Identification and Analysis of the Expression of the PIP5K Gene Family in Tomatoes
Abstract
1. Introduction
2. Results
2.1. Characterization of Members of the SlPIP5K Gene Family in Tomato
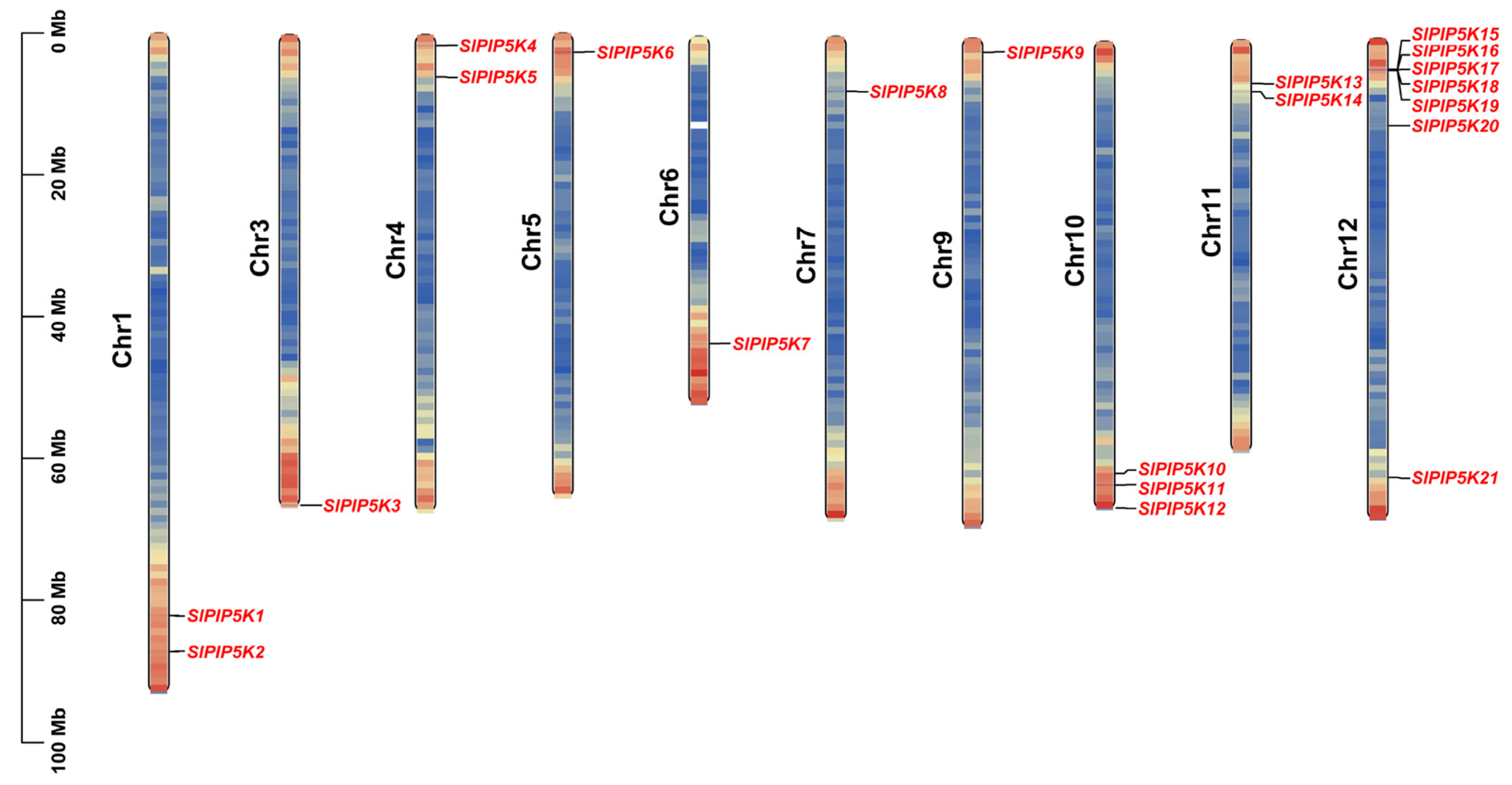
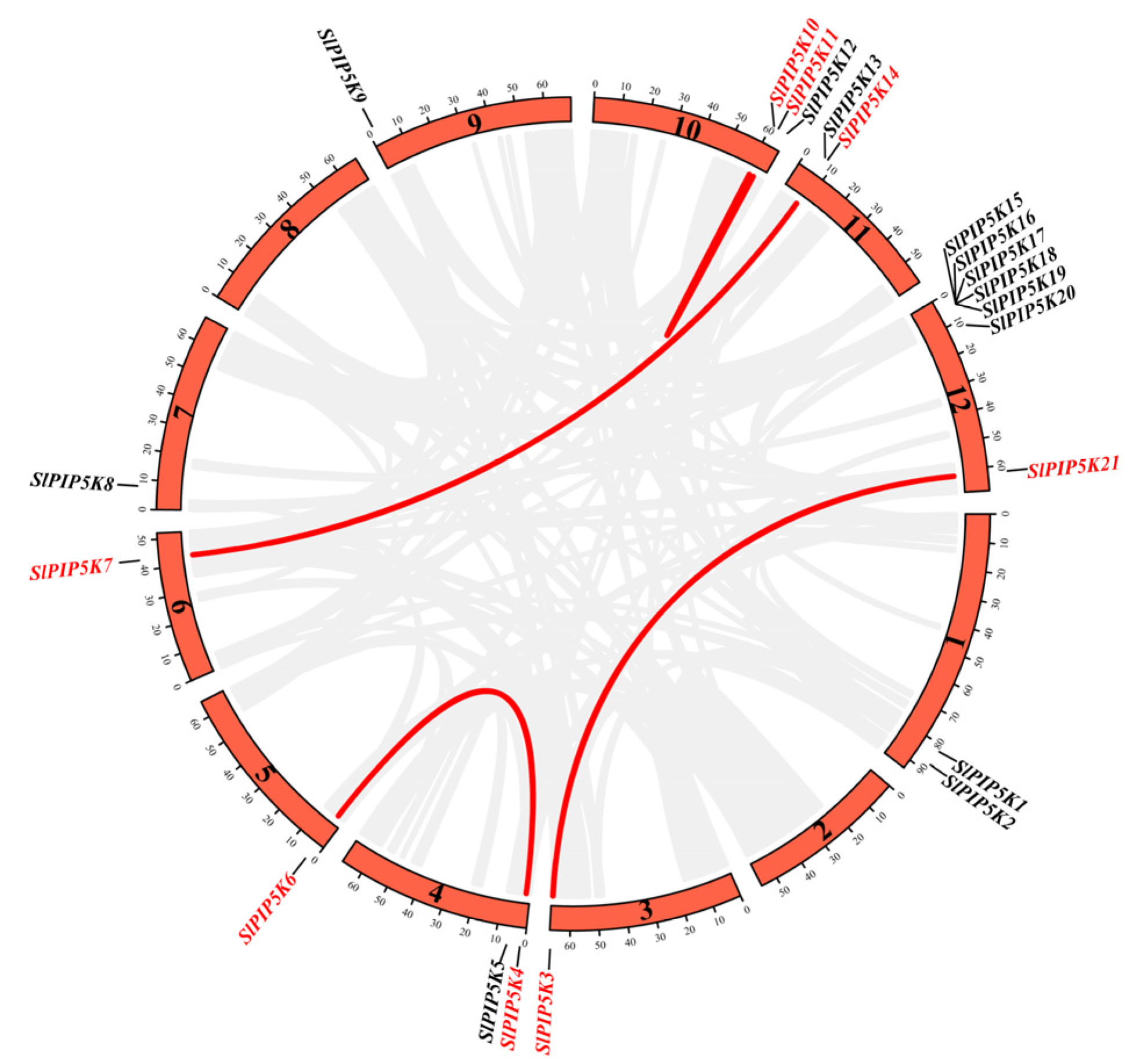
2.2. Chromosomal Localization of the SlPIP5K Gene Family in Tomato
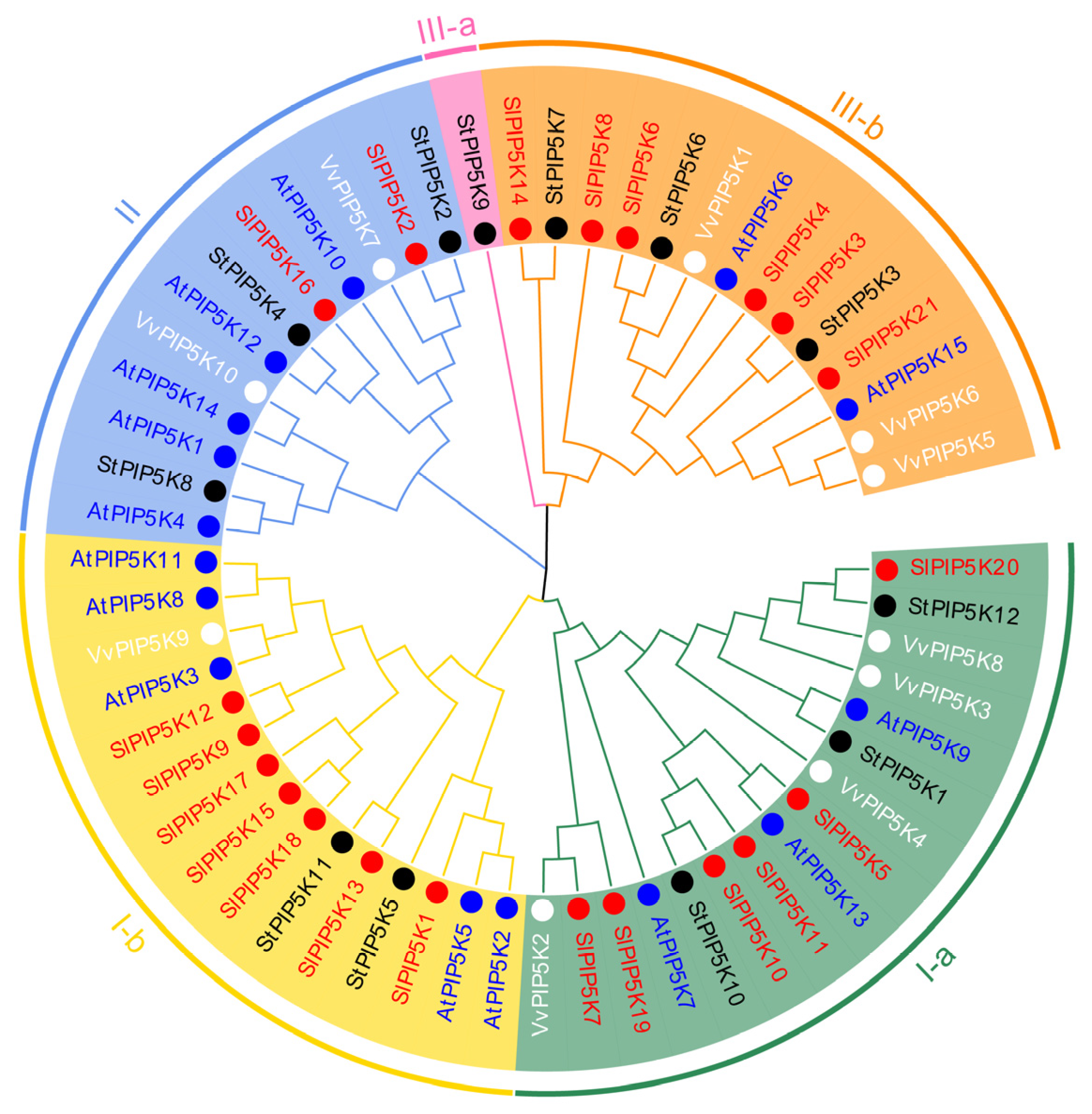
2.3. Phylogenetic Classification of the SlPIP5K Gene in Tomato
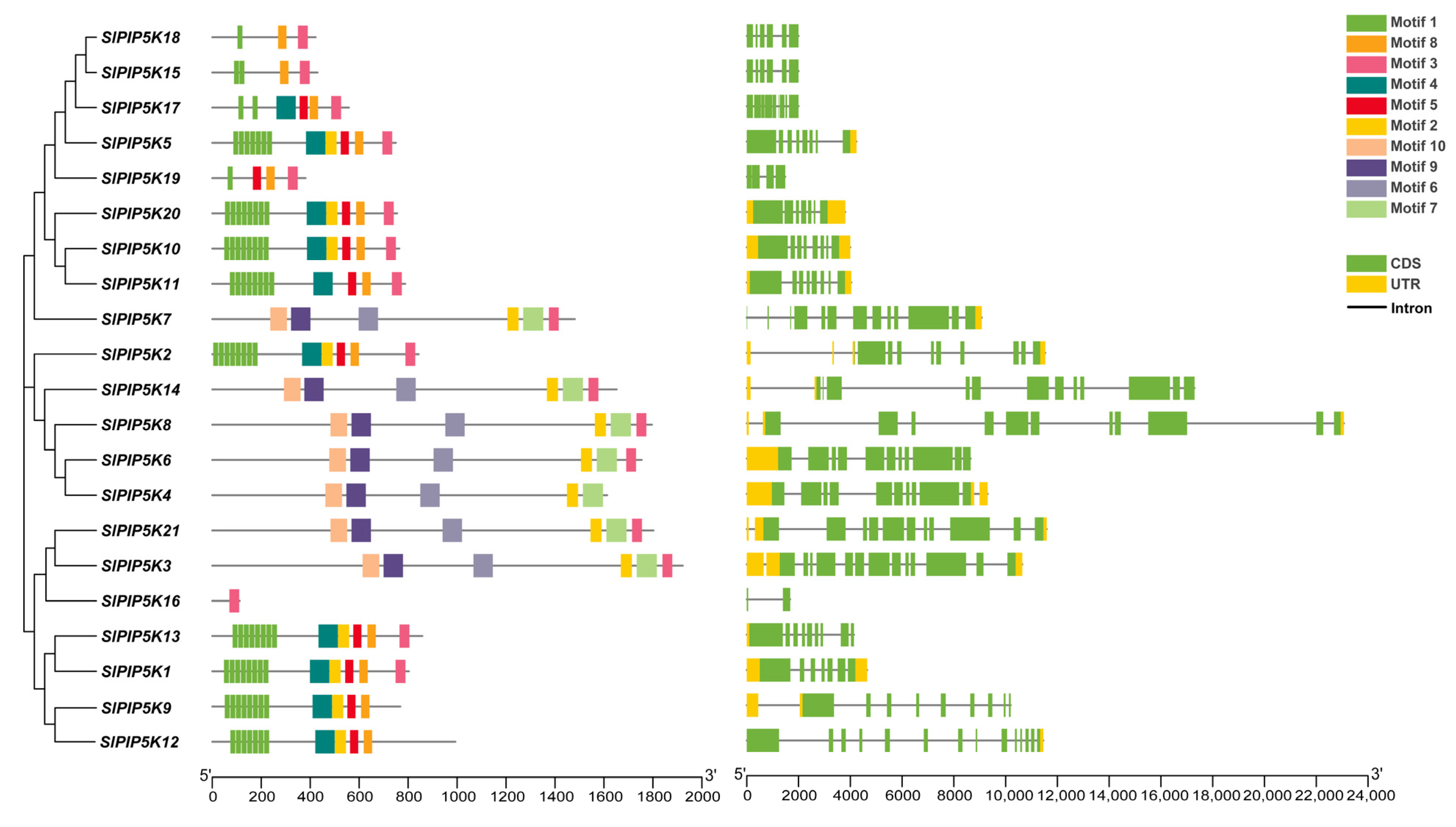
2.4. Tomato SlPIP5K Gene Family Member Motifs and Gene Structures
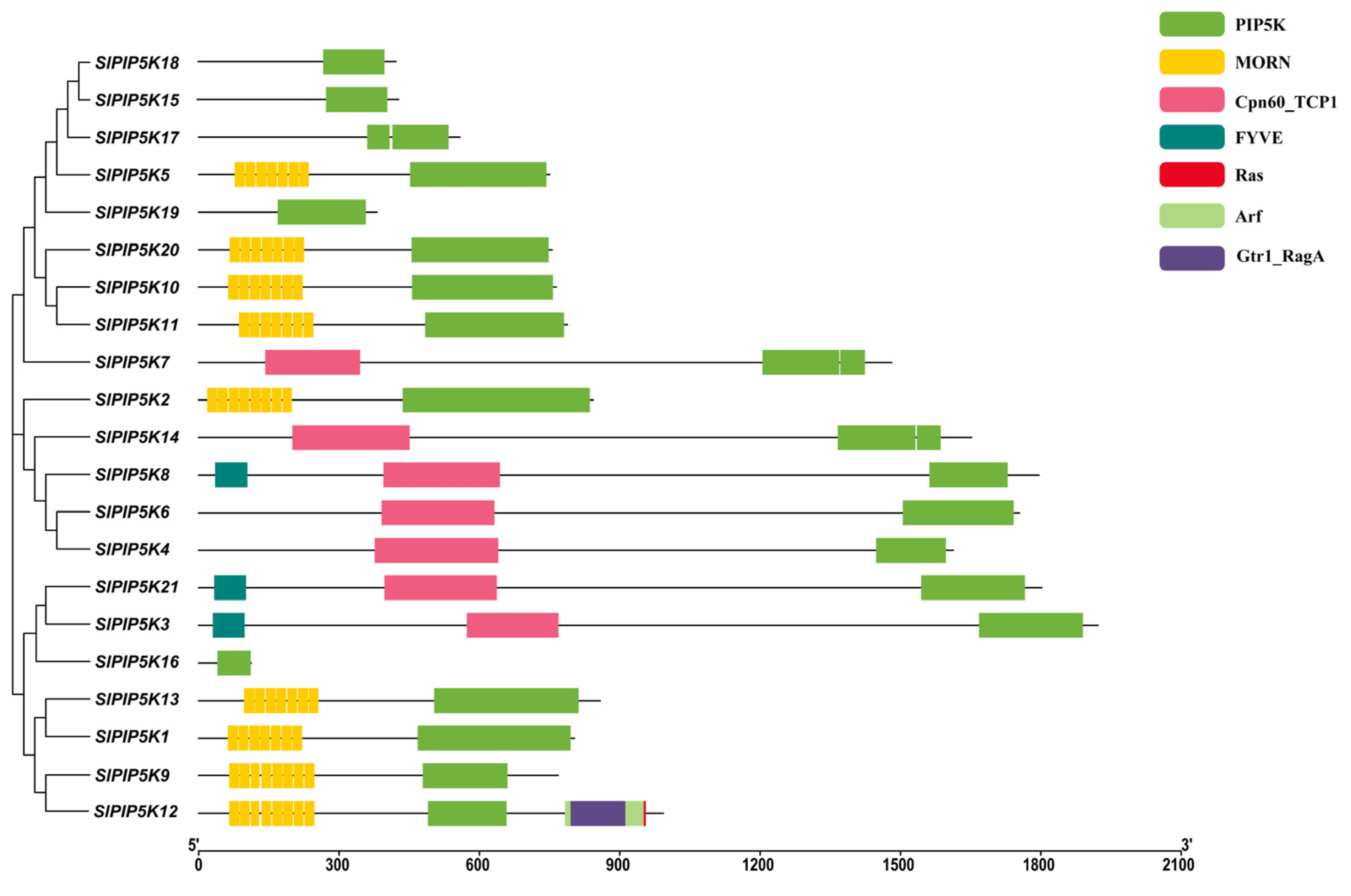
2.5. Analysis of Protein Structural Domains of Tomato SlPIP5K Gene Family Members
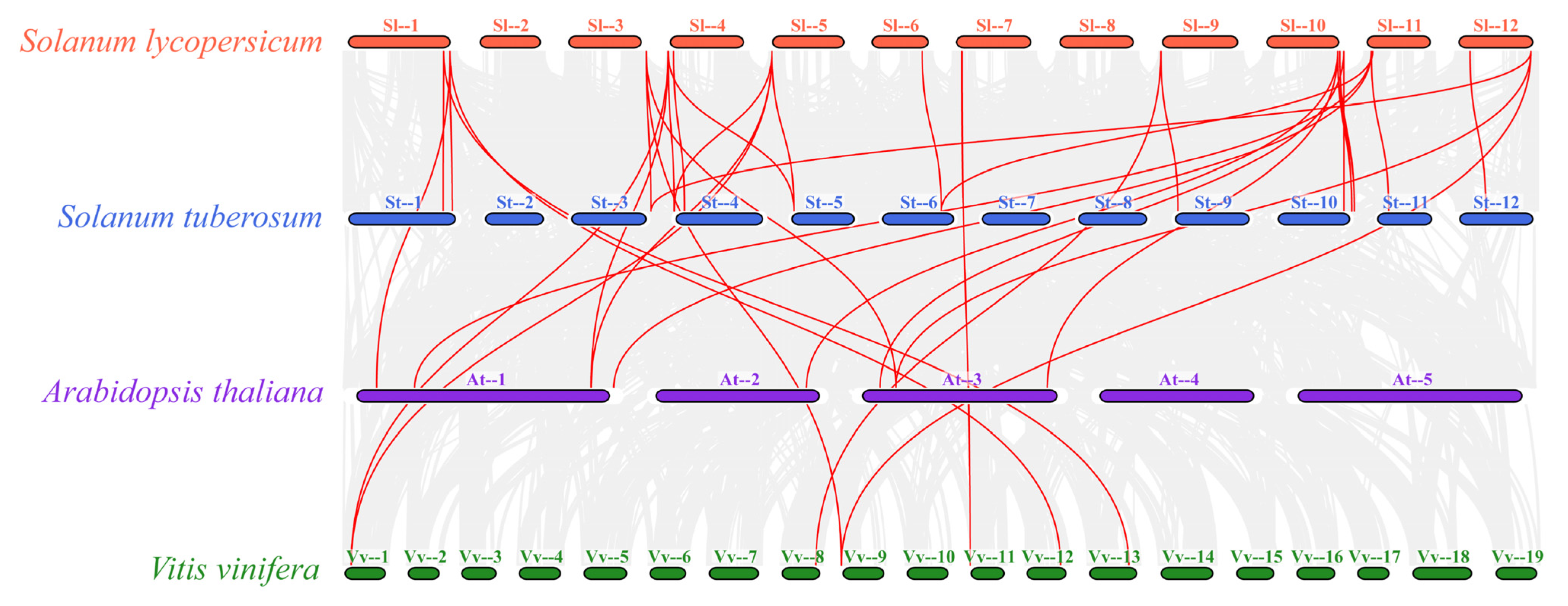
2.6. Analysis of Covariance and Evolutionary Pressure Analysis for Members of the SlPIP5K Gene Family in Tomato
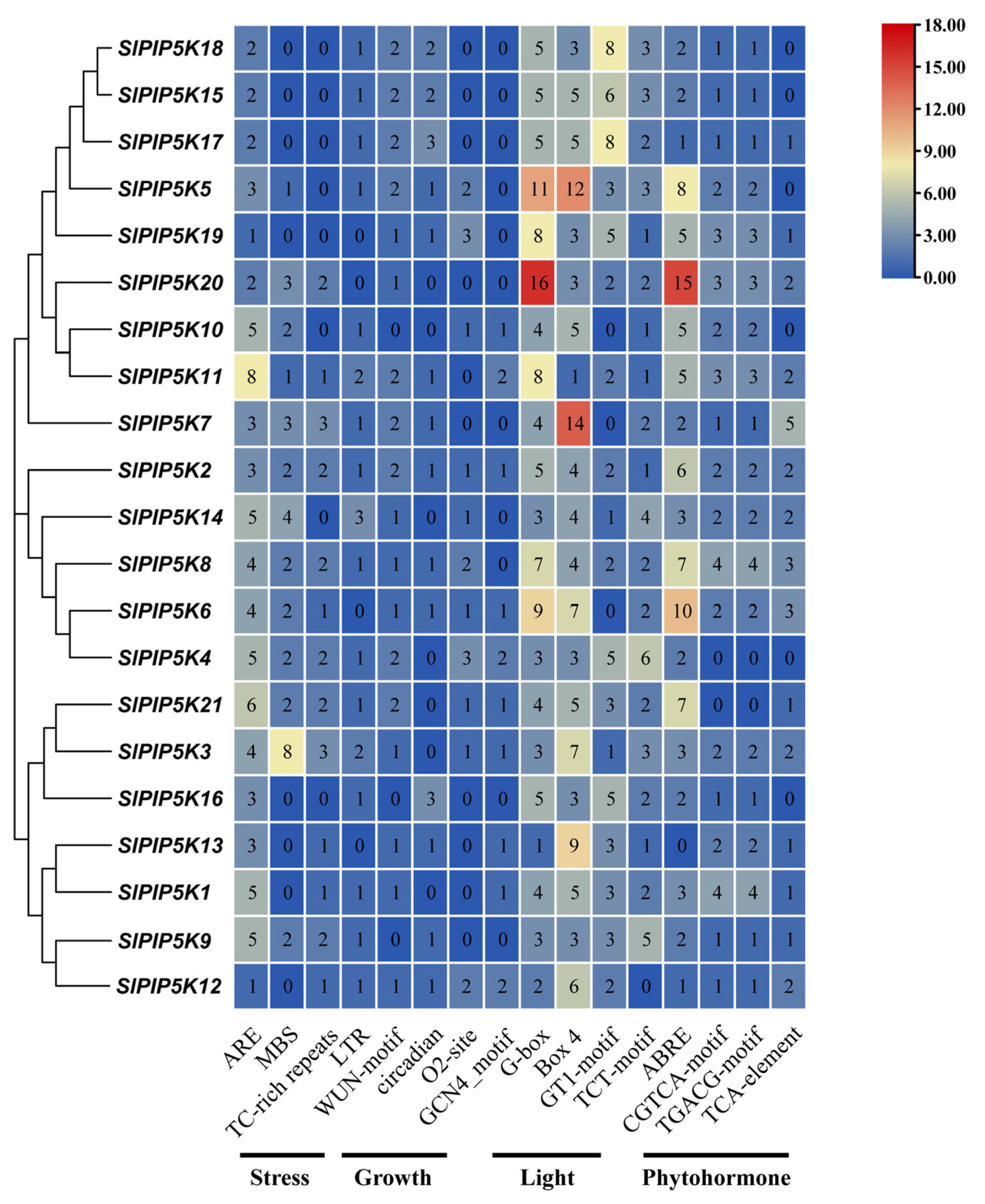
2.7. Analysis of Cis-Acting Elements of the Tomato SlPIP5K Gene Family Promoter
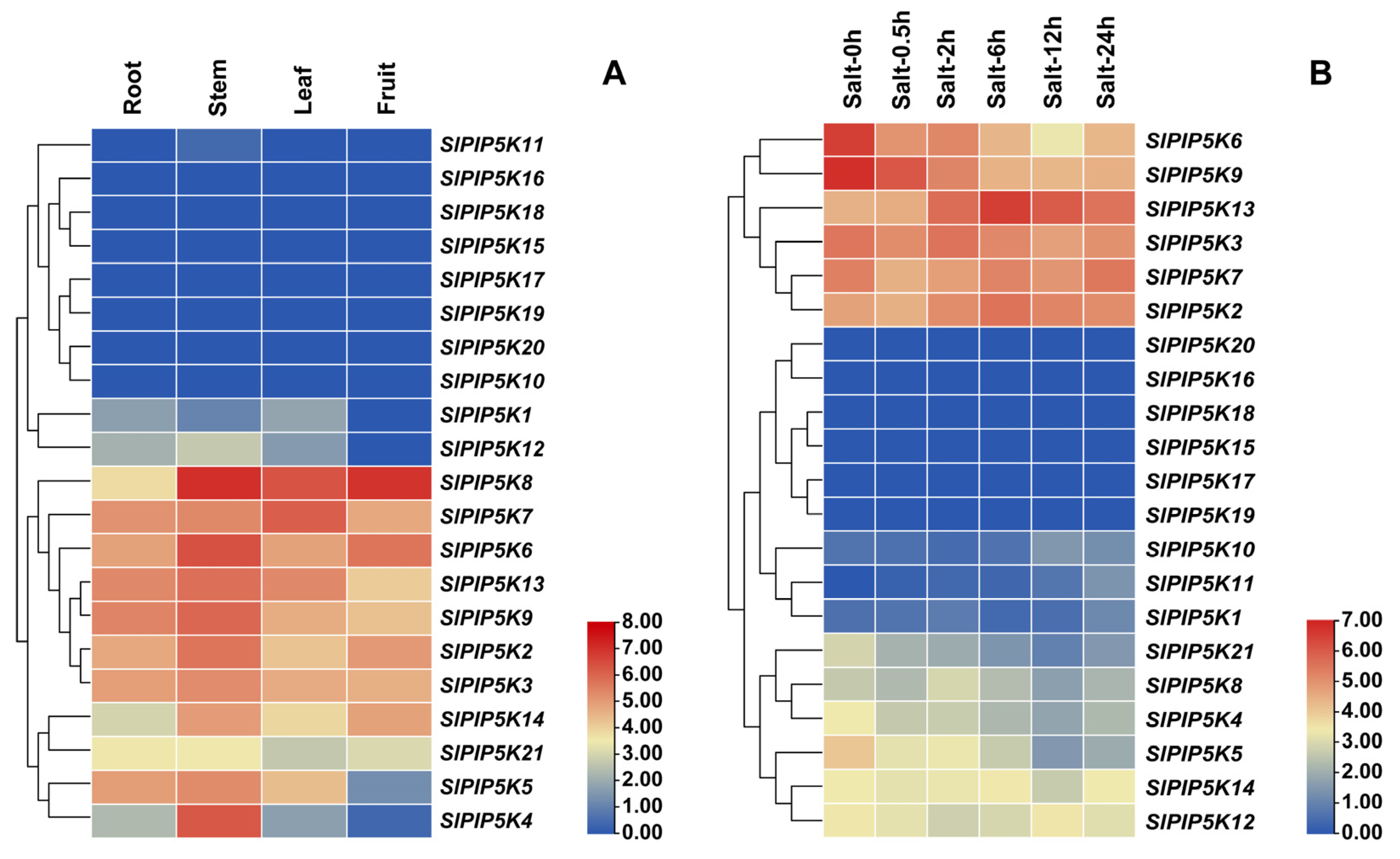
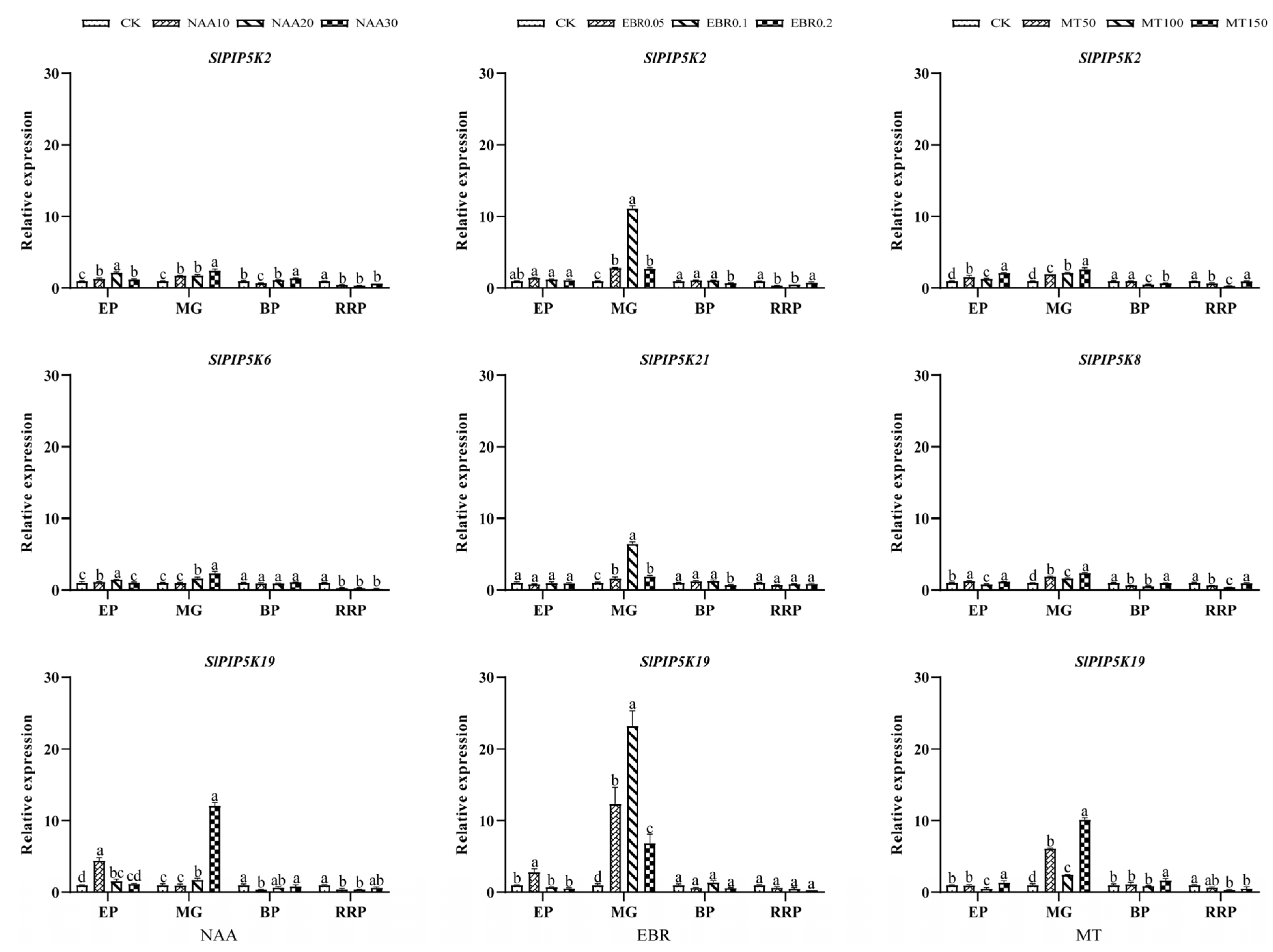
2.8. Analysis of the Expression Pattern of the SlPIP5K Gene Family in Tomato
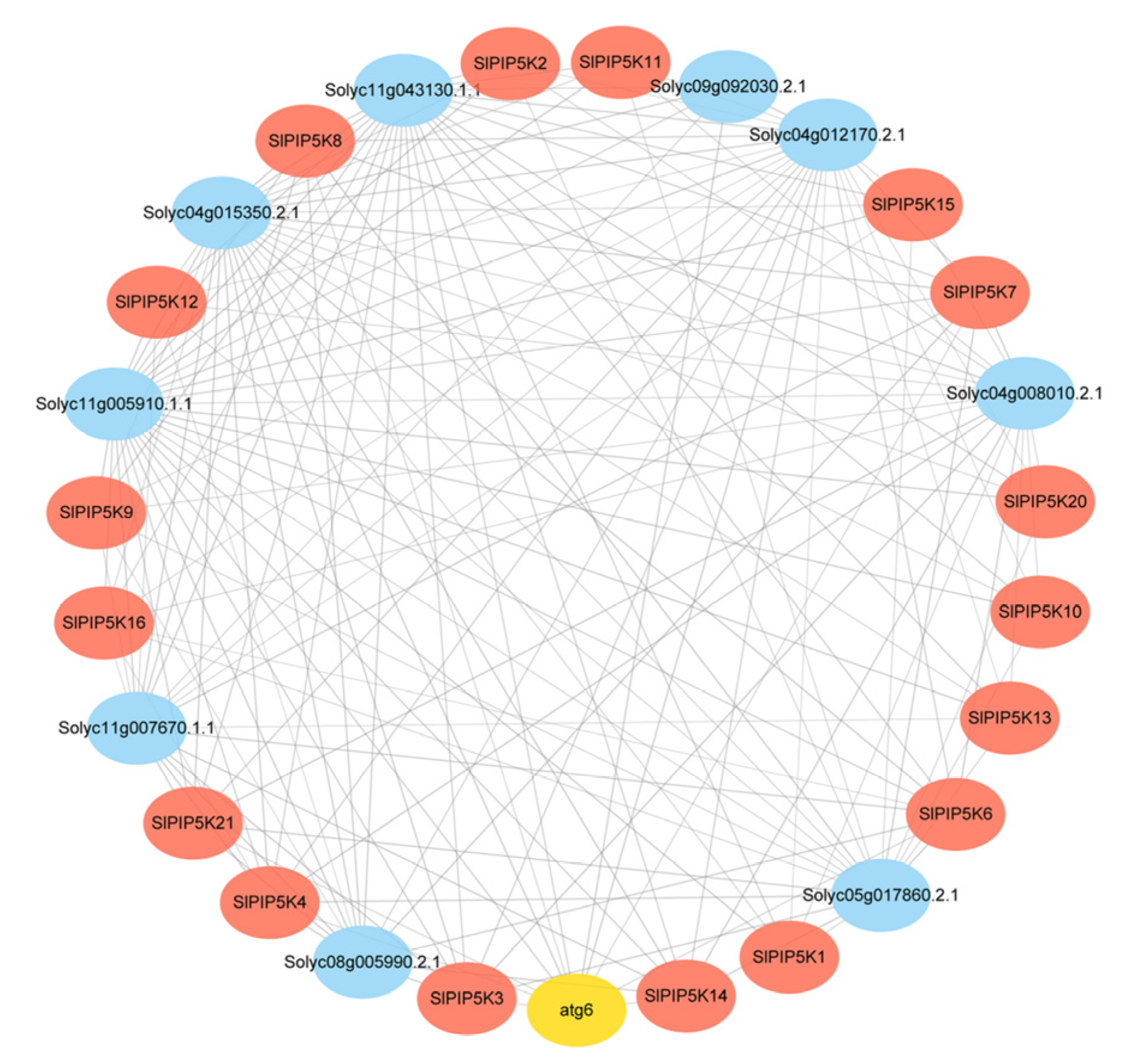
2.9. Analysis of the SlPIP5K Protein Interaction Network
2.10. Quantitative Fluorescence Analysis of the SlPIP5K Gene in Tomato
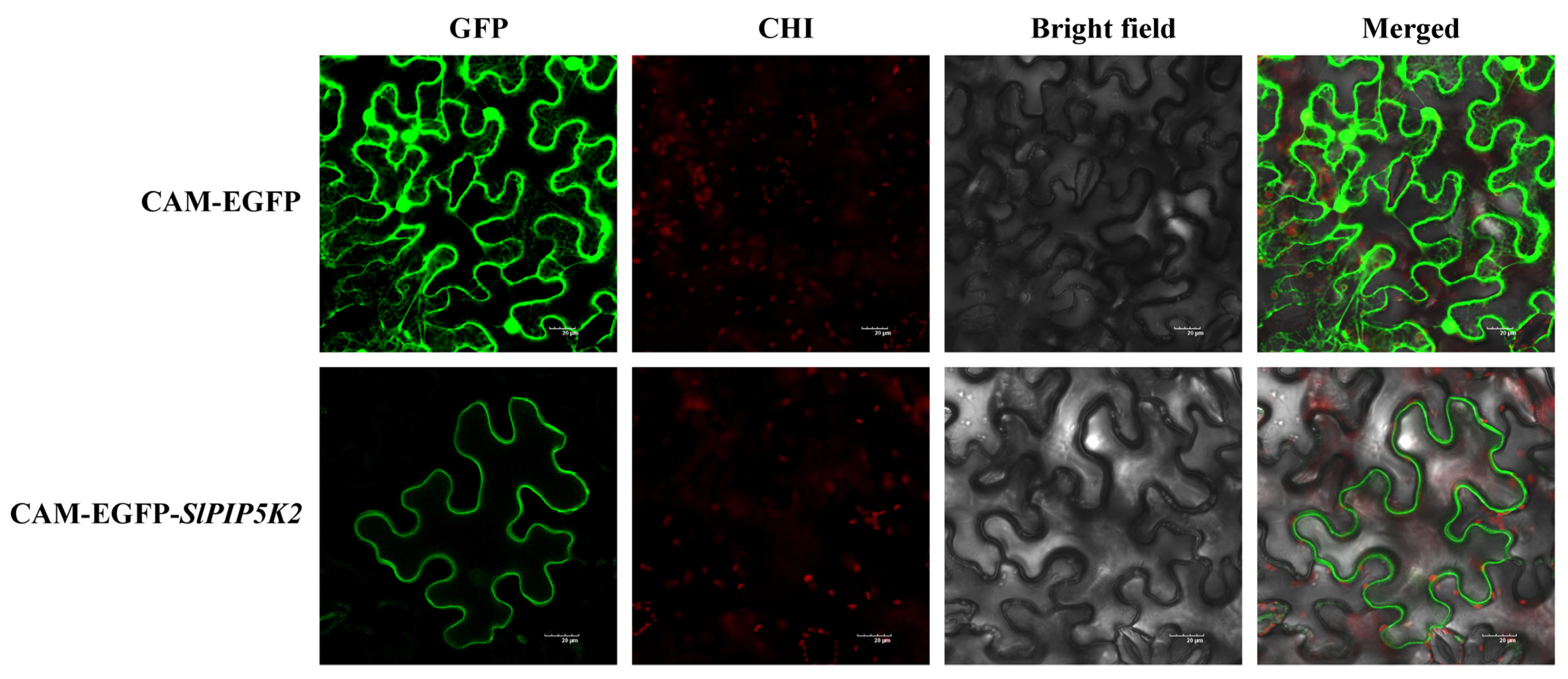
2.11. Analysis of the Subcellular Localization of SlPIP5K2 in Tomato
3. Discussion
4. Materials and Methods
4.1. Test Materials
4.2. Identification of the Tomato SlPIP5K Family
4.3. Physiochemical Analysis of SlPIP5K Gene Family Proteins in Tomato
4.4. Structural Analysis of the SlPIP5K Gene Family in Tomato
4.5. Chromosome Location, Collinearity and Ka/Ks Analysis of the SlPIP5K Gene in Tomato
4.6. Cis-Element Analysis of the SlPIP5K Gene in Tomato
4.7. Analysis of the Expression Pattern of the SlPIP5K Gene in Tomato
4.8. Analysis of the SlPIP5K Protein Interaction Network
4.9. Real-Time Fluorescence Quantitative PCR
4.10. Subcellular Localization of the Tomato SlPIP5K2 Gene
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevenson, J.M.; Perera, I.Y.; Heilmann, I.; Persson, S.; Boss, W.F. Inositol signaling and plant growth. Trends Plant Sci. 2000, 5, 252–258. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Z.; Hu, L.; Yue, Z. Genome-wide identification of PIP5K in wheat and its relationship with anther male sterility induced by high temperature. BMC Plant Biol. 2021, 21, 598. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.N.; Zhang, Y.X.; Li, L.L.; Yang, L.F.; Cang, R.H.; Jiang, T.T.; Sun, H.L.; Sui, J.X.; Huang, F.L. Progress of the regulatory function of PIP5K gene family in plants. Guizhou Agric. Sci. 2018, 46, 23–26. [Google Scholar]
- Zhang, G.Y.; Ding, Q.; Wei, B.Q. Identification of the PIP5K gene family in watermelon and its expression analysis in male sterile flower buds. Northwest J. Agric. 2021, 30, 883–893. [Google Scholar]
- Sun, C.; Yao, Y.; Geng, M.T.; Wang, Y.L.; Shang, L.; Hu, X.W.; Guo, J.C. Cloning and expression analysis of the cassava phosphatidylinositol phosphate 5-kinase PIP5K9 gene. Mol. Plant Breed. 2016, 14, 2290–2296. [Google Scholar]
- Zhang, Z.; Li, Y.; Huang, K.; Xu, W.; Zhang, C.; Yuan, H. Genome-wide systematic characterization and expression analysis of the phosphatidylinositol 4-phosphate 5-kinases in plants. Gene 2020, 756, 144915. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Xu, S.P.; Luo, D.; Xu, Z.H.; Xue, H.W. OsPIPK 1, a rice phosphatidylinositol monophosphate kinase, regulates rice heading by modifying the expression of floral induction genes. Plant Mol. Biol. 2004, 54, 295–310. [Google Scholar] [CrossRef]
- Sharma, S.; Bhattacharya, S.; Bhattacharya, A. PtdIns(4,5)P2 is generated by a novel phosphatidylinositol 4-phosphate 5-kinase in the protist parasite Entamoeba Histolytica. FEBS J. 2019, 286, 2216–2234. [Google Scholar] [CrossRef]
- Bout, I.V.D.; Divecha, N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009, 122, 3837–3850. [Google Scholar] [CrossRef]
- Khrongyut, S.; Rawangwong, A.; Pidsaya, A.; Sakagami, H.; Kondo, H.; Hipkaeo, W. Localization of phosphatidylinositol 4-phosphate 5-kinase (PIP5K) α, β, γ in the three major salivary glands in situ of mice and their response to β-adrenoceptor stimulation. J. Anat. 2019, 234, 502–514. [Google Scholar] [CrossRef]
- Muftuoglu, Y.; Xue, Y.; Gao, X.; Wu, D.; Ha, Y. Mechanism of substrate specificity of phosphatidylinositol phosphate kinases. Proc. Natl. Acad Sci. USA 2016, 113, 8711–8716. [Google Scholar] [CrossRef]
- Desrivières, S.; Cooke, F.T.; Parker, P.J.; Hall, M.N. MSS4, a phosphatidylinositol-4-phosphate 5-kinase needed for organization of the actin cytoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 1998, 273, 15787–15793. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, Y.; Martin, T.F.J. ARF6 regulates a plasma membrane pool of phosphatidylinositol(4,5)bisphosphate needed for regulated exocytosis. J. Cell Biol. 2003, 162, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Mellman, D.L.; Gonzales, M.L.; Song, C.; Barlow, C.A.; Wang, P.; Kendziorski, C.; Anderson, R.A. A PtdIns4,5P2-regulated nuclear poly(A) polymerase controls expression of select mRNAs. Nature 2008, 451, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Narkis, G.; Ofir, R.; Landau, D.; Manor, E.; Volokita, M.; Hershkowitz, R.; Elbedour, K.; Birk, O.S. Lethal Contractural Syndrome Type 3 (LCCS3) Is Caused by a Mutation in PIP5K1C, Which Encodes PIPKIγ of the Phophatidylinsitol Pathway. Am. J. Hum. Genet. 2007, 81, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Kusano, H.; Testerink, C.; Vermeer, J.M.; Tsuge, T.; Shimada, H.; Oka, A.; Munnik, T.; Aoyama, T. The Arabidopsis Phosphatidylinositol Phosphate 5-Kinase PIP5K3 Is a Key Regulator of Root Hair Tip Growth. Plant Cell 2008, 20, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Ugalde, J.M.; Rodriguez-Furlán, C.; Rycke, R.D.; Norambuena, L.; Friml, J.; León, G.; Tejos, R. Phosphatidylinositol 4-phosphate 5-kinases 1 and 2 are involved in the regulation of vacuole morphology during Arabidopsis thaliana pollen development. Plant Sci. 2016, 250, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ischebeck, T.; Stenzel, I.; Heilmann, I. Type B Phosphatidylinositol-4-Phosphate 5-Kinases Mediate Arabidopsis and Nicotiana tabacum Pollen Tube Growth by Regulating Apical Pectin Secretion. Plant Cell 2008, 20, 3312–3330. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Sanyal, S.; Ghosh, A.; Bhar, K.; Das, C.; Siddhanta, A. Phosphatidylinositol-4-phosphate 5-Kinase 1α Modulates Ribosomal RNA Gene Silencing through Its Interaction with Histone H3 Lysine 9 Trimethylation and Heterochromatin Protein HP1-α. J. Biol. Chem. 2015, 290, 20893–20903. [Google Scholar] [CrossRef]
- Zhou, Y.Q. Cloning and Functional Analysis of the Maize PIP5K Gene Promoter; Anhui Agricultural University: Hefei, China, 2012. [Google Scholar]
- Liu, Y.X. Identification of Tomato WRKY Gene Family Members and Expression Analysis; Shenyang Agricultural University: Shenyang, China, 2020. [Google Scholar]
- Li, N.; He, Q.; Wang, J.; Wang, B.K.; Zhao, J.T.; Huang, S.Y.; Yang, T.; Tang, Y.P.; Yang, S.B.; Patiguli, A.; et al. Superpangenome analyses highlight genomic diversity and structural variation across wild and cultivated tomato species. Nat. Genet. 2023, 55, 852–860. [Google Scholar] [CrossRef]
- Wang, B.K.; Wang, J.; Yang, T.; Wang, J.X.; Dai, Q.; Zhang, F.L.; Xi, R.; Yu, Q.H.; Li, N. The transcriptional regulatory network of hormones and genes under salt stress in tomato plants (Solanum lycopersicum L.). Front. Plant Sci. 2023, 14, 1115593. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Jeffryes, M.; Bateman, A.; Finn, R.D. The HMMER web server for protein sequence similarity search. Curr. Protoc. Bioinform. 2017, 60, 3.15.1–3.15.23. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; lvanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1579. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1191–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A Toolkit Incorporating Gamma-Series Methods and Sliding Window Strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Yao, Q.; Bai, Y.; Kumar, S.; Au, E.; Orfao, A.; Chim, C.S. Minimal residual disease detection by next-generation sequencing in multiple myeloma: An omparison with real-time quantitative PCR. Front. Oncol. 2020, 10, 611021. [Google Scholar] [CrossRef] [PubMed]
- Jouffrey, V.; Leonard, A.S.; Ahnert, S.E. Gene duplication and subsequent diversifification strongly affect phenotypic evolvability and robustness. R. Soc. Open Sci. 2021, 8, 201636. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional regulatory networks in response to abiotic stresses in Arabidopsis and grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, W.; Liu, J.G.; Li, Y.; Gai, J.Y.; Li, Y. Genome-wide characterization of the aldehyde dehydrogenase gene superfamily in soybean and its potential role in drought stress response. BMC Genom. 2017, 18, 518. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Q.; Chen, Q.S.; Lin, J.X.; Wang, Y.T.; Liu, H.L.; Liang, B.R.S.; Deng, Y.R.; Ren, C.Y.; Zhang, Y.X.; Yang, F.J.; et al. Identification of the DIR gene family in tomato and its analysis in response to abiotic stresses. Chin. Agric. Sci. 2022, 55, 3807–3824. [Google Scholar]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A novel family of junctional membrane complex proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [PubMed]
- Ma, H.; Lou, Y.; Lin, W.H.; Xue, H.W. MORN motifs in plant PIPKs are involved in the regulation of subcellular localization and phospholipid binding. Cell Res. 2006, 16, 466–478. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Im, Y.J.; Davis, A.J.; Perera, I.Y.; Johannes, E.; Allen, N.S.; Boss, W.F. The N-terminal Membrane Occupation and Recognition Nexus Domain of Arabidopsis Phosphatidylinositol Phosphate Kinase 1 Regulates Enzyme Activity. J. Biol. Chem. 2006, 282, 5443–5452. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2018, 47, 607–613. [Google Scholar] [CrossRef]
- Su, G.; Morris, J.H.; Demchak, B.; Bader, G.D. Biological Network Exploration with Cytoscape 3. Curr. Protoc. Bioinform. 2014, 47, 8.13.1–8.13.24. [Google Scholar] [CrossRef]
- Zeng, X.W. Identification and Functional Study of the Barley Autophagy Homologous Gene ATG6; Zhejiang University: Hangzhou, China, 2016. [Google Scholar]
- Liu, Y. Studies on the Structure and Function of Type IA Phosphatidylinositol-3-Kinase (PI3K) and Related Protein Complexes; Shandong University: Jinan, China, 2013. [Google Scholar]
- Yan, C. Study on the Mechanism of Drought Stress Signaling Response of Arabidopsis WD-40 Repeat Proteins AtARCA and AtAGB1; Yangzhou University: Yangzhou, China, 2005. [Google Scholar]
- Tang, Y.M.; Ren, L.F.; Huang, X.; Li, Q.; Chen, L. Subcellular localisation studies of DTL. Med. Res. Educ. 2023, 40, 1–7. [Google Scholar]


| Gene Name | Gene ID | Chromosome Location | Amino Acid | Molecular Weight | Isoelectric Point | Gravy | Protein Secondary Structure | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| SlPIP5K1 | Solyc01T003005.1 | chr1 | 802 | 90,622.79 | 8.61 | −0.647 | 26.18 | 8.73 | 47.76 |
| SlPIP5K2 | Solyc01T003618.1 | chr1 | 842 | 95,980.04 | 9.01 | −0.377 | 26.13 | 8.08 | 46.91 |
| SlPIP5K3 | Solyc03T003552.1 | chr3 | 1921 | 214,107.32 | 5.53 | −0.341 | 31.34 | 3.85 | 50.55 |
| SlPIP5K4 | Solyc04T000183.1 | chr4 | 1612 | 180,971.71 | 6.07 | −0.465 | 32.75 | 3.41 | 50.06 |
| SlPIP5K5 | Solyc04T000631.1 | chr4 | 749 | 84,051.01 | 5.70 | −0.638 | 22.96 | 9.08 | 49.80 |
| SlPIP5K6 | Solyc05T000316.1 | chr5 | 1753 | 196,588.79 | 5.71 | −0.463 | 33.31 | 4.28 | 48.20 |
| SlPIP5K7 | Solyc06T001582.1 | chr6 | 1480 | 165,198.92 | 5.25 | −0.443 | 33.65 | 3.92 | 49.73 |
| SlPIP5K8 | Solyc07T000618.2 | chr7 | 1795 | 200,276.95 | 6.08 | −0.434 | 31.59 | 3.84 | 50.97 |
| SlPIP5K9 | Solyc09T000252.1 | chr9 | 767 | 86,529.11 | 8.59 | −0.495 | 24.12 | 9.00 | 49.15 |
| SlPIP5K10 | Solyc10T002241.1 | chr10 | 763 | 87,038.01 | 6.47 | −0.639 | 23.98 | 9.31 | 48.49 |
| SlPIP5K11 | Solyc10T002463.1 | chr10 | 787 | 89,822.06 | 5.81 | −0.621 | 25.79 | 8.89 | 47.52 |
| SlPIP5K12 | Solyc10T002914.2 | chr10 | 992 | 110,916.05 | 9.12 | −0.450 | 24.80 | 8.77 | 46.67 |
| SlPIP5K13 | Solyc11T000703.1 | chr11 | 857 | 97,104.71 | 8.54 | −0.609 | 25.67 | 7.12 | 48.89 |
| SlPIP5K14 | Solyc11T000796.1 | chr11 | 1651 | 184,062.73 | 5.42 | −0.482 | 32.53 | 3.76 | 50.82 |
| SlPIP5K15 | Solyc12T000568.1 | chr12 | 429 | 48,871.65 | 4.90 | −0.647 | 27.97 | 10.72 | 41.03 |
| SlPIP5K16 | Solyc12T000573.1 | chr12 | 111 | 12,389.00 | 6.07 | −0.328 | 24.32 | 5.41 | 44.14 |
| SlPIP5K17 | Solyc12T000577.1 | chr12 | 557 | 62,248.04 | 5.31 | −0.560 | 25.31 | 9.52 | 45.96 |
| SlPIP5K18 | Solyc12T000581.1 | chr12 | 421 | 47,776.37 | 4.80 | −0.629 | 30.64 | 11.40 | 34.92 |
| SlPIP5K19 | Solyc12T000584.1 | chr12 | 380 | 42,934.34 | 4.90 | −0.485 | 35.79 | 6.84 | 39.21 |
| SlPIP5K20 | Solyc12T000991.1 | chr12 | 754 | 85,902.92 | 8.15 | −0.678 | 24.67 | 9.55 | 47.61 |
| SlPIP5K21 | Solyc12T002108.1 | chr12 | 1801 | 199,991.07 | 5.48 | −0.420 | 29.65 | 4.05 | 52.69 |
| Duplicate Gene Pairs | Ka | Ks | Ka/Ks | Duplicated Type |
|---|---|---|---|---|
| SlPIP5K10/SlPIP5K11 | 0.155179075 | 0.771386845 | 0.201168941 | Segmental |
| SlPIP5K14/SlPIP5K7 | 0.196236825 | 0.653379471 | 0.300341277 | Segmental |
| SlPIP5K21/SlPIP5K3 | 0.157812019 | 0.618222042 | 0.255267539 | Segmental |
| SlPIP5K4/SlPIP5K16 | 0.152163772 | 0.501943081 | 0.303149457 | Segmental |
| Treatment | Concentration |
|---|---|
| Control | Distilled water + 0.1% Tween 80 + 0.1% Ethanol |
| EBR | 0.05 mg·L−1 EBR + 0.1% Tween 80 + 0.1% Ethanol |
| EBR | 0.1 mg·L−1 EBR + 0.1% Tween 80 + 0.1% Ethanol |
| EBR | 0.2 mg·L−1 EBR + 0.1% Tween 80 + 0.1% Ethanol |
| MT | 50 μmol·L−1 MT + 0.1% Tween 80 + 0.1% Ethanol |
| MT | 100 μmol·L−1 MT + 0.1% Tween 80 + 0.1% Ethanol |
| MT | 150 μmol·L−1 MT + 0.1% Tween 80 + 0.1% Ethanol |
| NAA | 10 mg·L−1 + 0.1% Tween 80 + 0.1% Ethanol |
| NAA | 20 mg·L−1 + 0.1% Tween 80 + 0.1% Ethanol |
| NAA | 30 mg·L−1 + 0.1% Tween 80 + 0.1% Ethanol |
| Gene | Forward Primer Sequence (5′ → 3′) | Reverse Primer Sequence (5′ → 3′) |
|---|---|---|
| SlPIP5K1 | GCCAGATGGACAAGGGAGATA | CGTGCCATTCCCTTTAGGA |
| SlPIP5K2 | CATCACTGAAATGGTCCTCTCC | CAAACCGTCCTTCTCCAGACA |
| SlPIP5K3 | TGGGTGTGTTAGAGTCTCCTGG | CATCTTGGACATCGTTGGCT |
| SlPIP5K4 | TCCAGTATGCCGTCTTTGCG | TATGGTGTCTTGCCGAGGA |
| SlPIP5K5 | TCGCATTACGCCGACGAAT | CTTTACCACTCGCCTTCCCTCT |
| SlPIP5K6 | TTGGGCATTCTGTTCAGGAC | TGGTCTCGGTAAACGGACA |
| SlPIP5K7 | ATCGCCTGGAGAGAGTTGCT | ACAGCCCTCAATGAACAACAG |
| SlPIP5K8 | CCAGAGCCAGAAACAGAAGAGG | ATTGCCTTCCTATGCTCCG |
| SlPIP5K9 | TGGAGGATGCTATGAGTGCTC | ATGATTGCTTCACCTGGTCTCT |
| SlPIP5K11 | GCAGTTGGGAATCAGGCATAC | GAACTCACAAGACTGATGGCG |
| SlPIP5K13 | GAAGAAGAGGTCATCCGTGGA | AACCCAAAGCCATCCCTGT |
| SlPIP5K15 | TGCGTGAAACTGTGAAGAAACC | CATCATTCGTCGGCGTTATG |
| SlPIP5K17 | TGCGTGAAACTGTGAAGAAACC | ATCATCATTCGTCGGCGT |
| SlPIP5K18 | TGCGTGAAACTGTGAAGAAACC | CATCATTCGTCGGCGTTATG |
| SlPIP5K19 | TGGTGGTGGTAATGGTGGTC | TCCTCCTCCTCCTCATTCCTA |
| SlPIP5K21 | AGATGCCTGAGATGTCCACG | TGTAACGAATGCCCGCAA |
| Actin | CAGGGTGTTCTTCAGGAGCAA | GGTGTTATGGTCGGAATGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Z.; Li, X.; Chen, Z.; Liu, Y.; Zhang, F.; Dai, Q.; Yu, Q.; Li, N. Identification and Analysis of the Expression of the PIP5K Gene Family in Tomatoes. Int. J. Mol. Sci. 2024, 25, 159. https://doi.org/10.3390/ijms25010159
Wang Z, Wang Z, Li X, Chen Z, Liu Y, Zhang F, Dai Q, Yu Q, Li N. Identification and Analysis of the Expression of the PIP5K Gene Family in Tomatoes. International Journal of Molecular Sciences. 2024; 25(1):159. https://doi.org/10.3390/ijms25010159
Chicago/Turabian StyleWang, Zepeng, Zhongyu Wang, Xianguo Li, Zhaolong Chen, Yuxiang Liu, Fulin Zhang, Qi Dai, Qinghui Yu, and Ning Li. 2024. "Identification and Analysis of the Expression of the PIP5K Gene Family in Tomatoes" International Journal of Molecular Sciences 25, no. 1: 159. https://doi.org/10.3390/ijms25010159
APA StyleWang, Z., Wang, Z., Li, X., Chen, Z., Liu, Y., Zhang, F., Dai, Q., Yu, Q., & Li, N. (2024). Identification and Analysis of the Expression of the PIP5K Gene Family in Tomatoes. International Journal of Molecular Sciences, 25(1), 159. https://doi.org/10.3390/ijms25010159






