Mechanism Exploration of Amyloid-β-42 Disaggregation by Single-Chain Variable Fragments of Alzheimer’s Disease Therapeutic Antibodies
Abstract
1. Introduction
2. Results
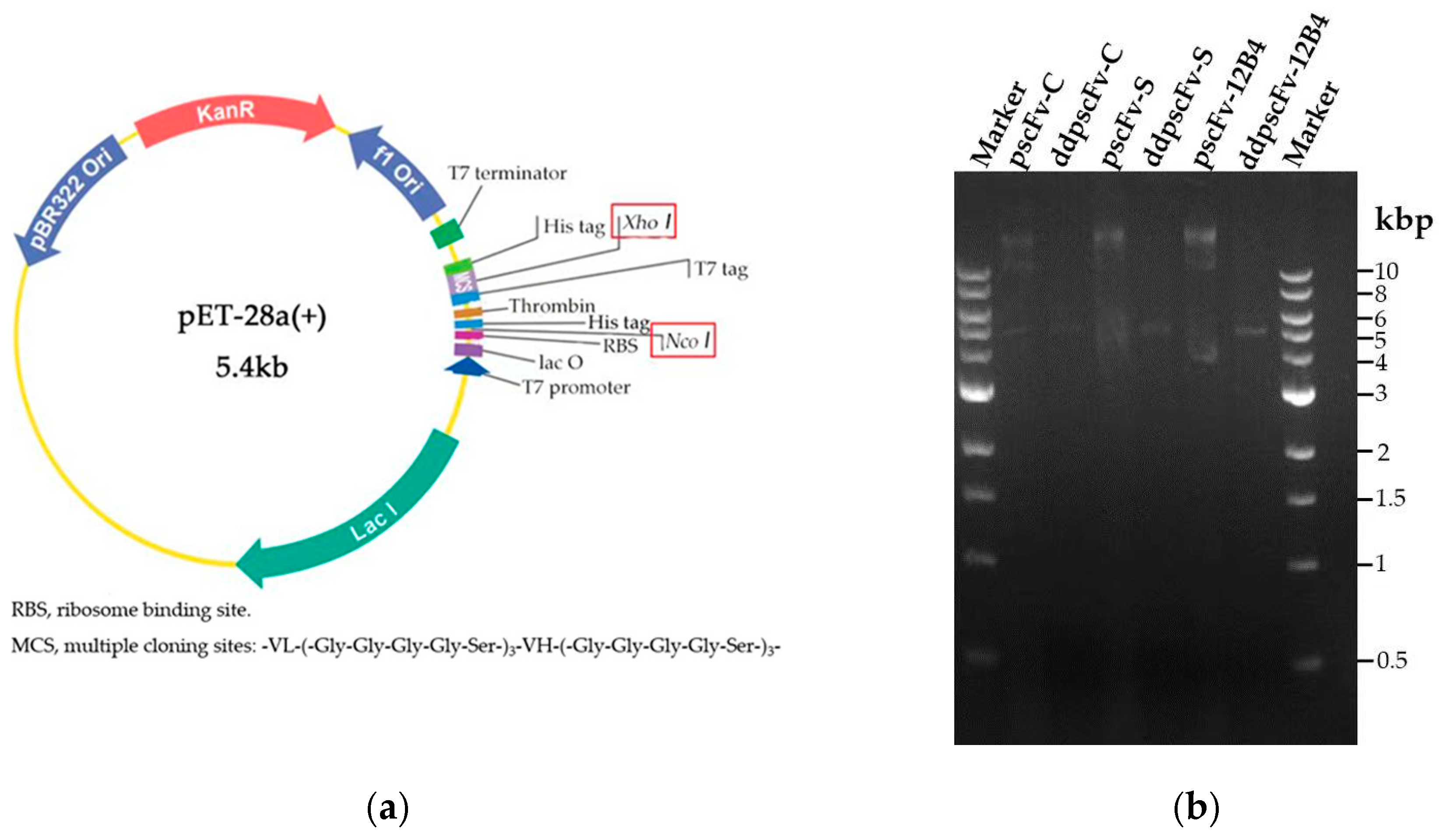
2.1. Construction and Identification of pscFvs
2.2. Expression and Purification of scFvs
2.3. scFv-C Could Give a Strong Binding Ability with Aβ42
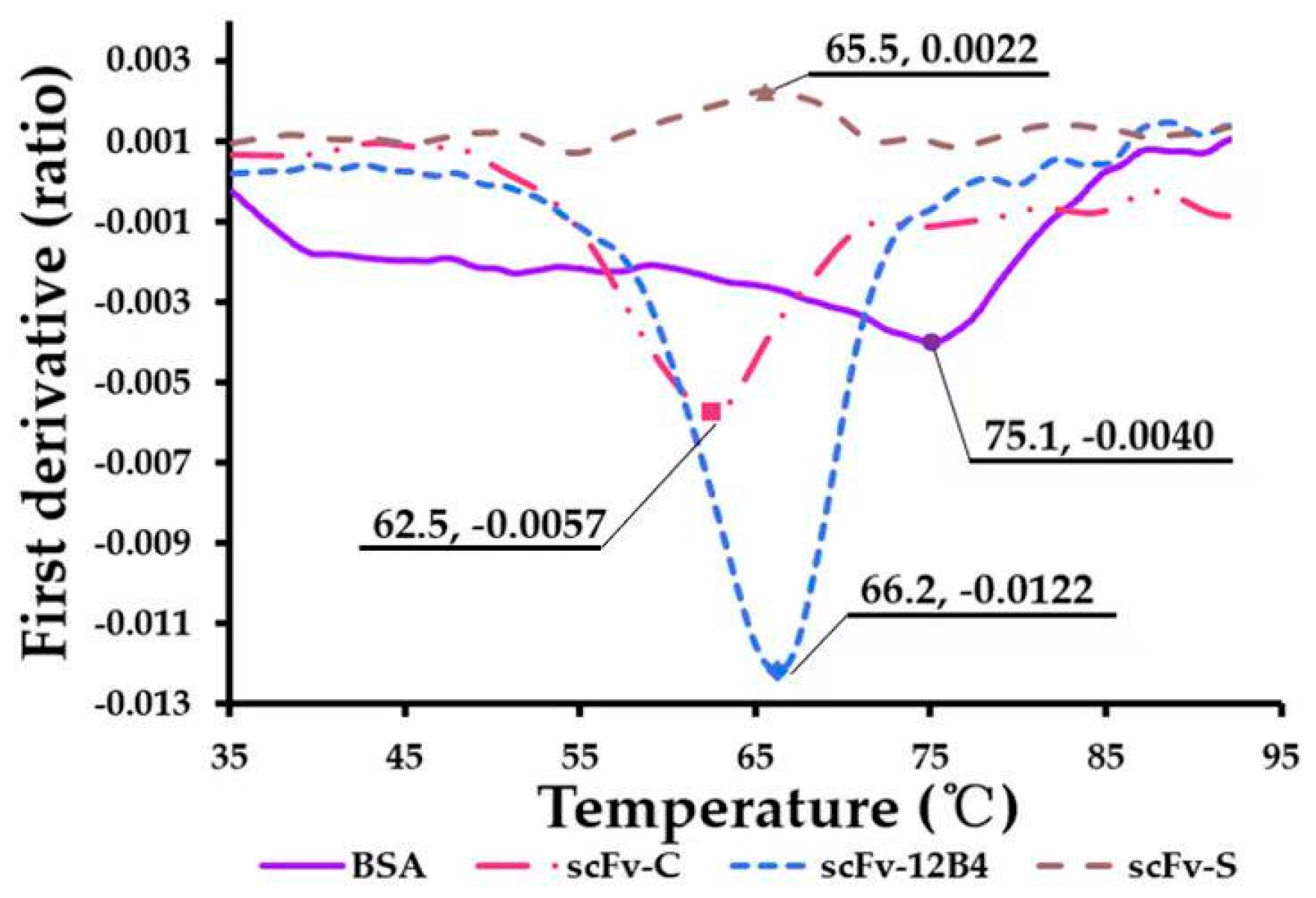
2.4. scFv Conformation Has Good Thermal Stability
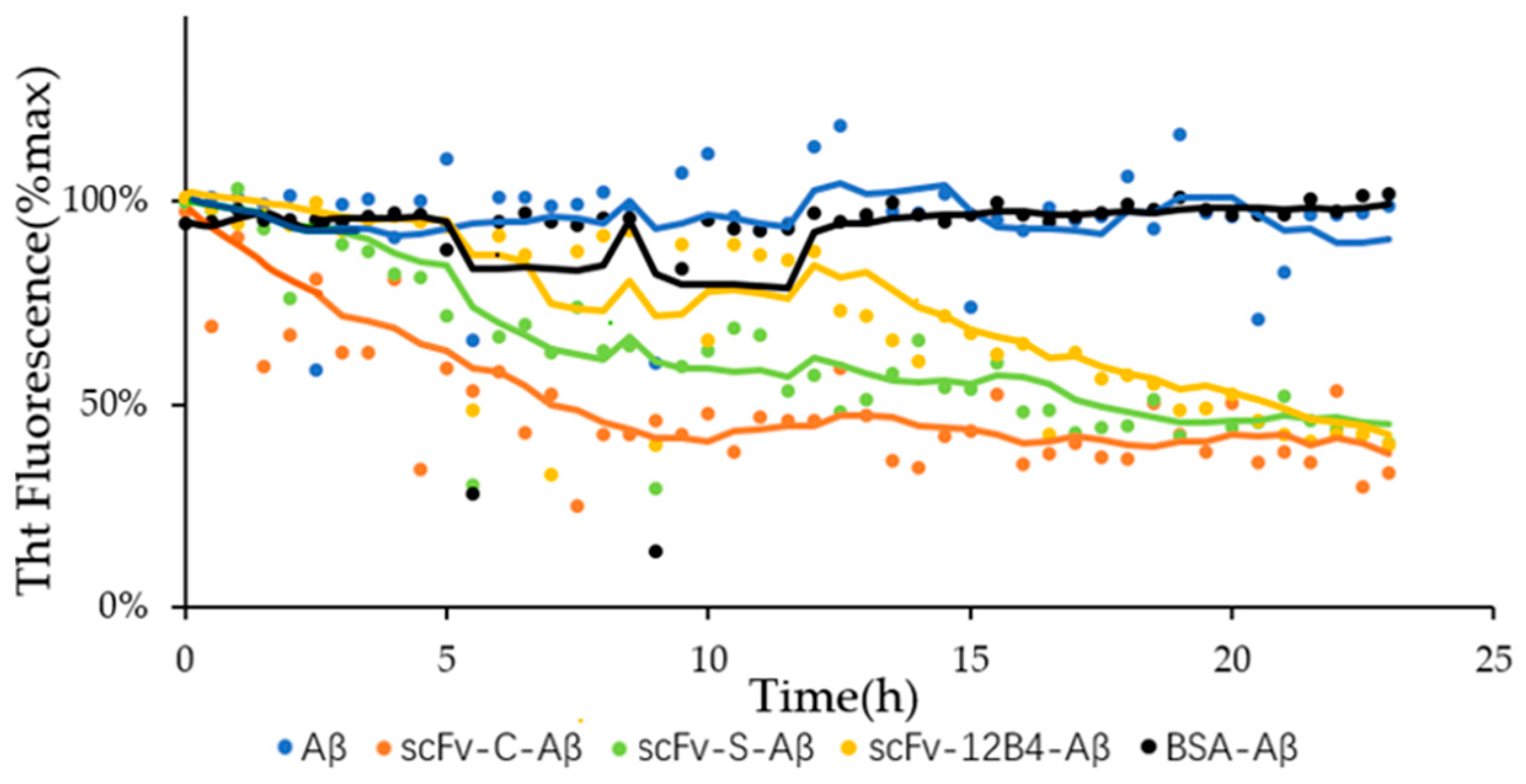
2.5. scFvs Can Promote Depolymerization of Aggregated Aβ42
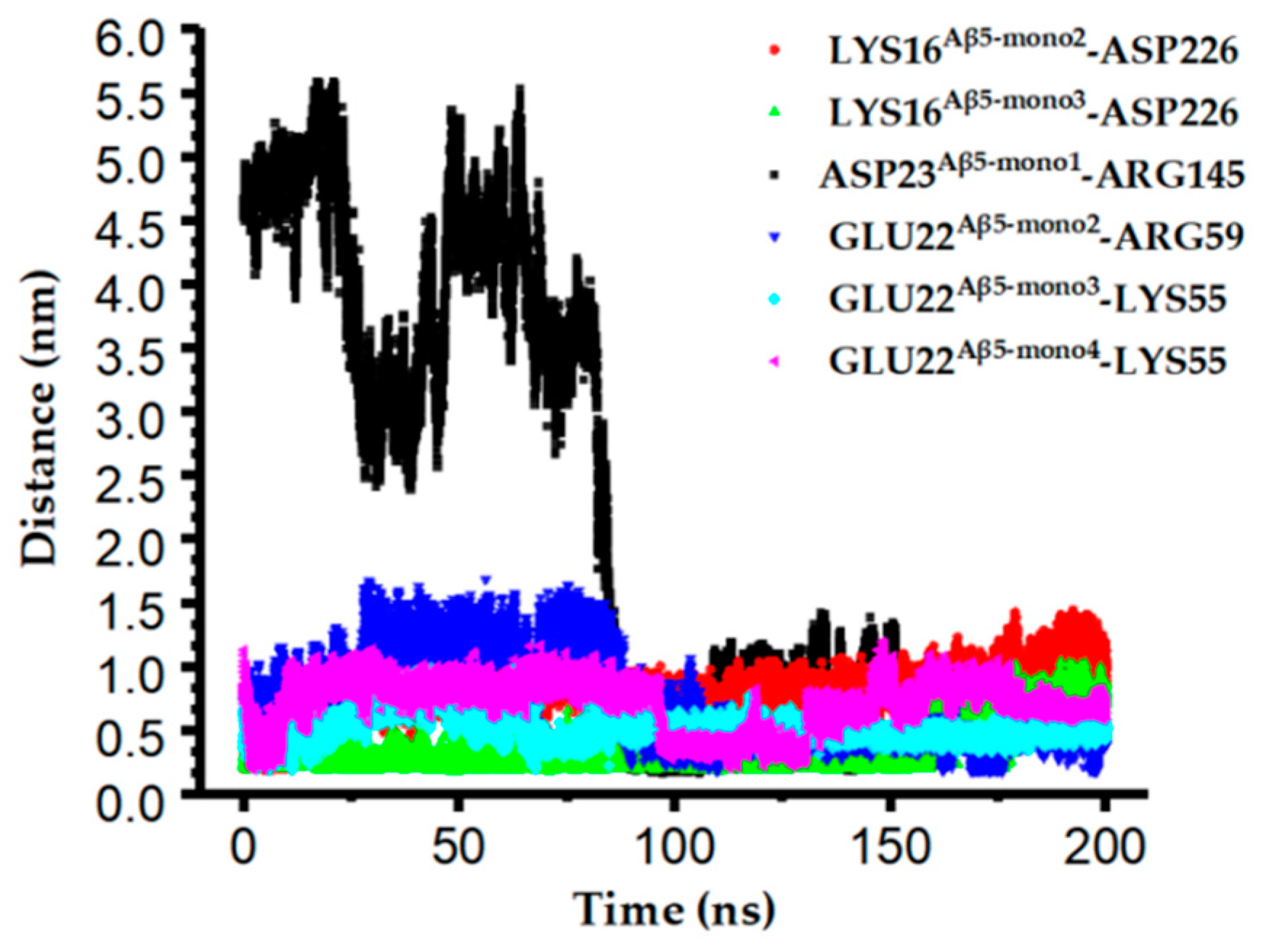
2.6. Potential Interaction between scFv-C and Aβ5
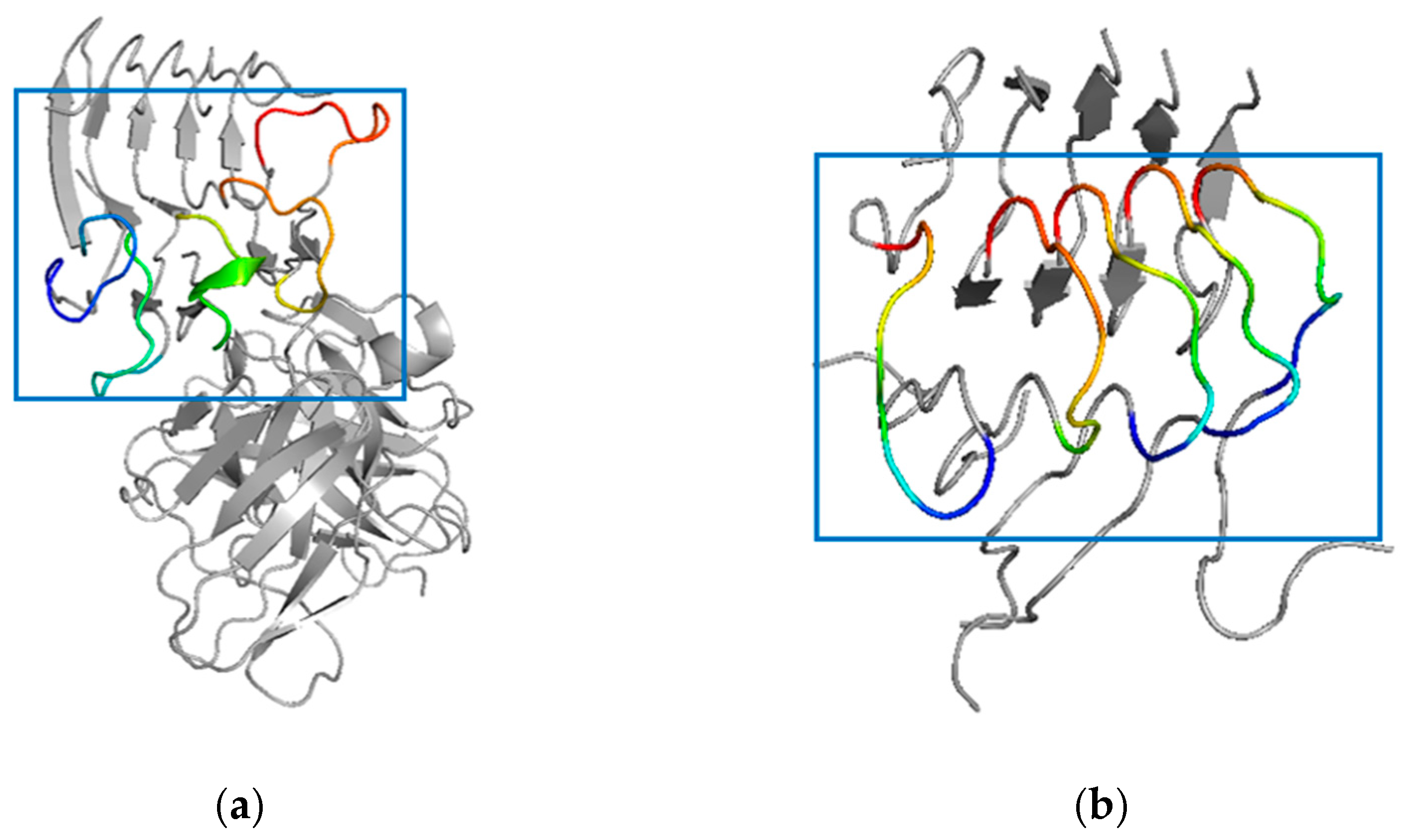
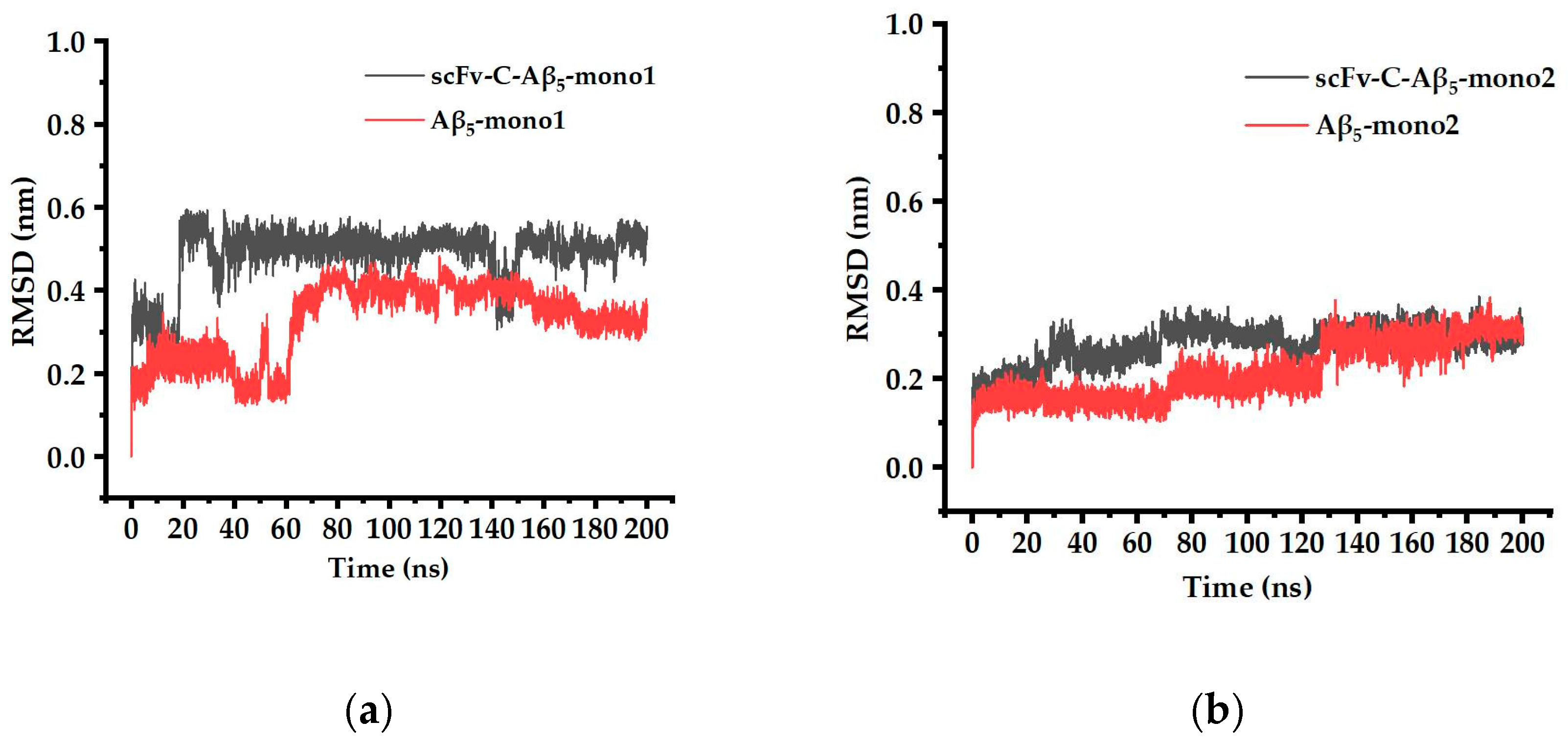
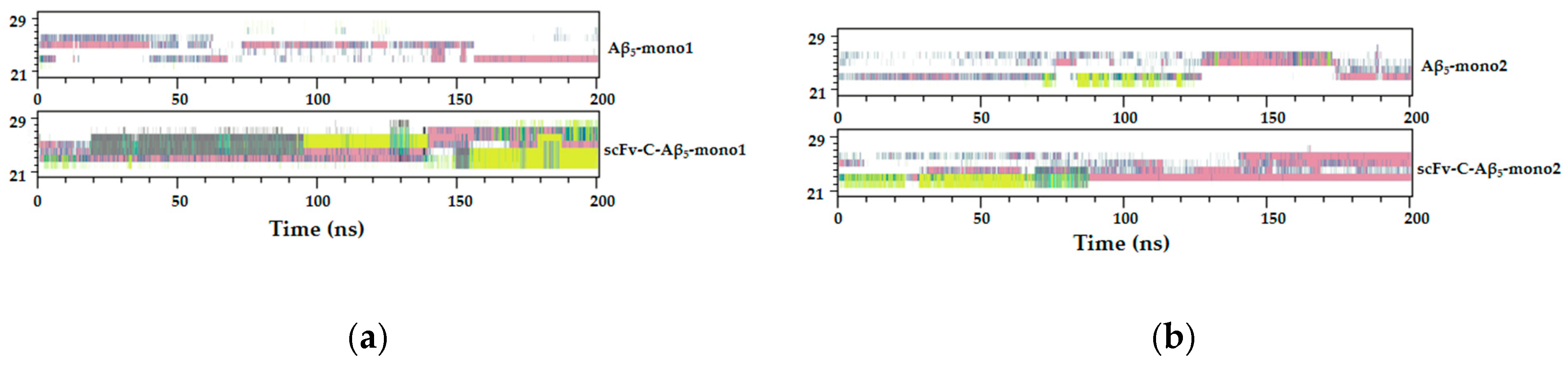
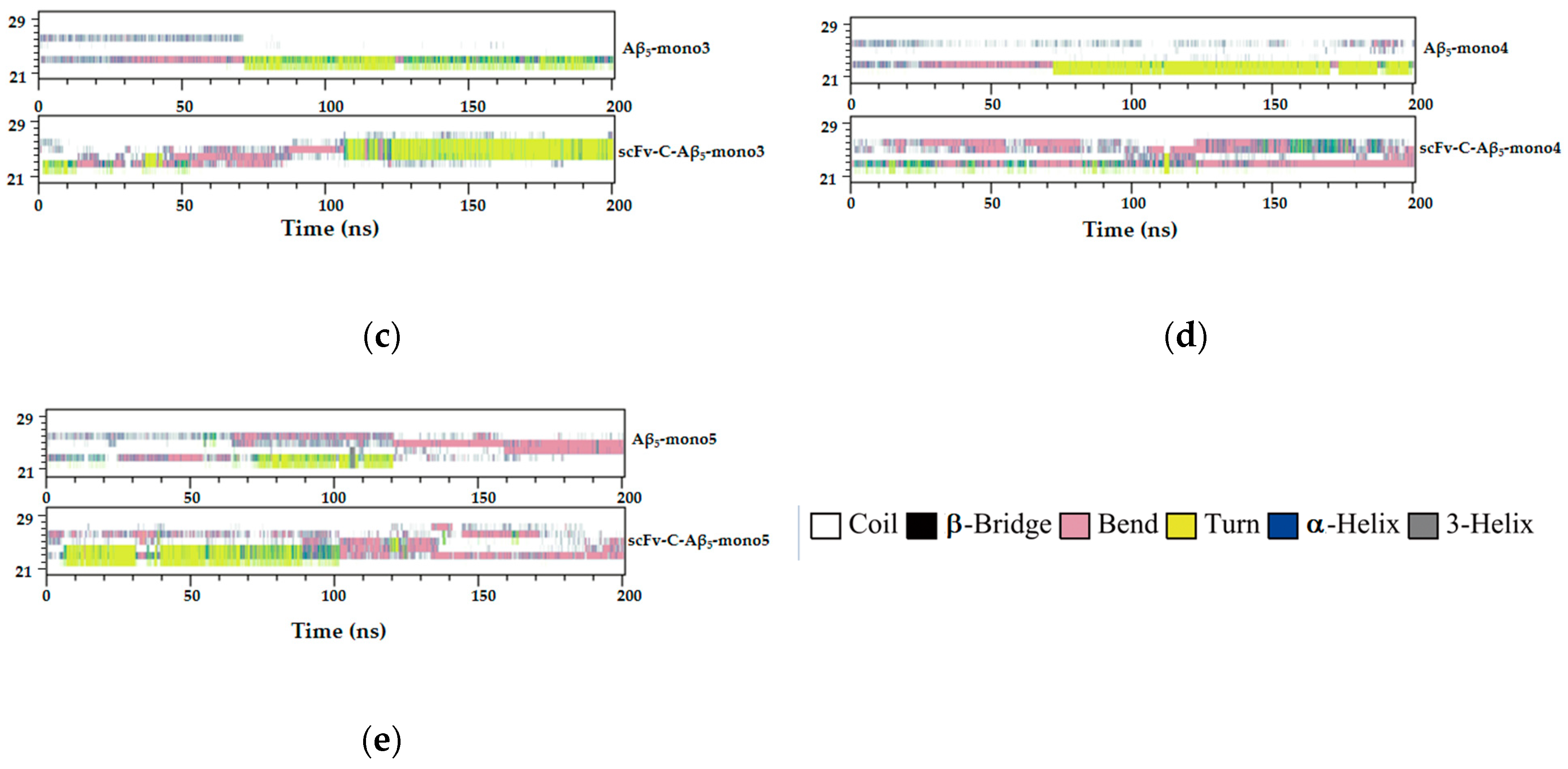
2.7. Simulated Structure Analysis of scFv-C-Aβ5 Complex and Aβ5 System
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Construction of scFv Plasmids (pscFvs)
4.2.2. Prokaryotic Expression of scFvs
4.2.3. Purification of Recombinant scFvs
4.2.4. Characterization of scFvs Based on Western Blotting
4.2.5. Characterization of scFvs’ Binding Activities Based on Enzyme-Linked Immunosorbent Assay (ELISA)
4.2.6. Characterization of scFvs’ Thermal Stability
4.2.7. Promoting Effect on Depolymerization of Aggregated Aβ42
4.2.8. Three-Dimensional Structure Construction of scFv-C and Aβ42 Pentamer (Aβ5) Complex System
4.2.9. Molecular Dynamics Simulation
4.2.10. Binding Energy Calculation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Manca, R.; Pardiñas, A.F.; Venneri, A. Alzheimer’s Disease Neuroimaging Initiative. The neural signatures of psychoses in Alzheimer’s disease: A neuroimaging genetics approach. Eur. Arch. Psychiatry. Clin. Neurosci. 2023, 273, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.R.; Silva, J.S.; Santos, J.; Rodrigues, M.S.; Madeira, D.; Oliveira, A.; Moreira-de-Sá, A.; Lourenço, V.S.; Gonçalves, F.Q.; Silva, H.B.; et al. Downregulation of Sirtuin 1 does not account for the impaired long-term potentiation in the prefrontal cortex of female APPswe/PS1dE9 mice modelling Alzheimer’s disease. Int. J. Mol. Sci. 2023, 24, 6968. [Google Scholar] [CrossRef] [PubMed]
- Gaugler, J.; James, B.; Johnson, T.; Reimer, J.; Solis, M.; Weuve, J.; Buckley, R.F.; Hohman, T.J. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, L.; Dong, C. A review of application of Aβ42/40 ratio in diagnosis and prognosis of Alzheimer’s disease. J. Alzheimers Dis. 2022, 90, 495–512. [Google Scholar] [CrossRef]
- Johnson, N.R.; Wang, A.C.; Coughlan, C.; Sillau, S.; Lucero, E.; Viltz, L.; Markham, N.; Allen, C.; Dhanasekaran, A.R.; Chial, H.J.; et al. Imipramine and olanzapine block apoE4-catalyzed polymerization of Aβ and show evidence of improving Alzheimer’s disease cognition. Alzheimers Res. Ther. 2022, 14, 88. [Google Scholar] [CrossRef]
- Bivona, G.; Iemmolo, M.; Piccoli, T.; Agnello, L.; Lo Sasso, B.; Ciaccio, M.; Ghersi, G. High cerebrospinal fluid CX3CL1 levels in Alzheimer’s disease patients but not in non-Alzheimer’s disease dementia. J. Clin. Med. 2022, 11, 5498. [Google Scholar] [CrossRef]
- Kabir, E.R.; Chowdhury, N.M.; Yasmin, H.; Kabir, M.T.; Akter, R.; Perveen, A.; Ashraf, G.M.; Akter, S.; Rahman, M.H.; Sweilam, S.H. Unveiling the potential of polyphenols as anti-amyloid molecules in Alzheimer’s disease. Curr. Neuropharmacol. 2022, 14, 787–807. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, V.P. Amelioration of Alzheimer’s disease pathology and cognitive deficits by immunomodulatory agents in animal models of Alzheimer’s disease. Neural Regen. Res. 2019, 14, 1158–1176. [Google Scholar]
- Cummings, J.L.; Goldman, D.P.; Simmons-Stern, N.R.; Ponton, E. The costs of developing treatments for Alzheimer’s disease: A retrospective exploration. Alzheimers Dement. 2022, 18, 469–477. [Google Scholar] [CrossRef]
- Brashear, H.R.; Ketter, N.; Bogert, J.; Di, J.; Salloway, S.P.; Sperling, R. Clinical evaluation of amyloid-related imaging abnormalities in bapineuzumab phase III studies. J. Alzheimer’s Dis. 2018, 66, 1409–1424. [Google Scholar] [CrossRef]
- Ultsch, M.; Li, B.; Maurer, T.; Mathieu, M.; Adolfsson, O.; Muhs, A.; Pfeifer, A.; Pihlgren, M.; Bainbridge, T.W.; Reichelt, M.; et al. Structure of crenezumab complex with Aβ shows loss of β-hairpin. Sci. Rep. 2016, 6, 39374. [Google Scholar] [CrossRef] [PubMed]
- Sink, K.; Warren, F.; Smith, J.; Schneider, A.; Fuji, R.N.; Quartino, A.; Mackey, H.; Rabbia, M.; Yule, S.; Fontoura, P.; et al. P1-046: Baseline characteristics from cread2: A phase III trial of crenezumab in early (prodromal-to-mild) Alzheimer’s disease. Alzheimers Dement. 2019, 15, P250. [Google Scholar] [CrossRef]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- Bard, F.; Barbour, R.; Cannon, C.; Carretto, R.; Fox, M.; Games, D.; Guido, T.; Hoenow, K.; Hu, K.; Johnson-Wood, K.; et al. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer’s disease-like neuropathology. Proc. Natl. Acad. Sci. USA 2003, 100, 2023–2028. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.; Hamid, M. ScFv antibody: Principles and clinical application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, H.; Alizadeh, M.; Shahidi, R.; Shobeiri, P.; Love, N.; Singhal, A. Subcortical signal alteration of corticospinal tracts. A radiologic manifestation of ARIA: A case report. Radiol. Case Rep. 2022, 18, 275–279. [Google Scholar] [CrossRef]
- Roytman, M.; Mashriqi, F.; Al-Tawil, K.; Schulz, P.E.; Zaharchuk, G.; Benzinger, T.L.S.; Franceschi, A.M. Amyloid-related imaging abnormalities: An update. AJR Am. J. Roentgenol. 2023, 220, 562–574. [Google Scholar] [CrossRef]
- Sun, Z.T.; Ma, C.; Li, G.J.; Zheng, J.Y.; Hao, Y.T.; Yang, Y.; Wang, X. Application of antibody fragments against Aβ with emphasis on combined application with nanoparticles in Alzheimer’s disease. Front. Pharmacol. 2021, 12, 654611. [Google Scholar] [CrossRef]
- Zhang, Y.; Qian, L.; Kuang, Y.; Liu, J.; Wang, D.; Xie, W.; Zhang, L.; Fu, L. An adeno-associated virus-mediated immunotherapy for Alzheimer’s disease. Mol. Immunol. 2022, 144, 26–34. [Google Scholar] [CrossRef]
- Liu, D.; Li, W.; Jiang, X.; Bai, S.; Liu, J.; Liu, X.; Shi, Y.; Kuai, Z.; Kong, W.; Gao, R. Using near-infrared enhanced thermozyme and scFv dual-conjugated Au nanorods for detection and targeted photothermal treatment of Alzheimer’s disease. Theranostics 2019, 9, 2268–2281. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, B. Molecular Dynamics Simulation: Fundamentals and Applications; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Schmidt, A.G.; Xu, H.; Khan, A.R.; O’Donnell, T.; Khurana, S.; King, L.R.; Manischewitz, J.; Golding, H.; Suphaphiphat, P.; Carfi, A.; et al. Preconfiguration of the antigen-binding site during affinity maturation of a broadly neutralizing influenza virus antibody. Proc. Natl. Acad. Sci. USA 2013, 110, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Bai, Y. Preparation of scFv stabilized chromatosomes for single-particle cryo-EM structure determination. STAR Protoc. 2021, 2, 100396. [Google Scholar] [CrossRef] [PubMed]
- Moser, A.C.; Trenhaile, S.; Frankenberg, K. Studies of antibody-antigen interactions by capillary electrophoresis: A review. Methods 2018, 146, 66–75. [Google Scholar] [CrossRef]
- Dulewicz, M.; Kulczyńska-Przybik, A.; Mroczko, P.; Kornhuber, J.; Lewczuk, P.; Mroczko, B. Biomarkers for the diagnosis of Alzheimer’s disease in clinical practice: The role of CSF biomarkers during the evolution of diagnostic criteria. Int. J. Mol. Sci. 2022, 23, 8598. [Google Scholar] [CrossRef]
- Bouter, Y.; Lopez Noguerola, J.S.; Tucholla, P.; Crespi, G.A.; Parker, M.W.; Wiltfang, J.; Miles, L.A.; Bayer, T.A. Abeta targets of the biosimilar antibodies of Bapineuzumab, Crenezumab, Solanezumab in comparison to an antibody against N-truncated Abeta in sporadic Alzheimer disease cases and mouse models. Acta Neuropathol. 2015, 130, 713–729. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Madrasi, K.; Abdul, H.; Young, C.; Park, J.; Burke, J.; Apgar, J.; Hua, F.; Gruenbaum, L. Quantitative systems pharmacology model of amyloid beta and plaque dynamics in Alzheimer’s disease upon treatment with Crenezumab, Solanezumab, and Bapineuzumab. Clin. Pharmacol. Ther. 2019, 105, S104. [Google Scholar]
- Imbimbo, B.P.; Triaca, V.; Imbimbo, C.; Nisticò, R. Investigational treatments for neurodegenerative diseases caused by inheritance of gene mutations: Lessons from recent clinical trials. Neural Regen. Res. 2023, 18, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Kojic, M.; Gaik, M.; Kiska, B.; Salerno-Kochan, A.; Hunt, S.; Tedoldi, A.; Mureev, S.; Jones, A.; Whittle, B.; Genovesi, L.A.; et al. Elongator mutation in mice induces neurodegeneration and ataxia-like behavior. Nat. Commun. 2018, 9, 3195. [Google Scholar] [CrossRef]
- Breitsprecher, D.; Fung, P.A.; Tschammer, N. Improving biosensor assay development by determining sample quality with Tycho NT.6. Nat. Methods 2018, 15, 298. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, Y.; Chen, J.; Jin, S.; Shan, Y. A biepitope, adjuvant-free, self-assembled influenza nanovaccine provides cross-protection against H3N2 and H1N1 viruses in mice. Nano Res. 2022, 15, 8304–8314. [Google Scholar] [CrossRef]
- Bertoni, M.; Kiefer, F.; Biasini, M.; Bordoli, L.; Schwede, T. Modeling protein quaternary structure of homo- and hetero-oligomers beyond binary interactions by homology. Sci. Rep. 2017, 7, 10480. [Google Scholar] [CrossRef]
- Sun, D.; Qiao, Y.; Jiang, X.; Li, P.; Kuai, Z.; Gong, X.; Liu, D.; Fu, Q.; Sun, L.; Li, H.; et al. Multiple Antigenic Peptide System Coupled with Amyloid Beta Protein Epitopes As An Immunization Approach to Treat Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 2794–2800. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository--new features and functionality. Nucleic Acids Res. 2017, 45, 313–319. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T.; Elofsson, A. QMEANDisCo―distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 2647. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.M.; Peng, H.P.; Chen, I.C.; Lee, Y.C.; Chen, J.B.; Tsai, K.C.; Chen, C.T.; Chang, J.Y.; Yang, E.W.; Hsu, P.C.; et al. Rationalization and Design of the Complementarity Determining Region Sequences in an Antibody-Antigen Recognition Interface. PLoS ONE 2012, 7, e33340. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Hess, B.; van der Vegt, N.F. Hydration thermodynamic properties of amino acid analogues: A systematic comparison of biomolecular force fields and water models. J. Phys. Chem. B 2006, 110, 17616–17626. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; van der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef]
- Safarizadeh, H.; Garkani-Nejad, Z. Molecular docking, molecular dynamics simulations and QSAR studies on some of 2-arylethenylquinoline derivatives for inhibition of Alzheimer’s amyloid-beta aggregation: Insight into mechanism of interactions and parameters for design of new inhibitors. J. Mol. Graph. Model. 2019, 87, 129–143. [Google Scholar] [CrossRef]
- Bachmann, S.J.; Van Gunsteren, W.F. Structural and energetic effects of the use of polarisable water to solvate proteins. Mol. Phys. 2015, 113, 2815–2828. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, S.; Luo, Z.; Han, F.; Han, W.; Wang, S.; Zhang, H. How different substitution positions of F, Cl atoms in benzene ring of 5-methylpyrimidine pyridine derivatives affect the inhibition ability of EGFRL858R/T790M/C797S inhibitors: A molecular dynamics simulation study. Molecules 2020, 25, 895–920. [Google Scholar]
- Pan, Y.; Lu, Z.; Li, C.; Qi, R.; Chang, H.; Han, L.; Han, W. Molecular dockings and molecular dynamics simulations reveal the potency of different inhibitors against Xanthine oxidase. ACS Omega 2021, 6, 11639–11649. [Google Scholar] [CrossRef] [PubMed]
- Rampogu, S.; Baek, A.; Park, C.; Son, M.; Parate, S.; Parameswaran, S.; Park, Y.; Shaik, B.; Kim, J.H.; Park, S.J.; et al. Discovery of small molecules that target vascular endothelial growth factor receptor-2 signalling pathway employing molecular modelling studies. Cells 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Qin, H.; Chen, J.; Wang, F.; He, C.; He, S.; Hong, B.; Liu, K.; Qiao, R.; Fan, H.; et al. Computational design of ultrashort peptide inhibitors of the receptor-binding domain of the SARS-CoV-2 S protein. Brief. Bioinform. 2021, 22, 243. [Google Scholar] [CrossRef] [PubMed]
- Sadr, A.S.; Eslahchi, C.; Ghassempour, A.; Kiaei, M. In silico studies reveal structural deviations of mutant profilin-1 and interaction with riluzole and edaravone in amyotrophic lateral sclerosis. Sci. Rep. 2021, 11, 6849. [Google Scholar] [CrossRef]
- Qian, M.; Guan, S.; Shan, Y.; Zhang, H.; Wang, S. Structural and molecular basis of cellulase Cel48F by computational modeling: Insight into catalytic and product release mechanism. J. Struct. Biol. 2016, 194, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wong, C.F. Rank-ordering protein-ligand binding affinity by a quantum mechanics/molecular mechanics/Poisson-Boltzmann-surface area model. J. Chem. Phys. 2007, 126, 026101. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery Consortium; Lynn, A. G-mmpbsa -A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, S.; Gu, L.; Zhou, J.; Li, Y.; Zheng, X. Computer-aided design of glucoside brain-targeted molecules based on 4PYP. J. Mol. Graph. Model. 2021, 103, 107819. [Google Scholar] [CrossRef] [PubMed]



| Donor | Acceptor | Probability |
|---|---|---|
| ASN58scFv-C-Sidechain | GLU22Aβ5-mono2-Sidechain | 12.87% |
| SER72scFv-C-Sidechain | GLU22Aβ5-mono4-Sidechain | 7.92% |
| THR36scFv-C-Sidechain | GLU22Aβ5-mono4-Sidechain | 7.92% |
| ASN178scFv-C-Sidechain | GLN15Aβ5-mono5-Sidechain | 22.77% |
| SER179scFv-C-Sidechain | GLN15Aβ5-mono4-Sidechain | 7.43% |
| ASN180scFv-C-Sidechain | HIS13Aβ5-mono4-Sidechain | 13.86% |
| GLY73scFv-C-Mainchain | GLU22Aβ5-mono4-Sidechain | 5.45% |
| TYR185scFv-C-Sidechain | GLN15Aβ5-mono5-Sidechain | 9.41% |
| HIS13Aβ5-mono5-Sidechain | SER179scFv-C-Sidechain | 9.90% |
| TYR37scFv-C-Sidechain | VAL18Aβ5-mono3-Mainchain | 5.45% |
| SER57scFv-C-Sidechain | PHE20Aβ5-mono2-Mainchain | 11.88% |
| GLY71scFv-C-Mainchain | GLU22Aβ5-mono3-Sidechain | 9.41% |
| ASN33scFv-C-Sidechain | GLU22Aβ5-mono4-Sidechain | 9.41% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Xu, L.; Zhang, J.; Wang, Y.; Wu, Z.; Sun, W.; Yao, X.; Wang, X.; Guan, S.; Shan, Y. Mechanism Exploration of Amyloid-β-42 Disaggregation by Single-Chain Variable Fragments of Alzheimer’s Disease Therapeutic Antibodies. Int. J. Mol. Sci. 2023, 24, 8371. https://doi.org/10.3390/ijms24098371
Fan X, Xu L, Zhang J, Wang Y, Wu Z, Sun W, Yao X, Wang X, Guan S, Shan Y. Mechanism Exploration of Amyloid-β-42 Disaggregation by Single-Chain Variable Fragments of Alzheimer’s Disease Therapeutic Antibodies. International Journal of Molecular Sciences. 2023; 24(9):8371. https://doi.org/10.3390/ijms24098371
Chicago/Turabian StyleFan, Xing, Lipeng Xu, Jianhao Zhang, Yidan Wang, Zirui Wu, Wenjing Sun, Xin Yao, Xu Wang, Shanshan Guan, and Yaming Shan. 2023. "Mechanism Exploration of Amyloid-β-42 Disaggregation by Single-Chain Variable Fragments of Alzheimer’s Disease Therapeutic Antibodies" International Journal of Molecular Sciences 24, no. 9: 8371. https://doi.org/10.3390/ijms24098371
APA StyleFan, X., Xu, L., Zhang, J., Wang, Y., Wu, Z., Sun, W., Yao, X., Wang, X., Guan, S., & Shan, Y. (2023). Mechanism Exploration of Amyloid-β-42 Disaggregation by Single-Chain Variable Fragments of Alzheimer’s Disease Therapeutic Antibodies. International Journal of Molecular Sciences, 24(9), 8371. https://doi.org/10.3390/ijms24098371






