An Effectively Uncoupled Gd8 Cluster Formed through Fixation of Atmospheric CO2 Showing Excellent Magnetocaloric Properties
Abstract
:1. Introduction
2. Results and Discussion
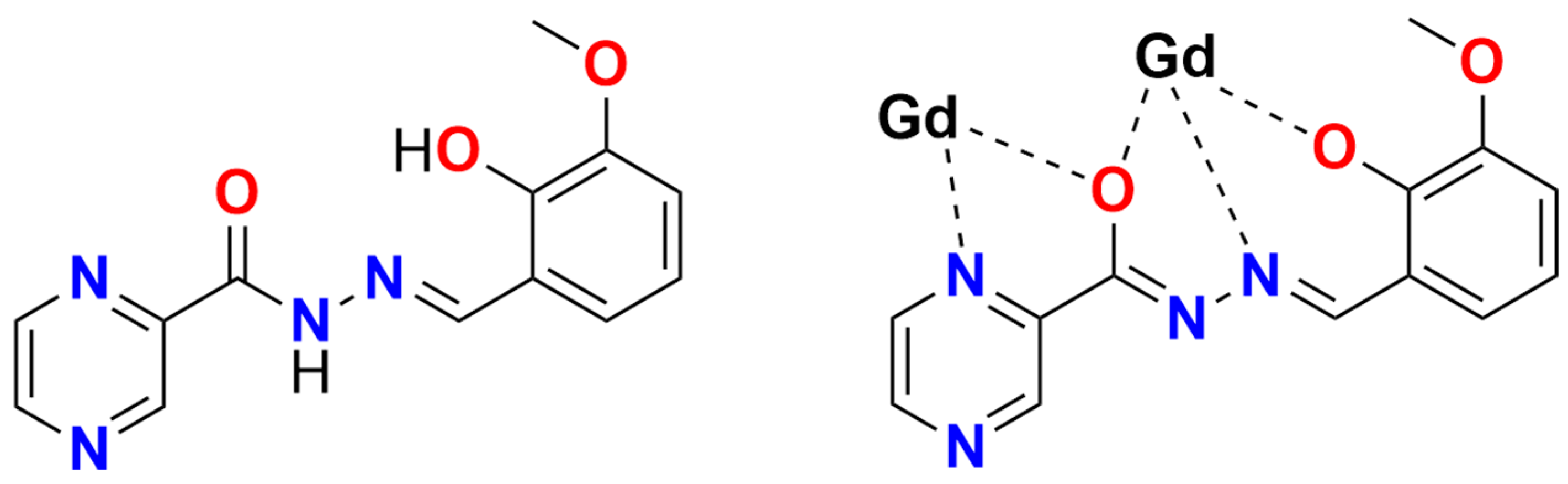
2.1. Crystallography
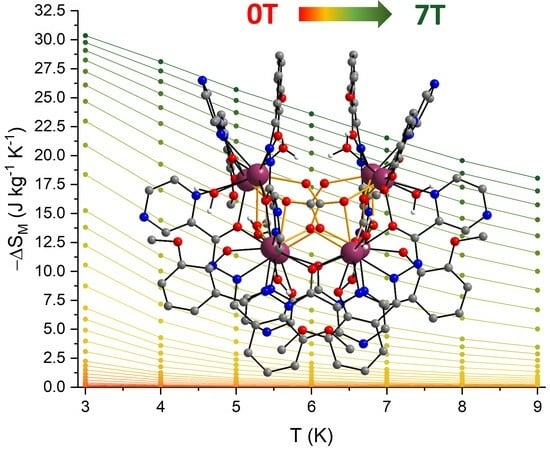
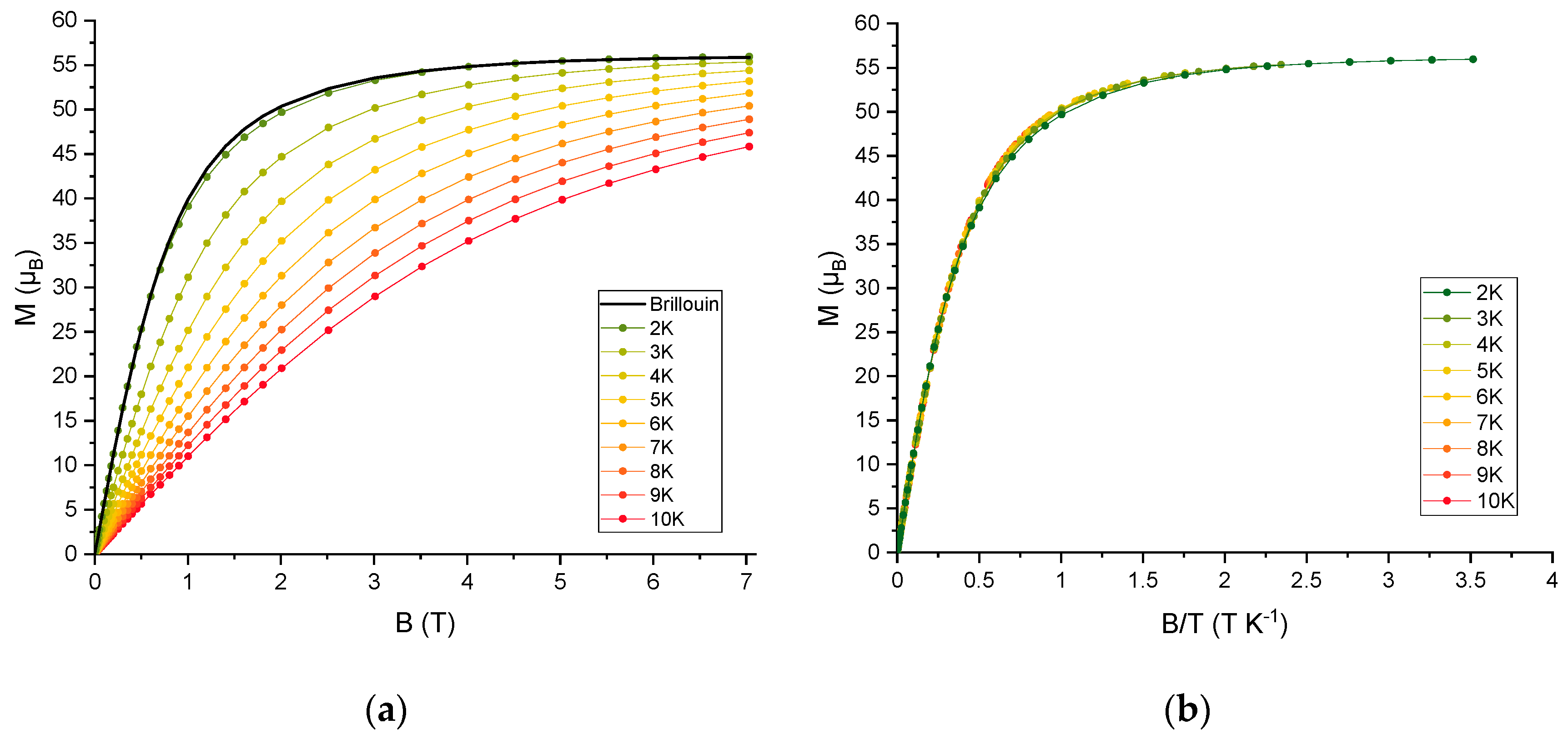
2.2. Magnetic Properties
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tishin, A.M.; Spichkin, Y.I. The Magnetocaloric Effect and Its Application; IOP Publishing: Bristol, UK; Philadelphia, PA, USA, 2003. [Google Scholar]
- Evangelisti, M.; Luis, F.; de Jongh, L.J.; Affronte, M. Magnetothermal properties of molecule-based materials. J. Mater. Chem. 2006, 16, 2534–2549. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Zhou, G.J.; Zheng, Z.; Winpenny, R.E. Molecule-based magnetic coolers. Chem. Soc. Rev. 2014, 43, 1462–1475. [Google Scholar] [CrossRef]
- Warburg, E. Magnetische Untersuchungen. Ann. Phys. 1881, 249, 141–164. [Google Scholar] [CrossRef]
- Sharples, J.W.; Zheng, Y.Z.; Tuna, F.; McInnes, E.J.; Collison, D. Lanthanide discs chill well and relax slowly. Chem. Commun. 2011, 47, 7650–7652. [Google Scholar] [CrossRef]
- Sharples, J.W.; Collison, D.; McInnes, E.J.L.; Schnack, J.; Palacios, E.; Evangelisti, M. Quantum signatures of a molecular nanomagnet in direct magnetocaloric measurements. Nat. Commun. 2014, 5, 5321–5326. [Google Scholar] [CrossRef]
- Lorusso, G.; Sharples, J.W.; Palacios, E.; Roubeau, O.; Brechin, E.K.; Sessoli, R.; Rossin, A.; Tuna, F.; McInnes, E.J.; Collison, D.; et al. A dense metal-organic framework for enhanced magnetic refrigeration. Adv. Mater. 2013, 25, 4653–4656. [Google Scholar] [CrossRef]
- Mo, Z.; Gong, J.; Xie, H.; Zhang, L.; Fu, Q.; Gao, X.; Li, Z.; Shen, J. Giant low-field cryogenic magnetocaloric effect in polycrystalline LiErF4 compound. Chin. Phys. B 2023, 32, 027503. [Google Scholar] [CrossRef]
- Tziotzi, T.G.; Gracia, D.; Dalgarno, S.J.; Schnack, J.; Evangelisti, M.; Brechin, E.K.; Milios, C.J. A Gd12Na6 Molecular Quadruple-Wheel with a Record Magnetocaloric Effect at Low Magnetic Fields and Temperatures. J. Am. Chem. Soc. 2023, 145, 7743–7747. [Google Scholar] [CrossRef]
- Ibrahim, M.; Peng, Y.; Moreno-Pineda, E.; Anson, C.E.; Schnack, J.; Powell, A.K. Gd3 Triangles in a Polyoxometalate Matrix: Tuning Molecular Magnetocaloric Effects in {Gd30M8} Polyoxometalate/Cluster Hybrids Through Variation of M2+. Small Struct. 2021, 2, 2100052. [Google Scholar] [CrossRef]
- Tian, H.; Zhao, L.; Guo, Y.N.; Guo, Y.; Tang, J.; Liu, Z. Quadruple-CO32− bridged octanuclear dysprosium(III) compound showing single-molecule magnet behaviour. Chem. Commun. 2012, 48, 708–710. [Google Scholar] [CrossRef]
- Tian, H.; Ungur, L.; Zhao, L.; Ding, S.; Tang, J.; Chibotaru, L.F. Exchange Interactions Switch Tunneling: A Comparative Experimental and Theoretical Study on Relaxation Dynamics by Targeted Metal Ion Replacement. Chem. Eur. J. 2018, 24, 9928–9939. [Google Scholar] [CrossRef]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Natrajan, L.; Pecaut, J.; Mazzanti, M. Fixation of atmospheric CO2 by a dimeric lanthanum hydroxide complex; assembly of an unusual hexameric carbonate. Dalton Trans. 2006, 1002–1005. [Google Scholar] [CrossRef]
- Tang, X.-L.; Wang, W.-H.; Dou, W.; Jiang, J.; Liu, W.-S.; Qin, W.-W.; Zhang, G.-L.; Zhang, H.-R.; Yu, K.-B.; Zheng, L.-M. Olive-shaped chiral supramolecules: Simultaneous self-assembly of heptameric lanthanum clusters and carbon dioxide fixation. Angew. Chem. Int. Ed. 2009, 48, 3499–3502. [Google Scholar] [CrossRef]
- Langley, S.K.; Moubaraki, B.; Murray, K.S. Magnetic properties of hexanuclear lanthanide(III) clusters incorporating a central µ6-carbonate ligand derived from atmospheric CO2 fixation. Inorg. Chem. 2012, 51, 3947–3949. [Google Scholar] [CrossRef]
- Pineda, E.M.; Lorusso, G.; Zangana, K.H.; Palacios, E.; Schnack, J.; Evangelisti, M.; Winpenny, R.E.P.; McInnes, E.J.L. Observation of the influence of dipolar and spin frustration effects on the magnetocaloric properties of a trigonal prismatic Gd7 molecular nanomagnet. Chem. Sci. 2016, 7, 4891–4895. [Google Scholar] [CrossRef]
- Goura, J.; Colacio, E.; Herrera, J.M.; Suturina, E.A.; Kuprov, I.; Lan, Y.; Wernsdorfer, W.; Chandrasekhar, V. Heterometallic Zn3Ln3 Ensembles Containing (µ6-CO3) Ligand and Triangular Disposition of Ln3+ ions: Analysis of Single-Molecule Toroic (SMT) and Single-Molecule Magnet (SMM) Behavior. Chem. Eur. J. 2017, 23, 16621–16636. [Google Scholar] [CrossRef]
- Bala, S.; Adhikary, A.; Bhattacharya, S.; Bishwas, M.S.; Poddar, P.; Mondal, R. Ln8 (Ln = Gd, Ho, Er, Yb) Butterfly Core-Exhibiting Magnetocaloric Effect and Field-Induced SMM Behavior for Er Analogue. ChemistrySelect 2017, 2, 11341–11345. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.L.; Zhao, L.; Guo, M.; Tang, J. Enhancement of Magnetocaloric Effect through Fixation of Carbon Dioxide: Molecular Assembly from Ln4 to Ln4 Cluster Pairs. Inorg. Chem. 2017, 56, 4104–4111. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Ju, W.; Luo, X.; Lin, Q.; Cao, J.; Xu, Y. A Series of Lanthanide Compounds Constructed from Ln8 Rings Exhibiting Large Magnetocaloric Effect and Interesting Luminescence. Inorg. Chem. 2018, 57, 8608–8614. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-F.; Kuang, W.-W.; Li, Y.-M.; Zhu, L.-L.; Xu, Y.; Yang, P.-P. A series of new octanuclear Ln8 clusters: Magnetic studies reveal a significant cryogenic magnetocaloric effect and slow magnetic relaxation. New J. Chem. 2019, 43, 1617–1625. [Google Scholar] [CrossRef]
- Kalita, P.; Goura, J.; Nayak, P.; Colacio, E.; Chandrasekhar, V. Octanuclear {Ln8} complexes: Magneto-caloric effect in the {Gd8} analogue. J. Chem. Sci. 2021, 133, 82. [Google Scholar] [CrossRef]
- Li, J.N.; Li, N.F.; Wang, J.L.; Liu, X.M.; Ping, Q.D.; Zang, T.T.; Mei, H.; Xu, Y. A new family of boat-shaped Ln8 clusters exhibiting the magnetocaloric effect and slow magnetic relaxation. Dalton Trans. 2021, 50, 13925–13931. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Qin, L.; Meng, Z.-S.; Yang, D.-F.; Wu, C.; Fu, Z.; Zheng, Y.-Z.; Liu, J.-L.; Tarasenko, R.; Orendac, M.; et al. Study of a magnetic-cooling material Gd(OH)CO3. J. Mater. Chem. A 2014, 2, 9851–9858. [Google Scholar] [CrossRef]
- Vergara, F.M.; Lima, C.H.; Henriques, M.; Candea, A.L.; Lourenco, M.C.; de, L. Ferreira, M.; Kaiser, C.R.; de Souza, M.V. Synthesis and antimycobacterial activity of N’-[(E)-(monosubstituted-benzylidene)]-2-pyrazinecarbohydrazide derivatives. Eur. J. Med. Chem. 2009, 44, 4954–4959. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]



| Compound | MW (g/mol) | −ΔSM (J/kgK) | B (T) | T (K) | Reference |
|---|---|---|---|---|---|
| [Gd8(opch)8(CO3)4(H2O)8]·4H2O·10MeCN | 4286.76 | 30.36 (89%) | 7 | 3 | this work |
| [Gd8(µ3-OH)4(L1)4(DEA)4Cl4](DMF)2(MeOH) | 3404.00 | 31.4 (77%) | 9 | 3 | [19] |
| [Gd8(μ3-OH)4(CO3)2L4(PhCOO)8] | 3982.60 | 28.38 (78%) | 7 | 2 | [20] |
| [Gd8(CH2OHCH2OH)8(SO4)12]·2(C2H7N)·2H2O | 3012.30 | 36.86 (80%) | 7 | 2 | [21] |
| [Gd8(µ3-O)4(L)8(CH3COO)4(CO3)2]·15H2O | 3463.85 | 32.49 (81%) | 7 | 2 | [22] |
| [Gd8(HL)6(L)2(µ3-OH)4(µ2-OH)2(H2O)4] | 3777.88 | 25.5 (73%) | 5 | 3 | [23] |
| [Gd8(IN)14(μ3-OH)8(μ2-OH)2(H2O)8]·11H2O | 3479.81 | 31.77 (80%) | 7 | 2 | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, J.; Seufert, D.; Anson, C.E.; Tang, J.; Powell, A.K. An Effectively Uncoupled Gd8 Cluster Formed through Fixation of Atmospheric CO2 Showing Excellent Magnetocaloric Properties. Int. J. Mol. Sci. 2024, 25, 264. https://doi.org/10.3390/ijms25010264
Braun J, Seufert D, Anson CE, Tang J, Powell AK. An Effectively Uncoupled Gd8 Cluster Formed through Fixation of Atmospheric CO2 Showing Excellent Magnetocaloric Properties. International Journal of Molecular Sciences. 2024; 25(1):264. https://doi.org/10.3390/ijms25010264
Chicago/Turabian StyleBraun, Jonas, Daniel Seufert, Christopher E. Anson, Jinkui Tang, and Annie K. Powell. 2024. "An Effectively Uncoupled Gd8 Cluster Formed through Fixation of Atmospheric CO2 Showing Excellent Magnetocaloric Properties" International Journal of Molecular Sciences 25, no. 1: 264. https://doi.org/10.3390/ijms25010264
APA StyleBraun, J., Seufert, D., Anson, C. E., Tang, J., & Powell, A. K. (2024). An Effectively Uncoupled Gd8 Cluster Formed through Fixation of Atmospheric CO2 Showing Excellent Magnetocaloric Properties. International Journal of Molecular Sciences, 25(1), 264. https://doi.org/10.3390/ijms25010264