miR-145-3p Inhibits MuSCs Proliferation and Mitochondria Mass via Targeting MYBL1 in Jianzhou Big-Eared Goats
Abstract
1. Introduction
2. Results
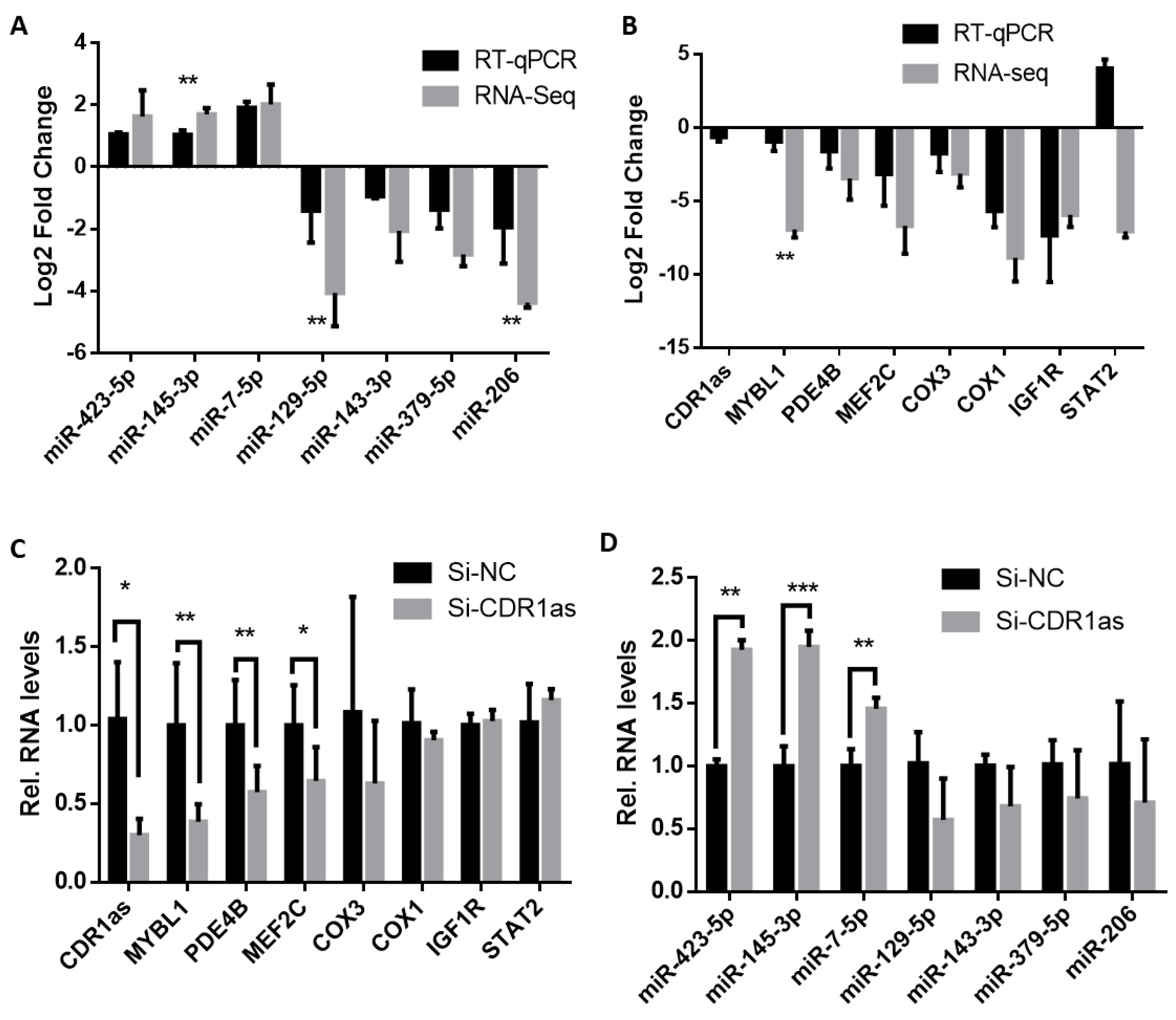
2.1. miR-145-3p Is Negatively Associated with Levels of MYBL1 mRNA in CDR1as-Perturbed Myocytes
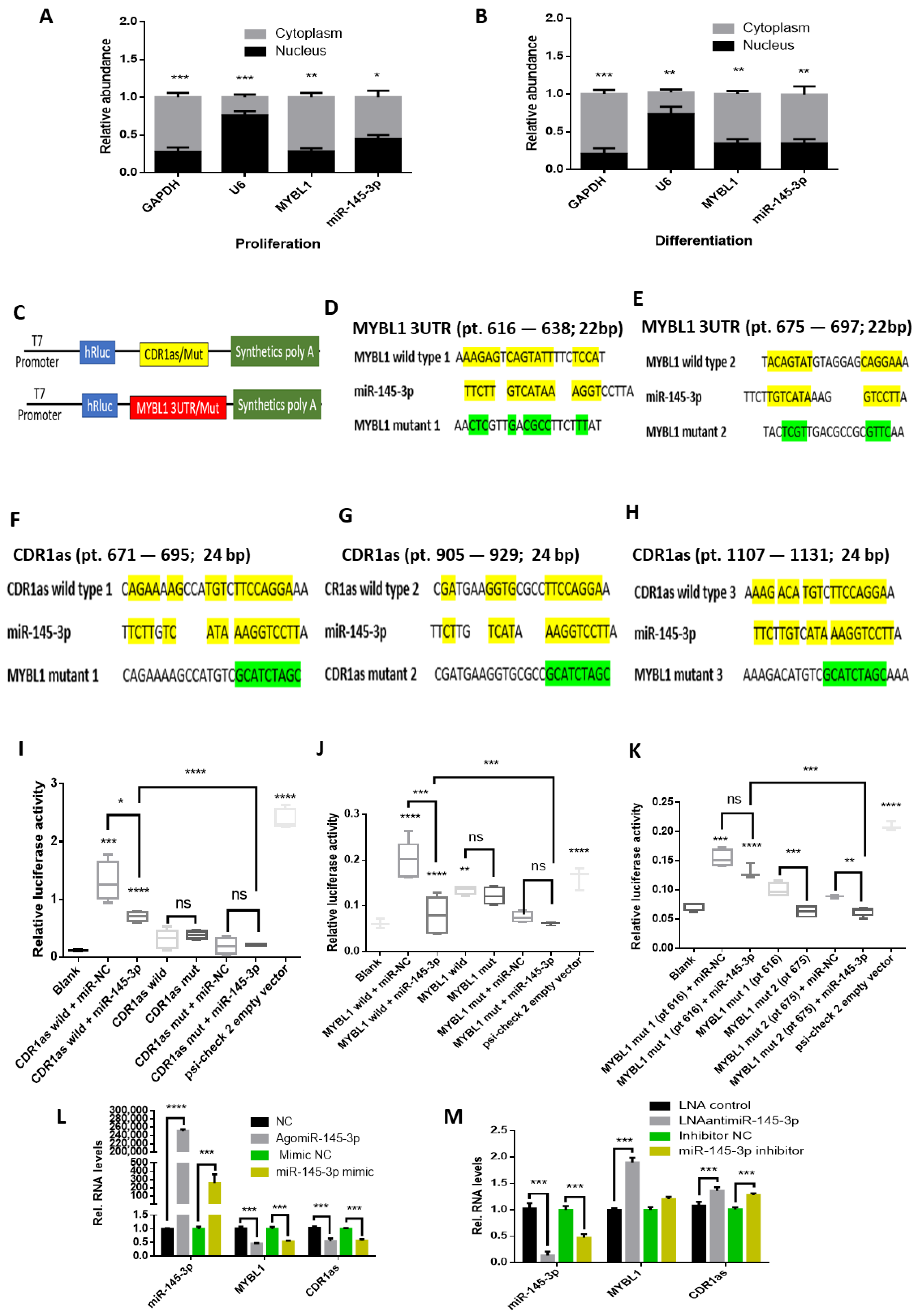
2.2. MYBL1 and CDR1as Are Targeted by miR-145-3p
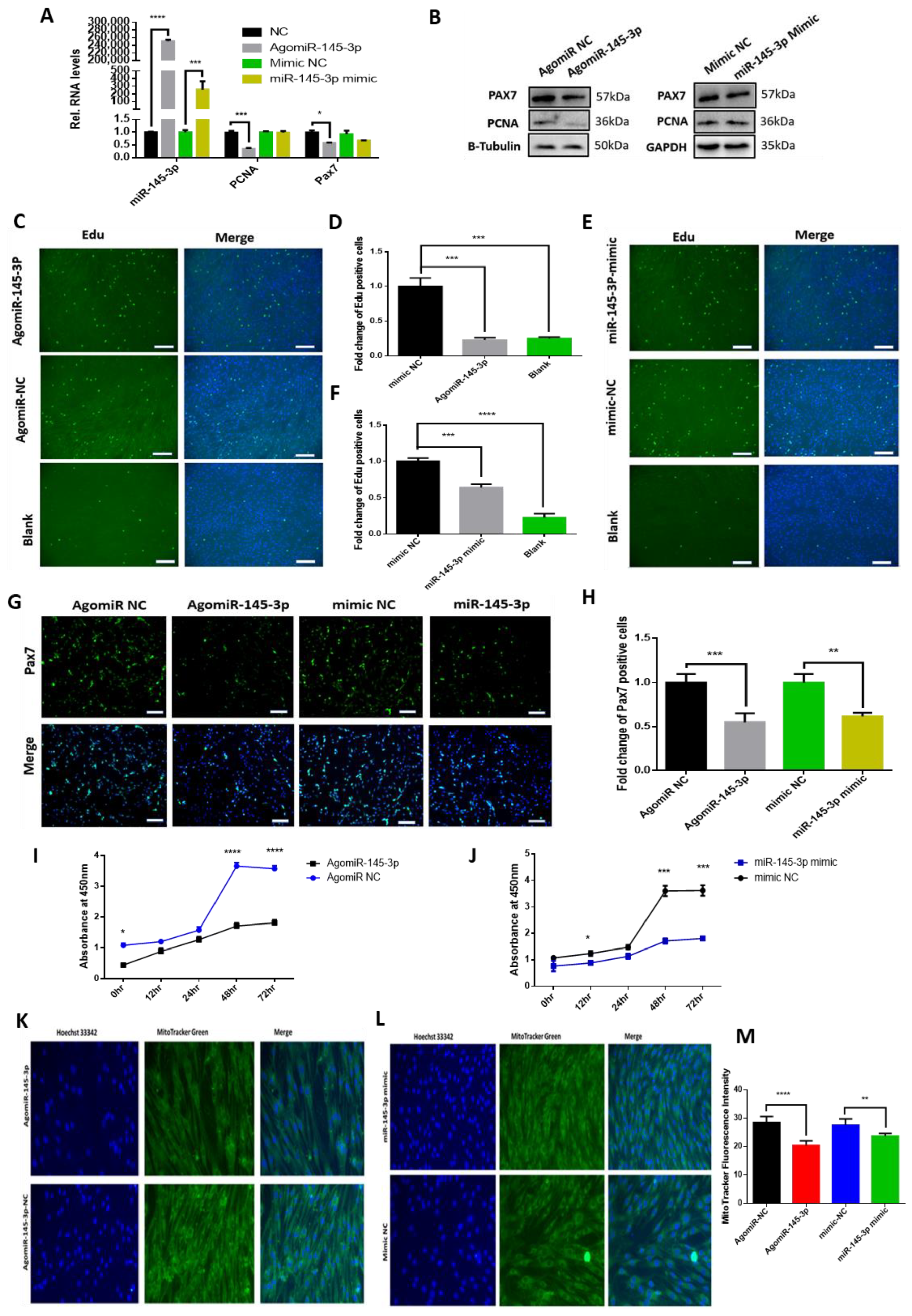
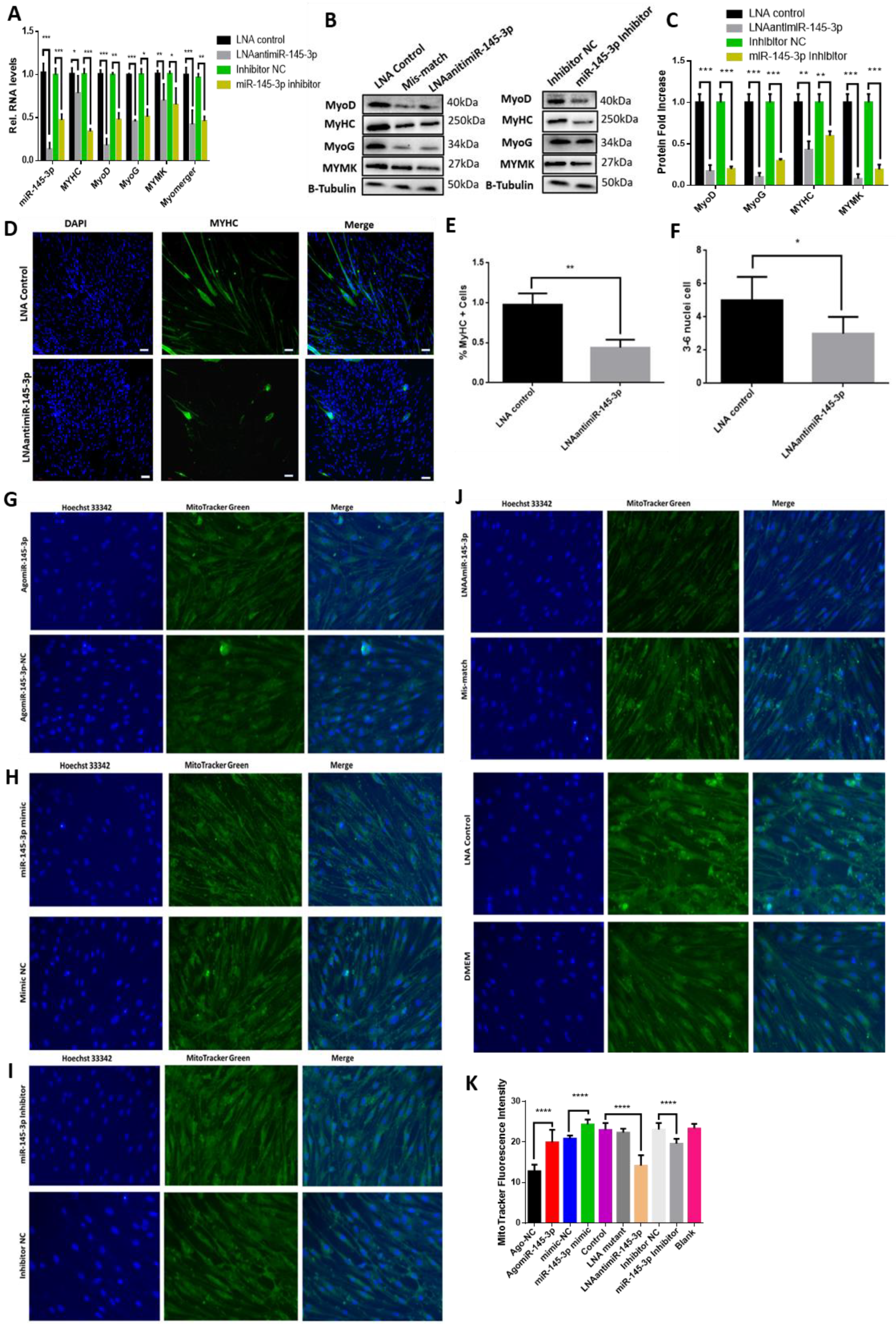
2.3. miRNA-145-3p Inhibits Myogenic Proliferation, Differentiation, and Mitochondrial Mass
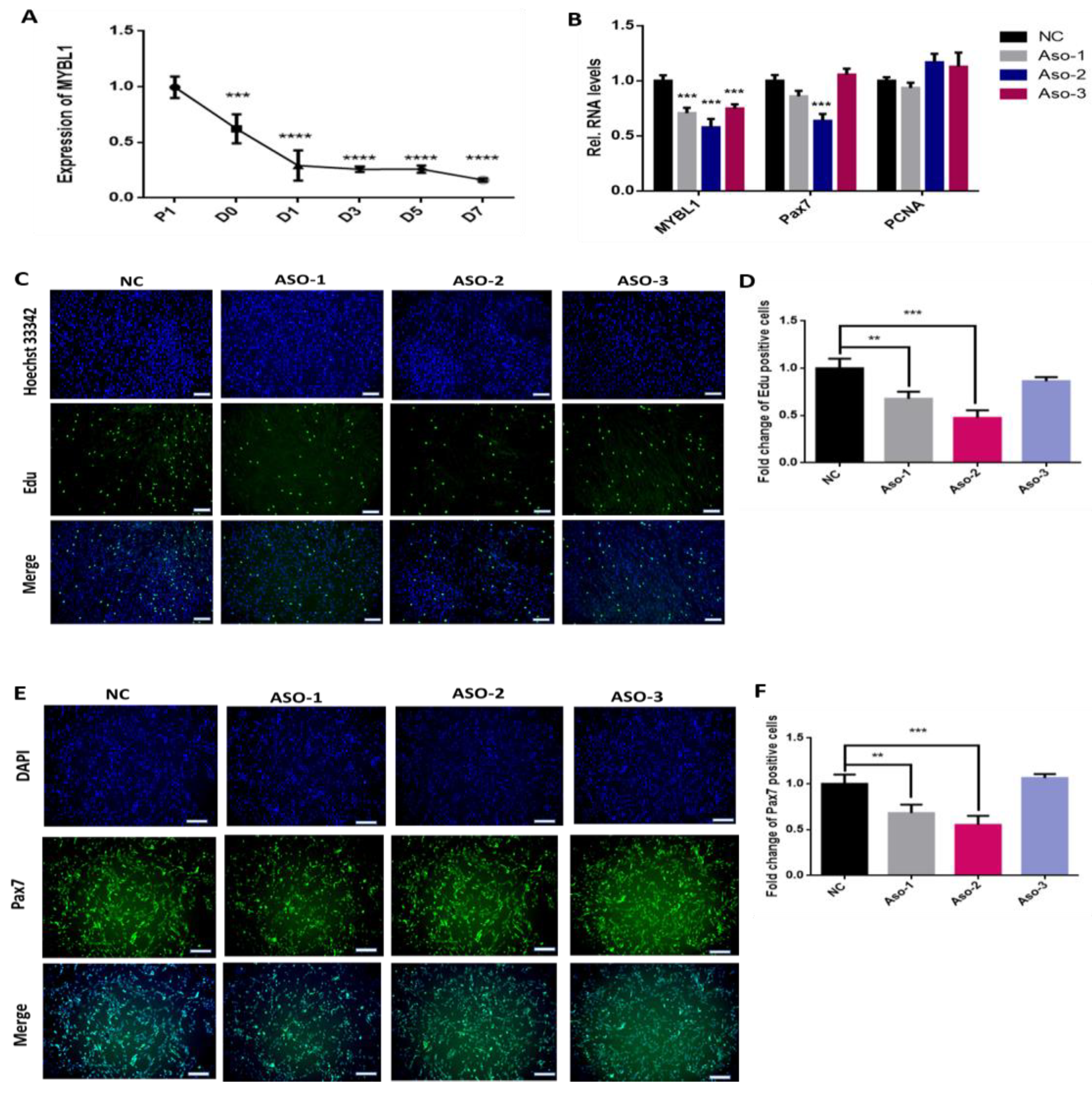
2.4. The Knockdown of MYBL1 Simulates the miR-145-3p’s Inhibition on MuSCs Proliferation
2.5. MYBL1 Mediates the Regulation of miR-145-3p on Expression of Vexin, VCPIP1, COX1, and COX2
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Goat MuSC Isolation and Culture
4.3. RNA Extraction and qRT-PCR
4.4. Validation of RNA-Seq Data
4.5. Quantification of the Expression Profiles of Some Myogenic Regulatory Genes in MuSCs
4.6. Plasmid Construction
4.7. Cell Culture and Treatments
4.8. Cell Number Assay
4.9. Luciferase Activity Assay
4.10. Immunofluorescence Staining
4.11. Western Blotting Analysis
4.12. Mitochondria Mass Assay
4.13. Nuclear and Cytoplasmic Extraction
4.14. LNAantimR-145-3p and MYBL1-ASO-2′MOE siRNAs Design
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| circRNA | Circular RNA |
| lncRNA | Long noncoding RNA |
| MEF2 | Myocyte enhancer factor 2 |
| miRNA | Micro RNA |
| MRFs | Myogenic regulatory factors |
| Pax: | Paired box |
| PBS | Phosphate buffer saline |
| RT-PCR | Reverse Transcription PCR |
| RT-qPCR | Real-time Quantitative PCR |
| MuSCs | Skeletal muscle satellite cells |
| UTR | Untranslated region |
| LNAantimiR | Lock nucleic Acid anti-miRNA |
| ASO-2′MOE | Antisense oligonucleotides 2′-O-methoxyethyl |
| PCNA | Proliferating Cell Nuclear Antigen |
| MyoD | Myogenic Differentiation 1 |
| MyoG | MyoGenin |
| MYMK | Myomaker |
| STAT2 | Signal transducer and activator of transcription 2 |
| MYBL1 | Myb proto-oncogene like 1 |
| COX | Cytochrome c oxidase subunit |
| ANOVA | Analysis of Variance |
| CDR1as | Cerebellar degeneration-related protein 1 antisense RNA |
| CCK-8 | Cell Counting Kit-8 |
| DMSO | Dimethyl Sulfoxide |
| DAPI | 4′, 6′-diamidino-2-phenylindole, blue |
| MyHC | Myosin heavy chain-embryonic |
| VCPIP1 | Valosin containing protein (p97)/p47 complex interacting protein 1 |
| CSNK1A1 | Casein kinase 1 alpha 1 |
References
- Chang, N.C.; Rudnicki, M.A. Satellite Cells: The Architects of Skeletal Muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar] [PubMed]
- Bailey, P.; Holowacz, T.; Lassar, A.B. The Origin of Skeletal Muscle Stem Cells in the Embryo and the Adult. Curr. Opin. Cell Biol. 2001, 13, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, F.; Rudnicki, M.A. Skeletal Muscle Satellite Cells and Adult Myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M. Myogenic Progenitor Cells and Skeletal Myogenesis in Vertebrates. Curr. Opin. Genet. Dev. 2006, 16, 525–532. [Google Scholar] [CrossRef]
- Zhou, Y.; Ness, S.A. Myb Proteins: Angels and Demons in Normal and Transformed Cells. Front. Biosci. 2011, 16, 1109–1131. [Google Scholar] [CrossRef]
- Gene Card Expression for MYBL1 Gene. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=MYBL1 (accessed on 30 October 2021).
- Arsura, M.; Hofmann, C.S.; Golay, J.; Introna, M.; Sonenshein, G.E. A-Myb Rescues Murine B-Cell Lymphomas from IgM-Receptor–Mediated Apoptosis through c-Myctranscriptional Regulation. Blood 2000, 96, 1013–1020. [Google Scholar] [CrossRef]
- Golay, J.; Broccoli, V.; Lamorte, G.; Bifulco, C.; Parravicini, C.; Pizzey, A.; Thomas, N.S.B.; Delia, D.; Ferrauti, P.; Vitolo, D.; et al. The A-Myb Transcription Factor Is a Marker of Centroblasts In Vivo. J. Immunol. 1998, 160, 2786. [Google Scholar] [CrossRef]
- Oh, I.-H.; Reddy, E.P. The Myb Gene Family in Cell Growth, Differentiation and Apoptosis. Oncogene 1999, 18, 3017–3033. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, W.; Wang, R.; Lei, C.; Zhou, R.; Tang, Z.; Li, K. Wnt Antagonist, Secreted Frizzled-Related Protein 1, Is Involved in Prenatal Skeletal Muscle Development and Is a Target of MiRNA-1/206 in Pigs. BMC Mol. Biol. 2015, 16, 4. [Google Scholar] [CrossRef]
- Liu, N.; Williams, A.H.; Maxeiner, J.M.; Bezprozvannaya, S.; Shelton, J.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. MicroRNA-206 Promotes Skeletal Muscle Regeneration and Delays Progression of Duchenne Muscular Dystrophy in Mice. J. Clin. Invest. 2012, 122, 2054–2065. [Google Scholar] [CrossRef]
- Goto, Y.; Kurozumi, A.; Arai, T.; Nohata, N.; Kojima, S.; Okato, A.; Kato, M.; Yamazaki, K.; Ishida, Y.; Naya, Y.; et al. Impact of Novel MiR-145-3p Regulatory Networks on Survival in Patients with Castration-Resistant Prostate Cancer. Br. J. Cancer 2017, 117, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Leng, J.; Zhang, X.; Capellini, T.D.; Chen, Y.; Yang, L.; Chen, Z.; Zheng, S.; Zhang, X.; Zhan, S.; et al. Identification of IGF2BP1-Related LncRNA-MiRNA-MRNA Network in Goat Skeletal Muscle Satellite Cells. Anim. Sci. J. 2021, 92, e13631. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Nie, L.; Ding, X.; Zhang, X.; Zhao, W.; Xu, X.; Kyei, B.; Dai, D.; Zhan, S.; et al. MyoD-Induced Circular RNA CDR1as Promotes Myogenic Differentiation of Skeletal Muscle Satellite Cells. Biochim. Biophys. Acta-Gene Regul. Mech. 2019, 1862, 807–821. [Google Scholar] [CrossRef]
- Kyei, B.; Li, L.; Yang, L.; Zhan, S.; Li, J.; Chen, Y.; Zhang, X.; Zheng, S.; Xu, X.; Odame, E.; et al. CDR1as Related MiRNA-MRNA Networks in Differentiating Goat Skeletal Muscle Satellite Cells. Res. Sq. 2020. [Google Scholar]
- Li, P.; Yang, X.; Yuan, W.; Yang, C.; Zhang, X.; Han, J.; Wang, J.; Deng, X.; Yang, H.; Li, P.; et al. CircRNA-Cdr1as Exerts Anti-Oncogenic Functions in Bladder Cancer by Sponging MicroRNA-135a. Cell. Physiol. Biochem. 2018, 46, 1606–1616. [Google Scholar] [CrossRef] [PubMed]
- Piwecka, M.; Glažar, P.; Hernandez-Miranda, L.R.; Memczak, S.; Wolf, S.A.; Rybak-Wolf, A.; Filipchyk, A.; Klironomos, F.; Cerda Jara, C.A.; Fenske, P.; et al. Loss of a Mammalian Circular RNA Locus Causes MiRNA Deregulation and Affects Brain Function. Science 2017, 357, eaam8526. [Google Scholar] [CrossRef]
- Remels, A.H.V.; Langen, R.C.J.; Schrauwen, P.; Schaart, G.; Schols, A.M.W.J.; Gosker, H.R. Regulation of Mitochondrial Biogenesis during Myogenesis. Mol. Cell. Endocrinol. 2010, 315, 113–120. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Kotake, N.; Yamada, S. Muscle Regeneration Occurs to Coincide with Mitochondrial Biogenesis. Mol. Cell. Biochem. 2011, 349, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Rochard, P.; Rodier, A.; Casas, F.; Cassar-Malek, I.; Marchal-Victorion, S.; Daury, L.; Wrutniak, C.; Cabello, G. Mitochondrial Activity Is Involved in the Regulation of Myoblast Differentiation through Myogenin Expression and Activity of Myogenic Factors*. J. Biol. Chem. 2000, 275, 2733–2744. [Google Scholar] [CrossRef]
- Seyer, P.; Grandemange, S.; Busson, M.; Carazo, A.; Gamaléri, F.; Pessemesse, L.; Casas, F.; Cabello, G.; Wrutniak-Cabello, C. Mitochondrial Activity Regulates Myoblast Differentiation by Control of C-Myc Expression. J. Cell. Physiol. 2006, 207, 75–86. [Google Scholar] [CrossRef]
- Kim, B.-W.; Lee, J.-W.; Choo, H.-J.; Lee, C.S.; Jung, S.-Y.; Yi, J.-S.; Ham, Y.-M.; Lee, J.-H.; Hong, J.; Kang, M.-J.; et al. Mitochondrial Oxidative Phosphorylation System Is Recruited to Detergent-Resistant Lipid Rafts during Myogenesis. Proteomics 2010, 10, 2498–2515. [Google Scholar] [CrossRef] [PubMed]
- Leary, S.C.; Battersby, B.J.; Hansford, R.G.; Moyes, C.D. Interactions between Bioenergetics and Mitochondrial Biogenesis. Biochim. Biophys. Acta-Bioenerg. 1998, 1365, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Dennerlein, S.; Rehling, P. Human Mitochondrial COX1 Assembly into Cytochrome <Em>C</Em> Oxidase at a Glance. J. Cell Sci. 2015, 128, 833. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, G.; Zhang, X.; Hüttemann, P.P.; Qiu, Y.; Liu, J.; Mitchell, A.; Lee, I.; Zhang, C.; Lee, J.-S.; et al. COX7AR Is a Stress-Inducible Mitochondrial COX Subunit That Promotes Breast Cancer Malignancy. Sci. Rep. 2016, 6, 31742. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, N.H.; Zwart, R.; Wolterman, R.A.; Ruiter, J.P.N.; Wanders, R.J.A.; Bolhuis, P.A.; van den Bogert, C. Differentiation and Proliferation of Respiration-Deficient Human Myoblasts. Biochim. Biophys. Acta-Mol. Basis Dis. 1993, 1181, 63–67. [Google Scholar] [CrossRef]
- Korohoda, W.; Pietrzkowski, Z.R.K. Chloramphenicol, an Inhibitor of Mitochondrial Protein Synthesis, Inhibits Myoblast Fusion and Myotube Differentiation. Folia Histochem Cytobiol. 1993, 31, 9–13. [Google Scholar]
- Scarpulla, R.C. Transcriptional Paradigms in Mammalian Mitochondrial Biogenesis and Function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Hock, M.B.; Kralli, A. Transcriptional Control of Mitochondrial Biogenesis and Function. Annu. Rev. Physiol. 2009, 71, 177–203. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Metabolic Control of Mitochondrial Biogenesis through the PGC-1 Family Regulatory Network. Biochim. Biophys. Acta-Mol. Cell Res. 2011, 1813, 1269–1278. [Google Scholar] [CrossRef]
- Handschin, C.; Rhee, J.; Lin, J.; Tarr, P.T.; Spiegelman, B.M. An Autoregulatory Loop Controls Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1alpha Expression in Muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 7111–7116. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Yanos, M.E.; Kayser, E.-B.; Quintana, A.; Sangesland, M.; Castanza, A.; Uhde, L.; Hui, J.; Wall, V.Z.; Gagnidze, A.; et al. MTOR Inhibition Alleviates Mitochondrial Disease in a Mouse Model of Leigh Syndrome. Science 2013, 342, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.H.; Lee, J.I.; Suh, Y.H.; Kim, W.; Song, J.H.; Jung, M.H. Mitochondrial Dysfunction Induces Aberrant Insulin Signalling and Glucose Utilisation in Murine C2C12 Myotube Cells. Diabetologia 2006, 49, 1924–1936. [Google Scholar] [CrossRef]
- Kyei, B.; Odame, E.; Li, L.; Yang, L.; Zhan, S.; Li, J.; Chen, Y.; Dai, D.; Cao, J.; Guo, J.; et al. Knockdown of CDR1as Decreases Differentiation of Goat Skeletal Muscle Satellite Cells via Upregulating MiR-27a-3p to Inhibit ANGPT1. Genes 2022, 13, 663. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Sheriff, S.; Friend, L.A.; James, J.H. Phosphodiesterase 4B Knockout Prevents Skeletal Muscle Atrophy in Rats with Burn Injury. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R417–R421. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Liu, Y.; Zhao, Z.; Liu, Q.; Wang, X.; Xie, Y.; Liu, Y.; Liu, Y.; Yang, Y.; Long, J.; et al. MYB Proto-Oncogene-like 1-TWIST1 Axis Promotes Growth and Metastasis of Hepatocellular Carcinoma Cells. Mol. Ther. Oncolytics 2020, 18, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Rong, Z.-H.; Chang, N.-B.; Yao, Q.-P.; Li, T.; Zhu, X.-L.; Cao, Y.; Jiang, M.-J.; Cheng, Y.-S.; Jiang, R.; Jiang, J. Suppression of CircDcbld1 Alleviates Intimal Hyperplasia in Rat Carotid Artery by Targeting MiR-145-3p/Neuropilin-1. Mol. Ther. Nucleic Acids 2019, 18, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Filipowicz, W.; Jaskiewicz, L.; Kolb, F.A.; Pillai, R.S. Post-Transcriptional Gene Silencing by SiRNAs and MiRNAs. Curr. Opin. Struct. Biol. 2005, 15, 331–341. [Google Scholar] [CrossRef]
- Gorbatenko, A.; Søkilde, R.; Sorensen, E.E.; Newie, I.; Persson, H.; Morancho, B.; Arribas, J.; Litman, T.; Rovira, C.; Pedersen, S.F. HER2 and P95HER2 Differentially Regulate MiRNA Expression in MCF-7 Breast Cancer Cells and Downregulate MYB Proteins through MiR-221/222 and MiR-503. Sci. Rep. 2019, 9, 3352. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: MicroRNA Target Prediction Easy, Fast and Flexible. Nucleic Acids Res. 2006, 34, W451–W454. [Google Scholar] [CrossRef]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs Activate Gene Transcription Epigenetically as an Enhancer Trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.L.; Achuthan, P.; Allen, J.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Azov, A.G.; Bennett, R.; Bhai, J.; et al. Ensembl 2021. Nucleic Acids Res. 2021, 49, D884–D891. [Google Scholar] [CrossRef] [PubMed]
- Koutsoulidou, A.; Mastroyiannopoulos, N.P.; Furling, D.; Uney, J.B.; Phylactou, L.A. Expression of MiR-1, MiR-133a, MiR-133b and MiR-206 Increases during Development of Human Skeletal Muscle. BMC Dev. Biol. 2011, 11, 34. [Google Scholar] [CrossRef]
- Geng, H.-H.; Li, R.; Su, Y.-M.; Xiao, J.; Pan, M.; Cai, X.-X.; Ji, X.-P. The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of MiR-7a on Its Target Genes Expression. PLoS ONE 2016, 11, e0151753. [Google Scholar] [CrossRef]
- Odame, E.; Chen, Y.; Zheng, S.; Dai, D.; Kyei, B.; Zhan, S.; Cao, J.; Guo, J.; Zhong, T.; Wang, L.; et al. Enhancer RNAs: Transcriptional Regulators and Workmates of NamiRNAs in Myogenesis. Cell. Mol. Biol. Lett. 2021, 26, 4. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chen, X.; Yu, B.; He, J.; Chen, D. MicroRNA-27a Promotes Myoblast Proliferation by Targeting Myostatin. Biochem. Biophys. Res. Commun. 2012, 423, 265–269. [Google Scholar] [CrossRef]
- Wu, G.; Yu, W.; Zhang, M.; Yin, R.; Wu, Y.; Liu, Q. MicroRNA-145-3p Suppresses Proliferation and Promotes Apotosis and Autophagy of Osteosarcoma Cell by Targeting HDAC4. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–586. [Google Scholar] [CrossRef]
- Na, Y.-R.; Yoon, Y.-N.; Son, D.-I.; Seok, S.-H. Cyclooxygenase-2 Inhibition Blocks M2 Macrophage Differentiation and Suppresses Metastasis in Murine Breast Cancer Model. PLoS ONE 2013, 8, e63451. [Google Scholar] [CrossRef]
- Wu, W.K.K.; Sung, J.J.Y.; Wu, Y.C.; Li, H.T.; Yu, L.; Li, Z.J.; Cho, C.H. Inhibition of Cyclooxygenase-1 Lowers Proliferation and Induces Macroautophagy in Colon Cancer Cells. Biochem. Biophys. Res. Commun. 2009, 382, 79–84. [Google Scholar] [CrossRef]
- Oh, I.H.; Reddy, E.P. Murine A-Myb Gene Encodes a Transcription Factor, Which Cooperates with Ets-2 and Exhibits Distinctive Biochemical and Biological Activities from c-Myb. J. Biol. Chem. 1997, 272, 21432–21443. [Google Scholar] [CrossRef]
- Naguibneva, I.; Ameyar-Zazoua, M.; Polesskaya, A.; Ait-Si-Ali, S.; Groisman, R.; Souidi, M.; Cuvellier, S.; Harel-Bellan, A. The MicroRNA MiR-181 Targets the Homeobox Protein Hox-A11 during Mammalian Myoblast Differentiation. Nat. Cell Biol. 2006, 8, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Place, R.F.; Li, L.-C.; Pookot, D.; Noonan, E.J.; Dahiya, R. MicroRNA-373 Induces Expression of Genes with Complementary Promoter Sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Malinska, D.; Kudin, A.P.; Bejtka, M.; Kunz, W.S. Changes in Mitochondrial Reactive Oxygen Species Synthesis during Differentiation of Skeletal Muscle Cells. Mitochondrion 2012, 12, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.L. The Whereabouts of MicroRNA Actions: Cytoplasm and Beyond. Trends Cell Biol. 2015, 25, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Wagatsuma, A.; Sakuma, K. Mitochondria as a Potential Regulator of Myogenesis. Sci. World J. 2013, 2013, 593267. [Google Scholar] [CrossRef]
- Bondesen, B.A.; Mills, S.T.; Kegley, K.M.; Pavlath, G.K. The COX-2 Pathway Is Essential during Early Stages of Skeletal Muscle Regeneration. Am. J. Physiol. Physiol. 2004, 287, C475–C483. [Google Scholar] [CrossRef]
- Wang, J.; He, F.; Chen, L.; Li, Q.; Jin, S.; Zheng, H.; Lin, J.; Zhang, H.; Ma, S.; Mei, J.; et al. Resveratrol Inhibits Pulmonary Fibrosis by Regulating MiR-21 through MAPK/AP-1 Pathways. Biomed. Pharmacother. 2018, 105, 37–44. [Google Scholar] [CrossRef]
- Liu, B.; Li, R.; Zhang, J.; Meng, C.; Zhang, J.; Song, X.; Lv, C. MicroRNA-708-3p as a Potential Therapeutic Target via the ADAM17-GATA/STAT3 Axis in Idiopathic Pulmonary Fibrosis. Exp. Mol. Med. 2018, 50, e465. [Google Scholar] [CrossRef]
- Farrell, R.E. Chapter 18—RT-PCR: A Science and an Art Form. In RNA Methodologies; Academic Press: San Diego, CA, USA, 2010; pp. 385–448. ISBN 978-0-12-374727-3. [Google Scholar]
- Magenta, A.; Rossini, A.; Fasanaro, P.; Pompilio, G.; Capogrossi, M.C. Chapter 35—MicroRNAs in Cardiac Regeneration. In Circulation Research; Academic Press: Oxford, UK, 2015; pp. 917–942. ISBN 978-0-12-405544-5. [Google Scholar]
- Kocerha, J.; Faghihi, M.A.; Lopez-Toledano, M.A.; Huang, J.; Ramsey, A.J.; Caron, M.G.; Sales, N.; Willoughby, D.; Elmen, J.; Hansen, H.F.; et al. MicroRNA-219 Modulates NMDA Receptor-Mediated Neurobehavioral Dysfunction. Proc. Natl. Acad. Sci. USA 2009, 106, 3507–3512. [Google Scholar] [CrossRef]
- Prediger, E. Antisense Oligonucleotides (ASOs). Available online: https://sg.idtdna.com/pages/education/decoded/article/antisense-oligonucleotides-(asos) (accessed on 4 October 2021).
- Wang, B. An ASO Modification That Enhances Nuclease Resistance, Lowers Toxicity, and Increases Binding Affinity. Available online: https://sg.idtdna.com/pages/education/decoded/article/an-aso-modification-that-enhances-nuclease-resistance-lowers-toxicity-and-increases-binding-affinity (accessed on 4 October 2021).
- Pallan, P.S.; Allerson, C.R.; Berdeja, A.; Seth, P.P.; Swayze, E.E.; Prakash, T.P.; Egli, M. Structure and Nuclease Resistance of 2′,4′-Constrained 2′-O-Methoxyethyl (CMOE) and 2′-O-Ethyl (CEt) Modified DNAs. Chem. Commun. 2012, 48, 8195–8197. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odame, E.; Li, L.; Nabilla, J.A.; Cai, H.; Xiao, M.; Ye, J.; Chen, Y.; Kyei, B.; Dai, D.; Zhan, S.; et al. miR-145-3p Inhibits MuSCs Proliferation and Mitochondria Mass via Targeting MYBL1 in Jianzhou Big-Eared Goats. Int. J. Mol. Sci. 2023, 24, 8341. https://doi.org/10.3390/ijms24098341
Odame E, Li L, Nabilla JA, Cai H, Xiao M, Ye J, Chen Y, Kyei B, Dai D, Zhan S, et al. miR-145-3p Inhibits MuSCs Proliferation and Mitochondria Mass via Targeting MYBL1 in Jianzhou Big-Eared Goats. International Journal of Molecular Sciences. 2023; 24(9):8341. https://doi.org/10.3390/ijms24098341
Chicago/Turabian StyleOdame, Emmanuel, Li Li, Joshua Abdulai Nabilla, He Cai, Miao Xiao, Jiangfeng Ye, Yuan Chen, Bismark Kyei, Dinghui Dai, Siyuan Zhan, and et al. 2023. "miR-145-3p Inhibits MuSCs Proliferation and Mitochondria Mass via Targeting MYBL1 in Jianzhou Big-Eared Goats" International Journal of Molecular Sciences 24, no. 9: 8341. https://doi.org/10.3390/ijms24098341
APA StyleOdame, E., Li, L., Nabilla, J. A., Cai, H., Xiao, M., Ye, J., Chen, Y., Kyei, B., Dai, D., Zhan, S., Cao, J., Guo, J., Zhong, T., Wang, L., & Zhang, H. (2023). miR-145-3p Inhibits MuSCs Proliferation and Mitochondria Mass via Targeting MYBL1 in Jianzhou Big-Eared Goats. International Journal of Molecular Sciences, 24(9), 8341. https://doi.org/10.3390/ijms24098341






