Concentrations of Selected Adipocytokines in the Blood Plasma in Proximal Suspensory Desmopathy of Horses, with a Focus on Their Physical Activity—A Pilot Study
Abstract
1. Introduction
Adipocytokines in Musculoskeletal Diseases
2. Results
2.1. Parameters of Clinical Characteristics
2.2. Ultrasound Examination
2.3. Relationship between the Concentration Levels of the Studied Cytokines and Clinical Characteristic Parameters—Laboratory Studies
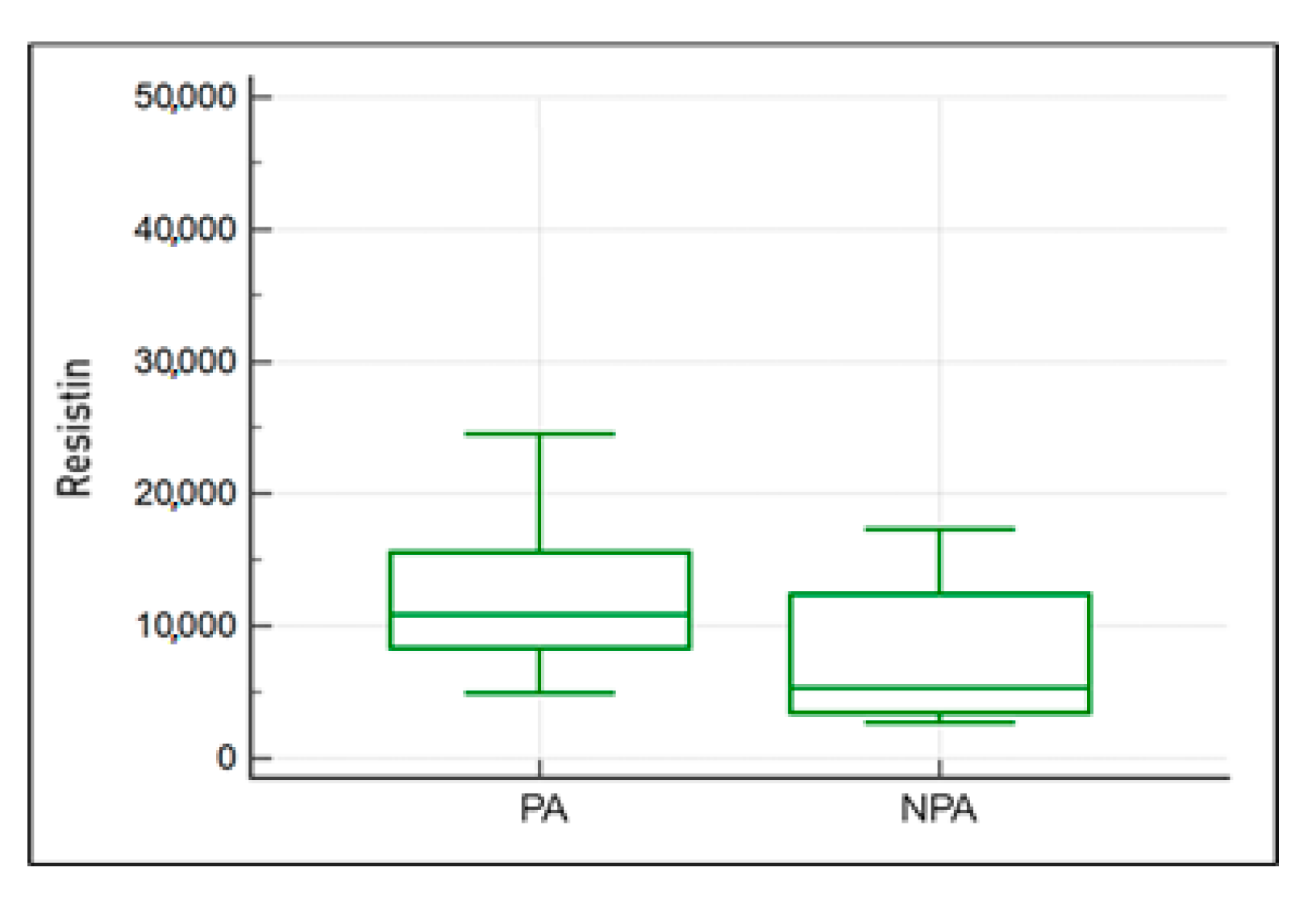
2.4. Impact of Desmopathy of the Proximal Suspensory Ligament on Resistin, Wisfatin, Leptin, and IL-8 Concentration in the Blood Plasma
2.5. Impact of the Activity Musculoskeletal System on Resistin, Wisfatin, Leptin, and IL-8 Concentration in the Blood Plasma
2.6. Correlations between Leptin Concentration and Resistin, Visfatin, and IL-8 Concentrations in the Blood Plasma among the Isolated Groups: Study Group (PSD) and Control Group (C)
3. Discussion
4. Materials and Methods
4.1. Scheme of the Study Group Eligibility Test
4.2. Veterinary Medical History, Clinical and Imaging Studies
4.3. Laboratory Analysis
4.4. Method Used to Assess Adipokine and Interleukin 8 Levels
4.5. Experiment Process
4.6. Statistical Analysis
5. Conclusions
Limitations of the Study
- Extended radiological diagnostics using magnetic resonance imaging;
- Supplementing with additional markers including tumour necrosis factor, interleukin-6, and vascular endothelial growth factor.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dyson, S. Proximal suspensory desmitis in the hindlimb: 42 cases. Br. Vet. J. 1994, 150, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Dyson, S. Magnetic resonance anatomy of the proximal metacarpal region of the horse described from images acquired from low- and high-field magnets. Vet. Radiol. Ultrasound 2009, 50, 595–605. [Google Scholar] [CrossRef]
- Williams, M.R.; Arnoczky, S.P.; Pease, A.P.; Stick, J.A. Microvasculature of the suspensory ligament of the forelimb of horses. Am. J. Vet. Res. 2013, 74, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Van Dijk, J. Application of a ridden horse ethogram to video recordings of 21 horses before and after diagnostic analgesia: Reduction in behaviour scores. Equine Vet. Educ. 2020, 32, 104–111. [Google Scholar] [CrossRef]
- Hardeman, A.; Egenvall, A.; Braganca, F.; Swagemakers, J.H.; Koene, M.; Roepstorff, L.; van Weeren, P.; Byström, A. Visual lameness assessment in comparison to quantitative gait analysis data in horses. Equine Vet. J. 2022, 54, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S.; Pinilla, M.J.; Bolas, N.; Murray, R. Proximal suspensory desmopathy in hindlimbs: Magnetic resonance imaging, gross post-mortem and histological study. Equine Vet. J. 2018, 50, 159–165. [Google Scholar] [CrossRef]
- Werpy, N.M. Review of Non-Weight-Bearing Proximal Suspensory Ligament Ultrasound for Alterations in the Muscle/Fat Indicating Pathologic Change. How to Maximaze the Use of Ultrasound in the Field. AAEP Proceedings, Volume 67, pp. 49–57. Available online: https://aaep.org/sites/default/files/2022-05/Werpy,%20Natasha.pdf (accessed on 24 November 2023).
- Denoix, J.-M.; Perrot, P.; Bousseau, B.; Sciciuna, C. Images echographiques des lésions du muscle interosseux III (ligament suspenseur du boulet). Prat. Vét. Equine 1991, 23, 23–33. [Google Scholar]
- Li, H.Y.; Hua, Y.H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. BioMed Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef]
- Shukunami, C.; Takimoto, A.; Oro, M.; Hiraki, Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Dev. Biol. 2006, 298, 234–247. [Google Scholar] [CrossRef]
- Pingel, J.; Petersen, M.C.; Fredberg, U.; Kjær, S.G.; Quistorff, B.; Langberg, H.; Hansen, J.B. Inflammatory and Metabolic Alterations of Kager’s Fat Pad in Chronic Achilles Tendinopathy. PLoS ONE 2015, 10, e0127811. [Google Scholar] [CrossRef]
- Falcão-Pires, I.; Castro-Chaves, P.; Miranda-Silva, D.; Lourenço, A.P.; Leite-Moreira, A.F. Physiological, pathological and potential therapeutic roles of adipokines. Drug Discov. Today 2012, 17, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nature reviews. Endocrinology 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Bonakdari, H.; Tardif, G.; Abram, F.; Pelletier, J.P.; Martel-Pelletier, J. Serum adipokines/related inflammatory factors and ratios as predictors of infrapatellar fat pad volume in osteoarthritis: Applying comprehensive machine learning approaches. Sci. Rep. 2020, 10, 9993. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Redman, S.; Milz, S.; Büttner, A.; Amin, A.; Moriggl, B.; Brenner, E.; Emery, P.; McGonagle, D.; Bydder, G. Adipose tissue at entheses: The rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann. Rheum. Dis. 2004, 63, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Codoñer-Franch, P.; Alonso-Iglesias, E. Resistin: Insulin resistance to malignancy. Clin. Chim. Acta Int. J. Clin. Chem. 2015, 438, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W.K. Resistin concentration in blood plasma of racing and rally horses. In Proceedings of the “Omnia Autem Animalia Sunt”: XVI Kongres Polskiego Towarzystwa Nauk Weterynaryjnych, Warszawa, Poland, 26–27 November 2021; Wydawnictwo SGGW: Warszawa, Poland, 2021. [Google Scholar]
- Fuentes-Romero, B.; Muñoz-Prieto, A.; Cerón, J.J.; Martín-Cuervo, M.; Iglesias-García, M.; Aguilera-Tejero, E.; Díez-Castro, E. Measurement of Plasma Resistin Concentrations in Horses with Metabolic and Inflammatory Disorders. Animals 2021, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- McGlothlin, J.R.; Gao, L.; Lavoie, T.; Simon, B.A.; Easley, R.B.; Ma, S.F.; Rumala, B.B.; Garcia, J.G.; Ye, S.Q. Molecular cloning and characterization of canine pre-B-cell colony-enhancing factor. Biochem. Genet. 2005, 43, 127–141. [Google Scholar] [CrossRef]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nature reviews. Endocrinology 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/visfatin in mammals. Curr. Opin. Gastroenterol. 2007, 23, 164–170. [Google Scholar] [CrossRef]
- Chiu, C.Z.; Wang, B.W.; Yu, Y.J.; Shyu, K.G. Hyperbaric oxygen activates visfatin expression and angiogenesis via angiotensin II and JNK pathway in hypoxic human coronary artery endothelial cells. J. Cell. Mol. Med. 2020, 24, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Gosset, M.; Berenbaum, F.; Salvat, C.; Sautet, A.; Pigenet, A.; Tahiri, K.; Jacques, C. Crucial role of visfatin/pre-B cell colony-enhancing factor in matrix degradation and prostaglandin E2 synthesis in chondrocytes: Possible influence on osteoarthritis. Arthritis Rheum. 2008, 58, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W.; Janczarek, I.; Wilk, I.; Staniszewska, M.; Kowalik, S. Plasma visfatin response to the intensity of exercise and training in race-horses. Pferdeheilkunde Equine Med. 2018, 34, 525–530. [Google Scholar] [CrossRef]
- Sweeney, G.; Keen, J.; Somwar, R.; Konrad, D.; Garg, R.; Klip, A. High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in l6 rat skeletal muscle cells. Endocrinology 2001, 142, 4806–4812. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rademacher, J.; Tietz, L.M.; Le, L.; Hartl, A.; Hermann, K.A.; Sieper, J.; Mansmann, U.; Rudwaleit, M.; Poddubnyy, D. Added value of biomarkers compared with clinical parameters for the prediction of radiographic spinal progression in axial spondyloarthritis. Rheumatology 2019, 58, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Abel, E.D.; Litwin, S.E.; Sweeney, G. Cardiac remodeling in obesity. Physiol. Rev. 2008, 88, 389–419. [Google Scholar] [CrossRef]
- Kleine, S.A.; Sanderson, S.L.; George, C.; Roth, I.; Gogal, R.M.; Thaliath, M.A.; Budsberg, S.C. Correlation of serum and synovial leptin concentrations with body condition scores in healthy and osteoarthritic dogs. Vet. Surg. VS 2019, 48, 780–785. [Google Scholar] [CrossRef]
- Bas, S.; Finckh, A.; Puskas, G.J.; Suva, D.; Hoffmeyer, P.; Gabay, C.; Lübbeke, A. Adipokines correlate with pain in lower limb osteoarthritis: Different associations in hip and knee. Int. Orthop. 2014, 38, 2577–2583. [Google Scholar] [CrossRef]
- Kędzierski, W. Changes in plasma leptin concentration during different types of exercises performed by horses. Anim. Int. J. Anim. Biosci. 2014, 8, 1456–1461. [Google Scholar] [CrossRef]
- Heidemann, J.; Ogawa, H.; Dwinell, M.B.; Rafiee, P.; Maaser, C.; Gockel, H.R.; Otterson, M.F.; Ota, D.M.; Lugering, N.; Domschke, W.; et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J. Biol. Chem. 2003, 278, 8508–8515. [Google Scholar] [CrossRef]
- Lee, H.G.; Choi, J.Y.; Park, J.W.; Park, T.S.; Song, K.D.; Shin, D.; Cho, B.W. Effects of exercise on myokine gene expression in horse skeletal muscles. Asian-Australas. J. Anim. Sci. 2019, 32, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.H.; Carey, A.L.; Watt, M.J.; Febbraio, M.A. Cytokine gene expression in human skeletal muscle during concentric contraction: Evidence that IL-8, like IL-6, is influenced by glycogen availability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R322–R327. [Google Scholar] [CrossRef] [PubMed]
- Beavers, K.M.; Hsu, F.C.; Isom, S.; Kritchevsky, S.B.; Church, T.; Goodpaster, B.; Pahor, M.; Nicklas, B.J. Long-term physical activity and inflammatory biomarkers in older adults. Med. Sci. Sports Exerc. 2010, 42, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Dyson, S. The Ridden Horse Pain Ethogram. Equine Vet. Educ. 2021, 34, 372–380. [Google Scholar] [CrossRef]
- Crass, J.R.; Genovense, R.L.; Render, J.A.; Bellon, E.M. Magnetic resonance, ultrasound and histopathologic correlation of acute and healing tendon injuries. Vet. Radiol. Ultrasound 1992, 33, 206–216. [Google Scholar] [CrossRef]
- Dyson, S.; Murray, R. Management of hindlimb proximal suspensory desmopathy by neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy: 155 horses (2003–2008). Equine Vet. J. 2012, 44, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Docheva, D.; Müller, S.A.; Majewski, M.; Evans, C.H. Biologics for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 222–239. [Google Scholar] [CrossRef]
- Durgam, S.; Stewart, M. Cellular and Molecular Factors Influencing Tendon Repair. Tissue Eng. Part B Rev. 2017, 23, 307–317. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. et Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Dakin, S.G.; Werling, D.; Hibbert, A.; Abayasekara, D.R.; Young, N.J.; Smith, R.K.; Dudhia, J. Macrophage sub-populations and the lipoxin A4 receptor implicate active inflammation during equine tendon repair. PLoS ONE 2012, 7, e32333. [Google Scholar] [CrossRef]
- de Castro Pochini, A.; Ejnisman, B.; de Seixas Alves, M.T.; Uyeda, L.F.; Nouailhetas, V.L.; Han, S.W.; Cohen, M.; Albertoni, W.M. Overuse of training increases mechanoreceptors in supraspinatus tendon of rats SHR. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2011, 29, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Gilles, C.L. Rehabilitation of tendon and ligament injuries. Proc. Am. Assoc. Equine Pract. Cong Orlando US 2016, 43, 306–309. [Google Scholar]
- Lui, P.P.Y.; Yung, P.S.H. Inflammatory mechanisms linking obesity and tendinopathy. J. Orthop. Transl. 2021, 31, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Maleitzke, T.; Geissler, S.; Hildebrandt, A.; Fleckenstein, F.N.; Niemann, M.; Fischer, H.; Perka, C.; Duda, G.N.; Winkler, T. Source and hub of inflammation: The infrapatellar fat pad and its interactions with articular tissues during knee osteoarthritis. J. Orthop. Res. 2022, 40, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Shetty, G.K.; Economides, P.A.; Horton, E.S.; Mantzoros, C.S.; Veves, A. Circulating adiponectin and resistin levels in relation to metabolic factors, inflammatory markers, and vascular reactivity in diabetic patients and subjects at risk for diabetes. Diabetes Care 2004, 27, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Calabro, P.; Samudio, I.; Willerson, J.T.; Yeh, E.T. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation 2004, 110, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Li, S.H.; Wang, C.H.; Fedak, P.W.; Li, R.K.; Weisel, R.D.; Mickle, D.A. Resistin promotes endothelial cell activation: Further evidence of adipokine-endothelial interaction. Circulation 2003, 108, 736–740. [Google Scholar] [CrossRef]
- Kougias, P.; Chai, H.; Lin, P.H.; Lumsden, A.B.; Yao, Q.; Chen, C. Adipocyte-derived cytokine resistin causes endothelial dysfunction of porcine coronary arteries. J. Vasc. Surg. 2005, 41, 691–698. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, S.E.; Lee, H.C.; Hur, J.; Lee, S.; Youn, S.W.; Lee, J.; Lee, H.J.; Lee, T.K.; Park, J.; et al. Adipokine resistin is a key player to modulate monocytes, endothelial cells, and smooth muscle cells, leading to progression of atherosclerosis in rabbit carotid artery. J. Am. Coll. Cardiol. 2011, 57, 99–109. [Google Scholar] [CrossRef]
- Steppan, C.M.; Brown, E.J.; Wright, C.M.; Bhat, S.; Banerjee, R.R.; Dai, C.Y.; Enders, G.H.; Silberg, D.G.; Wen, X.; Wu, G.D.; et al. A family of tissue-specific resistin-like molecules. Proc. Natl. Acad. Sci. USA 2001, 98, 502–506. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Chatzinikolaou, A.; Tournis, S.; Nikolaidis, M.G.; Jamurtas, A.Z.; Douroudos, I.I.; Papassotiriou, I.; Thomakos, P.M.; Taxildaris, K.; Mastorakos, G.; et al. Intensity of resistance exercise determines adipokine and resting energy expenditure responses in overweight elderly individuals. Diabetes Care 2009, 32, 2161–2167. [Google Scholar] [CrossRef]
- Van Pelt, D.W.; Guth, L.M.; Horowitz, J.F. Aerobic exercise elevates markers of angiogenesis and macrophage IL-6 gene expression in the subcutaneous adipose tissue of overweight-to-obese adults. J. Appl. Physiol. 2017, 123, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Ihalainen, J.K.; Schumann, M.; Eklund, D.; Hämäläinen, M.; Moilanen, E.; Paulsen, G.; Häkkinen, K.; Mero, A.A. Combined aerobic and resistance training decreases inflammation markers in healthy men. Scand. J. Med. Sci. Sports 2018, 28, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, A.; Kulikova, V.; Ziegler, M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Lee, H.G.; Sp, N.; Kang, D.Y.; Jang, K.J.; Lee, H.K.; Cho, B.W.; Yang, Y.M. Validation of exercise-response genes in skeletal muscle cells of Thoroughbred racing horses. Asian-Australas. J. Anim. Sci. 2021, 34, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Buchbinder, R.; van Tulder, M.; Öberg, B.; Costa, L.M.; Woolf, A.; Schoene, M.; Croft, P. Lancet Low Back Pain Series Working Group. Low back pain: A call for action. Lancet 2018, 391, 2384–2388. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Ackermann, P.W.; Hart, D.A. General Overview and Summary of Concepts Regarding Tendon Disease Topics Addressed Related to Metabolic Disorders. Adv. Exp. Med. Biol. 2016, 920, 293–298. [Google Scholar] [CrossRef]
- Sui, Y.; Lee, J.H.; DiMicco, M.A.; Vanderploeg, E.J.; Blake, S.M.; Hung, H.H.; Plaas, A.H.; James, I.E.; Song, X.Y.; Lark, M.W.; et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009, 60, 2985–2996. [Google Scholar] [CrossRef]
- Rechardt, M.; Viikari-Juntura, E.; Shiri, R. Adipokines as predictors of recovery from upper extremity soft tissue disorders. Rheumatology 2014, 53, 2238–2242. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, J.; Li, H.; Ouyang, X.; Sun, X.; Tang, Y.; Chen, H.; Wang, B.; Li, X. Leptin Upregulates Peripheral CD4+CXCR5+ICOS+ T Cells via Increased IL-6 in Rheumatoid Arthritis Patients. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2018, 38, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Kędzierski, W. Leptin fluctuations in trained horses, during a work season. J. Equine Vet. Sci. 2016, 43, 12–17. [Google Scholar] [CrossRef]
- Filková, M.; Haluzík, M.; Gay, S.; Senolt, L. The role of resistin as a regulator of inflammation: Implications for various human pathologies. Clin. Immunol. 2009, 133, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Keen, J.A.; Fordham, T.; Morgan, R.A. Adipose tissue dysfunction in obese horses with equine metabolic syndrome. Equine Vet. J. 2019, 51, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.A.; Dugdale, A.H.A. Assessment of body condition and bodyweight. Equine Appl. Clin. Nutr. 2013, 12, 393–404. [Google Scholar] [CrossRef]
- Werpy, N.M.; Denoix, J.M.; McIlwraith, C.W.; Frisbie, D.D. Comparison between standard ultrasonography, angle contrast ultrasonography, and magnetic resonance imaging characteristics of the normal equine proximal suspensory ligament. Vet. Radiol. Ultrasound 2013, 54, 536–547. [Google Scholar] [CrossRef]
- Giardullo, L.; Corrado, A.; Maruotti, N.; Cici, D.; Mansueto, N.; Cantatore, F.P. Adipokine role in physiopathology of inflammatory and degenerative musculoskeletal diseases. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211015034. [Google Scholar] [CrossRef]
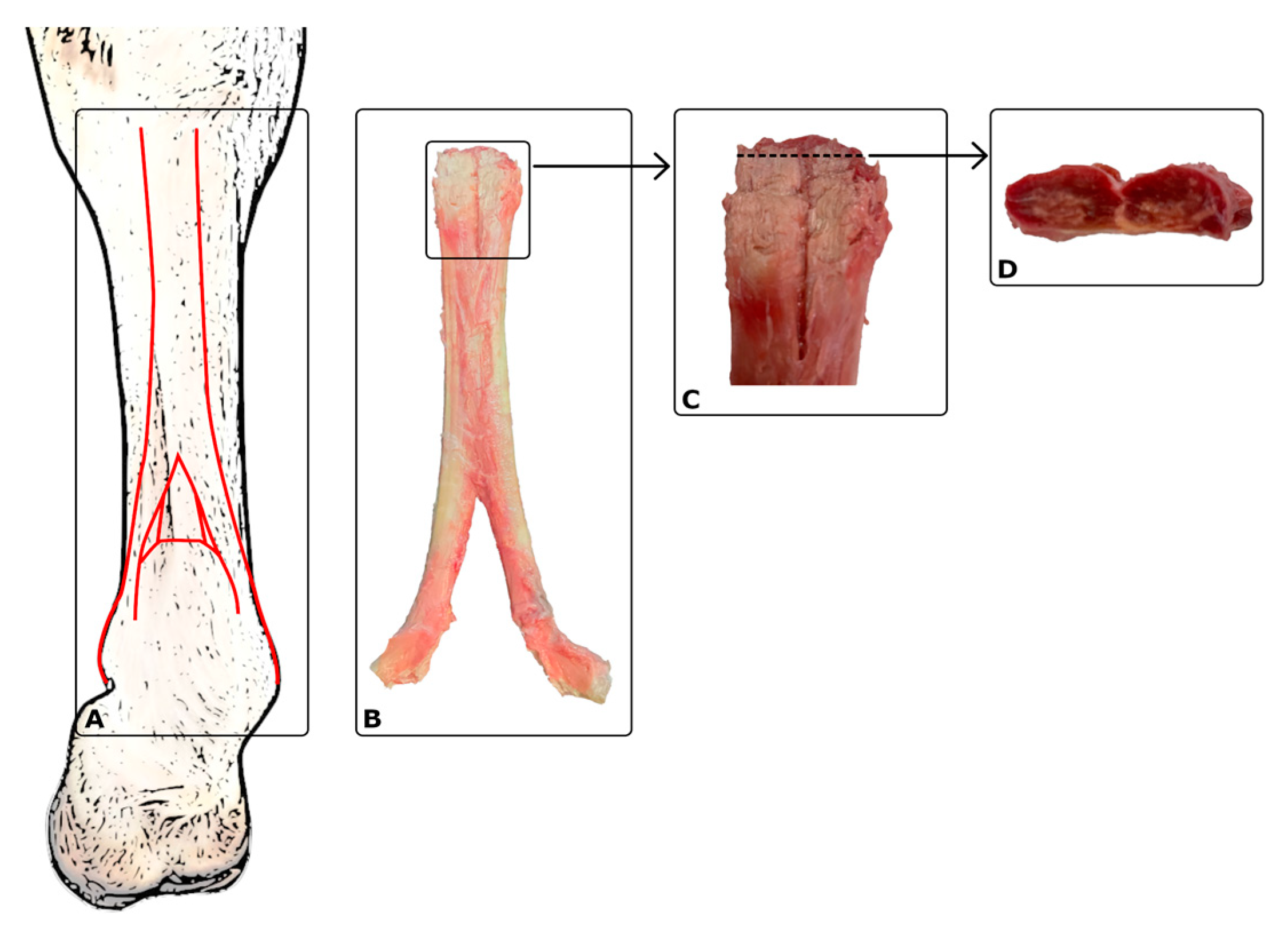

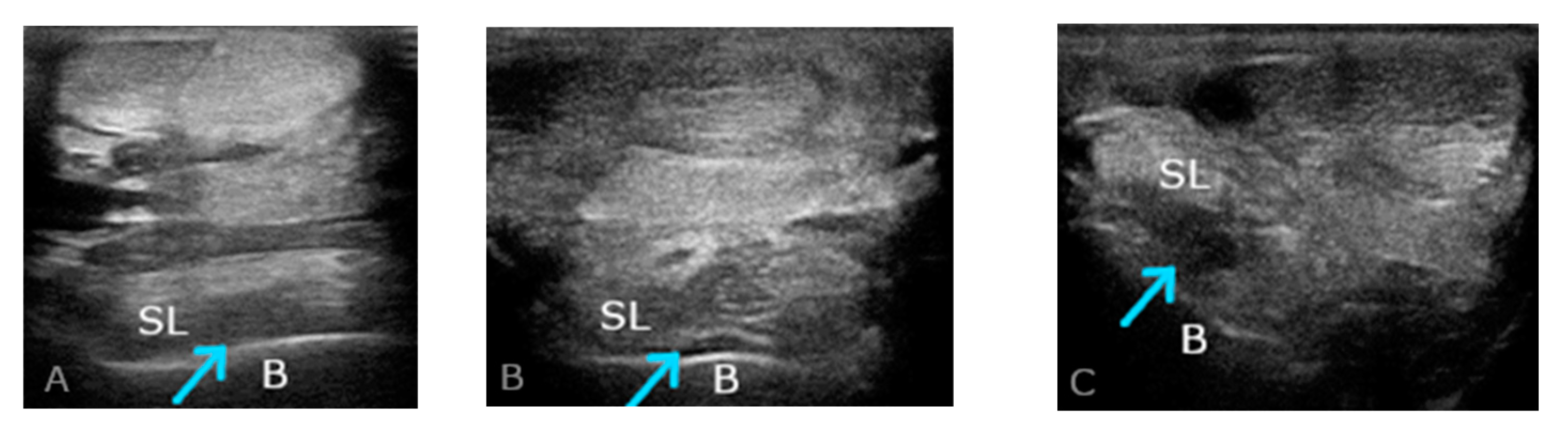

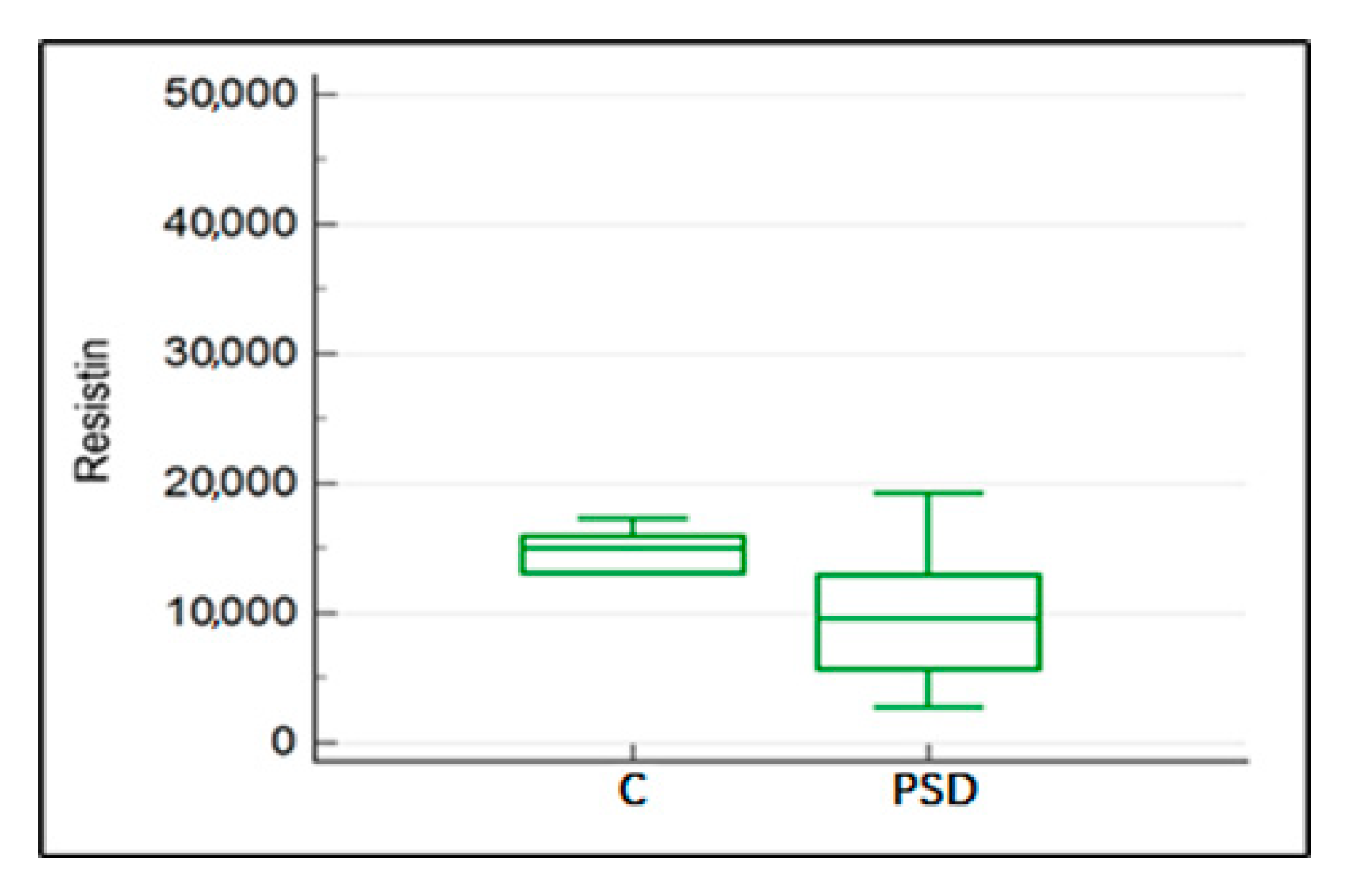

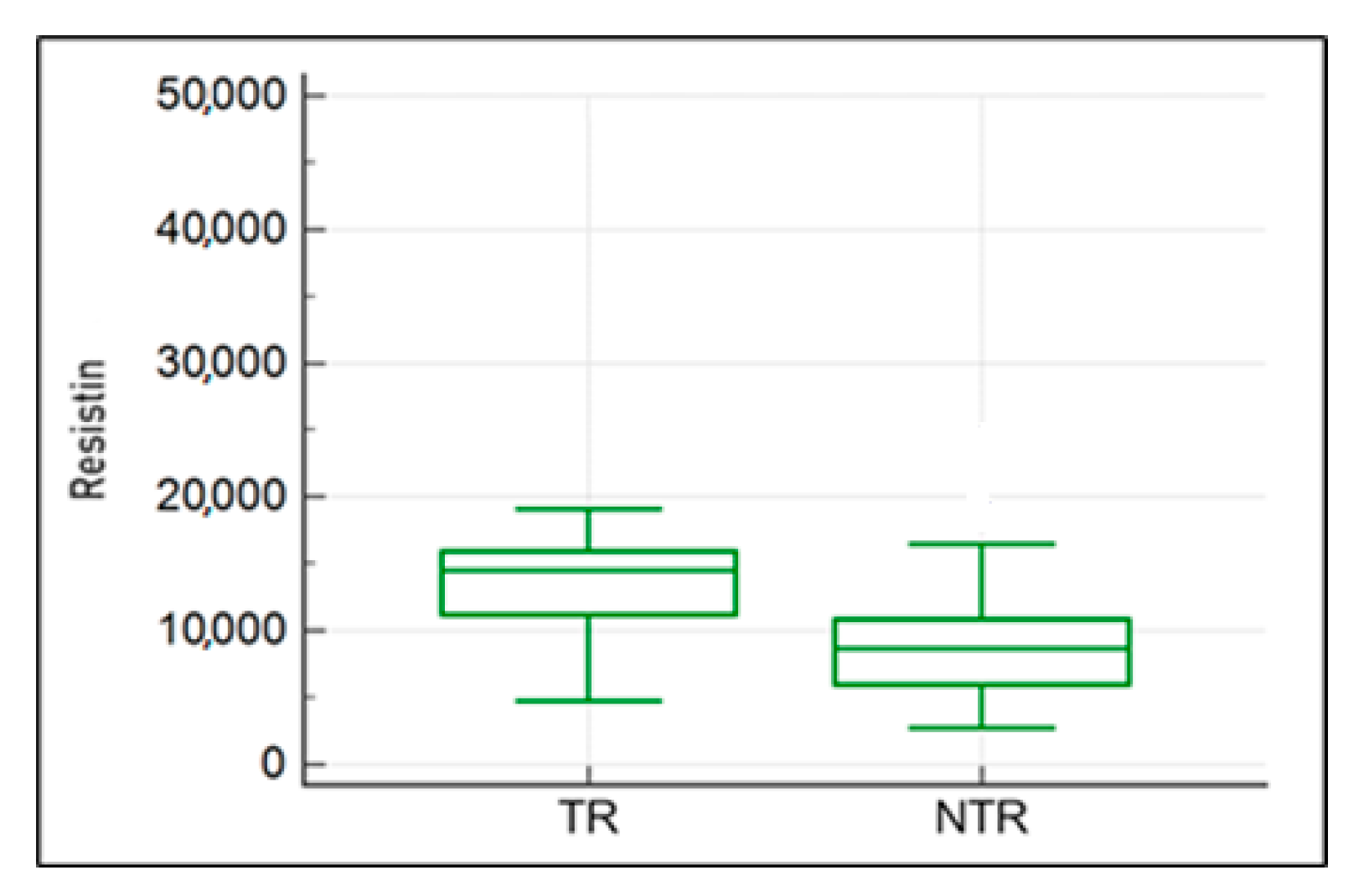
| Term | Definitions of the Characteristic Terms Used |
|---|---|
| Lameness | A change in the horse’s gait, usually caused by pain or mechanical restriction. |
| Chronic condition | Disease symptoms that may occur with PSD, including but not limited to lameness, lasting no less than 21 days. |
| Maintenance method | Determines the conditions of the horses’ daily living, i.e., whether they had access to a paddock and the time they were out of the stable. |
| Paddocking | This means that the horse has been in the paddock, regardless of its surface area, at least once a day for a minimum of 2 h at least 4 days a week. |
| No paddocking | This means that the conditions for keeping the horse did not meet the criteria given for the term “paddocking”; leaving the stable only for training, walking in hand, and/or using the carousel qualified as “no paddocking”. |
| Method of use | Defines the conditions of use of the horse by the rider, i.e., regular work under the saddle; used for recreational riding or sporting recreation. |
| Training | Daily work under the saddle, lasting a minimum of 40 to 60 min a day, at least 4 times a week, regardless of the riding discipline and the level of training of the horse and rider. |
| No training | This means that the conditions of use of the horse by the rider did not meet the criteria given for the term “training”. |
| Feature | PSD Group | Control Group | ||
|---|---|---|---|---|
| n | (%) | n | (%) | |
| Gender mare | 7 | 25 | 2 | 22 |
| Gelding | 14 | 53 | 7 | 78 |
| Stallion | 6 | 22 | - | - |
| Age (years) | 13 | 7 | ||
| median | (9–20) | (4–18) | ||
| Breed—half-bred horse | 25 | 93 | 7 | 78 |
| Wielkopolska | 2 | 7 | - | - |
| Other | - | - | 2 | 22 |
| BCS 2 | 22 | 81 | 5 | 55 |
| BCS 3 | 7 | 19 | 4 | 45 |
| History | PSD Group n % | Control Group n % | ||
|---|---|---|---|---|
| Lameness | 27 (100%) | 0 | ||
| Right front limb | 12 | 44 | - | - |
| Left front limb | 11 | 11 | - | - |
| Right hind limb | 1 | 4 | - | - |
| Left hind limb | 3 | 11 | - | - |
| Housing method (all horses) | 36 (100%) | |||
| Paddock | 22 | 61 | 6 | 26 |
| No paddock | 5 | 14 | 3 | 8 |
| Usage method (all horses) | 36 (100%) | |||
| Training (working) | 18 | 50 | 6 | 17 |
| No training (non-working) | 9 | 25 | 3 | 8 |
| Resistin | All Horses | PSD | C | |||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Age | 0.12 | 0.50 | 0.29 | 0.15 | 0.38 | 0.32 |
| RBC | 0.09 | 0.63 | 0.12 | 0.95 | 0.33 | 0.38 |
| HGB | 0.12 | 0.52 | 0.15 | 0.47 | 0.37 | 0.33 |
| MCH | 0.44 | 0.01 | 0.45 | 0.03 | 0.01 | 0.98 |
| MCHC | 0.43 | 0.01 | 0.30 | 0.14 | 0.14 | 0.73 |
| MCV | 0.28 | 0.12 | 0.39 | 0.05 | 0.08 | 0.85 |
| WBC | 0.36 | 0.03 | 0.49 | 0.01 | 0.37 | 0.33 |
| NEU | 0.40 | 0.02 | 0.59 | 0.002 | 0.30 | 0.44 |
| LIMF | 0.04 | 0.84 | 0.44 | 0.03 | 0.47 | 0.21 |
| NLR | 0.28 | 0.11 | 0.50 | 0.01 | 0.10 | 0.79 |
| MLR | 0.21 | 0.23 | 0.41 | 0.04 | 0.03 | 0.93 |
| PLT | 0.02 | 0.89 | 0.001 | 0.99 | 0.35 | 0.36 |
| Leptin | n | All Horses (ng/mL) | n | PSD Group (ng/mL) | n | C Group (ng/mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| Resistin | 36 | r | 0.15 | 27 | r | 0.15 | 9 | r | 0.024 |
| p | 0.412 | p | 0.412 | p | 0.96 | ||||
| Visfatin | 36 | r | 0.63 | 27 | r | 0.63 | 9 | r | 0.47 |
| p | 0.001 | p | 0.001 | p | 0.24 | ||||
| IL-8 | 36 | r | 0.12 | 27 | r | 0.12 | 9 | r | 0.89 |
| p | 0.58 | p | 0.58 | p | 0.003 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowicka, B.; Torres, A.; Polkowska, I.; Jackow-Nowicka, J.; Przewozny, M.; Jackow-Malinowska, J. Concentrations of Selected Adipocytokines in the Blood Plasma in Proximal Suspensory Desmopathy of Horses, with a Focus on Their Physical Activity—A Pilot Study. Int. J. Mol. Sci. 2024, 25, 205. https://doi.org/10.3390/ijms25010205
Nowicka B, Torres A, Polkowska I, Jackow-Nowicka J, Przewozny M, Jackow-Malinowska J. Concentrations of Selected Adipocytokines in the Blood Plasma in Proximal Suspensory Desmopathy of Horses, with a Focus on Their Physical Activity—A Pilot Study. International Journal of Molecular Sciences. 2024; 25(1):205. https://doi.org/10.3390/ijms25010205
Chicago/Turabian StyleNowicka, Beata, Anna Torres, Izabela Polkowska, Jagoda Jackow-Nowicka, Maciej Przewozny, and Joanna Jackow-Malinowska. 2024. "Concentrations of Selected Adipocytokines in the Blood Plasma in Proximal Suspensory Desmopathy of Horses, with a Focus on Their Physical Activity—A Pilot Study" International Journal of Molecular Sciences 25, no. 1: 205. https://doi.org/10.3390/ijms25010205
APA StyleNowicka, B., Torres, A., Polkowska, I., Jackow-Nowicka, J., Przewozny, M., & Jackow-Malinowska, J. (2024). Concentrations of Selected Adipocytokines in the Blood Plasma in Proximal Suspensory Desmopathy of Horses, with a Focus on Their Physical Activity—A Pilot Study. International Journal of Molecular Sciences, 25(1), 205. https://doi.org/10.3390/ijms25010205






