Non-Alcoholic Fatty Liver Disease and Bone Tissue Metabolism: Current Findings and Future Perspectives
Abstract
1. Introduction
2. Methods
- Article type: Classical Article, Clinical Study, Clinical Trial, Comparative Study, Controlled Clinical Trial, Multicenter Study, Meta-Analysis, Observational Study, and Randomized Controlled Trial, Preprint;
- Species: Humans, Other Animals;
- Article language: English;
- Age: Child, Adolescent, Adult;
- Publication date: 5 years.
3. NAFLD and Decreased Bone Mineral Density: Some Epidemiological Evidence
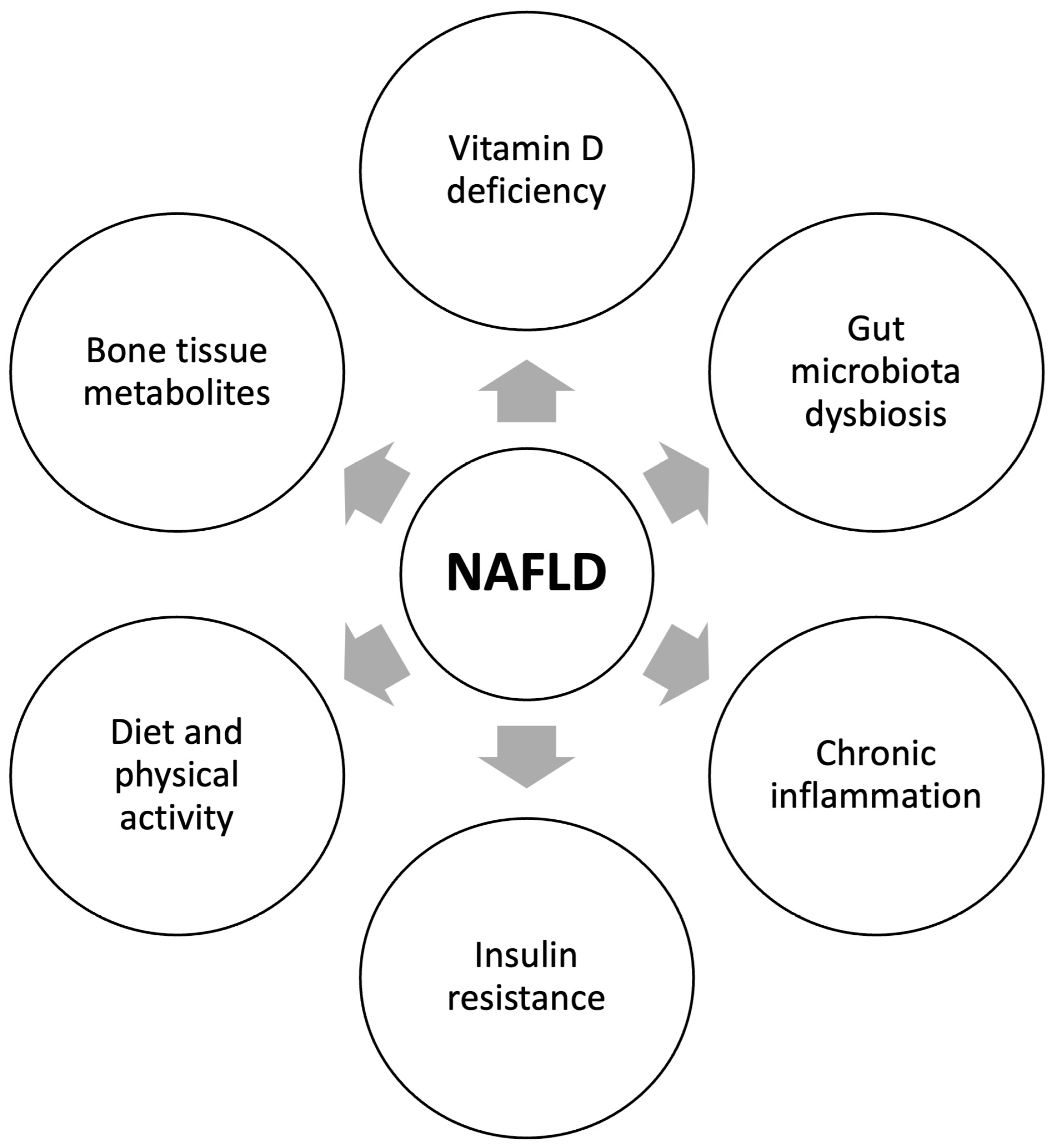
4. Pathogenetic Mechanisms of the Relationship between NAFLD and Reduced Bone Mineral Density
4.1. Vitamin D
4.2. Chronic Inflammation
4.3. Gut Microbiota
4.4. Diet and Physical Activity
4.5. Biologically Active Substances
4.5.1. Osteopontin
4.5.2. Procollagen Type 1 N-Terminal Propeptide
4.5.3. Osteoprotegerin
4.5.4. Adiponectin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parameswaran, M.; Hasan, H.A.; Sadeque, J.; Jhaveri, S.; Avanthika, C.; Arisoyin, A.E.; Dhanani, M.B.; Rath, S.M. Factors That Predict the Progression of Non-alcoholic Fatty Liver Disease (NAFLD). Cureus 2021, 13, e20776. [Google Scholar] [CrossRef] [PubMed]
- Juanola, O.; Martínez-López, S.; Francés, R.; Gómez-Hurtado, I. Non-Alcoholic Fatty Liver Disease: Metabolic, Genetic, Epigenetic and Environmental Risk Factors. Int. J. Environ. Res. Public Health 2021, 18, 5227. [Google Scholar] [CrossRef] [PubMed]
- Rosato, V.; Masarone, M.; Dallio, M.; Federico, A.; Aglitti, A.; Persico, M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int. J. Environ. Res. Public Health 2019, 16, 3415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Touros, A.; Kim, W.R. Nonalcoholic Fatty Liver Disease and Metabolic Syndrome. Clin. Liver Dis. 2018, 22, 133–140. [Google Scholar] [CrossRef]
- Pouwels, S.; Sakran, N.; Graham, Y.; Leal, A.; Pintar, T.; Yang, W.; Kassir, R.; Singhal, R.; Mahawar, K.; Ramnarain, D. Non-alcoholic fatty liver disease (NAFLD): A review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 2022, 22, 63. [Google Scholar] [CrossRef]
- Van Wagner, L.B.; Rinella, M.E. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Curr. Hepatol. Rep. 2016, 15, 75–85. [Google Scholar] [CrossRef]
- Li, A.; Ahmed, A.; Kim, D. Extrahepatic Manifestations of Nonalcoholic Fatty Liver Disease. Gut Liver 2020, 14, 168–178. [Google Scholar] [CrossRef]
- Maevskaya, M.V.; Kotovskaya, Y.V.; Ivashkin, V.T.; Tkacheva, O.N.; Troshina, E.A.; Shestakova, M.V.; Breder, V.V.; Geyvandova, N.I.; Doshchitsin, V.L.; Dudinskaya, E.N.; et al. The National Consensus statement on the management of adult patients with non-alcoholic fatty liver disease and its main comorbidities. Ther. Arch. 2022, 94, 216–253. (In Russian) [Google Scholar] [CrossRef]
- Li, A.A.; Kim, D.; Ahmed, A. Association of Sarcopenia and NAFLD: An Overview. Clin. Liver Dis. 2020, 16, 73–76. [Google Scholar] [CrossRef]
- Mikami, K.; Endo, T.; Sawada, N.; Igarashi, G.; Kimura, M.; Hasegawa, T.; Iino, C.; Sawada, K.; Nakaji, S.; Ishibashi, Y.; et al. Association of bone metabolism with fatty liver disease in the elderly in Japan: A community-based study. Intern. Med. 2020, 59, 1247–1256. [Google Scholar] [CrossRef]
- Chen, H.J.; Yang, H.Y.; Hsueh, K.C.; Shen, C.C.; Chen, R.Y.; Yu, H.C.; Wang, T.L. Increased risk of osteoporosis in patients with nonalcoholic fatty liver disease: A population-based retrospective cohort study. Medicine 2018, 97, e128352018. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Z.; Xu, Q.M.; Wu, X.X.; Cai, C.; Zhang, L.J.; Shi, K.Q.; Shi, H.Y.; Li, L.J. The combined effect of nonalcoholic fatty liver disease and metabolic syndrome on osteoporosis in postmenopausal females in Eastern China. Int. J. Endocrinol. 2018, 2018, 2314769. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, D.C.; Kim, C.O. Association Between 10-Year Fracture Probability and Nonalcoholic Fatty Liver Disease With or Without Sarcopenia in Korean Men: A Nationwide Population-Based Cross-Sectional Study. Front. Endocrinol. 2021, 12, 599339. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Cen, L.; Chen, X.; Pan, J.; Li, Y.; Chen, W.; Yu, C. Increased risk of low bone mineral density in patients with non-alcoholic fatty liver disease: A cohort study. Eur. J. Endocrinol. 2020, 182, 157–164. [Google Scholar] [CrossRef]
- Pan, B.; Cai, J.; Zhao, P.; Liu, J.; Fu, S.; Jing, G.; Niu, Q.; Li, Q. Relationship between prevalence and risk of osteoporosis or osteoporotic fracture with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2275–2286. [Google Scholar] [CrossRef]
- Sung, J.; Ryu, S.; Song, Y.M.; Cheong, H.K. Relationship between non-alcoholic fatty liver disease and decreased bone mineral density: A retrospective cohort study in Korea. J. Prev. Med. Public Health 2020, 53, 342–352. [Google Scholar] [CrossRef]
- Khan, S.; Kalkwarf, H.J.; Hornung, L.; Siegel, R.; Arce-Clachar, A.C.; Sheridan, R.; Ippisch, H.M.; Xanthakos, S.A. Histologic Severity of Nonalcoholic Fatty Liver Disease Associates with Reduced Bone Mineral Density in Children. Dig. Dis. Sci. 2023, 68, 644–655. [Google Scholar] [CrossRef]
- Mantovani, A.; Gatti, D.; Zoppini, G.; Lippi, G.; Bonora, E.; Byrne, C.D.; Nobili, V.; Targher, G. Association Between Nonalcoholic Fatty Liver Disease and Reduced Bone Mineral Density in Children: A Meta-Analysis. Hepatology 2019, 70, 812–823. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Zhang, Y.; Yang, Y.; Qin, G. Association between vitamin D. and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: Results from a meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 17221–17234. [Google Scholar]
- Sun, S.; Xu, M.; Zhuang, P.; Chen, G.; Dong, K.; Dong, R.; Zheng, S. Effect and mechanism of vitamin D. activation disorder on liver fibrosis in biliary atresia. Sci. Rep. 2021, 11, 19883. [Google Scholar] [CrossRef]
- He, R.; Fan, L.; Song, Q.; Diao, H.; Xu, H.; Ruan, W.; Ma, L.; Wang, D. [Protective effect of active vitamin D on liver fibrosis induced by sodium arsenite in SD rats]. Wei Sheng Yan Jiu 2022, 51, 926–933. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.N.; Khalifa, A.A.; Hemead, D.A.; Alsemeh, A.E.; Habib, M.A. 1,25-Dihydroxycholecalciferol down-regulates 3-mercaptopyruvate sulfur transferase and caspase-3 in rat model of non-alcoholic fatty liver disease. J. Mol. Histol. 2023, 54, 119–134. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.G.; Liu, Y.X.; Wang, H.; Wang, B.P.; Qu, H.Q.; Wang, B.L.; Zhu, M. Active form of vita-min D ameliorates non-alcoholic fatty liver disease by alleviating oxidative stress in a high-fat diet rat model. Endocr. J. 2017, 64, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Alshaibi, H.F.; Bakhashab, S.; Almuhammadi, A.; Althobaiti, Y.S.; Baghdadi, M.A.; Alsolami, K. Protective Effect of Vitamin D against Hepatic Molecular Apoptosis Caused by a High-Fat Diet in Rats. Curr. Issues Mol. Biol. 2023, 45, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.L.; Jiang, L.; Adams, J.S.; Xu, Z.Z.; Shen, J.; Janssen, S.; Ackermann, G.; Vanderschueren, D.; Pauwels, S.; Knight, R.; et al. Vitamin D metabolites and the gut microbiome in older men. Nat. Commun. 2020, 11, 5997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Chen, L.; Yang, J.; Zhao, S.S.; Jin, S.; Ao, N.; Yang, J.; Liu, H.X.; Du, J. Vitamin D alleviates non-alcoholic fatty liver disease via restoring gut microbiota and metabolism. Front. Microbiol. 2023, 14, 1117644. [Google Scholar] [CrossRef]
- Saberi, B.; Dadabhai, A.; Nanavati, J.; Wang, L.; Shinohara, R.T.; Mullin, G.E. Vitamin D levels do not predict the stage of hepatic fibrosis in patients with non-alcoholic fatty liver disease: A PRISMA compliant systematic review and meta-analysis of pooled data. World J. Hepatol. 2018, 10, 142–154. [Google Scholar] [CrossRef]
- Jaruvongvanich, V.; Ahuja, W.; Sanguankeo, A.; Wijarnpreecha, K.; Upala, S. Vitamin D and histologic severity of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Dig. Liver Dis. 2017, 49, 618–622. [Google Scholar] [CrossRef]
- Kumar, M.; Parchani, A.; Kant, R.; Das, A. Relationship Between Vitamin D Deficiency and Non-alcoholic Fatty Liver Disease: A Cross-Sectional Study From a Tertiary Care Center in Northern India. Cureus 2023, 15, e34921. [Google Scholar] [CrossRef]
- da Silva, T.B.P.; Luiz, M.M.; Delinocente, M.L.B.; Steptoe, A.; de Oliveira, C.; da Silva Alexandre, T. Is Abdominal Obesity a Risk Factor for the Incidence of Vitamin D Insufficiency and Deficiency in Older Adults? Evidence from the ELSA Study. Nutrients 2022, 14, 4164. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Meng, Y.; Yu, Q.; Wang, Q.; Xu, H.; Yuan, H.; Li, X.; Chen, L. Effects of Vitamin D Supplementation in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. Metab. 2020, 18, e97205. [Google Scholar] [CrossRef] [PubMed]
- Nobili, V.; Giorgio, V.; Liccardo, D.; Bedogni, G.; Morino, G.; Alisi, A.; Cianfarani, S. Vitamin D levels and liver histological alterations in children with nonalcoholic fatty liver disease. Eur. J. Endocrinol. 2014, 170, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.; Elangovan, H.; Stokes, R.; Gunton, J.E. Vitamin D and the liver-correlation or cause? Nutrients 2018, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Y.; Li, Y.; Li, K.; Hou, S.; Ding, M.; Tan, J.; Zhu, Z.; Tang, Y.; Liu, Y.; et al. Vitamin D receptor (VDR) mediates the quiescence of activated hepatic stellate cells (aHSCs) by regulating M2 macrophage exosomal smooth muscle cell-associated protein 5 (SMAP-5). J. Zhejiang Univ. Sci. B 2023, 24, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.; Neff, G.; Patil, R.; Behling, C.; Hu, C.; Shringarpure, R.; de Temple, B.; Fong, E. A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis. JHEP Rep. 2022, 5, 100563. [Google Scholar] [CrossRef] [PubMed]
- Srikanthan, K.; Feyh, A.; Visweshwar, H.; Shapiro, J.I.; Sodhi, K. Systematic review of metabolic syndrome biomarkers: A panel for early detection, management, and risk stratification in the West Virginian population. Int. J. Med. Sci. 2016, 13, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi-Oyelere, B.L.; Schollum, L.; Kuhn-Sherlock, B.; McConnell, M.; Mros, S.; Coad, J.; Roy, N.C.; Kruger, M.C. Inflammatory markers and bone health in postmenopausal women: A cross-sectional overview. Immun. Ageing 2019, 16, 15. [Google Scholar] [CrossRef]
- Chin, K.Y.; Wong, S.K.; Ekeuku, S.O.; Pang, K.L. Relationship Between Metabolic Syndrome and Bone Health-An Evaluation of Epidemiological Studies and Mechanisms Involved. Diabetes Metab. Syndr. Obes. 2020, 13, 3667–3690. [Google Scholar] [CrossRef]
- Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, X.Y.; Jiang, Y.P.; Zhang, J.B.; Zhang, Q.Y.; Wang, N.N.; Xin, H.L. Monotropein attenuates oxidative stress via Akt/mTOR-mediated autophagy in osteoblast cells. Biomed. Pharmacother. 2020, 121, 109566. [Google Scholar] [CrossRef]
- Lu, L.; Chen, X.; Liu, Y.; Yu, X. Gut microbiota and bone metabolism. FASEB J. 2021, 35, e21740. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cai, L.; Guo, W. New Advances in Improving Bone Health Based on Specific Gut Microbiota. Front. Cell. Infect. Microbiol. 2022, 12, 821429. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, K.; Fang, X.; Lu, F.; Zhang, W.; Song, X.; Chen, L.; Sun, J.; Chen, H. Inhibition of Lipopolysaccharide-Induced Inflammatory Bone Loss by Saikosaponin D is Associated with Regulation of the RANKL/RANK Pathway. Drug Des. Devel Ther. 2021, 15, 4741–4757. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Rao, S.; Cheng, Y.; Zhuo, X.; Deng, C.; Xu, N.; Zhang, H.; Yang, L. Microbial osteoporosis: The interplay between the gut microbiota and bones via host metabolism and immunity. Microbiologyopen 2019, 8, e00810. [Google Scholar] [CrossRef] [PubMed]
- Grüner, N.; Ortlepp, A.L.; Mattner, J. Pivotal Role of Intestinal Microbiota and Intraluminal Metabolites for the Maintenance of Gut-Bone Physiology. Int. J. Mol. Sci. 2023, 24, 5161. [Google Scholar] [CrossRef]
- Ji, Y.; Yin, Y.; Li, Z.; Zhang, W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 2019, 11, 1712. [Google Scholar] [CrossRef]
- Van der Windt, D.J.; Sud, V.; Zhang, H.; Tsung, A.; Huang, H. The Effects of Physical Exercise on Fatty Liver Disease. Gene Expr. 2018, 18, 89–101. [Google Scholar] [CrossRef]
- Nachit, M.; Leclercq, I.A. Emerging awareness on the importance of skeletal muscle in liver diseases: Time to dig deeper into mechanisms! Clin. Sci. 2019, 133, 465–481. [Google Scholar] [CrossRef]
- Qiao, J.; Wu, Y.; Ren, Y. The impact of a high fat diet on bones: Potential mechanisms. Food Funct. 2021, 12, 963–975. [Google Scholar] [CrossRef]
- Hafner, H.; Chang, E.; Carlson, Z.; Zhu, A.; Varghese, M.; Clemente, J.; Abrishami, S.; Bagchi, D.P.; MacDougald, O.A.; Singer, K.; et al. Lactational High-Fat Diet Exposure Programs Metabolic Inflammation and Bone Marrow Adiposity in Male Offspring. Nutrients 2019, 11, 1393. [Google Scholar] [CrossRef]
- Montalvany-Antonucci, C.C.; Zicker, M.C.; Ferreira, A.V.M.; Macari, S.; Ramos-Junior, E.S.; Gomez, R.S.; Pereira, T.S.F.; Madeira, M.F.M.; Fukada, S.Y.; Andrade, I., Jr.; et al. High-fat diet disrupts bone remodeling by inducing local and systemic alterations. J. Nutr. Biochem. 2018, 59, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarín, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef] [PubMed]
- Marini, S.; Barone, G.; Masini, A.; Dallolio, L.; Bragonzoni, L.; Longobucco, Y.; Maffei, F. The Effect of Physical Activity on Bone Biomarkers in People With Osteoporosis: A Systematic Review. Front. Endocrinol. 2020, 11, 585689. [Google Scholar] [CrossRef]
- Zubareva, E.Y.; Senchukova, M.A. Prognostic and predictive significance of osteopontin in malignant neoplasms. Adv. Mol. Oncology 2021, 8, 23–28. [Google Scholar] [CrossRef]
- Denhardt, D.T.; Guo, X. Osteopontin: A protein with diverse functions. FASEB J. 1993, 7, 1475–1482. [Google Scholar] [CrossRef]
- Berezin, A.E.; Panasenko, T.A.; Koretskaya, E.Y. Osteopontin as a new biological marker of cardiovascular remodeling. Ukr. J. Cardiol. 2010, 4, 98–102. [Google Scholar] [CrossRef]
- Shibanova, I.A.; Khryachkova, O.N. The use of biomarkers of calcium and phosphorus metabolism for the diagnosis and risk stratification in patients with coronary heart disease. Russ. Med. J. 2017, 20, 1409–1414. [Google Scholar]
- Gimba, E.; Brum, M.; Moraes, G. Fulllengthosteopontin and its splice variants as modulators of chemoresistance and radioresistance (Review). Int. J. Oncol. 2019, 54, 420–430. [Google Scholar] [CrossRef]
- Szulc, P. Bone turnover: Biology and assessment tools. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 725–738. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Wrona, A.; Gruszewska, E.; Cylwik, B.; Panasiuk, A.; Flisiak, R.; Chrostek, L. Serum level of interleukin-6 (IL-6) and N-terminal propeptide of procollagen type I (PINP) in patients with liver diseases. Scand. J. Clin. Lab. Investig. 2018, 78, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Zhang, W.; Li, P.; Zhou, J.; Li, Z.; Zhou, Y.; Li, M. Upregulation of osteoprotegerin inhibits tert-butyl hydroperoxide-induced apoptosis of human chondrocytes. Exp. Ther. Med. 2022, 24, 470. [Google Scholar] [CrossRef] [PubMed]
- Khattar, V.; Wang, L.; Peng, J.B. Calcium selective channel TRPV6: Structure, function, and implications in health and disease. Gene 2022, 817, 146192. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Shan, C.; Fu, J.R.; Zhang, Y.; Wang, Y.Y.; Zhu, Y.C.; Yu, J.; Cai, J.; Li, S.X.; Tao, T.; et al. RANKL Is Independently Associated with Increased Risks of Non-Alcoholic Fatty Liver Disease in Chinese Women with PCOS: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 451. [Google Scholar] [CrossRef] [PubMed]
- de CirizaVillacampa, C.P. Osteoprotegerin: A promising biomarker in the metabolic syndrome-Newperspectives. Biochem. Anal. Biochem. 2016, 5, 1–3. [Google Scholar] [CrossRef]
- Karalazou, P.; Ntelios, D.; Chatzopoulou, F.; Fragou, A.; Taousani, M.; Mouzaki, K.; Galli-Tsinopoulou, A.; Kouidou, S.; Tzimagiorgis, G. OPG/RANK/RANKL signaling axis in patients with type I diabetes: Associations with parathormone and vitamin D. Ital. J. Pediatr. 2019, 45, 161. [Google Scholar] [CrossRef]
- Omran, F.; Kyrou, I.; Osman, F.; Lim, V.G.; Randeva, H.S.; Chatha, K. Cardiovascular Biomarkers: Lessons of the Past and Prospects for the Future. Int. J. Mol. Sci. 2022, 23, 5680. [Google Scholar] [CrossRef]
- Tai, T.Y.; Chen, C.L.; Tsai, K.S.; Tu, S.T.; Wu, J.S.; Yang, W.S. A longitudinal analysis of serum adiponectin levels and bone mineral density in postmenopausal women in Taiwan. Sci. Rep. 2022, 12, 8090. [Google Scholar] [CrossRef]
- Yatagai, T.; Nagasaka, S.; Taniguchi, A.; Fukushima, M.; Nakamura, T.; Kuroe, A.; Nakai, Y.; Ishibashi, S. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism 2003, 52, 1274–1278. [Google Scholar] [CrossRef]
- Sandhya, N.; Gokulakrishnan, K.; Ravikumar, R.; Mohan, V.; Balasubramanyam, M. Association of hypoadiponectinemia with hypoglutathionemia in NAFLD subjects with and without type 2 diabetes. Dis. Markers 2010, 29, 213–221. [Google Scholar] [CrossRef]
- Ramezani-Moghadam, M.; Wang, J.; Ho, V.; Iseli, T.J.; Alzahrani, B.; Xu, A.; Van der Poorten, D.; Qiao, L.; George, J.; Hebbard, L. Adiponectin reduces hepatic stellate cell migration by promoting tissue inhibitor of metalloproteinase-1 (TIMP-1) secretion. J. Biol. Chem. 2015, 290, 5533–5542. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, L.M.; Bucher, C.H.; Löffler, J.; Rinne, C.; Duda, G.N.; Geissler, S.; Schulz, T.J.; Schmidt-Bleek, K. The benefits of adipocyte metabolism in bone health and regeneration. Front. Cell. Dev. Biol. 2023, 11, 1104709. [Google Scholar] [CrossRef] [PubMed]
- Gamberi, T.; Magherini, F.; Modesti, A.; Fiaschi, T. Adiponectin Signaling Pathways in Liver Diseases. Biomedicines 2018, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Graupera, I.; Isus, L.; Coll, M.; Pose, E.; Díaz, A.; Vallverdú, J.; Rubio-Tomás, T.; Martínez-Sánchez, C.; Huelin, P.; Llopis, M.; et al. Molecular characterization of chronic liver disease dynamics: From liver fibrosis to acute-on-chronic liver failure. JHEP Rep. 2022, 4, 100482. [Google Scholar] [CrossRef]
- Gómez-Santos, B.; Saenz de Urturi, D.; Nuñez-García, M.; Gonzalez-Romero, F.; Buque, X.; Aurrekoetxea, I.; Gutiérrez de Juan, V.; Gonzalez-Rellan, M.J.; García-Monzón, C.; González-Rodríguez, Á.; et al. Liver osteopontin is required to prevent the progression of age-related nonalcoholic fatty liver disease. Aging Cell 2020, 19, e13183. [Google Scholar] [CrossRef]
- Caserza, L.; Casula, M.; Elia, E.; Bonaventura, A.; Liberale, L.; Bertolotto, M.; Artom, N.; Minetti, S.; Contini, P.; Verzola, D.; et al. Serum osteopontin predicts glycaemic profile improvement in metabolic syndrome: A pilot study. Eur. J. Clin. Investig. 2021, 51, e13403. [Google Scholar] [CrossRef]
- Luger, M.; Kruschitz, R.; Kienbacher, C.; Traussnigg, S.; Langer, F.B.; Schindler, K.; Würger, T.; Wrba, F.; Trauner, M.; Prager, G.; et al. Prevalence of Liver Fibrosis and its Association with Non-invasive Fibrosis and Metabolic Markers in Morbidly Obese Patients with Vitamin D Deficiency. Obes. Surg. 2016, 26, 2425–2432. [Google Scholar] [CrossRef]
- Bukhari, T.; Jafri, L.; Majid, H.; Khan, A.H.H.; Siddiqui, I. Determining Bone Turnover Status in Patients With Chronic Liver Disease. Cureus 2021, 13, e14479. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Chen, X. Bone turnover markers and probable advanced nonalcoholic fatty liver disease in middle-aged and elderly men and postmenopausal women with type 2 diabetes. Front. Endocrinol. 2020, 10, 926. [Google Scholar] [CrossRef]
- Veidal, S.S.; Vassiliadis, E.; Bay-Jensen, A.C.; Tougas, G.; Vainer, B.; Karsdal, M.A. Procollagen type I N-terminal propeptide (PINP) is a marker for fibrogenesis in bile duct ligation-induced fibrosis in rats. Fibrogenesis Tissue Repair 2010, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Jiang, L.; Liu, Q.; Zhang, J.; Niu, W.; Liu, J.; Zhang, Q. Low Bone Turnover Markers in Young and Middle-Aged Male Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 6191468. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Liu, L.; Zhang, L.; Li, M.; Liu, R.; Li, T.; Chen, E.; Liu, S. Relationships of Serum Bone Turnover Markers With Metabolic Syndrome Components and Carotid Atherosclerosis in Patients With Type 2 Diabetes Mellitus. Front. Cardiovasc. Med. 2022, 9, 824561. [Google Scholar] [CrossRef] [PubMed]
- Nan, R.; Grigorie, D.; Cursaru, A.; Șucaliuc, A.; Drăguț, R.; Rusu, E.; Mușat, M.; Radulian, G. Bisphosphonates-A good choice for women with type 2 diabetes and postmenopausal osteoporosis? Farmacia 2016, 64, 257–261. [Google Scholar]
- Yang, M.; Liu, Y.; Zhou, G.; Guo, X.; Zou, S.; Liu, S.; Jiang, L.; Liu, Y.; Zhu, L.; Guo, C.; et al. Value of serum osteoprotegerin in noninvasive diagnosis of nonalcoholic steatohepatitis. Zhonghua Gan Zang Bing Za Zhi 2016, 24, 96–101. (In Chinese) [Google Scholar] [CrossRef]
- Piñar-Gutierrez, A.; García-Fontana, C.; García-Fontana, B. Obesity and Bone Health: A Complex Relationship. Int. J. Mol. Sci. 2022, 23, 8303. [Google Scholar] [CrossRef]
- Alzahrani, B.; Iseli, T.; Ramezani-Moghadam, M.; Ho, V.; Wankell, M.; Sun, E.J.; Qiao, L.; George, J.; Hebbard, L.W. The role of AdipoR1 and AdipoR2 in liver fibrosis. Biochim. Biophys. Acta 2018, 1864, 700–708. [Google Scholar] [CrossRef]
- Bastos, A.C.S.D.F.; Gomes, A.V.P.; Silva, G.R.; Emerenciano, M.; Ferreira, L.B.; Gimba, E.R.P. The Intracellular and Secreted Sides of Osteopontin and Their Putative Physiopathological Roles. Int. J. Mol. Sci. 2023, 24, 2942. [Google Scholar] [CrossRef]
- Shao, Z.; Bi, S. Endocrine regulation and metabolic mechanisms of osteopontin in the development and progression of osteosarcoma, metastasis and prognosis. Front. Endocrinol. 2023, 13, 1100063. [Google Scholar] [CrossRef]
- Bai, R.J.; Li, Y.S.; Zhang, F.J. Osteopontin, a bridge links osteoarthritis and osteoporosis. Front. Endocrinol. 2022, 13, 1012508. [Google Scholar] [CrossRef]
- Lamort, A.S.; Giopanou, I.; Psallidas, I.; Stathopoulos, G.T. Osteopontin as a link between inflammation and cancer: The thorax in the spotlight. Cells 2019, 8, 815. [Google Scholar] [CrossRef] [PubMed]
- Moorman, H.R.; Poschel, D.; Klement, J.D.; Lu, C.; Redd, P.S.; Liu, K. Osteopontin: A key regulator of tumor progression and immunomodulation. Cancers 2020, 12, 3379. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Cui, Y.; Lane, J.; Jia, S.; Ji, J.; Jiang, W.G. Distinctive Prognostic Value and Cellular Functions of Osteopontin Splice Variants in Human Gastric. Cancer Cells 2021, 10, 1820. [Google Scholar] [CrossRef] [PubMed]
- Bruha, R.; Vitek, L.; Smid, V. Osteopontin-A potential biomarker of advanced liver disease. Ann. Hepatol. 2020, 19, 344–352. [Google Scholar] [CrossRef]
- Song, Z.; Chen, W.; Athavale, D.; Ge, X.; Desert, R.; Das, S.; Han, H.; Nieto, N. Osteopontin Takes Center Stage in Chronic Liver Disease. Hepatology 2021, 73, 1594–1608. [Google Scholar] [CrossRef]
- Remmerie, A.; Martens, L.; Thoné, T.; Castoldi, A.; Seurinck, R.; Pavie, B.; Roels, J.; Vanneste, B.; De Prijck, S.; Vanhockerhout, M.; et al. Osteopontin Expression Identifies a Subset of Recruited Macrophages Distinct from Kupffer Cells in the Fatty Liver. Immunity 2020, 53, 641–657. [Google Scholar] [CrossRef]
- Aguilera, A.A.; Diaz, G.H.; Barcelata, M.L.; Guerrero, O.A.; Ros, R.M. Effects of fish oil on hypertension, plasma lipids, and tumor necrosis factor-alpha in rats with sucrose-induced metabolic syndrome. J. Nutr. Biochem. 2004, 15, 350–357. [Google Scholar] [CrossRef]
- Diemar, S.S.; Dahl, S.S.; West, A.S.; Simonsen, S.A.; Iversen, H.K.; Jørgensen, N.R. A Systematic Review of the Circadian Rhythm of Bone Markers in Blood. Calcif. Tissue Int. 2023, 112, 126–147. [Google Scholar] [CrossRef]
- Krzanowski, M.; Krzanowska, K.; Dumnicka, P.; Gajda, M.; Woziwodzka, K.; Fedak, D.; Grodzicki, T.; Litwin, J.A.; Sułowicz, W. Elevated Circulating Osteoprotegerin Levels in the Plasma of Hemodialyzed Patients With Severe Artery Calcification. Ther. Apher. Dial. 2018, 22, 519–529. [Google Scholar] [CrossRef]
- Adhyatmika, A.; Beljaars, L.; Putri, K.S.S.; Habibie, H.; Boorsma, C.E.; Reker-Smit, C.; Luangmonkong, T.; Guney, B.; Haak, A.; Mangnus, K.A.; et al. Osteoprotegerin Is more than a Possible Serum Marker in Liver Fibrosis: A Study into Its Function in Human and Murine Liver. Pharmaceutics 2020, 12, 471. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, Q.; Song, N.; Yan, Z.; Lin, R.; Wu, S.; Jiang, L.; Hong, S.; Xie, J.; Zhou, H.; et al. AdipoR1/AdipoR2 dual agonist recovers nonalcoholic steatohepatitis and related fibrosis via endoplasmic reticulum-mitochondria axis. Nat. Commun. 2020, 11, 5807, Erratum in Nat. Commun. 2021, 12, 2036. [Google Scholar] [CrossRef] [PubMed]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M.; et al. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Yoon, H.E.; Shin, S.J.; Choi, B.S.; Kim, Y.S.; Chang, Y.S.; Park, C.W. The Adiponectin Receptor Agonist AdipoRon Ameliorates Diabetic Nephropathy in a Model of Type 2 Diabetes. J. Am. Soc. Nephrol. 2018, 29, 1108–1127. [Google Scholar] [CrossRef] [PubMed]
- Alkhathami, K.; Soman, A.; Chandy, S.; Ramamoorthy, B.; Alqahtani, B. Comparing the effects of retro and forward walking on serum adiponectin levels in obese young adults. J. Taibah Univ. Med. Sci. 2023, 18, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Park, O.J.; Kim, J.; Han, S.; Yang, Y.; Yun, C.H.; Han, S.H. Adiponectin Deficiency Triggers Bone Loss by Up-Regulation of Osteoclastogenesis and Down-Regulation of Osteoblastogenesis. Front. Endocrinol. 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Huang, R.; Tang, P.T.; Tu, M.; Guo, X.L. Perirenalfat thickness is associated with bone turnover markers and bone mineral density in postmenopausal women with type 2 diabetes mellitus. Front. Endocrinol. 2022, 13, 990667. [Google Scholar] [CrossRef] [PubMed]
- Kleerekoper, M.; Nelson, D.A.; Peterson, E.L.; Wilson, P.S.; Jacobsen, G.; Longcope, C. Body composition and gonadal steroids in older white and black women. J. Clin. Endocrinol. Metab. 1994, 79, 775–779. [Google Scholar] [CrossRef]
- Di, F.; Gao, D.; Yao, L.; Zhang, R.; Qiu, J.; Song, L. Differences in metabonomic profiles of abdominal subcutaneous adipose tissue in women with polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1077604. [Google Scholar] [CrossRef]
- Ward, L.J.; Nilsson, S.; Hammar, M.; Lindh-Åstrand, L.; Berin, E.; Lindblom, H.; Spetz Holm, A.C.; Rubér, M.; Li, W. Resistance training decreases plasma levels of adipokines in postmenopausal women. Sci. Rep. 2020, 10, 19837. [Google Scholar] [CrossRef]
- Azizieh, F.Y.; Shehab, D.; Al Jarallah, K.; Mojiminiyi, O.; Gupta, R.; Raghupathy, R. Circulatory pattern of cytokines, adipokines and bone markers in postmenopausal women with low BMD. J. Inflamm. Res. 2019, 12, 99–108. [Google Scholar] [CrossRef]
- Jo, D.; Son, Y.; Yoon, G. Role of Adiponectin and Brain Derived Neurotrophic Factor in Metabolic Regulation Involved in Adiposity and Body Fat Browning. J. Clin. Med. 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-H.; Guo, L.-J.; Yuan, L.-Q.; Xie, H.; Zhou, H.D.; Wu, X.P.; Liao, E.Y. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp. Cell Res. 2005, 309, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ma, B.; Cui, C.; Zhao, J.; Liu, S.; Qiu, Y.; Zheng, Y.; Gao, M.; Luan, X. Protective effects of AdipoRon on the liver of Huoyan goose fed a high-fat diet. Poult. Sci. 2022, 101, 101708. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Smith, T.; Rahman, K. Adiponectin agonist ADP355 attenuates CCl4-induced liver fibrosis in mice. PLoS ONE 2014, 9, e110405. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Afifi, N.; Yassin, N.Z.; Abdel-Rahman, R.F.; Abd El-Rahman, S.S.; Fayed, H.M. Mesalazine, an osteopontin inhibitor: The potential prophylactic and remedial roles in induced liver fibrosis in rats. Chem. Biol. Interact. 2018, 289, 109–118. [Google Scholar] [CrossRef]
- Leung, T.M.; Wang, X.; Kitamura, N. Osteopontin delays resolution of liver fibrosis. Lab. Investig. 2013, 93, 1082–1089. [Google Scholar] [CrossRef]
| Biologically Active Substance | Encoding Gene | Tissue Distribution/Main Sources | Functions | Role in Bone Tissue Metabolism | References |
|---|---|---|---|---|---|
| Osteopontin (OPN) | Secreted phosphoprotein 1 (SPP1) | Bone cells (preosteoblasts, osteoclasts/osteoblasts, osteocytes), hepatocytes, epithelial cells, endothelial cells, fibroblasts, renal tubular cells, smooth muscle cells, immune cells (T cells, macrophages, and natural killer cells), malignantly transformed epithelial cell lines | Regulation of extracellular matrix metabolism and bone biomineralization; participation in immune response, inflammation, and tissue repair | Osteopontin participates in the regulation of bone mass through the neuron- and endocrine-mediated mechanisms | [55,56,57,58,59] |
| N-terminal propeptide of procollagen type 1 (P1NP) | type 1 collagen (COL1) | Osteoblasts, hepatic stellate cells | N-terminal and C-terminal propeptides of procollagen type 1 represent the parts of the procollagen molecule. Procollagen type 1 is secreted into the extracellular space where the N-terminal and C-terminal propeptides are cleaved by propeptidases, generating the basic functional heterotrimeric unit of collagen 1. Collagen 1 contributes around 90% of the total organic component of bone matrix | The dynamic and sensitive marker of changes in bone formation | [60,61] |
| Osteoprotegerin (OPG) | TNF receptor superfamily member 11b (TNFRSF11B) | Osteoblasts, medullary thymic epithelial cells, intestinal microfold cells, fibroblasts, vascular endothelial cells, and some cancer cells (e.g., breast and prostate cancers) | Participation in bone homeostasis and regulation of calcium-phosphorus metabolism. It also plays a role in lymphnode organogenesis, immune modulation, fibrosis, angiogenesis, and vascular calcification. Currently the diagnostic, prognostic, and therapeutic value of osteoprotegerin in various cancers, especially bone metastasis, is widely discussed in the literature. | Osteoprotegerin is a negative regulator of bone resorption. It performs its function primarily through the impact on osteoclast development, activation, and apoptosis | [62,63,64,65,66,67] |
| Adiponectin | Adiponectin, C1Q and collagen domain containing | Exclusively adipose tissue, mainly visceral adipose tissue and to a lesser extent subcutaneous adipose tissue | Functions as a homeostatic factor for regulating glucose levels, lipid metabolism, and insulin sensitivity through its anti-inflammatory, anti-fibrotic, and antioxidant effects | Adiponectin may have a pro-osteogenic role by stimulating osteoblast differentiation and activity, alongside inhibiting the osteoclastogenesis. | [68,69,70,71,72,73] |
| Biologically Active Substance | Possible Role in Liver Diseases | Association with Metabolic Syndrome | References |
|---|---|---|---|
| Osteopontin (OPN) | Participation in liver fibrosis development and progression via activation of stellate cells | Elevated serum level of OPN is associated with metabolic syndrome and its different components (systolic and diastolic blood pressure, fasting blood glucose, serum concentrations of total cholesterol, low density lipoprotein cholesterol [LDL-c], triglycerides, HbA1c, fasting plasma insulin, and homeostatic model assessment of insulin resistance [HOMA-IR]) | [74,75,76,77] |
| N-terminal propeptide of procollagen type 1 (P1NP) | Production of type 1 collagen during liver fibrosis increases up to 8-fold. The P1NP seems to be related to liver fibrosis development and progression in NAFLD and some other chronic liver diseases, such as primary biliary cholangitis, and alcoholic liver disease. | Serum concentration of P1NP appears to be higher in patients with metabolic syndrome, compared to healthy subjects. At the same time, it was shown that elevated serum levels of P1NP were negatively correlated with triglyceride levels. | [61,78,79,80,81,82,83] |
| Osteoprotegerin (OPG) | High serum osteoprotegerin levels are associated with liver fibrosis. Elevated serum osteoprotegerin levels were shown in patients with alcoholic liver fibrosis, primary biliary cholangitis, and viral cirrhosis compared to respective controls. Osteoprotegerin may stimulate fibrogenesis in the liver through transforming growth factor beta 1 (TGFβ1) and is associated with the degree of fibrogenesis. | Serum levels of osteoprotegerin are increased in patients with metabolic syndrome. High serum levels of osteoprotegerin are associated with the poor control of type 2 diabetes mellites and the development of its complications. It was shown that osteoprotegerin levels were negatively correlated with body weight, waist circumference, HOMA-IR, and fasting plasma insulin, whereas they were positively correlated with insulin sensitivity. Taken together these data suggest that osteoprotegerin may serve as an independent predictor of metabolic syndrome. | [62,84,85,86] |
| Adiponectin | Adiponectin has been demonstrated to have an anti-fibrotic action in the liver by blocking the activation of the expression of pro-fibrotic genes. Increased circulating adiponectin levels were found to be associated with the development of liver fibrosis. In contrast, both serum levels and hepatic adiponectin receptor expression were shown to be decreased in NAFLD. Adiponectin reduces the transport of fatty acids and the accumulation of triglycerides in the liver. | Adiponectin induces an increase in circulating insulin levels, promotes the consumption of glucose by muscle cells and adipocytes, and inhibits the formation of glucose and glycogen in the liver and skeletal muscles. It also controls lipid metabolism by promoting the transport of fatty acids and β-oxidation in muscle cells by inhibiting hepatic lipogenesis and by stimulating the storage function of adipose tissue. | [69,73,87] |
| Biologically Active Substance | Analog of the Substance, Agonist/Antagonist of Its Receptor Used for the Treatment | Animal Species/Study Population | Main Findings | Reference |
|---|---|---|---|---|
| Adiponectin | AdipoRon (an orally active synthetic adiponectin receptor agonist) | Wild-type mice, Adipor1−/−, Adipor2−/−, Adipor1−/− Adipor2−/− knock-out mice, and the db/db mice | AdipoRon ameliorated insulin resistance and glucose intolerance in mice fed a high-fat diet. AdipoRon ameliorated diabetes of genetically obese rodent model db/dbmice and prolonged the shortened lifespan of db/db mice on a high-fat diet. | [102] |
| 27 patients with T2DM and 6 healthy controls C57BLKS/J db/db mice | AdipoR1/AdipoR2 expression was significantly decreased in the glomerulus of human diabetic kidneys compared with that of nondiabetic control kidneys, even in the earliest chronic kidney disease stage. AdipoRon ameliorated diabetes-induced renal damage by reducing intrarenal lipotoxicity and oxidative stress | [103] | ||
| Huoyan geese | AdipoRon could alter the expression of lipid metabolism-related genes, inflammatory factors, apoptosis and autophagy genes, and adiponectin and its receptor genes in liver tissues; reduce the lipid content in blood and liver tissues of geese fed high-fat diets; improve liver antioxidant capacity; regulate apoptosis and autophagy of hepatocytes; and reduce liver inflammatory injury | [113] | ||
| JT003 (AdipoR1/AdipoR2 dual agonist) | C57BL/6J mice | Adiponectin-based agonist JT003 potently improves insulin resistance in high fat diet induced NASH mice. JT003 treatment significantly improves endoplasmic reticulum–mitochondria axis function, which contributes to the reduced hepatic stellate cell activation in CCl4-induced liver fibrosis | [101] | |
| ADP355 (adiponectin analog, capable of mimicking its active site) | C57BL/6J mice | ADP355 is a potent anti-fibrotic agent that attenuates CCl4-induced liver fibrosis in mice | [114] | |
| Osteopontin (OPN) | Mesalazine (osteopontin inhibitor) | Wistar rats | In rats with TAA-induced liver fibrosis, mesalazine exerted an anti-fibrotic action through limiting the oxidative damage and altering the TNF-ɑ pathway, along with downregulating transforming growth factor β1 (TGF-β1), OPN, alpha-smooth muscle actin, and caspase-3 signaling pathways in the liver | [115] |
| Wild-type and OPN-knockout (Opn(−/−)) mice | Osteopontin delays liver fibrosis resolution following TAA cessation in the livers of wild-type mice due to sustained fibrillar collagen-I deposition. Inhibiting OPN could be an effective therapeutic strategy for resolving liver fibrosis. | [116] | ||
| Osteoprotegerin (OPG) | Balb/c mice C57BL/6 mice Cirrhotic human liver tissue | Liver fibrosis is accompanied by a higher production of OPG in liver tissue, particularly in response to TGFβ1. Hepatic stellate cells and scar-associated myofibroblasts appear to be an important source of OPG in human tissue, while in murine liver tissue (mouse model of CCl4-induced liver fibrosis) scar-associated cells appear to be the main source. Osteoprotegerin has profibrotic abilities through neutralization of RANKL and/or TRAIL and upregulation of TGFβ1 expression. Spontaneous or drug-induced resolution of fibrosis is accompanied by lower expression of OPG. | [100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drapkina, O.M.; Elkina, A.Y.; Sheptulina, A.F.; Kiselev, A.R. Non-Alcoholic Fatty Liver Disease and Bone Tissue Metabolism: Current Findings and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 8445. https://doi.org/10.3390/ijms24098445
Drapkina OM, Elkina AY, Sheptulina AF, Kiselev AR. Non-Alcoholic Fatty Liver Disease and Bone Tissue Metabolism: Current Findings and Future Perspectives. International Journal of Molecular Sciences. 2023; 24(9):8445. https://doi.org/10.3390/ijms24098445
Chicago/Turabian StyleDrapkina, Oxana M., Anastasia Yu. Elkina, Anna F. Sheptulina, and Anton R. Kiselev. 2023. "Non-Alcoholic Fatty Liver Disease and Bone Tissue Metabolism: Current Findings and Future Perspectives" International Journal of Molecular Sciences 24, no. 9: 8445. https://doi.org/10.3390/ijms24098445
APA StyleDrapkina, O. M., Elkina, A. Y., Sheptulina, A. F., & Kiselev, A. R. (2023). Non-Alcoholic Fatty Liver Disease and Bone Tissue Metabolism: Current Findings and Future Perspectives. International Journal of Molecular Sciences, 24(9), 8445. https://doi.org/10.3390/ijms24098445






