The Synthesis and Biological Activity of Organotin Complexes with Thio-Schiff Bases Bearing Phenol Fragments
Abstract
1. Introduction
2. Results
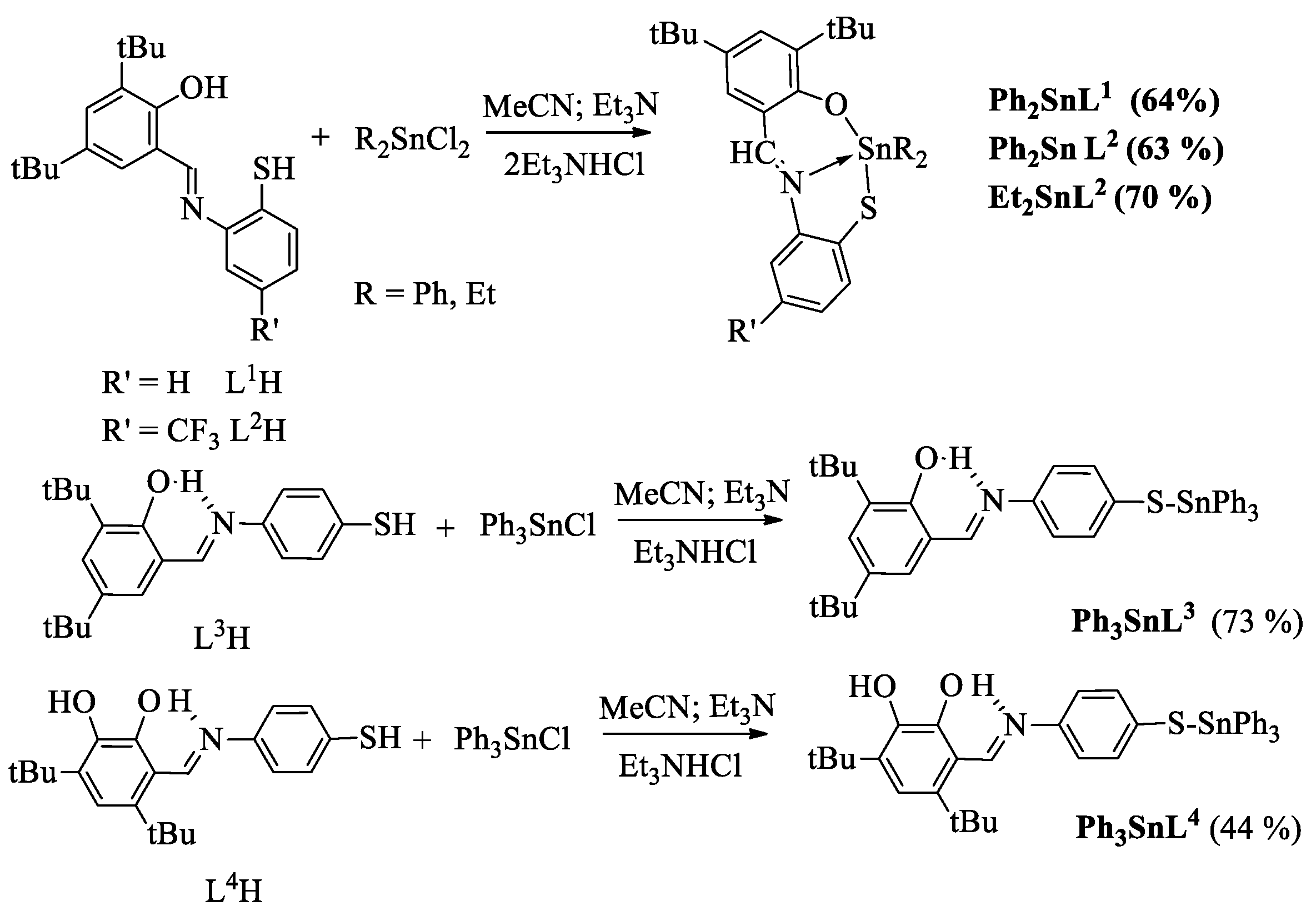
2.1. Synthesis and Characterization
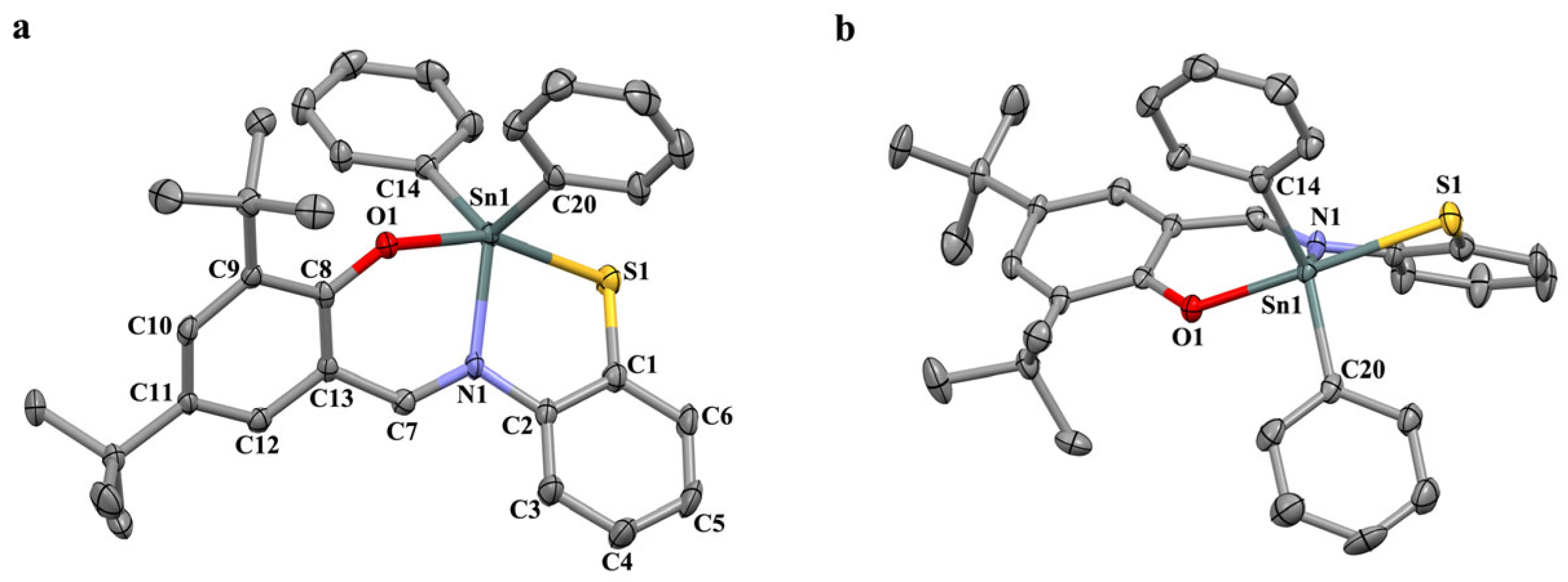
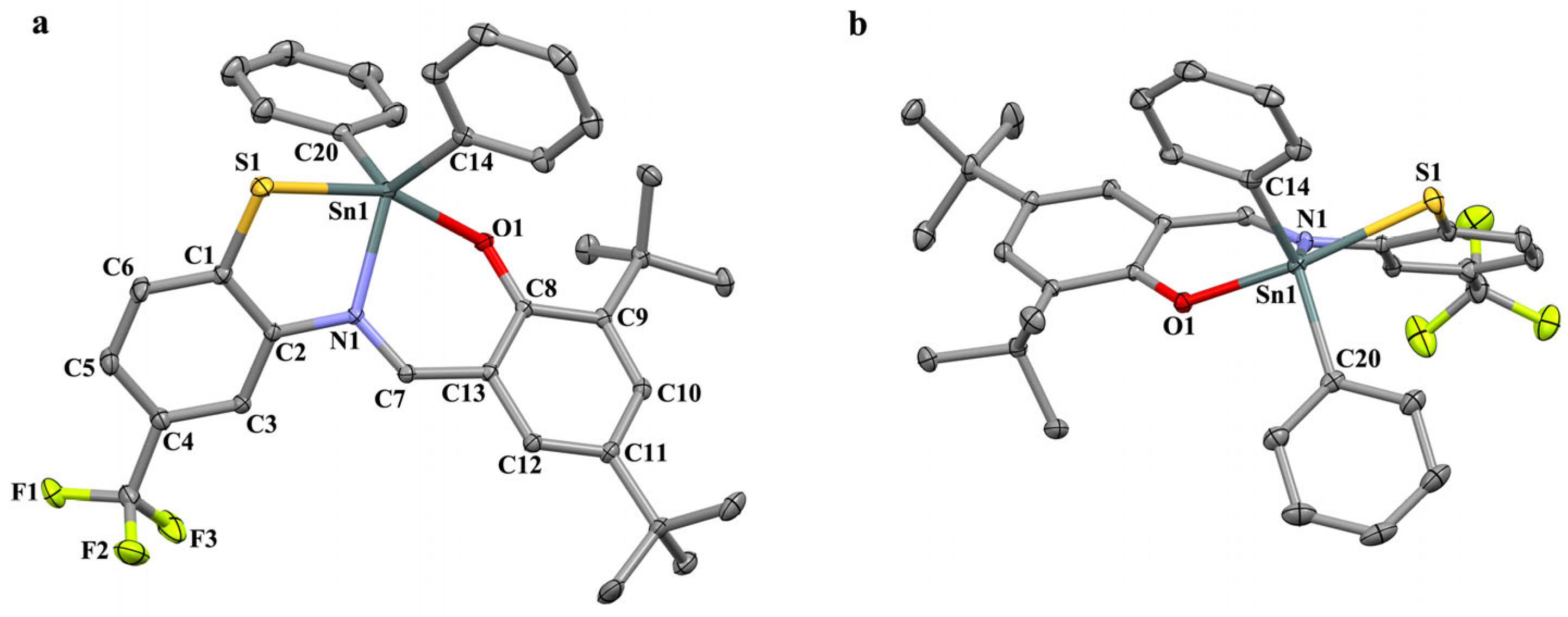
2.2. X-ray Analysis
2.3. Radical Scavenging Activity
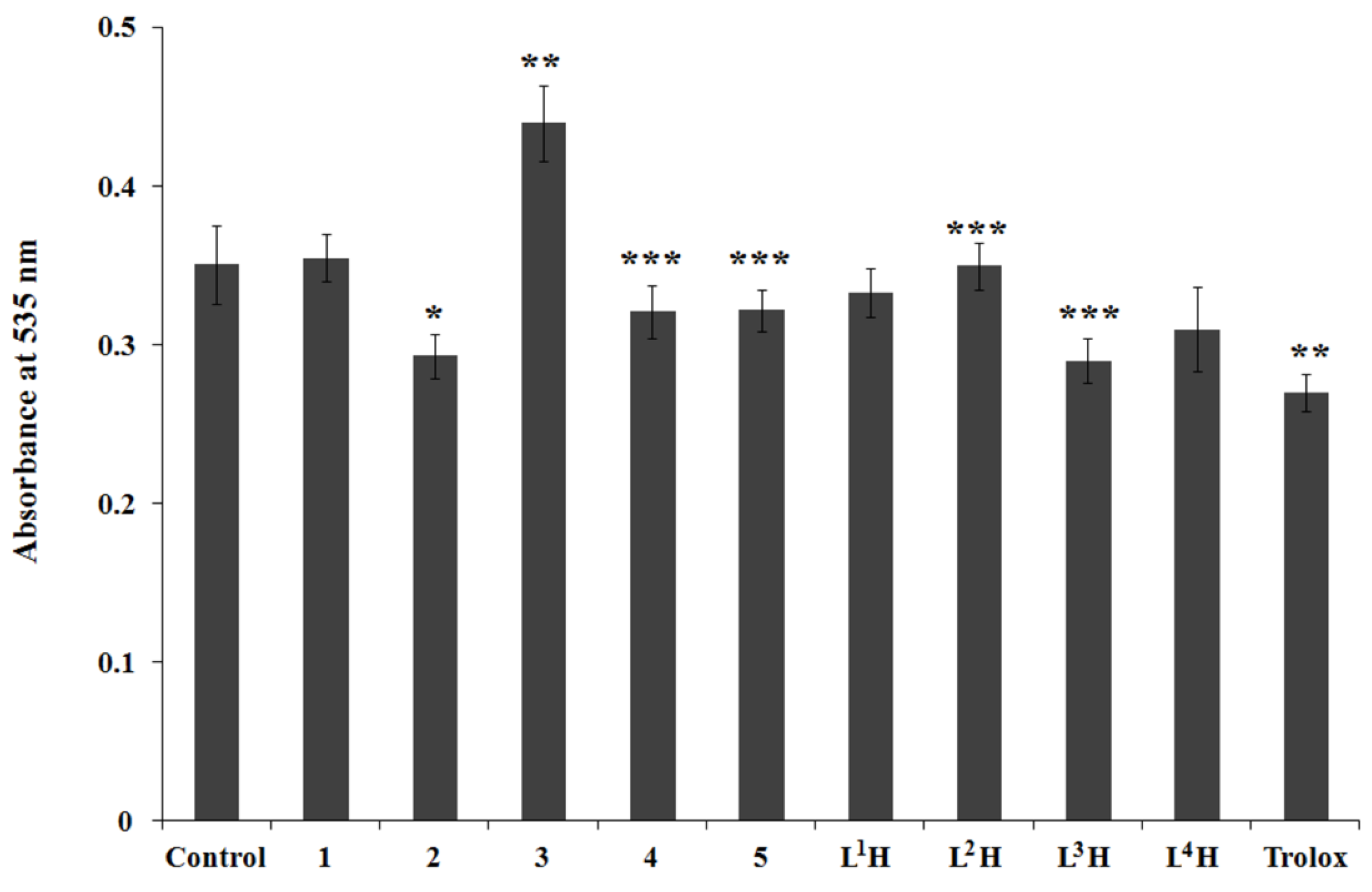
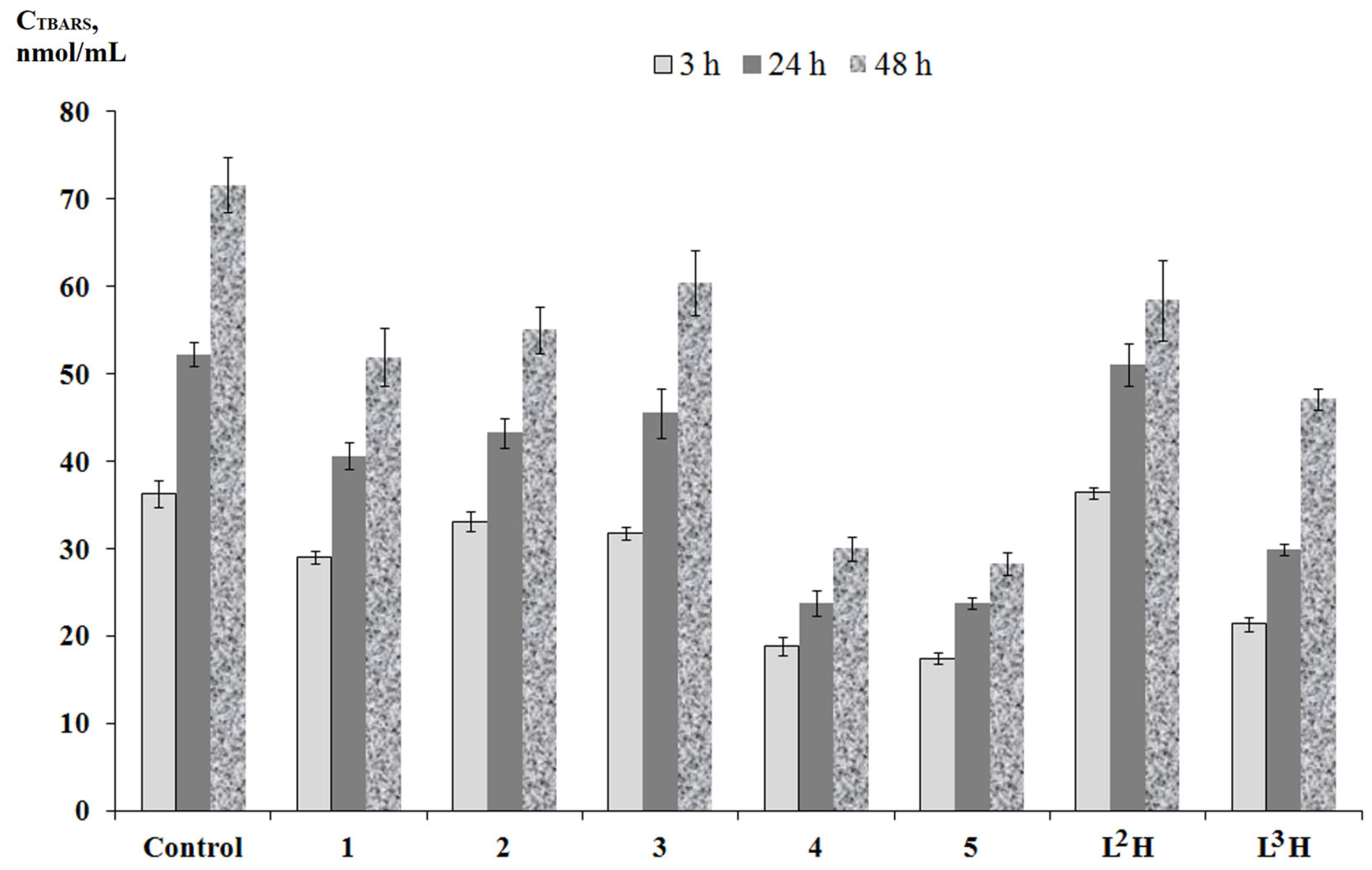
2.4. AAPH-Induced Oxidation of DNA and Lipid Peroxidation
2.5. Antiproliferative Activity
3. Materials and Methods
3.1. General
3.2. Syntheses
3.3. The DPPH Radical Scavenging Activity Assay
3.4. ABTS Assay
3.5. Inhibition of Superoxide Radical Anion Formation by Xanthine Oxidase (NBT Assay)
3.6. AAPH-Induced Oxidation of the DNA Assay
3.7. Lipid Peroxidation of Rat Liver Homogenate
3.8. In Vitro Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zafar, W.; Sumrra, S.H.; Chohan, Z.H. A review: Pharmacological aspects of metal based 1,2,4-triazole derived Schiff bases. Eur. J. Med. Chem. 2021, 222, 113602. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Tanaka, K.; Chujo, Y. Vapochromic Luminescent π-Conjugated Systems with Reversible Coordination-Number Control of Hypervalent Tin(IV)-Fused Azobenzene Complexes. Chem. Eur. J. 2021, 27, 7561–7571. [Google Scholar] [CrossRef] [PubMed]
- Cantón-Diaz, A.; Muñoz-Flores, B.M.; Berrones-Reyes, J.; Moggio, I.; Arias, E.; Turlakov, G.; Santillán, R.; Jiménez-Pérez, V.M. Organotin compounds bearing C3-symmetric Schiff base: Microwave-assisted multicomponent synthesis and their photophysical properties. J. Organomet. Chem. 2021, 122111, 954–955. [Google Scholar] [CrossRef]
- Cantón-Díaz, A.M.; Muñoz-Flores, B.M.; Moggio, I.; Arias, E.; de León, A.; García-López, M.C.; Santillán, R.; Ochoa, M.E.; Jiménez-Pérez, V.M. One-pot microwave-assisted synthesis of organotin Schiff bases: An optical and electrochemical study towards their effect in organic solar cells. New J. Chem. 2018, 42, 14586–14596. [Google Scholar] [CrossRef]
- Lopez-Espejel, V.; Gomez-Trevino, A.; Munoz-Flores, B.M.; Treto-Suarez, M.A.; Schott, E.; Paez-Hernandez, D.; Zarate, X.; Jimenez-Perez, V.M. Organotin Schiff bases as halofluorochromic dyes: Green synthesis, chemio-photophysical characterization, DFT, and their fluorescent bioimaging in vitro. Mater. Chem. B 2021, 9, 7698–7712. [Google Scholar] [CrossRef]
- Vinayak, R.; Dey, D.; Ghosh, D.; Chattopadhyay, D.; Ghosh, A.; Nayek, H.P. Schiff base supported mononuclear organotin(IV) complexes: Syntheses, structures and fluorescence cell imaging. Appl. Organometal. Chem. 2017, 32, e4122. [Google Scholar] [CrossRef]
- Yenişehirli, G.; Öztaş, N.A.; Şahin, E.; Çelebier, M.; Ancın, N.; Öztaş, S.G. Synthesis, Characterization, and In Vitro Antimicrobial Activities of Organotin(IV)Complexes of Schiff Bases with ONO-type Donor Atoms. Heteroatom. Chem. 2010, 21, 373–385. [Google Scholar] [CrossRef]
- Devi, J.; Pachwania, S.; Yadav, J.; Kumar, A. Pentacoordinated diorganotin(IV) complexes resulting from tridentate (NOO) donor Schiff bases: Synthesis, characterization, in vitro antioxidant, antimicrobial activities, and QSAR studies. Phosphorus Sulfur. Silicon Relat. Elem. 2021, 196, 119–132. [Google Scholar] [CrossRef]
- Gonzalez, A.; Gomez, E.; Cortes-Lozada, A.; Hernandez, S.; Ramirez-Apan, T.; Nieto-Camacho, A. Heptacoordinate Tin(IV) Compounds Derived from Pyridine Schiff Bases: Synthesis, Characterization, in Vitro Cytotoxicity, Anti-inflammatory and Antioxidant Activity. Chem. Pharm. Bull. 2009, 57, 5–15. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.; Luna-García, R.; Cortés-Lozada, A.; Hernández, S.; Ramírez-Apan, T.; Nieto-Camacho, A.; Gómez, E. Dinuclear heptacoordinate dibutyltin (IV) complexes derived from Schiff bases and dicarboxylates: Synthesis, cytotoxicity, and antioxidant activity. J. Organomet. Chem. 2013, 738, 10–19. [Google Scholar] [CrossRef]
- Beltran, H.I.; Damian-Zea, C.; Hernandez-Ortega, S.; Nieto-Camacho, A.; Ramírez-Apan, M.T. Synthesis and characterization of di-phenyl-tinIV-salicyliden-ortho-aminophenols: Analysis of in vitro antitumor/antioxidant activities and molecular structures. J. Inorg. Biochem. 2007, 101, 1070–1085. [Google Scholar] [CrossRef]
- Jiang, W.; Qin, Q.; Xiao, X.; Tan, Y. Diorganotin(IV) complexes based on tridentate ONO ligands as potential anticancer agents. J. Inorg. Biochem. 2022, 232, 111808. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Song, Q.; Liu, X.; Li, C.; Wang, H.; Li, C.; Hong, M. Synthesis and in vitro cytotoxicity study of three di-organotin(IV) Schiff base di-acylhydrazone complexes. J. Inorg. Biochem. 2022, 236, 111983. [Google Scholar] [CrossRef]
- Galvan-Hidalgo, J.M.; Gomez, E.; Ramırez-Apan, T.; Nieto-Camacho, A.; Hernandez-Ortega, S. Synthesis and cytotoxic activity of dibutyltin complexes derived from pyridoxamine and salicylaldehydes. Med. Chem. Res. 2015, 24, 3621–3631. [Google Scholar] [CrossRef]
- Roy, N.; Sproules, S.; Weyhermuller, T.; Wieghardt, K. Trivalent Iron and Ruthenium Complexes with a Redox Noninnocent (2-Mercaptophenylimino)-methyl-4,6-di-tert-butylphenolate(2-) Ligand. Inorg. Chem. 2009, 48, 3783–3791. [Google Scholar] [CrossRef]
- Roy, N.; Sproules, S.; Bothe, E.; Weyhermüller, T.; Wieghardt, K. Polynuclear Complexes Containing the Redox Noninnocent Schiff Base Ligand 2-[(E)-2-Mercaptophenylimino]methyl-4,6-di-tert-butylphenolate(2–). Eur. J. Inorg. Chem. 2009, 18, 2655–2663. [Google Scholar] [CrossRef]
- Donzelli, A.; Metushi, I.; Potvin, P.G. Titanium(IV) Complexes of Disulfide-Linked Schiff Bases. Inorg. Chem. 2012, 51, 5138−5145. [Google Scholar] [CrossRef]
- Donzelli, A.; Potvin, P.G. TiIV Complexes of Redox-Active Schiff Bases. Eur. J. Inorg. Chem. 2012, 4, 741–750. [Google Scholar] [CrossRef]
- Ji, J.; Chen, X.; Lin, H.; Jia, A.-Q.; Zhang, Q.-F. Ruthenium(II) complexes with substituted 2-(methylthio)-phenylsalicylaldimine Schiff-base ligands. Inorganica Chim. Acta 2019, 494, 105–111. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Burmistrova, D.A.; Voronina, J.K.; Pomortseva, N.P.; Fokin, V.A.; Tselukovskaya, E.D.; Ananyev, I.V.; Berberova, N.T.; Eremenko, I.L. Heteroligand α-Diimine-Zn(II) Complexes with O,N,O′- and O,N,S-Donor Redox-Active Schiff Bases Synthesis, Structure and Electrochemical Properties. Molecules 2022, 27, 8216. [Google Scholar] [CrossRef]
- Showkot Hossain, A.M.; Mendez-Arriaga, J.M.; Xia, C.; Xie, J.; Gomez-Ruiz, S. Metal complexes with ONS donor Schiff bases. A review. Polyhedron 2022, 217, 115692. [Google Scholar] [CrossRef]
- Tyagi, P.; Tyagi, M.; Agrawal, S.; Chandra, S.; Ojha, H.; Pathak, M. Synthesis, characterization of 1,2,4-triazole Schiff base derived 3d-metal complexes: Inducescytotoxicity in HepG2, MCF-7 cell line, BSA binding fluorescence and DFT study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 171, 246–257. [Google Scholar] [CrossRef]
- Basu Baul, T.S.; Addepalli, M.R.; Hlychho, B.; Lycka, A.; Vamadevan, P.; Saurav, S.; Manna, S.K.; Fatima, M.; Guedes da Silva, C. O,N,S-tris-chelating ligand scaffolds flanked with cyclohexyl or adamantyl substituents anchored with diorganotin(IV) moieties: Synthesis, structures and cytotoxicity. Inorganica Chim. Acta 2022, 537, 120935. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K.; Sismanoglu, T.; Hadjiliadis, N. Anti-proliferative and antitumor activity of organotin(IV) compounds. An overview of the last decade and future perspectives. J. Inorg. Biochem. 2019, 194, 114–152. [Google Scholar] [CrossRef] [PubMed]
- Shpakovsky, D.B.; Banti, C.N.; Mukhatova, E.M.; Gracheva, Y.A.; Osipova, V.P.; Berberova, N.T.; Albov, D.V.; Antonenko, T.A.; Aslanov, L.A.; Milaeva, E.R.; et al. Synthesis, antiradical activity and in vitro cytotoxicity of novel organotin complexes based on 2,6-di-tert-butyl-4-mercaptophenol. Dalton Trans. 2014, 43, 6880–6890. [Google Scholar] [CrossRef]
- Milaeva, E.R.; Shpakovsky, D.B.; Gracheva, Y.A.; Antonenko, T.A.; Osolodkin, D.I.; Palyulin, A.; Shevtsov, P.N.; Neganova, M.E.; Vinogradova, D.V.; Shevtsova, E.F. Some insight into the mode of cytotoxic action of organotin compounds with protective 2,6-di-tert-butylphenol fragments. J. Organomet. Chem. 2015, 782, 96–102. [Google Scholar] [CrossRef]
- Antonenko, T.A.; Shpakovsky, D.B.; Vorobyov, M.A.; Gracheva, Y.A.; Kharitonashvili, E.V.; Dubova, L.G.; Shevtsova, E.F.; Tafeenko, V.A.; Aslanov, L.A.; Iksanova, A.G.; et al. Antioxidative vs cytotoxic activities of organotin complexes bearing 2,6-di-tert-butylphenol moieties. Appl. Organomet. Chem. 2018, 32, e4381. [Google Scholar] [CrossRef]
- Milaeva, E.R.; Shpakovsky, D.B.; Gracheva, Y.A.; Antonenko, T.A.; Ksenofontova, T.D.; Nikitin, E.A.; Berseneva, D.A. Novel selective anticancer agents based on Sn and Au complexes. Mini-review. Pure Appl. Chem. 2020, 92, 1201–1216. [Google Scholar] [CrossRef]
- Protasenko, N.A.; Baryshnikova, S.V.; Cherkasov, A.V.; Poddel’skii, A.I. Pentacoordinated Complexes of Triphenyltin(IV) with Bidentate N-Phenyl-o-iminophenols. Russ. J. Coord. Chem. 2022, 48, 478–486. [Google Scholar] [CrossRef]
- Protasenko, N.A.; Baryshnikova, S.V.; Astaf’eva, T.V.; Cherkasov, A.V.; Poddel’sky, A.I. Mono- and Binuclear Zinc Complexes with a Bidentate Phenol-Containing 2-Benzylideneamino-5-Methylphenol Schiff Base. Russ. J. Coord. Chem. 2021, 47, 417–423. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Poddel’sky, A.I.; Bellan, E.V.; Smolyaninov, I.V.; Cherkasov, A.V.; Fukin, G.K.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Ferrocene-containing tin(IV) complexes based on o-benzoquinone and o-iminobenzoquinone ligands. Synthesis, molecular structure and electrochemical properties. Inorg. Chem. 2020, 59, 6774–6784. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Bellan, E.V.; Poddel’skii, A.I.; Smolyaninov, I.V.; Berberova, N.T.; Abakumov, G.A. Synthesis, structure, and properties of a new multiredox-active Sn(IV) complex based on 3,6-di-tert-butyl-o-benzoquinone and ferrocenylaldimine phenol. Dokl. Chem. 2017, 474, 101–104. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Bellan, E.V.; Poddel’sky, A.I.; Arsenyev, M.V.; Smolyaninov, I.V.; Fukin, G.K.; Piskunov, A.V.; Berberova, N.T.; Cherkasov, V.K.; Abakumov, G.A. Tin(IV) and antimony(V) complexes bearing catecholate ligand connected to ferrocene. Synthesis, molecular structure and electrochemical properties. Eur. J. Inorg. Chem. 2016, 33, 5230–5241. [Google Scholar] [CrossRef]
- Baryshnikova, S.V.; Bellan, E.V.; Poddel’sky, A.I.; Fukin, G.K.; Abakumov, G.A. The synthesis and structure of new tin(II) complexes based on ferrocenyl-containing o-iminophenols. Inorg. Chem. Commun. 2016, 69, 94–97. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Burmistrova, D.A.; Pomortseva, N.P.; Voronina, Y.K.; Poddel’sky, A.I.; Berberova, N.T.; Eremenko, I.L. Complexes R2Sn(IV)L with O,N,O’-Donor Schiff Bases: Synthesis, Structures, and Redox Properties. Russ. J. Coord. Chem. 2023, 49, 124–141. [Google Scholar] [CrossRef]
- Chans, G.M.; Nieto-Camacho, A.; Ramırez-Apan, T.; Hernandez-Ortega, S.; Alvarez-Toledano, C.; Gomez, E. Synthetic, Spectroscopic, Crystallographic, and Biological Studies of Seven-Coordinated Diorganotin(IV) Complexes Derived from Schiff Bases and Pyridinic Carboxylic Acids. Aust. J. Chem. 2015, 69, 279–290. [Google Scholar] [CrossRef]
- Xanthopoulou, M.N.; Hadjikakou, S.K.; Hadjiliadis, N.; Milaeva, E.R.; Gracheva, J.A.; Tyurin, V.Y.; Kourkoumelis, N.; Christoforidis, K.C.; Metsios, A.K.; Karkabounas, S.; et al. Biological studies of new organotin(IV) complexes of thioamide ligands. Eur. J. Med. Chem. 2008, 43, 327–335. [Google Scholar] [CrossRef]
- Chegerev, M.G.; Piskunov, A.V. Chemistry of Complexes of Group 14 Elements Based on Redox-Active Ligands of the o-Iminoquinone Type. Russ. J. Coord. Chem. 2018, 44, 258–271. [Google Scholar] [CrossRef]
- Antonova, N.A.; Kolyada, M.N.; Osipova, V.P.; Pimenov, Y.T.; Berberova, N.T.; Tyurin, V.Y.; Gracheva, Y.A.; Milaeva, E.R. Study of the Antioxidant Properties of Phosphorylated Phenols. Dokl. Chem. 2008, 419, 62–64. [Google Scholar] [CrossRef]
- Petrosyan, V.D.; Milaeva, E.R.; Gracheva, Y.A.; Grigoriev, E.V.; Tyurin, V.Y.; Pimenov, Y.T.; Berberova, N.T. The promoting effect of organotin compounds upon peroxidation of oleic acid. Appl. Organomet. Chem. 2002, 16, 655–659. [Google Scholar] [CrossRef]
- Mukhatova, E.M.; Osipova, V.P.; Kolyada, M.N.; Movchan, N.O.; Shpakovsky, D.B.; Gracheva, Y.A.; Orlova, S.I.; Milaeva, E.R. Synthesis and Antioxidant Activity of New Organotin Compounds Containing a 2,6-Di-tert-butylphenol Moiety. Dokl. Chem. 2013, 451, 177–180. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Burmistrova, D.A.; Arsenyev, M.V.; Almyasheva, N.R.; Ivanova, E.S.; Smolyaninova, S.A.; Pashchenko, K.P.; Poddel’sky, A.I.; Berberova, N.T. Catechol- and Phenol-Containing Thio-Schiff Bases: Synthesis, Electrochemical Properties and Biological Evaluation. ChemistrySelect 2021, 6, 10609–10618. [Google Scholar] [CrossRef]
- Antonenko, T.A.; Shpakovsky, D.B.; Berseneva, D.A.; Gracheva, Y.A.; Dubova, L.G.; Shevtsov, P.N.; Redkozubova, O.M.; Shevtsova, E.F.; Tafeenko, V.A.; Aslanov, L.A.; et al. Cytotoxic activity of organotin carboxylates based on synthetic phenolic antioxidants and polycyclic bile acids. J. Organomet. Chem. 2020, 909, 121089. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon: Oxford, UK, 1980. [Google Scholar]
- Sheldrick, M. SADABS; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanism of antioxidant activity using the DPPH free radical method. Food. Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Smolyaninov, I.V.; Poddel’sky, A.I.; Antonova, N.A.; Smolyaninova, S.A.; Osipova, V.P.; Berberova, N.T. Radical scavenging activity of sterically hindered catecholate and o-amidophenolate complexes of LSbVPh3 type. J. Organomet. Chem. 2011, 696, 2611–2620. [Google Scholar] [CrossRef]
- Re, R.; Pellergrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Toda, S.; Kumura, M.; Ohnishi, M. Effects of Phenolcarboxylic Acids on Superoxide Anion and Lipid Peroxidation Induced by Superoxide Anion. Planta Med. 1991, 57, 8–10. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Z.-Q. The protective effect of hydroxyl-substituted Schiff bases on the radical-induced oxidation of DNA. J. Phys. Org. Chem. 2009, 22, 791–798. [Google Scholar] [CrossRef]
- Stroev, E.N.; Makarova, V.G. Praktikum po Biologiheskoy Khimii [Practical Work in Biological Chemistry]; Vushaya Shkola: Moscow, Russia, 1986. (In Russian) [Google Scholar]
| N | Compound | IC50 (DPPH), µM | IC50 (ABTS∙+), µM | ABTCTEAC | IC50 (O2∙−), µM |
|---|---|---|---|---|---|
| 1 | Ph2SnL1 | >200 | 28.8 ± 0.9 | 0.62 ± 0.09 | 7.80 ± 0.23 |
| 2 | Ph2SnL2 | >200 | 25.0 ± 1.3 | 0.79 ± 0.11 | 5.16 ± 0.08 |
| 3 | Et2SnL2 | 143.7 ± 10.3 | 35.4 ± 1.8 | 0.57 ± 0.04 | 24.02 ± 0.10 |
| 4 | Ph3SnL3 | 44.7 ± 1.1 | 17.2 ± 0.5 | 1.25 ± 0.11 | pr.a * |
| 5 | Ph3SnL4 | 16.1 ± 0.4 | 22.1 ± 0.7 | 0.93 ± 0.13 | pr.a * |
| L1H | 42.1 ± 1.9 | 16.9 ± 0.8 | 1.02 ± 0.10 | 89.06 ± 3.10 | |
| L2H | 43.5 ± 1.5 | 14.2 ± 0.6 | 1.16 ± 0.12 | 74.07 ± 1.06 | |
| L3H | 30.0 ± 1.3 | 17.7 ± 1.2 | 1.07 ± 0.09 | >100 | |
| L4H | 10.0 ± 0.5 | 8.8 ± 0.6 | 1.88 ± 0.10 | 68.69 ± 1.72 | |
| Trolox | 12.0 ± 0.5 | 16.0 ± 1.0 | 1.00 ± 0.03 | 62.7 ± 0.60 |
| N | Compound | IC50, µM | ||
|---|---|---|---|---|
| A 549 | HCT-116 | MCF-7 | ||
| 1 | Ph2SnL1 | 167.88 ± 9.32 | 54.6 ± 8.92 | 120.84 ± 5.44 |
| 2 | Ph2SnL2 | 28.89 ± 1.15 | 11.56 ± 0.87 | 5.91 ± 0.03 |
| 3 | Et2SnL2 | 72.06 ± 3.01 | 38.91 ± 1.74 | 75.09 ± 1.06 |
| 4 | Ph3SnL3 | 0.42 ± 0.01 | 0.19 ± 0.02 | 0.23 ± 0.03 |
| 5 | Ph3SnL4 | 0.32 ± 0.04 | 0.23 ± 0.01 | 0.31 ± 0.03 |
| a L3H | 30.63 ± 1.35 | 25.50 ± 0.99 | 43.19 ± 4.27 | |
| a L4H | 3.53 ± 0.31 | 11.03 ± 0.17 | 7.43 ± 0.70 | |
| b cisplatin | 9.02 ± 0.90 | 11.20 ± 1.90 | 15.2 ± 1.10 | |
| Doxorubicin | 0.13 ± 0.02 | 0.35 ± 0.05 | 0.62 ± 0.07 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smolyaninov, I.V.; Poddel’sky, A.I.; Burmistrova, D.A.; Voronina, Y.K.; Pomortseva, N.P.; Polovinkina, M.A.; Almyasheva, N.R.; Zamkova, M.A.; Berberova, N.T.; Eremenko, I.L. The Synthesis and Biological Activity of Organotin Complexes with Thio-Schiff Bases Bearing Phenol Fragments. Int. J. Mol. Sci. 2023, 24, 8319. https://doi.org/10.3390/ijms24098319
Smolyaninov IV, Poddel’sky AI, Burmistrova DA, Voronina YK, Pomortseva NP, Polovinkina MA, Almyasheva NR, Zamkova MA, Berberova NT, Eremenko IL. The Synthesis and Biological Activity of Organotin Complexes with Thio-Schiff Bases Bearing Phenol Fragments. International Journal of Molecular Sciences. 2023; 24(9):8319. https://doi.org/10.3390/ijms24098319
Chicago/Turabian StyleSmolyaninov, Ivan V., Andrey I. Poddel’sky, Daria A. Burmistrova, Yulia K. Voronina, Nadezhda P. Pomortseva, Maria A. Polovinkina, Nailya R. Almyasheva, Maria A. Zamkova, Nadezhda T. Berberova, and Igor L. Eremenko. 2023. "The Synthesis and Biological Activity of Organotin Complexes with Thio-Schiff Bases Bearing Phenol Fragments" International Journal of Molecular Sciences 24, no. 9: 8319. https://doi.org/10.3390/ijms24098319
APA StyleSmolyaninov, I. V., Poddel’sky, A. I., Burmistrova, D. A., Voronina, Y. K., Pomortseva, N. P., Polovinkina, M. A., Almyasheva, N. R., Zamkova, M. A., Berberova, N. T., & Eremenko, I. L. (2023). The Synthesis and Biological Activity of Organotin Complexes with Thio-Schiff Bases Bearing Phenol Fragments. International Journal of Molecular Sciences, 24(9), 8319. https://doi.org/10.3390/ijms24098319







