Geometry and UV-Vis Spectra of Au3+ Complexes with Hydrazones Derived from Pyridoxal 5′-Phosphate: A DFT Study
Abstract
1. Introduction
2. Results and Discussion
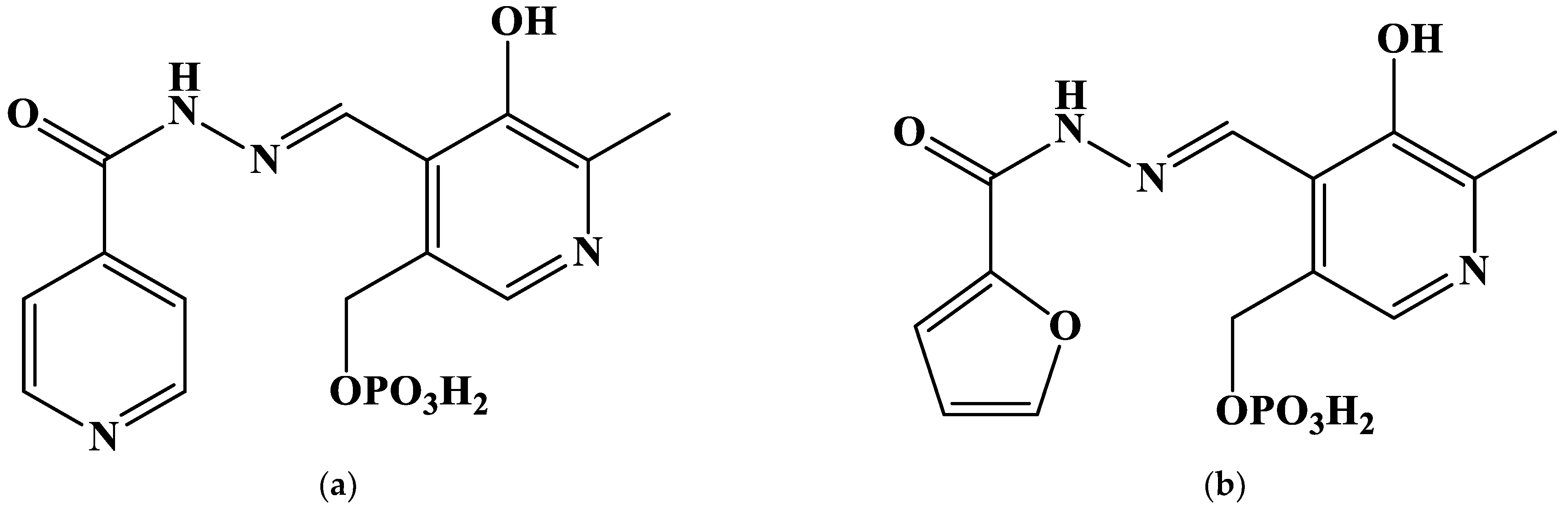
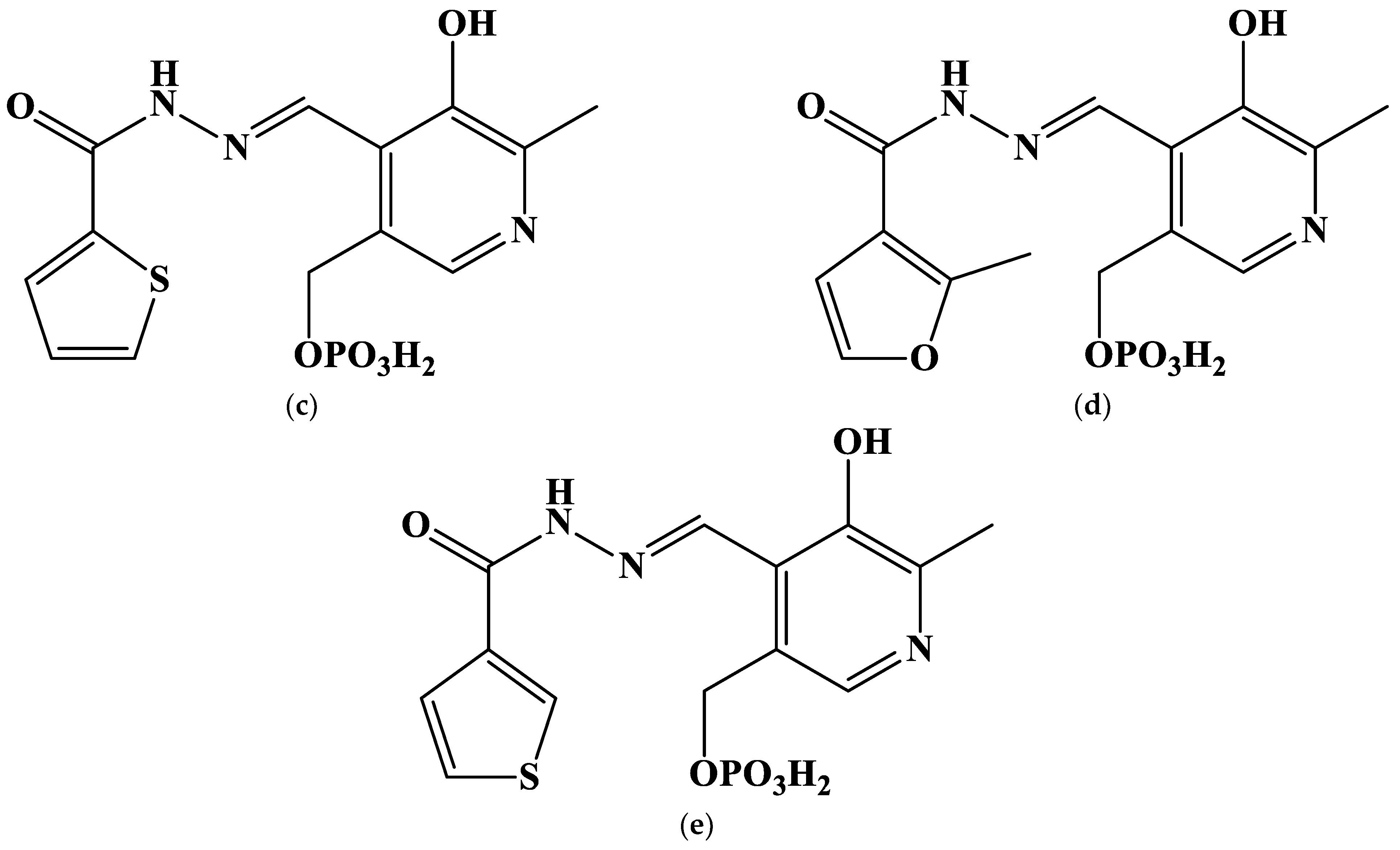
2.1. The Search of Probable Molecular Models
2.2. The Geometry of Gold(III) Complexes
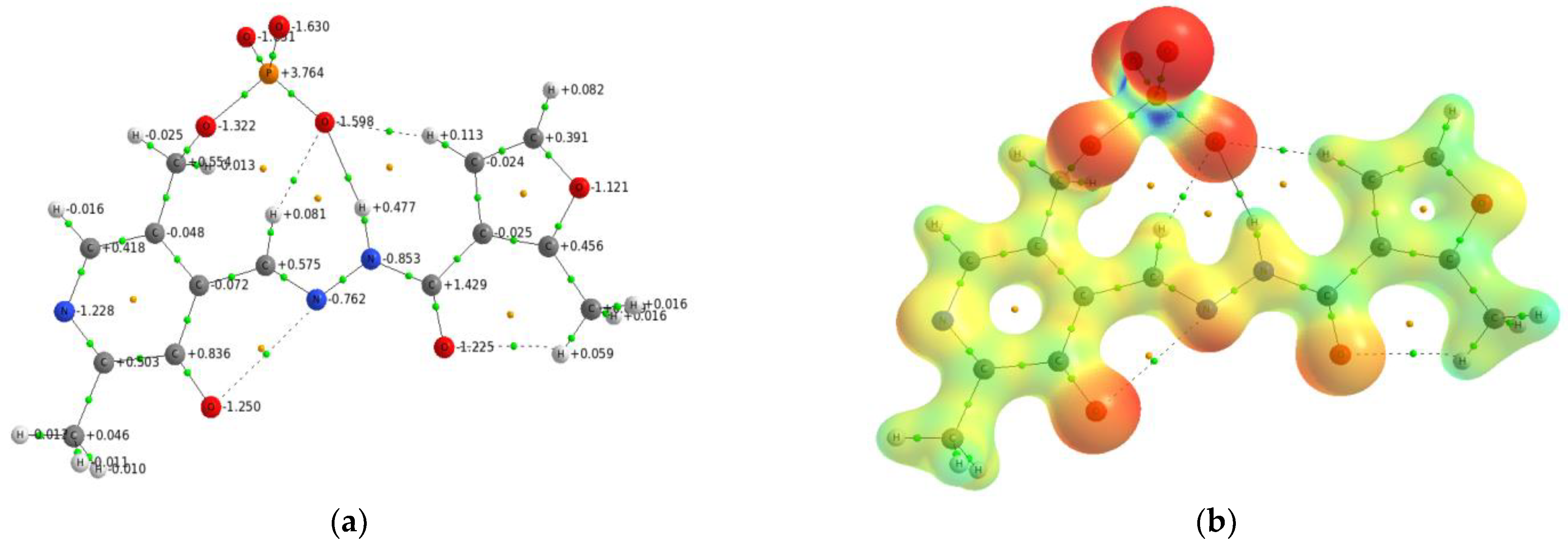
2.3. The Electron Density Distribution Analysis
2.4. The UV–Vis Absorption Spectra Calculations
3. Materials and Methods
Calculation Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Radisavljević, S.; Ćoćić, D.; Jovanović, S.; Šmit, B.; Petković, M.; Milivojević, N.; Planojević, N.; Marković, S.; Petrović, B. Synthesis, Characterization, DFT Study, DNA/BSA-Binding Affinity, and Cytotoxicity of Some Dinuclear and Trinuclear Gold(III) Complexes. J. Biol. Inorg. Chem. 2019, 24, 1057–1076. [Google Scholar] [CrossRef] [PubMed]
- Parish, R.V.; Howe, B.P.; Wright, J.P.; Mack, J.; Pritchard, R.G.; Buckley, R.G.; Elsome, A.M.; Fricker, S.P. Chemical and Biological Studies of Dichloro(2-((Dimethylamino)Methyl)Phenyl)Gold(III). Inorg. Chem. 1996, 35, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Parish, R.V.; Mack, J.; Hargreaves, L.; Wright, J.P.; Buckley, R.G.; Elsome, A.M.; Fricker, S.P.; Theobald, B.R.C. Chemical and Biological Reactions of Diacetato[2-(Dimethylaminomethyl)-Phenyl]Gold(III), [Au(O2CMe)2(Dmamp)]. J. Chem. Soc. Dalton Trans. 1996, 1, 69–74. [Google Scholar] [CrossRef]
- Buckley, R.G.; Elsome, A.M.; Fricker, S.P.; Henderson, G.R.; Theobald, B.R.C.; Parish, R.V.; Howe, B.P.; Kelland, L.R. Antitumor Properties of Some 2-[(Dimethylamino)Methyl]Phenylgold(III) Complexes. J. Med. Chem. 1996, 39, 5208–5214. [Google Scholar] [CrossRef]
- Bertrand, B.; Williams, M.R.M.; Bochmann, M. Gold(III) Complexes for Antitumor Applications: An Overview. Chem. Eur. J. 2018, 24, 11840–11851. [Google Scholar] [CrossRef]
- Tong, K.-C.; Hu, D.; Wan, P.-K.; Lok, C.-N.; Che, C.-M. Anticancer Gold(III) Compounds With Porphyrin or N-Heterocyclic Carbene Ligands. Front. Chem. 2020, 8, 587207. [Google Scholar] [CrossRef]
- Gurba, A.; Taciak, P.; Sacharczuk, M.; Młynarczuk-Biały, I.; Bujalska-Zadrożny, M.; Fichna, J. Gold(III) Derivatives in Colon Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 724. [Google Scholar] [CrossRef]
- Abyar, F.; Tabrizi, L. New Cyclometalated Gold (III) Complex Targeting Thioredoxin Reductase: Exploring as Cytotoxic Agents and Mechanistic Insights. Biometals 2020, 33, 107–122. [Google Scholar] [CrossRef]
- Büssing, R.; Karge, B.; Lippmann, P.; Jones, P.G.; Brönstrup, M.; Ott, I. Gold(I) and Gold(III) N-Heterocyclic Carbene Complexes as Antibacterial Agents and Inhibitors of Bacterial Thioredoxin Reductase. ChemMedChem 2021, 16, 3402–3409. [Google Scholar] [CrossRef]
- Saggioro, D.; Rigobello, M.P.; Paloschi, L.; Folda, A.; Moggach, S.A.; Parsons, S.; Ronconi, L.; Fregona, D.; Bindoli, A. Gold(III)-Dithiocarbamato Complexes Induce Cancer Cell Death Triggered by Thioredoxin Redox System Inhibition and Activation of ERK Pathway. Chem. Biol. 2007, 14, 1128–1139. [Google Scholar] [CrossRef]
- Quero, J.; Cabello, S.; Fuertes, T.; Mármol, I.; Laplaza, R.; Polo, V.; Gimeno, M.C.; Rodriguez-Yoldi, M.J.; Cerrada, E. Proteasome versus Thioredoxin Reductase Competition as Possible Biological Targets in Antitumor Mixed Thiolate-Dithiocarbamate Gold(III) Complexes. Inorg. Chem. 2018, 57, 10832–10845. [Google Scholar] [CrossRef]
- Haeubl, M.; Reith, L.M.; Gruber, B.; Karner, U.; Müller, N.; Knör, G.; Schoefberger, W. DNA Interactions and Photocatalytic Strand Cleavage by Artificial Nucleases Based on Water-Soluble Gold(III) Porphyrins. J. Biol. Inorg. Chem. 2009, 14, 1037–1052. [Google Scholar] [CrossRef]
- Radisavljević, S.; Đeković Kesić, A.; Ćoćić, D.; Puchta, R.; Senft, L.; Milutinović, M.; Milivojević, N.; Petrović, B. Studies of the Stability, Nucleophilic Substitution Reactions, DNA/BSA Interactions, Cytotoxic Activity, DFT and Molecular Docking of Some Tetra- and Penta-Coordinated Gold(iii) Complexes. New J. Chem. 2020, 44, 11172–11187. [Google Scholar] [CrossRef]
- Glišić, B.Đ.; Djuran, M.I. Gold Complexes as Antimicrobial Agents: An Overview of Different Biological Activities in Relation to the Oxidation State of the Gold Ion and the Ligand Structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Kanthecha, D.N.; Bhatt, B.S.; Patel, M.N.; Vaidya, F.U.; Pathak, C. DNA Interaction, Anticancer, Cytotoxicity and Genotoxicity Studies with Potential Pyrazine-Bipyrazole Dinuclear µ-Oxo Bridged Au(III) Complexes. Mol. Divers. 2022, 26, 2085–2101. [Google Scholar] [CrossRef]
- Al-Khodir, F.A.I.; Refat, M.S. Synthesis, Spectroscopic, Thermal and Anticancer Studies of Metal-Antibiotic Chelations: Ca(II), Fe(III), Pd(II) and Au(III) Chloramphenicol Complexes. J. Mol. Struct. 2016, 1119, 157–166. [Google Scholar] [CrossRef]
- Radulović, N.S.; Stojanović, N.M.; Glišić, B.Đ.; Randjelović, P.J.; Stojanović-Radić, Z.Z.; Mitić, K.V.; Nikolić, M.G.; Djuran, M.I. Water-Soluble Gold(III) Complexes with N-Donor Ligands as Potential Immunomodulatory and Antibiofilm Agents. Polyhedron 2018, 141, 164–180. [Google Scholar] [CrossRef]
- Hussain, I.; Ullah, A.; Khan, A.U.; Khan, W.U.; Ullah, R.; Almoqbil, A.; Naser, A.A.S.; Mahmood, H.M. Synthesis, Characterization and Biological Activities of Hydrazone Schiff Base and Its Novel Metals Complexes. Sains Malays 2019, 48, 1439–1446. [Google Scholar] [CrossRef]
- Abou Melha, K.S.A.; Al-Hazmi, G.A.A.; Refat, M.S. Synthesis of Nano-Metric Gold Complexes with New Schiff Bases Derived from 4-Aminoantipyrene, Their Structures and Anticancer Activity. Russ. J. Gen. Chem. 2017, 87, 3043–3051. [Google Scholar] [CrossRef]
- Alibrahim, K.A.; Al-Saif, F.A.; Bakhsh, H.A.; Refat, M.S. Synthesis, Physicochemical, and Biological Studies of New Pyridoxine HCl Mononuclear Drug Complexes of V(III), Ru(III), Pt(II), Se(IV), and Au(III) Metal Ions. Russ. J. Gen. Chem. 2018, 88, 2400–2409. [Google Scholar] [CrossRef]
- Ratia, C.; Sueiro, S.; Soengas, R.G.; Iglesias, M.J.; López-Ortiz, F.; Soto, S.M. Gold(III) Complexes Activity against Multidrug-Resistant Bacteria of Veterinary Significance. Antibiotics 2022, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Đurović, M.D.; Bugarčić, Ž.D.; van Eldik, R. Stability and Reactivity of Gold Compounds—From Fundamental Aspects to Applications. Coord. Chem. Rev. 2017, 338, 186–206. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; Zhang, J.-J.; Che, C.-M. Chemical Biology of Anticancer Gold(III) and Gold(I) Complexes. Chem. Soc. Rev. 2015, 44, 8786–8801. [Google Scholar] [CrossRef] [PubMed]
- Patanjali, P.; Kumar, R.; Sourabh; Kumar, A.; Chaudhary, P.; Singh, R. Reviewing Gold(III) Complexes as Effective Biological Operators. MGC 2018, 17, 35–52. [Google Scholar] [CrossRef]
- Warżajtis, B.; Glišić, B.Đ.; Živković, M.D.; Rajković, S.; Djuran, M.I.; Rychlewska, U. Different Reaction Products as a Function of Solvent: NMR Spectroscopic and Crystallographic Characterization of the Products of the Reaction of Gold(III) with 2-(Aminomethyl)Pyridine. Polyhedron 2015, 91, 35–41. [Google Scholar] [CrossRef]
- Palanichamy, K.; Sreejayan, N.; Ontko, A.C. Overcoming Cisplatin Resistance Using Gold(III) Mimics: Anticancer Activity of Novel Gold(III) Polypyridyl Complexes. J. Inorg. Biochem. 2012, 106, 32–42. [Google Scholar] [CrossRef]
- Lum, C.T.; Wong, A.S.-T.; Lin, M.C.; Che, C.-M.; Sun, R.W.-Y. A Gold(III) Porphyrin Complex as an Anti-Cancer Candidate to Inhibit Growth of Cancer-Stem Cells. Chem. Commun. 2013, 49, 4364–4366. [Google Scholar] [CrossRef]
- Al-Jaroudi, S.S.; Monim-ul-Mehboob, M.; Altaf, M.; Al-Saadi, A.A.; Wazeer, M.I.M.; Altuwaijri, S.; Isab, A.A. Synthesis, Spectroscopic Characterization, Electrochemical Behavior and Computational Analysis of Mixed Diamine Ligand Gold(III) Complexes: Antiproliferative and in Vitro Cytotoxic Evaluations against Human Cancer Cell Lines. Biometals 2014, 27, 1115–1136. [Google Scholar] [CrossRef]
- Radisavljević, S.; Bratsos, I.; Scheurer, A.; Korzekwa, J.; Masnikosa, R.; Tot, A.; Gligorijević, N.; Radulović, S.; Rilak Simović, A. New Gold Pincer-Type Complexes: Synthesis, Characterization, DNA Binding Studies and Cytotoxicity. Dalton Trans. 2018, 47, 13696–13712. [Google Scholar] [CrossRef]
- Murphy, T.B.; Johnson, D.K.; Rose, N.J.; Aruffo, A.; Schomaker, V. Structural Studies of Iron(III) Complexes of the New Iron-Binding Drug, Pyridoxal Isonicotinoyl Hydrazone. Inorg. Chim. Acta 1982, 66, L67–L68. [Google Scholar] [CrossRef]
- Back, D.F.; Manzoni de Oliveira, G.; Roman, D.; Ballin, M.A.; Kober, R.; Piquini, P.C. Synthesis of Symmetric N,O-Donor Ligands Derived from Pyridoxal (Vitamin B6): DFT Studies and Structural Features of Their Binuclear Chelate Complexes with the Oxofilic Uranyl and Vanadyl(V) Cations. Inorg. Chim. Acta 2014, 412, 6–14. [Google Scholar] [CrossRef]
- Murašková, V.; Szabó, N.; Pižl, M.; Hoskovcová, I.; Dušek, M.; Huber, Š.; Sedmidubský, D. Self Assembly of Dialkoxo Bridged Dinuclear Fe(III) Complex of Pyridoxal Schiff Base with C C Bond Formation—Structure, Spectral and Magnetic Properties. Inorg. Chim. Acta 2017, 461, 111–119. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N. La3+, Ce3+, Eu3+, and Gd3+ Complex Formation with Hydrazones Derived from Pyridoxal 5’-Phosphate in a Neutral Tris–HCl Buffer. Russ. J. Inorg. Chem. 2021, 66, 1561–1568. [Google Scholar] [CrossRef]
- Brittenham, G.M. Pyridoxal Isonicotinoyl Hydrazone.: Effective Iron Chelation after Oral Administration. Ann. N. Y. Acad. Sci. 1990, 612, 315–326. [Google Scholar] [CrossRef]
- Shtyrlin, N.V.; Khaziev, R.M.; Shtyrlin, V.G.; Gilyazetdinov, E.M.; Agafonova, M.N.; Usachev, K.S.; Islamov, D.R.; Klimovitskii, A.E.; Vinogradova, T.I.; Dogonadze, M.Z.; et al. Isonicotinoyl Hydrazones of Pyridoxine Derivatives: Synthesis and Antimycobacterial Activity. Med. Chem. Res. 2021, 30, 952–963. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Zhernakov, M.A.; Gilyazetdinov, E.M.; Bukharov, M.S.; Islamov, D.R.; Usachev, K.S.; Klimovitskii, A.E.; Serov, N.Y.; Burilov, V.A.; Shtyrlin, V.G. Complexes of NiII, CoII, ZnII, and CuII with Promising Anti-Tuberculosis Drug: Solid-State Structures and DFT Calculations. Inorganics 2023, 11, 167. [Google Scholar] [CrossRef]
- Kuranova, N.N.; Yarullin, D.N.; Zavalishin, M.N.; Gamov, G.A. Complexation of Gold(III) with Pyridoxal 5′-Phosphate-Derived Hydrazones in Aqueous Solution. Molecules 2022, 27, 7346. [Google Scholar] [CrossRef]
- Behera, P.K.; Maity, L.; Kisan, H.K.; Dutta, B.; Isab, A.A.; Chandra, S.K.; Dinda, J. Gold(I) and Gold(III) Complexes Supported by a Pyrazine / Pyrimidine Wingtip N-Heterocyclic Carbene: Synthesis, Structure and DFT Studies. J. Mol. Struct. 2021, 1223, 129253. [Google Scholar] [CrossRef]
- Ramadan, R.M.; Noureldeen, A.F.H.; Abo-Aly, M.M.; El-Medani, S.M. Spectroscopic, DFT Analysis, Antimicrobial and Cytotoxicity Studies of Three Gold(III) Complexes. Inorg. Nano-Metal Chem. 2021, 52, 213–225. [Google Scholar] [CrossRef]
- Li, L.; Bai, F.; Zhang, H.; Li, H. DFT/TDDFT Investigation on Bis-Cyclometalated Alkynylgold(III) Complex: Comparison of Absorption and Emission Properties. Sci. China Chem. 2013, 56, 641–647. [Google Scholar] [CrossRef]
- Gabbiani, C.; Casini, A.; Messori, L.; Guerri, A.; Cinellu, M.A.; Minghetti, G.; Corsini, M.; Rosani, C.; Zanello, P.; Arca, M. Structural Characterization, Solution Studies, and DFT Calculations on a Series of Binuclear Gold(III) Oxo Complexes: Relationships to Biological Properties. Inorg. Chem. 2008, 47, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Sankarganesh, M.; Raja, J.D.; Revathi, N.; Solomon, R.V.; Kumar, R.S. Gold(III) Complex from Pyrimidine and Morpholine Analogue Schiff Base Ligand: Synthesis, Characterization, DFT, TDDFT, Catalytic, Anticancer, Molecular Modeling with DNA and BSA and DNA Binding Studies. J. Mol. Liq. 2019, 294, 111655. [Google Scholar] [CrossRef]
- Ayoub, N.A.; Browne, A.R.; Anderson, B.L.; Gray, T.G. Cyclometalated Gold(III) Trioxadiborrin Complexes: Studies of the Bonding and Excited States. Dalton Trans. 2016, 45, 3820–3830. [Google Scholar] [CrossRef] [PubMed]
- Zavalishin, M.N.; Gamov, G.A.; Pimenov, O.A.; Pogonin, A.E.; Aleksandriiskii, V.V.; Usoltsev, S.D.; Marfin, Y.S. Pyridoxal 5′-Phosphate 2-Methyl-3-Furoylhydrazone as a Selective Sensor for Zn2+ Ions in Water and Drug Samples. J. Photochem. Photobiol. A Chem. 2022, 432, 114112. [Google Scholar] [CrossRef]
- González-Arellano, C.; Corma, A.; Iglesias, M.; Sánchez, F. Homogeneous and Heterogenized Au(III) Schiff Base-Complexes as Selective and General Catalysts for Self-Coupling of Aryl Boronic Acids. Chem. Commun. 2005, 15, 1990–1992. [Google Scholar] [CrossRef]
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef]
- Cirera, J.; Ruiz, E.; Alvarez, S. Shape and Spin State in Four-Coordinate Transition-Metal Complexes: The Case of the D6 Configuration. Chem. Eur. J. 2006, 12, 3162–3167. [Google Scholar] [CrossRef]
- Jouhannet, R.; Dagorne, S.; Blanc, A.; Frémont, P. Chiral Gold(III) Complexes: Synthesis, Structure, and Potential Applications. Chem. Eur. J. 2021, 27, 9218–9240. [Google Scholar] [CrossRef]
- Borissova, A.O.; Korlyukov, A.A.; Antipin, M.Y.; Lyssenko, K.A. Estimation of Dissociation Energy in Donor−Acceptor Complex AuCl·PPh3 via Topological Analysis of the Experimental Electron Density Distribution Function. J. Phys. Chem. A 2008, 112, 11519–11522. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02 2016. Available online: https://www.scienceopen.com/document?vid=6be7271f-f651-464b-aee6-ef20b0743b6b (accessed on 18 April 2023).
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J.; Mennucci, B.; Tomasi, J.; Cammi, R.; Barone, V. Geometries and Properties of Excited States in the Gas Phase and in Solution: Theory and Application of a Time-Dependent Density Functional Theory Polarizable Continuum Model. J. Chem. Phys. 2006, 124, 094107. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A New Hybrid Exchange–Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Figgen, D.; Rauhut, G.; Dolg, M.; Stoll, H. Energy-Consistent Pseudopotentials for Group 11 and 12 Atoms: Adjustment to Multi-Configuration Dirac–Hartree–Fock Data. Chem. Phys. 2005, 311, 227–244. [Google Scholar] [CrossRef]
- Peterson, K.A.; Puzzarini, C. Systematically Convergent Basis Sets for Transition Metals. II. Pseudopotential-Based Correlation Consistent Basis Sets for the Group 11 (Cu, Ag, Au) and 12 (Zn, Cd, Hg) Elements. Theor. Chem. Acc. 2005, 114, 283–296. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron Affinities of the First-row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Caricato, M. Absorption and Emission Spectra of Solvated Molecules with the EOM–CCSD–PCM Method. J. Chem. Theory Comput. 2012, 8, 4494–4502. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Program for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com/ (accessed on 18 April 2023).
- Bader, R.F.W. The Quantum Mechanical Basis of Conceptual Chemistry. Monatsh. Chem. 2005, 136, 819–854. [Google Scholar] [CrossRef]
- AIMAll. Available online: https://aim.tkgristmill.com/ (accessed on 18 April 2023).
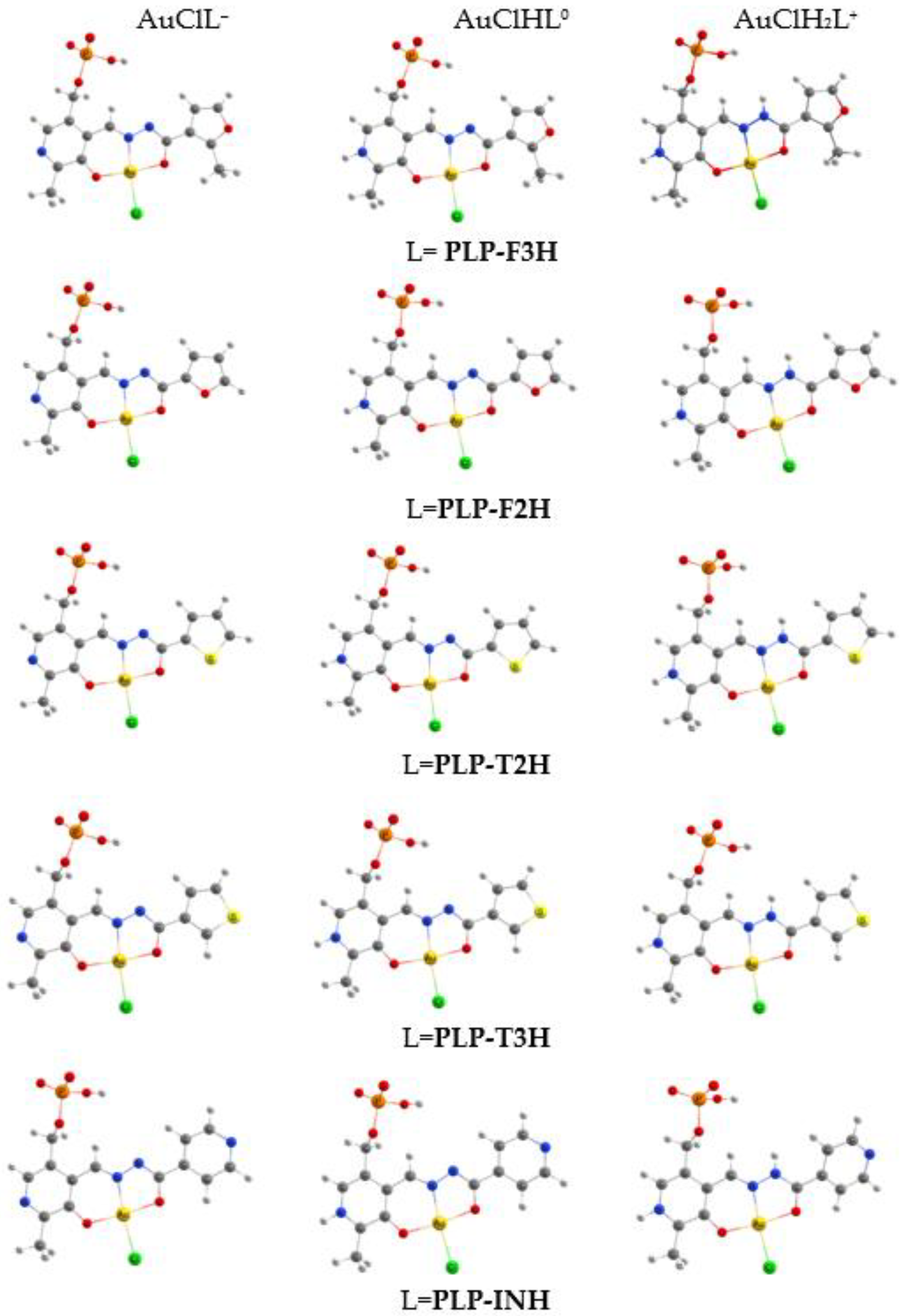
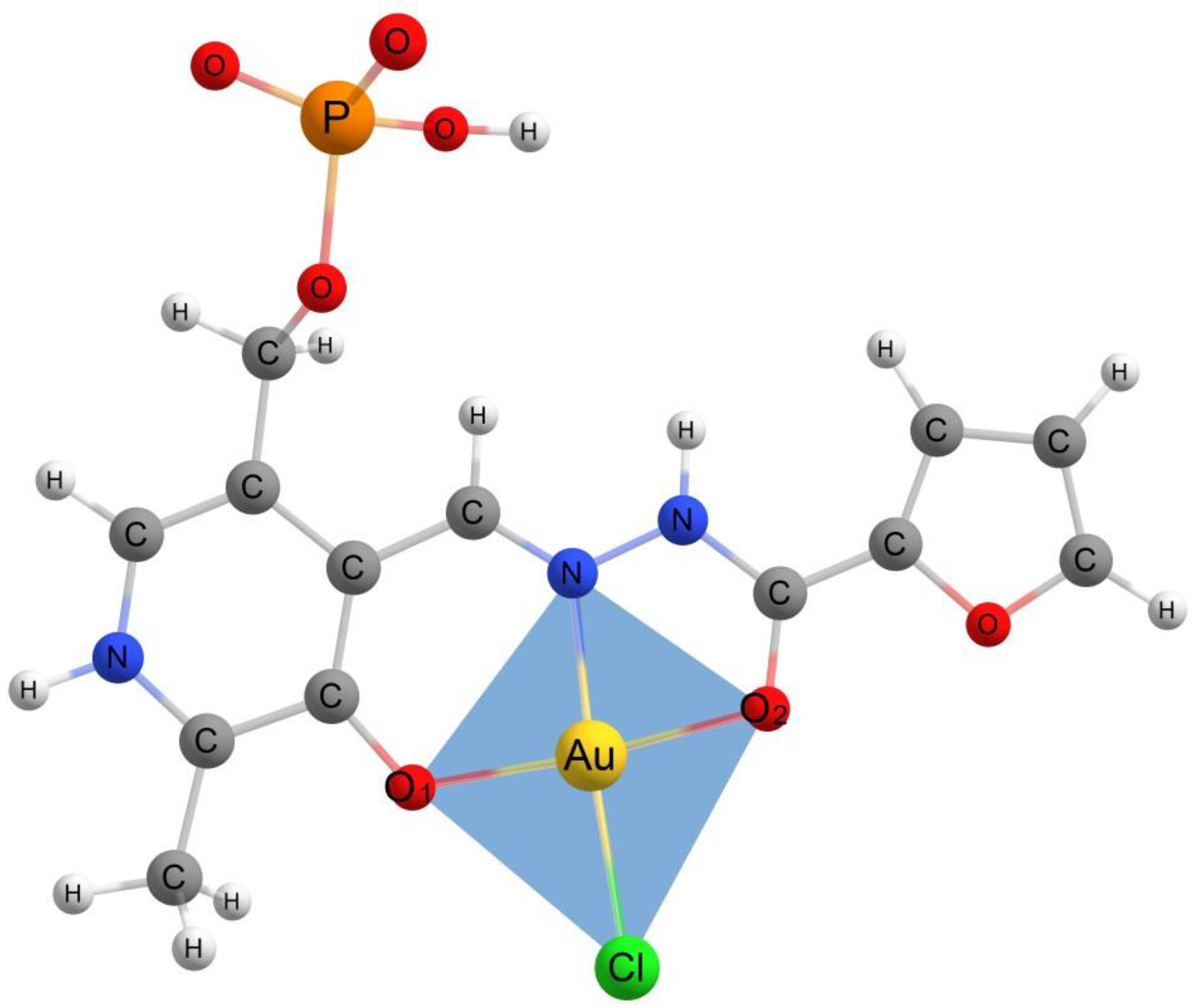
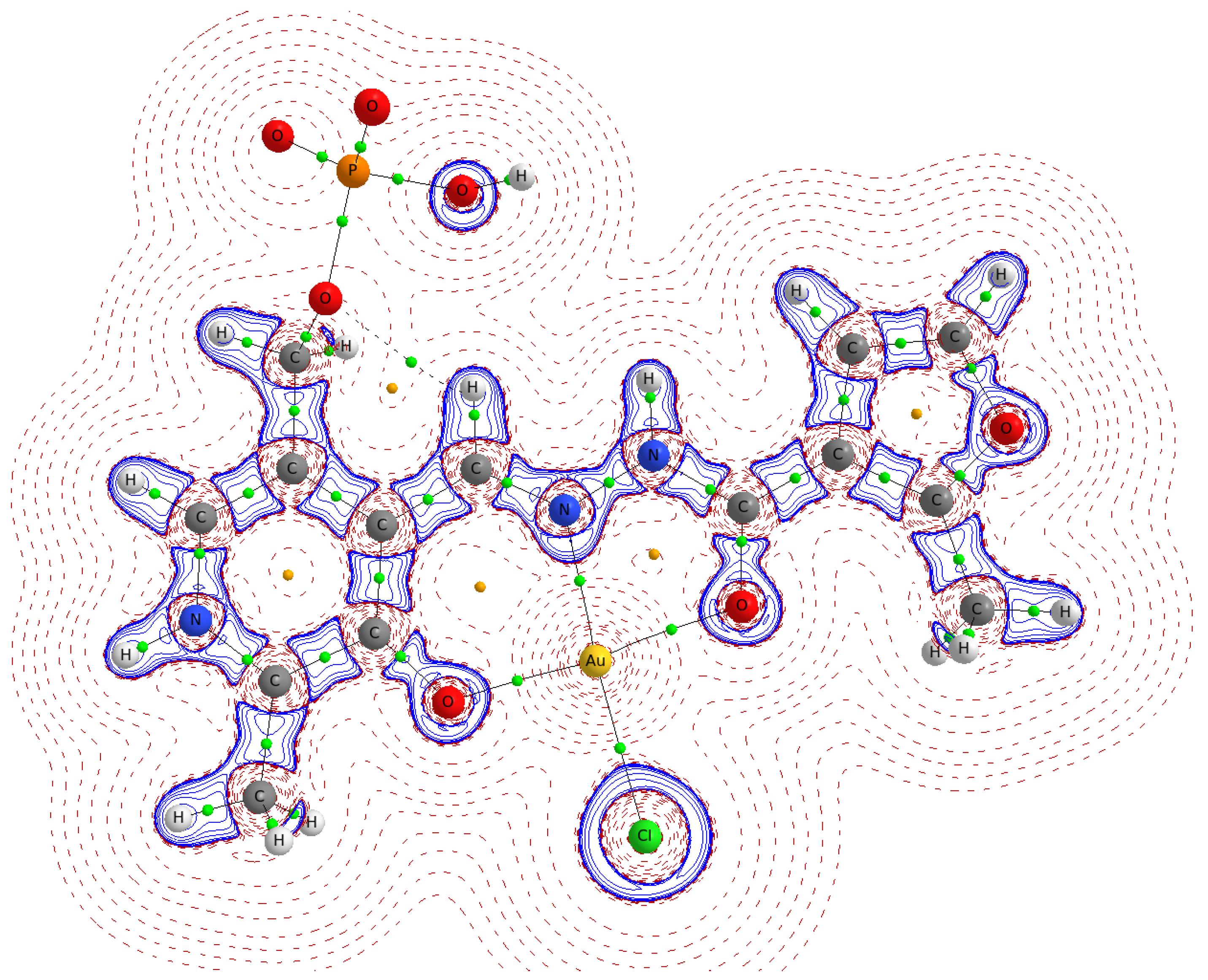
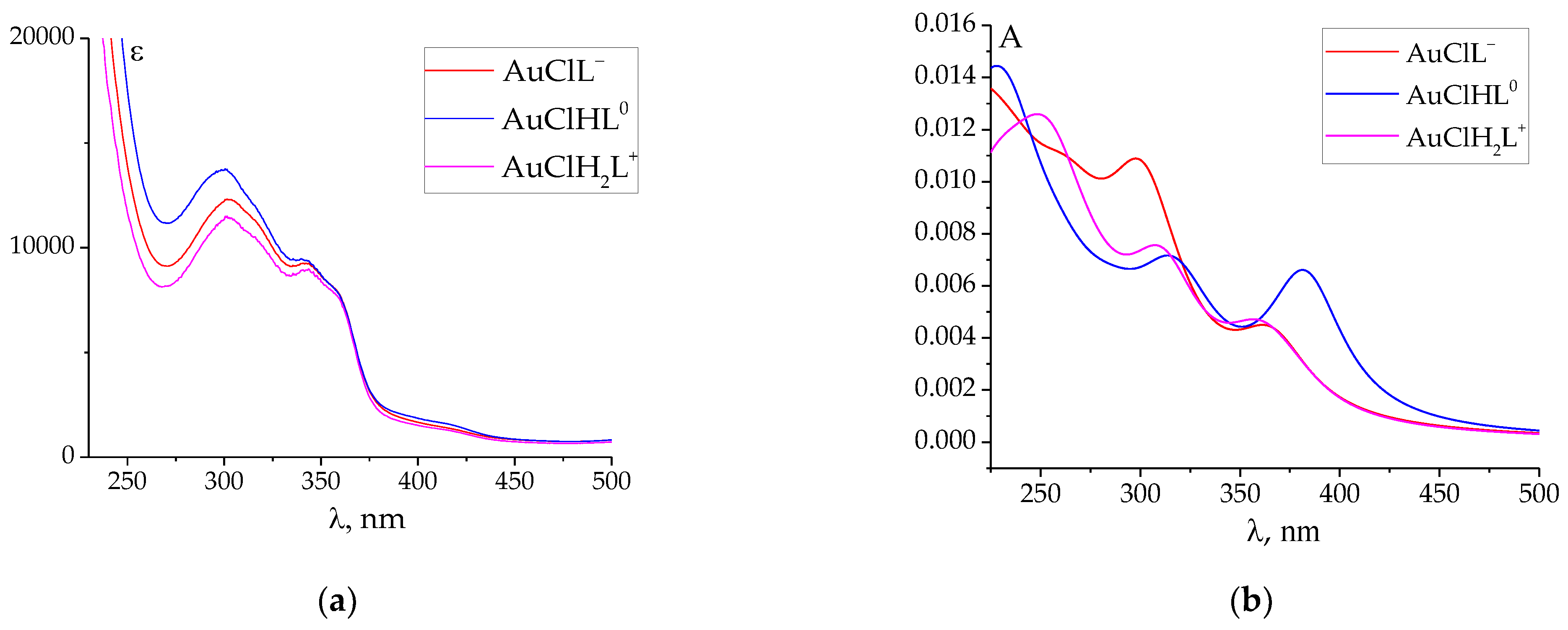
| Distance | AuCl(PLP-F3H) | AuCl(PLP-F2H) | AuCl(PLP-T3H) | AuCl(PLP-T2H) | AuCl(PLP-INH) |
|---|---|---|---|---|---|
| r(Au–Cl) | 2.334 * | 2.332 | 2.332 | 2.333 | 2.331 |
| 2.324 | 2.322 | 2.322 | 2.323 | 2.320 | |
| 2.294 | 2.293 | 2.292 | 2.294 | 2.292 | |
| r(Au–N) | 1.987 | 1.989 | 1.987 | 1.989 | 1.987 |
| 1.993 | 1.994 | 1.992 | 1.995 | 1.992 | |
| 2.000 | 2.000 | 1.999 | 2.000 | 2.001 | |
| r(Au–O1) | 1.981 | 1.978 | 1.981 | 1.979 | 1.979 |
| 1.983 | 1.981 | 1.984 | 1.981 | 1.982 | |
| 1.971 | 1.970 | 1.971 | 1.971 | 1.969 | |
| r(Au–O2) | 2.003 | 2.006 | 2.003 | 2.005 | 2.005 |
| 1.992 | 1.995 | 1.992 | 1.993 | 1.994 | |
| 2.015 | 2.017 | 2.014 | 2.016 | 2.023 | |
| r(O1…O2) | 3.980 | 3.981 | 3.980 | 3.980 | 3.981 |
| 3.971 | 3.972 | 3.972 | 3.971 | 3.972 | |
| 3.983 | 3.983 | 3.982 | 3.983 | 3.988 |
| Interaction | re | ρb | ∇2ρb | λ1 | λ2 | λ3 | ε | δ | Gb | Vb | Hb |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Au–Cl | 2.334 * | 0.662 | +4.123 | −0.097 | −0.094 | +0.363 | 0.032 | 0.885 | +0.0761 | −0.1095 | −0.0334 |
| 2.324 | 0.680 | +4.036 | −0.102 | −0.098 | +0.368 | 0.031 | 0.906 | +0.0770 | −0.1122 | −0.0352 | |
| 2.294 | 0.732 | +3.773 | −0.113 | −0.111 | +0.381 | 0.021 | 0.977 | +0.0796 | −0.1201 | −0.0405 | |
| Au–N | 1.987 | 1.007 | +7.919 | −0.199 | −0.190 | +0.717 | 0.047 | 0.828 | +0.1506 | −0.2195 | −0.0689 |
| 1.993 | 0.995 | +7.982 | −0.194 | −0.187 | +0.712 | 0.042 | 0.814 | +0.1499 | −0.2174 | −0.0675 | |
| 2.000 | 0.957 | +8.939 | −0.178 | −0.174 | +0.723 | 0.026 | 0.769 | +0.1554 | −0.2185 | −0.0631 | |
| Au–O1 | 1.981 | 0.917 | +10.112 | −0.179 | −0.178 | +0.777 | 0.003 | 0.819 | +0.1609 | −0.2174 | −0.0565 |
| 1.983 | 0.903 | +10.467 | −0.174 | −0.173 | +0.781 | 0.007 | 0.793 | +0.1626 | −0.2171 | −0.0545 | |
| 1.971 | 0.937 | +10.236 | −0.185 | −0.184 | +0.794 | 0.006 | 0.823 | +0.1644 | −0.2231 | −0.0587 | |
| Au–O2 | 2.003 | 0.879 | +9.761 | −0.172 | −0.168 | +0.745 | 0.027 | 0.767 | +0.1534 | −0.2060 | −0.0526 |
| 1.992 | 0.903 | +9.892 | −0.179 | −0.175 | +0.764 | 0.023 | 0.784 | +0.1577 | −0.2132 | −0.0555 | |
| 2.015 | 0.843 | +9.794 | −0.163 | −0.160 | +0.729 | 0.019 | 0.725 | +0.1496 | −0.1979 | −0.0483 |
| Protonated Species | q(Au) | q(Cl) | q(N) | q(O1) | q(O2) |
|---|---|---|---|---|---|
| AuClL− | +1.137 | −0.499 | −0.751 | −1.024 | −1.024 |
| AuClHL0 | +1.165 | −0.472 | −0.729 | −1.023 | −1.010 |
| AuClH2L+ | +1.219 | −0.396 | −0.725 | −0.991 | −1.012 |
| Complex | Protonated Form | Excited State | λcal (nm) | λexp max (nm) | Oscillator Strength (f) | Composition * | Character |
|---|---|---|---|---|---|---|---|
| AuCl(PLP-F3H) | AuL− | S3 | 365.64 | 340 | 0.2094 | 112 → 115 (48%), 98 → 114 (16%) | π→π * |
| S6 | 301.07 | 301 | 0.538 | 112 → 115 (59%), 111 → 115 (30%) | π→π * | ||
| AuHL0 | S2 | 382.48 | 341 | 0.4084 | 113 → 114 (61%), 112 → 114 (34%) | π→π * | |
| S6 | 315.34 | 301 | 0.2776 | 112 → 114 (36%), 109 → 114 (29%) | π→π * | ||
| AuH2L+ | S4 | 359.36 | 343 | 0.178 | 105 → 114(50%), 111 → 115 (26%) | dAu-Cl → dAu-p | |
| S6 | 310.78 | 302 | 0.3531 | 113 → 115 (45%), 111 → 115 (35%) | π→π * | ||
| Au(PLP-F2H) | AuL− | S3 | 371.03 | 345 | 0.3073 | 109 → 111 (87%) | π→π * |
| S6 | 308.57 | 320 | 0.5777 | 108 → 111 (82%) | π→π * | ||
| AuHL0 | S2 | 390.63 | 345 | 0.5444 | 109 → 110 (88%), 107 → 110 (12%) | π→π * | |
| S6 | 316.81 | 319 | 0.3263 | 107 → 110 (78%), 104 → 110 (11%) | π→π * | ||
| AuH2L+ | S4 | 362.11 | 347 | 0.2398 | 101 → 110(50%), 108 → 111 (40%) | dAu-Cl → dAu-p | |
| S6 | 305.77 | 321 | 0.7109 | 106 → 111 (64%), 108 → 112 (13%) | π→π * | ||
| Au(PLP-T3H) | AuL− | S3 | 366.18 | 344 | 0.2188 | 113 → 115 (94%) | π→π * |
| S6 | 300.15 | 305 | 0.5659 | 112 → 115 (84%) | π→π * | ||
| AuHL0 | S2 | 378.94 | 343 | 0.4116 | 113 → 114 (80%), 110 → 114 (10%) | π→π * | |
| S6 | 309.81 | 306 | 0.4295 | 110 → 114 (76%), 107 → 114 (13%) | π→π * | ||
| AuH2L+ | S4 | 359.5 | 344 | 0.1836 | 104 → 114 (49%), 112 → 115 (39%) | dAu-Cl → dAu-p | |
| S7 | 298.89 | 306 | 0.3441 | 109 → 115 (32%), 111 → 115 (20%) | π→π * | ||
| Au(PLP-T2H) | AuL− | S3 | 370.67 | 358 | 0.2977 | 113 → 115 (92%) | π→π * |
| S6 | 308.23 | 312 | 0.5955 | 112 → 115 (86%) | π→π * | ||
| AuHL0 | S2 | 388.13 | 346 | 0.5268 | 113 → 114 (89%) | π→π * | |
| S6 | 315.74 | 308 | 0.3514 | 110 → 114 (65%), 111 → 114 (14%) | π→π * | ||
| AuH2L+ | S4 | 361.33 | 349 | 0.2318 | 104 → 114 (53%), 112 → 115 (42%) | dAu-Cl → dAu-p | |
| S6 | 308.2 | 320 | 0.6769 | 110 → 115 (56%), 112 → 116 (18%) | π→π * | ||
| Au(PLP-INH) | AuL− | S3 | 367.67 | 358 | 0.1659 | 112 → 114 (88%) | π→π * |
| S6 | 298.85 | 305 | 0.4482 | 111 → 114 (90%), 103 → 113 (10%) | π→π * | ||
| AuHL0 | S2 | 371.42 | 347 | 0.2778 | 112 → 113 (93%) | π→π * | |
| S6 | 304.51 | 307 | 0.3935 | 108 → 113 (49%), 109 → 113 (18%) | π→π * | ||
| AuH2L+ | S4 | 361.58 | 346 | 0.1556 | 111 → 114 (56%), 103 → 113 (23%) | dAu-Cl → dAu-p | |
| S12 | 280.3 | 307 | 0.1657 | 110 → 114 (37%), 108 → 114 (18%) | π→π * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimenov, O.A.; Grazhdan, K.V.; Zavalishin, M.N.; Gamov, G.A. Geometry and UV-Vis Spectra of Au3+ Complexes with Hydrazones Derived from Pyridoxal 5′-Phosphate: A DFT Study. Int. J. Mol. Sci. 2023, 24, 8412. https://doi.org/10.3390/ijms24098412
Pimenov OA, Grazhdan KV, Zavalishin MN, Gamov GA. Geometry and UV-Vis Spectra of Au3+ Complexes with Hydrazones Derived from Pyridoxal 5′-Phosphate: A DFT Study. International Journal of Molecular Sciences. 2023; 24(9):8412. https://doi.org/10.3390/ijms24098412
Chicago/Turabian StylePimenov, Oleg A., Konstantin V. Grazhdan, Maksim N. Zavalishin, and George A. Gamov. 2023. "Geometry and UV-Vis Spectra of Au3+ Complexes with Hydrazones Derived from Pyridoxal 5′-Phosphate: A DFT Study" International Journal of Molecular Sciences 24, no. 9: 8412. https://doi.org/10.3390/ijms24098412
APA StylePimenov, O. A., Grazhdan, K. V., Zavalishin, M. N., & Gamov, G. A. (2023). Geometry and UV-Vis Spectra of Au3+ Complexes with Hydrazones Derived from Pyridoxal 5′-Phosphate: A DFT Study. International Journal of Molecular Sciences, 24(9), 8412. https://doi.org/10.3390/ijms24098412






