, 108633. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Xing, Y.M.; Ramakrishnan, M.; Chen, C.B.; Xie, F.F.; Hua, Q.Z.; Chen, J.N.; Zhang, R.; Zhao, J.T.; Hu, G.B.; et al. Transcriptomics-based identification and characterization of genes related to sugar metabolism in ‘Hongshuijing’ Pitaya. Hortic. Plant J. 2022, 8, 450–460. [Google Scholar] [CrossRef]
- Kötting, O.; Kossmann, J.; Zeeman, S.C.; Lloyd, J.R. Regulation of starch metabolism: The age of enlightenment. Curr. Opin. Plant Biol. 2010, 13, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Wang, H.; Yu, H.; Pang, E.; Lu, T.; Li, Y.; Jiang, W.; Li, Q. Girdling promotes tomato fruit enlargement by enhancing fruit sink strength and triggering cytokinin accumulation. Front. Plant Sci. 2023, 14, 1174403. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, C.; Li, Y.; Huang, R.; Guo, M.; Liu, J.; Sun, T.; Ge, Y. Postharvest melatonin dipping maintains quality of apples by mediating sucrose metabolism. Plant Physiol. Biochem. 2022, 174, 43–50. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, Y.L.; Wang, M.M.; Han, H.Y.; Luo, Y.S.; Ding, W.Q.; Xu, W.L.; Zhong, Y.J.; Huang, H.X.; Qu, S.P. Soluble sugars accumulation and related gene expression during fruit development in Cucurbita maxima Duchesne. Sci. Hortic. 2020, 272, 109520. [Google Scholar] [CrossRef]
- Li, X.B.; Li, C.N.; Sun, J.; Jackson, A. Dynamic changes of enzymes involved in sugar and organic acid level modification during blueberry fruit maturation. Food Chem. 2020, 309, 125617. [Google Scholar] [CrossRef]
- Yang, H.; Tian, C.; Ji, S.; Ni, F.; Fan, X.; Yang, Y.; Sun, C.; Gong, H.; Zhang, A. Integrative analyses of metabolome and transcriptome reveals metabolomic variations and candidate genes involved in sweet cherry (Prunus avium L.) fruit quality during development and ripening. PLoS ONE 2021, 16, e0260004. [Google Scholar] [CrossRef]
- Gao, Y.; Yao, Y.; Chen, X.; Wu, J.; Wu, Q.; Liu, S.; Guo, A.; Zhang, X. Metabolomic and transcriptomic analyses reveal the mechanism of sweet-acidic taste formation during pineapple fruit development. Front. Plant Sci. 2022, 13, 971506. [Google Scholar] [CrossRef]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Instrumental and sensory quality characteristics of blueberry fruit from twelve cultivars. Postharvest Biol. Technol. 2008, 49, 19–26. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Zhang, Q.; Dossett, M.; Polashock, J.J.; Rodriguez-Saona, C.; Vorsa, N.; Edger, P.P.; Ashrafi, H.; Babiker, E.; Finn, C.E.; et al. Breeding trait priorities of the blueberry industry in the United States and Canada. HortScience 2018, 53, 1021–1028. [Google Scholar] [CrossRef]
- Tesnière, C.; Verriès, C. Molecular cloning and expression of cDNAs encoding alcohol dehydrogenases from Vitis vinifera L. during berry development. Plant Sci. 2000, 157, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, S.; Jin, Y.; Tang, Y.; Qi, H. The relationship between CmADHs and the diversity of volatile organic compounds of three aroma types of melon (Cucumis melo). Front. Physiol. 2016, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.F.; Du, X.Y.; Zhang, Q.L.; Ma, F.W.; Luo, Z.R.; Yang, Y. DkPK genes promote natural deastringency in C-PCNA persimmon by up-regulating DkPDC and DkADH expression. Front. Plant Sci. 2017, 8, 149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lü, H.M.; Li, J.M.; Huang, Y.H.; Zhang, M.Y.; Zhang, S.L.; Wu, J. Genome-wide identification, expression and functional analysis of the phosphofructokinase gene family in Chinese white pear (Pyrus bretschneideri). Gene 2019, 702, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.-M.; Stitt, M.; et al. The sucros-trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef]
- Wingler, A.; Delatte, T.L.; Ó’Hara, L.E.; Primavesi, L.F.; Jhurreea, D.; Paul, M.J.; Schluepmann, H. Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiol. 2012, 158, 1241–1251. [Google Scholar] [CrossRef]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef]
- Plaxton, W.C. The organization and regulation of plant glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef]
- Basson, C.E.; Groenewald, J.-H.; Kossmann, J.; Cronjé, C.; Bauer, R. Upregulation of pyrophosphate: Fructose 6-phosphate 1-phosphotransferase (PFP) activity in strawberry. Transgenic Res. 2011, 20, 925–931. [Google Scholar] [CrossRef]
- Mei, Z.X.; Li, Z.Q.; Lu, X.; Zhang, S.H.; Liu, W.J.; Zou, Q.; Yu, L.; Fang, H.C.; Zhang, Z.Y.; Mao, Z.Q.; et al. Supplementation of natural light duration promotes accumulation of sugar and anthocyanins in apple (Malus domestica Borkh.) fruit. Environ. Exp. Bot. 2023, 205, 105133. [Google Scholar] [CrossRef]
- Ambasht, P.K.; Kayastha, A.M. Plant pyruvate kinase. Biol. Plant. 2002, 45, 1–10. [Google Scholar] [CrossRef]
- Qin, Q.P.; Kaas, Q.; Zhang, L.L.; Xu, K.; Li, N.Y.; Zheng, W.W.; Lai, Q.X. Isolation and characterization of a cytosolic pyruvate kinase cDNA from loquat (Eriobotrya japonica Lindl.). Plant Mol. Biol. Rep. 2012, 31, 109–119. [Google Scholar] [CrossRef]
- Kennedy, R.A.; Rumpho, M.E.; Fox, T.C. Anaerobic metabolism in plants. Plant Physiol. 1992, 100, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Drew, M.C. Oxygen deficiency and root metabolism: Injury and acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 223–250. [Google Scholar] [CrossRef] [PubMed]
- Strommer, J. The plant ADH gene family. Plant J. 2011, 66, 128–142. [Google Scholar] [CrossRef]
- Eicks, M.; Maurino, V.; Knappe, S.; Flügge, U.-I.; Fischer, K. The plastidic pentose phosphate translocator represents a link between the cytosolic and the plastidic pentose phosphate pathways in plants. Plant Physiol. 2002, 128, 512–522. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, J.; Lin, H.; Lin, M.; Lin, Y.; Wang, H.; Hung, Y.-C. Salicylic acid reduces the incidence of Phomopsis longanae Chi infection in harvested longan fruit by affecting the energy status and respiratory metabolism. Postharvest Biol. Technol. 2020, 160, 111035. [Google Scholar] [CrossRef]
- Tao, S.K.; Zhu, Y.; Pan, Y.G.; Zhang, Z.K.; Huang, L.J. Enhancement of respiratory metabolism of the pentose phosphate pathway (PPP) strengthens the chilling tolerance of postharvest papaya fruit stored at 1 °C. Postharvest Biol. Technol. 2022, 191, 111988. [Google Scholar] [CrossRef]
- Kunz, S.; Pesquet, E.; Kleczkowski, L.A. Functional dissection of sugar signals affecting gene expression in Arabidopsis thaliana. PLoS ONE 2014, 9, e100312. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.S.; Sharma, M.; Laxmi, A. Role of sugar and auxin crosstalk in plant growth and development. Physiol. Plant. 2022, 174, e13546. [Google Scholar] [CrossRef] [PubMed]
- Hao, N.J.; Zou, X.X.; Lin, X.X.; Cai, R.Q.; Xiao, W.J.; Tong, T.; Yin, H.P.; Sun, A.; Guo, X.H. LecRK-VIII.2 mediates the cross-talk between sugar and brassinosteroid during hypocotyl elongation in Arabidopsis. Reprod. Breed. 2021, 1, 55–63. [Google Scholar] [CrossRef]
- Li, J.; Quan, Y.; Wang, L.; Wang, S. Brassinosteroid promotes grape berry quality-focus on physicochemical qualities and their coordination with enzymatic and molecular processes: A Review. Int. J. Mol. Sci. 2023, 24, 445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Chen, L.; Li, S.; Zhang, Y.; Xu, R.; Liu, Z.; Liu, W.; Kong, J.; Huang, X.; Wang, Y.; et al. BRI1 and BAK1 interact with G proteins and regulate sugar-responsive growth and development in Arabidopsis. Nat. Commun. 2018, 9, 1522. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Wang, T.; Liu, W.; Zhang, J.; Fang, H.; Zhang, Z.; Peng, F.; Chen, X.; Wang, N. MdMYB305-MdbHLH33-MdMYB10 regulates sugar and anthocyanin balance in red-fleshed apple fruits. Plant J. 2023, 113, 1062–1079. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, S.; Lin, X.; Fang, H.; Shi, Y.; Grierson, D.; Chen, K. Transcription factor CitERF16 is involved in citrus fruit sucrose accumulation by activating CitSWEET11d. Front. Plant Sci. 2021, 12, 809619. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gu, K.D.; Zhang, L.L.; Sun, C.H.; Zhang, Q.Y.; Wang, J.H.; Wang, C.K.; Wang, W.Y.; Du, M.C.; Hu, D.G. MdbHLH3 modulates apple soluble sugar content by activating phosphofructokinase gene expression. J. Integr. Plant Biol. 2022, 64, 884–900. [Google Scholar] [CrossRef]
- Wei, X.; Liu, F.; Chen, C.; Ma, F.; Li, M. The Malus domestica sugar transporter gene family: Identifications based on genome and expression profiling related to the accumulation of fruit sugars. Front. Plant Sci. 2014, 5, 569. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Wang, J.; Zhang, J.; Miao, H.; Jia, C.; Wang, Z.; Xu, B.; Jin, Z. MuMADS1 and MaOFP1 regulate fruit quality in a tomato ovate mutant. Plant Biotechnol. J. 2018, 16, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.G.; Luo, G.H. Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol. Commun. 1990, 6, 55–57. [Google Scholar]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Stewart, R.R.C.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Maehly, A.C. Plant peroxidase. Methods Enzymol. 1995, 2, 801–813. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.W.; Breen, P.J. Activity of phenylalanine ammonialyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J. Am. Soc. Hortic. Sci. 1991, 116, 865–869. [Google Scholar] [CrossRef]
- Maas, J.L.; Wang, S.Y.; Galletta, G.J. Evaluation of strawberry cultivars for ellagic acid content. HortScience 1991, 26, 66–68. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef]
- Heischmann, S.; Quinn, K.; Cruickshank-Quinn, C.; Liang, L.-P.; Reisdorph, R.; Reisdorph, N.; Patel, M. Exploratory metabolomics profiling in the kainic acid rat model reveals depletion of 25-hydroxyvitamin D3 during epileptogenesis. Sci. Rep. 2016, 6, 31424. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Comprehensive resistance evaluation of 15 blueberry cultivars under high soil pH stress based on growth phenotype and physiological traits. Front. Plant Sci. 2022, 13, 1072621. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wei, Z.; Wu, Y.; Zhang, C.; Lyu, L.; Wu, W.; Li, W. Transcriptomic and Metabolomic Profiling Reveals the Variations in Carbohydrate Metabolism between Two Blueberry Cultivars. Int. J. Mol. Sci. 2024, 25, 293. https://doi.org/10.3390/ijms25010293
Yang H, Wei Z, Wu Y, Zhang C, Lyu L, Wu W, Li W. Transcriptomic and Metabolomic Profiling Reveals the Variations in Carbohydrate Metabolism between Two Blueberry Cultivars. International Journal of Molecular Sciences. 2024; 25(1):293. https://doi.org/10.3390/ijms25010293
Chicago/Turabian StyleYang, Haiyan, Zhiwen Wei, Yaqiong Wu, Chunhong Zhang, Lianfei Lyu, Wenlong Wu, and Weilin Li. 2024. "Transcriptomic and Metabolomic Profiling Reveals the Variations in Carbohydrate Metabolism between Two Blueberry Cultivars" International Journal of Molecular Sciences 25, no. 1: 293. https://doi.org/10.3390/ijms25010293
APA StyleYang, H., Wei, Z., Wu, Y., Zhang, C., Lyu, L., Wu, W., & Li, W. (2024). Transcriptomic and Metabolomic Profiling Reveals the Variations in Carbohydrate Metabolism between Two Blueberry Cultivars. International Journal of Molecular Sciences, 25(1), 293. https://doi.org/10.3390/ijms25010293






