Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin
Abstract
1. Introduction
2. Results
2.1. Morphology, Size Distribution, and Encapsulation Efficiency of NPs
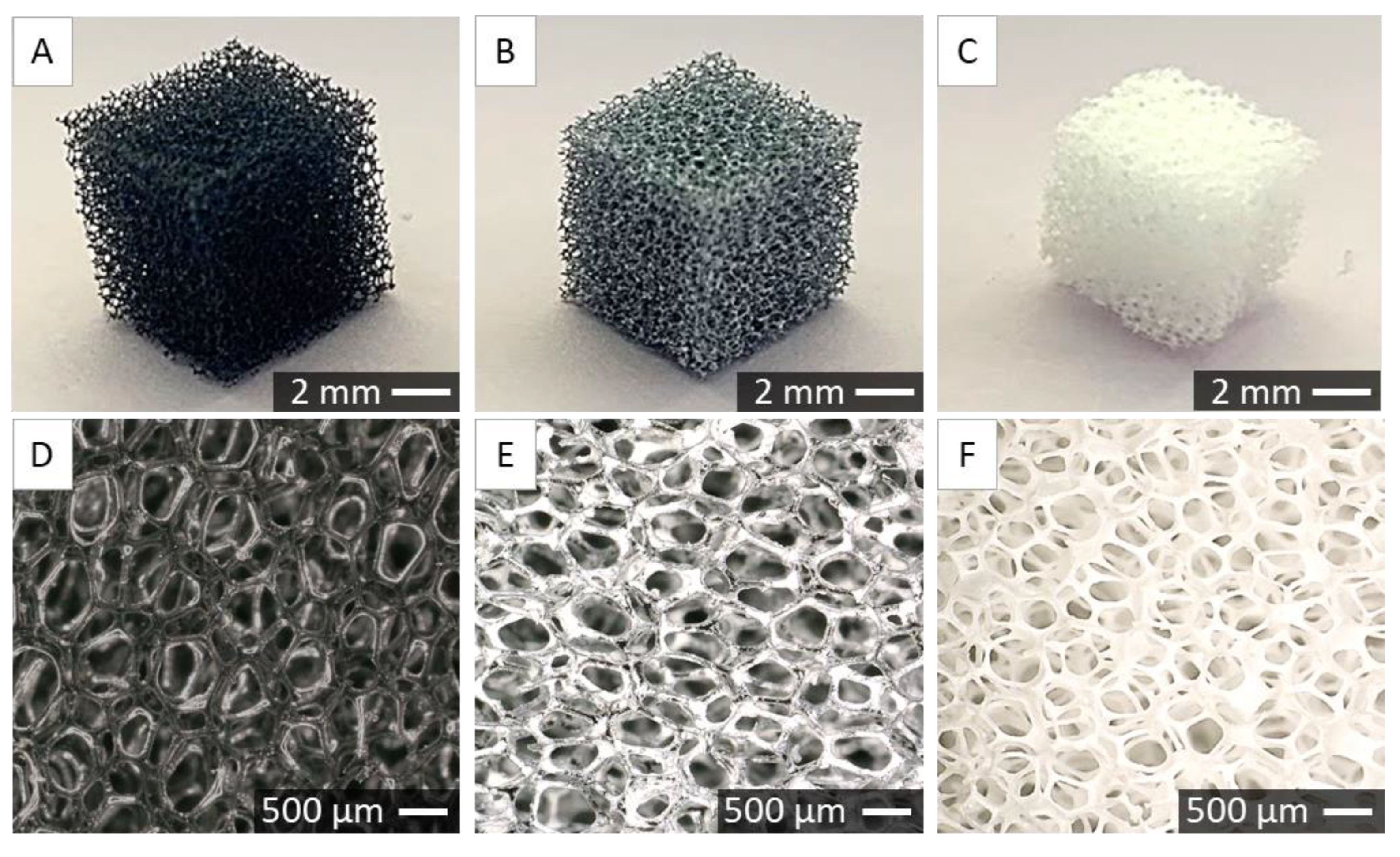
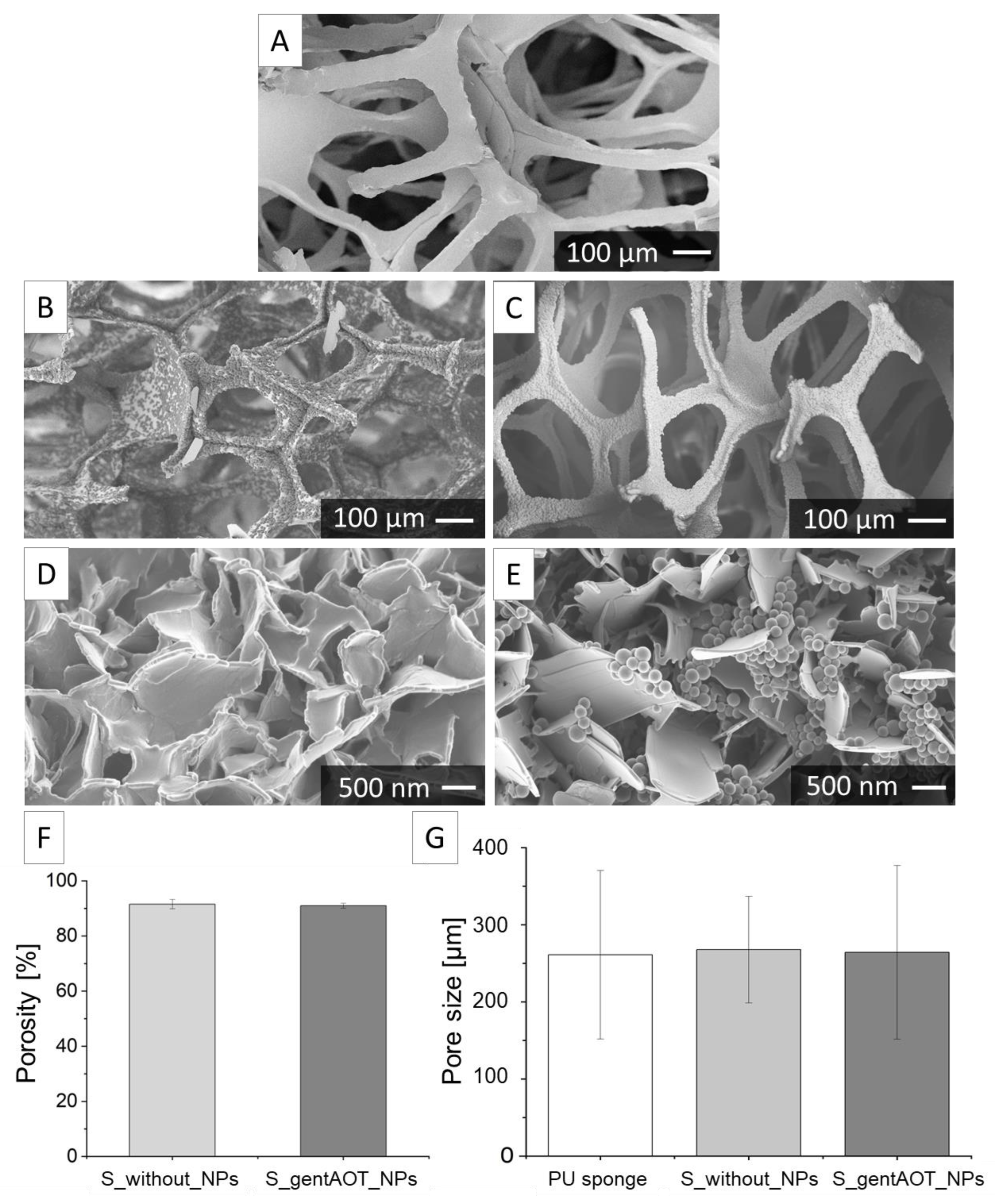
2.2. Characterisation of Porous Scaffolds
2.3. Drug Release
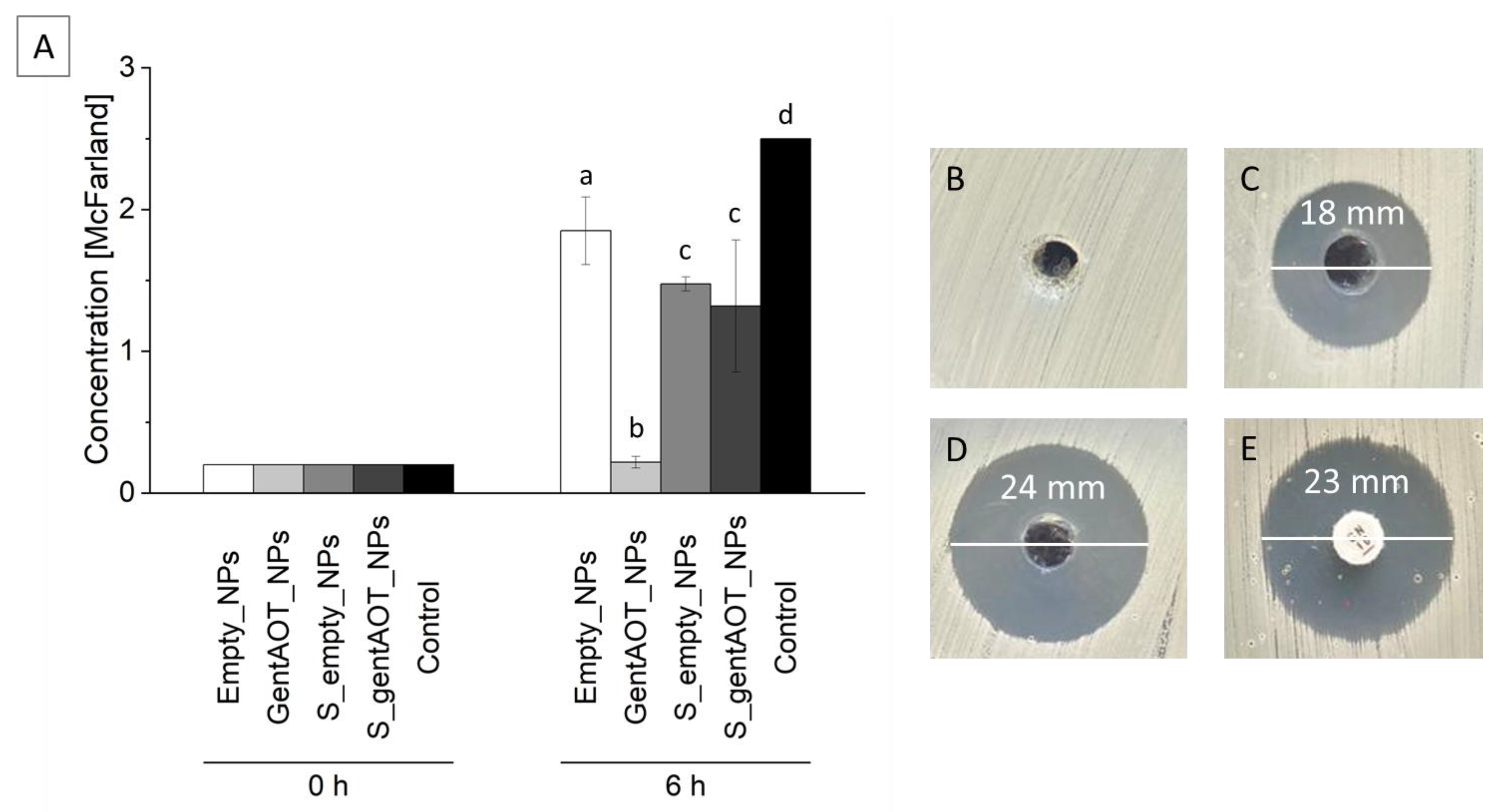
2.4. Antimicrobial Efficiency
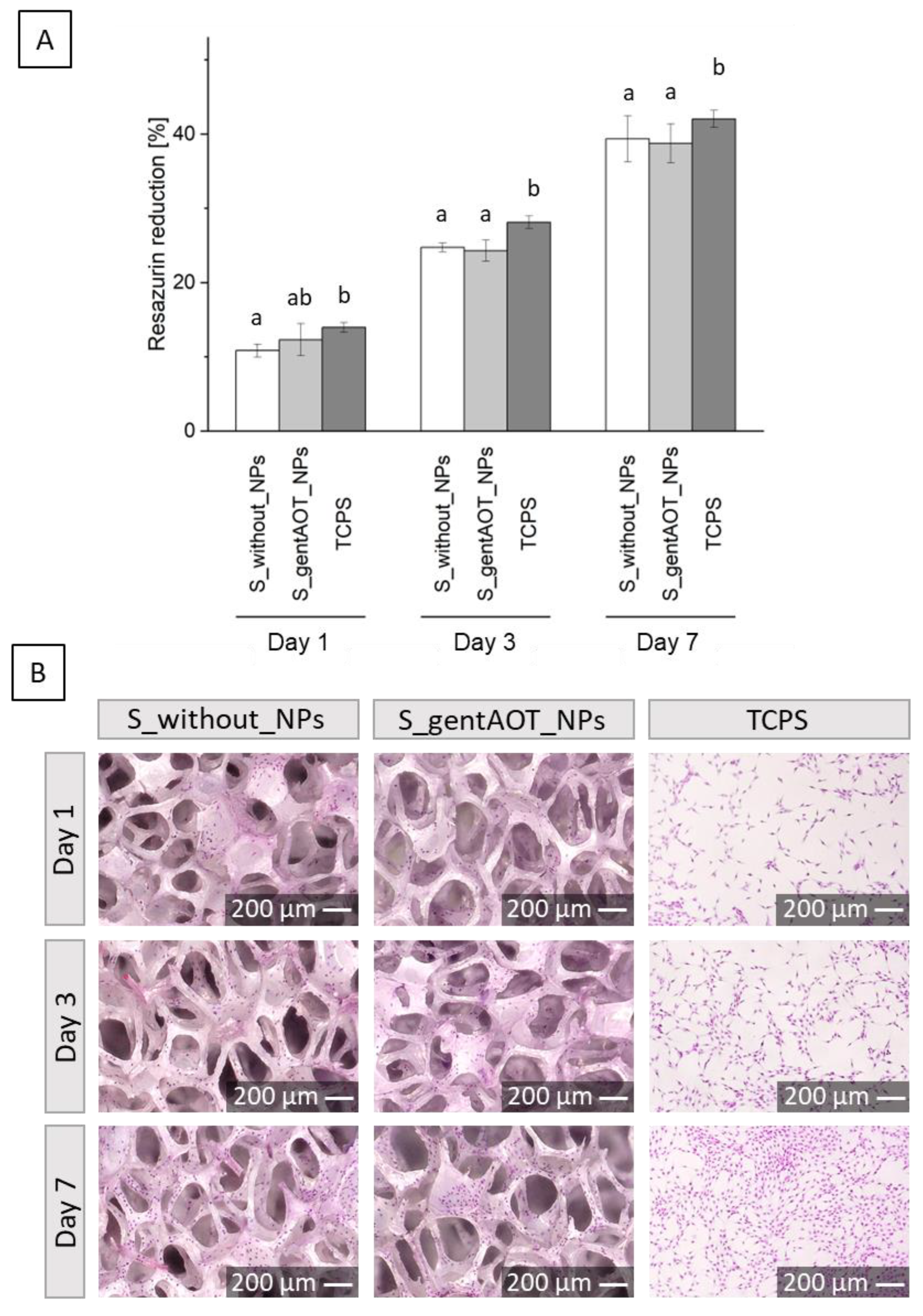
2.5. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Manufacturing of Nanoparticles Loaded with Hydrophobic Gentamicin
4.3. Nanoparticles Characterization
4.4. Zirconia Suspension Preparation
4.5. Porous Scaffold Fabrication
4.6. Precipitation of CaP
4.7. Scaffolds Characterization
4.8. Biological Evaluation
- Sx—fluorescence of the samples,
- Sblank—fluorescence of MEM with 10% AlamarBlue reagent, without cells (0% reduction of resazurin),
- Sreduced—fluorescence of MEM with 10% AlamarBlue reagent autoclaved at 121 °C for 15 min (100% reduction of resazurin).
4.9. Drug Release Profiles
4.10. Antimicrobial Effect
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumato, T.J.; An, S.H.; Ishimoto, T.; Nakano, T.; Matsumoto, T.; Imazato, S. Zirconia-hydroxyapatite composite material with micro porous structure. Dent. Mater 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Kumar, P.; Dehiya, B.S.; Sindhu, A.; Kumar, R.; Pruncu, C.I.; Yadav, A. Fabrication and characterization of silver nanorods incorporated calcium silicate scaffold using polymeric sponge replica technique. Mater. Des. 2020, 195, 109026. [Google Scholar] [CrossRef]
- Kwiecień, K.; Pudełko, I.; Knap, K.; Reczyńska-Kolman, K.; Krok-Borkowicz, M.; Ochońska, D.; Brzychczy-Włoch, M.; Pamuła, E. Insight in Superiority of the Hydrophobized Gentamycin in Terms of Antibiotics Delivery to Bone Tissue. Int. J. Mol. Sci. 2022, 23, 12077. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhi, P.; Liu, L.; Liu, C.; Fang, A. 3D printing method for bone tissue engineering scaffold. Med. Nov. Technol. Devices 2023, 17, 100205. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, D.; Lin, L.; Li, S.; Hou, W.; He, Y.; Sheng, L.; Zhang, X.; Li, H.; Zhao, W.; et al. Large-pore-size Ti6Al4V scaffolds with different pore structures for vascularized bone regeneration. Mater. Sci. Eng. C 2021, 131, 112499. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Xie, D.; He, Z.; Liang, H.; Shen, L.; Yang, Y.; Tian, Z.; Wu, G.; Wang, C. Additive manufacturing of Bio-inspired ceramic bone Scaffolds: Structural Design, mechanical properties and biocompatibility. Mater. Des. 2022, 217, 110610. [Google Scholar] [CrossRef]
- Winnett, J.; Jumbu, N.; Cox, S.; Gibbons, G.; Grover, L.M.; Warnett, J.; Willaims, M.; Dancer, C.; Mallick, K. In-Vitro viability of bone scaffolds fabricated using the adaptive foam reticulation technique. Biomater. Adv. 2022, 136, 212766. [Google Scholar] [CrossRef]
- Imani, S.M.; Rabiee, S.M.; Goudarzi, A.M.; Dardel, M.; Tayebi, L. Optimization of composite bone scaffolds prepared by a new modified foam replica technique. Mater. Today Commun. 2022, 31, 103293. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, D.; Li, M.; Zhou, J.; Yang, R.; Zhang, J.; Chai, W.; Wang, Y. Modification of hydroxyapatite (HA) powder by carboxymethyl chitosan (CMCS) for 3D printing bioceramic bone scaffolds. Ceram. Int. 2023, 49, 538–547. [Google Scholar] [CrossRef]
- Imani, S.M.; Rabiee, S.M.; Goudarzi, A.M.; Dardel, M. A novel modification for polymer sponge method to fabricate the highly porous composite bone scaffolds with large aspect ratio suitable for repairing critical-sized bone defects. Vacuum 2020, 176, 109316. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials approaches to treating implant-associated osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nie, B.; Huo, S.; Qu, X.; Guo, J.; Liu, X.; Hong, Q.; Wang, Y.; Yang, J.; Yue, B. Bone infection site targeting nanoparticle-antibiotics delivery vehicle to enhance treatment efficacy of orthopedic implant related infection. Bioact. Mater. 2022, 16, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.H.; Yang, J.; Fei, J. New perspectives on traumatic bone infections. Chin. J. Traumatol. 2020, 23, 314–318. [Google Scholar] [CrossRef]
- Alenezi, A.; Chrcanovic, B. Effects of the local administration of antibiotics on bone formation on implant surface in animal models: A systematic review and meta-analysis. Jpn. Dent. Sci. Rev. 2020, 56, 177–183. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Ariyachaokun, K.; Pansri, S. Enhanced bioactivity and antibacterial properties of anodized ZrO2 implant coatings via optimized nanoscale morphology and timed antibiotic release through PLGA overcoat. Ceram. Int. 2021, 47, 33775–33787. [Google Scholar] [CrossRef]
- Froiio, F.; Lammari, N.; Tarhini, M.; Alomari, M.; Louaer, W.; Meniai, A.H.; Paolino, D.; Fessi, H.; Elaissari, A. Polymer-based nanocontainers for drug delivery. In Smart Nanocontainers; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 271–285. [Google Scholar]
- Jarai, B.M.; Kolewe, E.L.; Stillman, Z.S.; Raman, N.; Fromen, C.A. Polymeric nanoparticles. In Nanoparticles for Biomedical Applications: Fundamental Concepts, Biological Interactions and Clinical Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 303–324. [Google Scholar]
- Akhtar, B.; Muhammad, F.; Aslam, B.; Saleemi, M.K.; Sharif, A. Biodegradable nanoparticle based transdermal patches for gentamicin delivery: Formulation, characterization and pharmacokinetics in rabbits. J. Drug Deliv. Sci. Technol. 2020, 57, 101680. [Google Scholar] [CrossRef]
- Akhtar, B.; Muhammad, F.; Aslam, B.; Saleemi, M.K.; Sharif, A. Pharmacokinetic profile of chitosan modified poly lactic co-glycolic acid biodegradable nanoparticles following oral delivery of gentamicin in rabbits. Int. J. Biol. Macromol. 2020, 164, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Greene, M.K.; Insua, J.L.; Pessoa, J.S.; Small, D.M.; Smyth, P.; McCann, A.; Cogo, F.; Bengoechea, J.; Taggart, C.; et al. Clearance of intracellular Klebsiella pneumoniae infection using gentamicin-loaded nanoparticles. J. Control. Release 2018, 279, 316–325. [Google Scholar] [CrossRef][Green Version]
- Kavruk, M.; Celikbicak, O.; Ozalp, V.C.; Borsa, B.A.; Hernandez, F.J.; Bayramoglu, G.; Salih, B.; Arica, M. Antibiotic loaded nanocapsules functionalized with aptamer gates for targeted destruction of pathogens. Chem. Comm. 2015, 51, 8492–8495. [Google Scholar] [CrossRef]
- Nader, D.; Yousef, F.; Kavanagh, N.; Ryan, B.K.; Kerrigan, S.W. Targeting internalized Staphylococcus aureus using vancomycin-loaded nanoparticles to treat recurrent bloodstream infections. Antibiotics 2021, 10, 8492–8495. [Google Scholar] [CrossRef]
- Chen, Y.H.; Li, T.J.; Tsai, B.Y.; Chen, L.K.; Lai, Y.H.; Li, M.J.; Tsai, C.; Shieh, D. Vancomycin-loaded nanoparticles enhance sporicidal and antibacterial efficacy for clostridium difficile infection. Front. Microbiol. 2019, 10, 1141. [Google Scholar] [CrossRef][Green Version]
- Takeuchi, I.; Kobayashi, S.; Hida, Y.; Makino, K. Estradiol-loaded PLGA nanoparticles for improving low bone mineral density of cancellous bone caused by osteoporosis: Application of enhanced charged nanoparticles with iontophoresis. Colloids Surf. B Biointerfaces 2017, 155, 35–40. [Google Scholar] [CrossRef]
- Lacoma, A.; Usón, L.; Mendoza, G.; Sebastián, V.; Garcia-Garcia, E.; Muriel-Moreno, B.; Domínguez, J.; Arruebo, M.; Prat, C. Novel intracellular antibiotic delivery system against Staphylococcus aureus: Cloxacillin-loaded poly(d,l-lactide-co-glycolide) acid nanoparticles. Nanomedicine 2020, 15, 1189–1203. [Google Scholar] [CrossRef]
- Imbuluzqueta, E.; Elizondo, E.; Gamazo, C.; Moreno-Calvo, E.; Veciana, J.; Ventosa, N.; Blanco-Prieto, M. Novel bioactive hydrophobic gentamicin carriers for the treatment of intracellular bacterial infections. Acta Biomater. 2011, 7, 1599–1608. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Zhang, B.; Yang, H.; Mushtaq, R.T.; Liu, M.; Bao, C.; Shi, Y.; Luo, Z.; Zhang, W. The design and evaluation of bionic porous bone scaffolds in fluid flow characteristics and mechanical properties. Comput. Methods Programs Biomed. 2022, 225, 107059. [Google Scholar] [CrossRef] [PubMed]
- Mantila Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. A 2010, 92, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.T. Porous scaffolds for bone regeneration. J. Sci.: Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Muhamad Nor, M.A.A.; Hong, L.C.; Arifin Ahmad, Z.; Akil, H. Preparation and characterization of ceramic foam produced via polymeric foam replication method. J. Mater. Process. Technol. 2008, 207, 235–239. [Google Scholar] [CrossRef]
- An, S.; Matsumoto, T.; Miyajina, H.; Kim, K.H.; Imazato, S. Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 2012, 28, 1221–1231. [Google Scholar] [CrossRef]
- Tapia-Lopez, L.; Esparza-Ponce, H.; Luna-Velasco, A.; Garcia-Casillas, P.; Castro-Carmona, H.; Castro, J. Bioactivation of zirconia surface with laminin protein coating via plasma etching and chemical modification. Surf. Coat. Technol. 2020, 402, 126307. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Pooshang Bagheri, K.; Shokrgozar, M.A. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater. Chem. Phys. 2021, 263, 124432. [Google Scholar] [CrossRef]
- Imbuluzqueta, E.; Gamazo, C.; Lana, H.; Campanero, M.Á.; Salas, D.; Gil, A.G.; Elizondo, E.; Ventosa, N.; Veciana, J.; Blanco-Prieto, M. Hydrophobic gentamicin-loaded nanoparticles are effective against Brucella melitensis infection in mice. Antimicrob. Agents Chemother. 2013, 57, 3326–3333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ter Boo, G.; Grijpma, D.; Richards, R.; Moriarty, T.; Eglin, D. Preparation of gentamicin dioctyl sulfosuccinate loaded poly(trimethylene carbonate) matrices intended for the treatment of orthopaedic infections. Clin. Hemorheol. Microcirc. 2015, 60, 89–98. [Google Scholar] [CrossRef]
- Varghese, N.M.; Venkatachalam, S. Potential nanocarriers for the delivery of drugs to the brain. Nanoengineered Biomaterials for Advanced Drug Delivery. In Nanoengineered Biomaterials for Advanced Drug Delivery; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; pp. 449–472. [Google Scholar]
- Irmak, G.; Öztürk, M.G.; Gümüşderelioğlu, M. Salinomycin encapsulated PLGA nanoparticles eliminate osteosarcoma cells via inducing/inhibiting multiple signaling pathways: Comparison with free salinomycin. J. Drug Deliv. Sci. Technol. 2020, 58, 101834. [Google Scholar] [CrossRef]
- Rotman, S.; Grijpma, D.; Richards, R.; Moriarty, T.; Eglin, D.; Guillaume, O. Drug delivery systems functionalized with bone mineral seeking agents for bone targeted therapeutics. J. Control. Release 2018, 269, 88–99. [Google Scholar] [CrossRef]
- Jiang, T.; Yu, X.; Carbone, E.; Nelson, C.; Kan, H.; Lo, K. Poly aspartic acid peptide-linked PLGA based nanoscale particles: Potential for bone-targeting drug delivery applications. Int. J. Pharm. 2014, 475, 547–557. [Google Scholar] [CrossRef]
- Posadowska, U.; Brzychczy-Włoch, M.; Pamuła, E. Gentamicin loaded PLGA nanoparticles as local drug delivery system for the osteomyelitis treatment. Acta Bioeng. Biomech. 2015, 17, 41–47. [Google Scholar] [PubMed]
- Abdelghany, S.; Quinn, D.; Ingram, R.; Gilmore, B.; Donnelly, R.; Taggart, C.; Scott, C. Gentamicin-loaded nanoparticles show improved antimicrobial effects towards Pseudomonas aeruginosa infection. Int. J. Nanomed. 2012, 7, 4053–4063. [Google Scholar]
- Tas, A.; Bhaduri, S. Rapid coating of Ti6Al4V at room temperature with a calcium phosphate solution similar to 10× simulated body fluid. J. Mater. Res. 2004, 19, 2742–2749. [Google Scholar] [CrossRef]
- Costa, D.; Allo, B.; Klassen, R.; Hutter, J.; Dixon, S.; Rizkalla, A. Control of surface topography in biomimetic calcium phosphate coatings. Langmuir 2012, 28, 3871–3880. [Google Scholar] [CrossRef]
- Desante, G.; Pudełko, I.; Krok-Borkowicz, M.; Pamuła, E.; Jacobs, P.; Kazek-Kęsik, A.; Nießen, J.; Rainer, T.; Gonzalez-Julian, J.; Schickle, K. Surface Multifunctionalization of Inert Ceramic Implants by Calcium Phosphate Biomimetic Coating Doped with Nanoparticles Encapsulating Antibiotics. ACS Appl. Mater. Interfaces 2023, 15, 21699–21718. [Google Scholar] [CrossRef] [PubMed]
- Soh, E.; Kolos, E.; Ruys, A. Foamed high porosity alumina for use as a bone tissue scaffold. Ceram. Int. 2015, 41, 1031–1047. [Google Scholar] [CrossRef]
- Kovacik, A.; Tvrda, E.; Fulopova, D.; Cupka, P.; Kovacikova, E.; Zbynovska, K.; Massanyi, P. In Vitro Assessment of Gentamicin Cytotoxicity on the Selected Mammalian Cell Line (Vero cells). Adv. Life Sci. 2017, 1, 111–116. [Google Scholar] [CrossRef][Green Version]
- Dobrzynski, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of biodegradable copolymers with the use of low toxic zirconium compounds. 1. Copolymerization of glycolide with L-lactide initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Wojak-Ćwik, I.; Rumian, Ł.; Krok-Borkowicz, M.; Hess, R.; Bernhardt, R.; Dobrzyński, P.; Möller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; et al. Synergistic effect of bimodal pore distribution and artificial extracellular matrices in polymeric scaffolds on osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2019, 97, 12–22. [Google Scholar] [CrossRef] [PubMed]


| Type of NPs | Size ± SD [nm] | Zeta Potential ± SD [mV] | Encapsulation Efficiency ± SD [%] | Drug Loading ± SD [%] |
|---|---|---|---|---|
| Empty_NPs | 214.6 ± 14.0 | −9.5 ± 1.0 | - | - |
| GentAOT_NPs | 236.7 ± 33.6 | −3.7 ± 0.2 | 99.9 ± 0.1 | 9.1 ± 0.1 |
| Ingredients | Mass [g] | Mass Percentage [%] |
|---|---|---|
| Basic suspension | 180.00 | 86.26 |
| Distilled water | 19.09 | 9.15 |
| CE 64 | 0.10 | 0.05 |
| Glycerine | 6.69 | 3.21 |
| Ethanol | 2.78 | 1.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudełko, I.; Moskwik, A.; Kwiecień, K.; Kriegseis, S.; Krok-Borkowicz, M.; Schickle, K.; Ochońska, D.; Dobrzyński, P.; Brzychczy-Włoch, M.; Gonzalez-Julian, J.; et al. Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin. Int. J. Mol. Sci. 2023, 24, 8400. https://doi.org/10.3390/ijms24098400
Pudełko I, Moskwik A, Kwiecień K, Kriegseis S, Krok-Borkowicz M, Schickle K, Ochońska D, Dobrzyński P, Brzychczy-Włoch M, Gonzalez-Julian J, et al. Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin. International Journal of Molecular Sciences. 2023; 24(9):8400. https://doi.org/10.3390/ijms24098400
Chicago/Turabian StylePudełko, Iwona, Anna Moskwik, Konrad Kwiecień, Sven Kriegseis, Małgorzata Krok-Borkowicz, Karolina Schickle, Dorota Ochońska, Piotr Dobrzyński, Monika Brzychczy-Włoch, Jesus Gonzalez-Julian, and et al. 2023. "Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin" International Journal of Molecular Sciences 24, no. 9: 8400. https://doi.org/10.3390/ijms24098400
APA StylePudełko, I., Moskwik, A., Kwiecień, K., Kriegseis, S., Krok-Borkowicz, M., Schickle, K., Ochońska, D., Dobrzyński, P., Brzychczy-Włoch, M., Gonzalez-Julian, J., & Pamuła, E. (2023). Porous Zirconia Scaffolds Functionalized with Calcium Phosphate Layers and PLGA Nanoparticles Loaded with Hydrophobic Gentamicin. International Journal of Molecular Sciences, 24(9), 8400. https://doi.org/10.3390/ijms24098400









