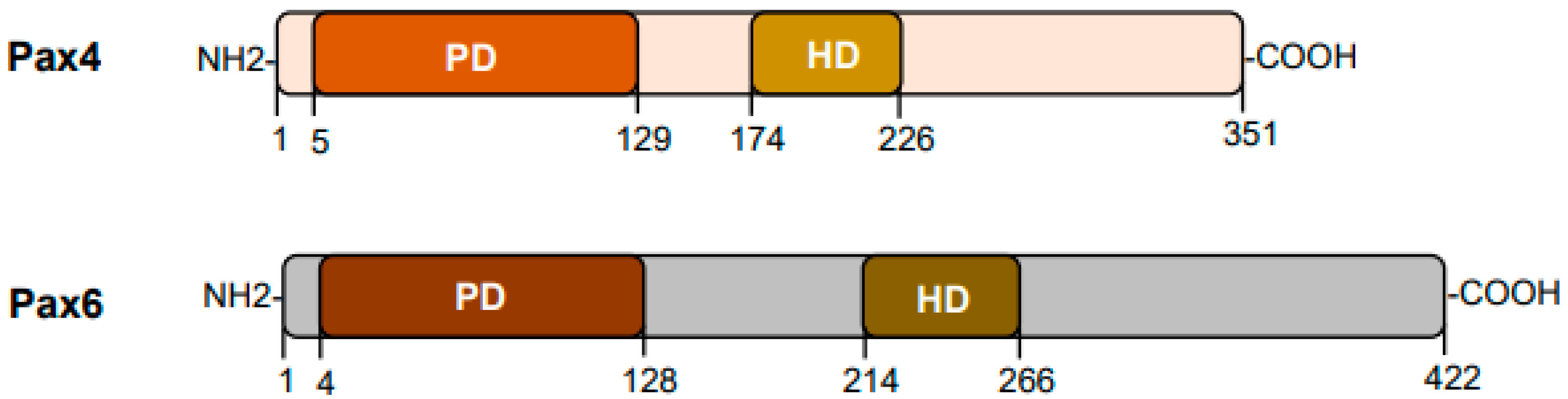
Pax4 in Health and Diabetes
Abstract
1. Introduction
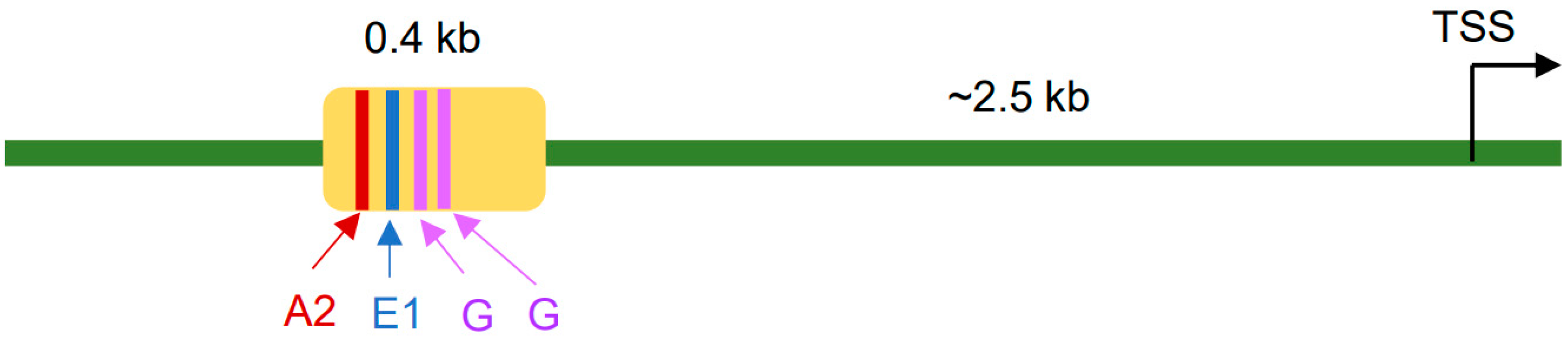
2. Pax4 Gene Expression in the Pancreas
3. The Role of Pax4 in the Embryonic Development of Pancreatic Islets: Determination of β Cell Lineage
4. Mechanisms of Pax4 Action in Islet Cell Development
5. The Role of Pax4 in Adult Islet Cells: Proliferation, Survival, and Transdifferentiation
6. The Role of Pax4 in the Development of Diabetes
| PAX4 Mutation | Associated Types of Diabetes | Phenotypes | References |
|---|---|---|---|
| R121W | T2D | Impaired DNA binding activity; Loss of Pax4 inhibition on Pax6-induced transcription; Family history of diabetes, impaired glucose tolerance, diabetic state varies from mild to severe | [12] |
| R133W | Ketosis-prone diabetes | Decreased repressor activity; Severe alteration of GSIS; Low C-peptide levels | [13] |
| R164W | MODY9 | Impaired transcriptional repression of insulin and glucagon promoters; Impaired glucose tolerance; Family history of diabetes | [14] |
| R164Q | MODY9 | Heterogeneous clinical manifestation; Age at diagnosis ranges from 24 years to 50 years | [57] |
| R163W | MODY9 | Low-frequency heterozygous missense mutation, likely autosomal dominant; Affects the DNA-binding homeodomain; Infantile-onset; Polydipsia, polyuria, and hyperglycemia | [58] |
| R192H | Early-onset T2D, MODY9 | Impaired repressor activity; Higher hemoglobin A1c levels; Decreased 2 h C-peptide levels in OGTT | [53,55,59] |
| R192S | Early-onset T2D | Single-nucleotide polymorphism associated with lower fasting C-peptide levels and 2 h C-peptide levels in OGTT; Higher BMI | [59] |
| P321H | T1D | Reduced repressor activity in α and β cell lines; Impaired DNA-binding activity; Diminished capacity for β cell proliferation; Positive for anti-islet cell antibodies with susceptibility to diabetic state | [52] |
| IVS7-1G>A | MODY9 | Causes aberrant mRNA splicing and Q250 deletion, impairing Pax4 repressor functions and increasing susceptibility to apoptosis at high glucose; Associated with early-onset renal complications | [15] |
7. Pax4 as a Potential Therapeutic Target for Diabetes Treatment
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Blake, J.A.; Ziman, M.R. Pax genes: Regulators of lineage specification and progenitor cell maintenance. Development 2014, 141, 737–751. [Google Scholar] [CrossRef]
- Chi, N.; Epstein, J.A. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 2002, 18, 41–47. [Google Scholar] [CrossRef]
- Thompson, B.; Davidson, E.A.; Liu, W.; Nebert, D.W.; Bruford, E.A.; Zhao, H.; Dermitzakis, E.T.; Thompson, D.C.; Vasiliou, V. Overview of PAX gene family: Analysis of human tissue-specific variant expression and involvement in human disease. Hum. Genet. 2021, 140, 381–400. [Google Scholar] [CrossRef]
- Bopp, D.; Burri, M.; Baumgartner, S.; Frigerio, G.; Noll, M. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell 1986, 47, 1033–1040. [Google Scholar] [CrossRef]
- Treisman, J.; Harris, E.; Desplan, C. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 1991, 5, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Walther, C.; Guenet, J.L.; Simon, D.; Deutsch, U.; Jostes, B.; Goulding, M.D.; Plachov, D.; Balling, R.; Gruss, P. Pax: A murine multigene family of paired box-containing genes. Genomics 1991, 11, 424–434. [Google Scholar] [CrossRef]
- Stapleton, P.; Weith, A.; Urbanek, P.; Kozmik, Z.; Busslinger, M. Chromosomal localization of seven PAX genes and cloning of a novel family member, PAX-9. Nat. Genet. 1993, 3, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.I.; Juarez-Vicente, F.; Cobo-Vuilleumier, N.; Garcia-Dominguez, M.; Gauthier, B.R. The Diabetes-Linked Transcription Factor PAX4: From Gene to Functional Consequences. Genes 2017, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Yamaoka, T.; Otsuka, S.; Moritani, M.; Matsumoto, T.; Itakura, M. Molecular cloning of mouse paired-box-containing gene (Pax)-4 from an islet beta cell line and deduced sequence of human Pax-4. Biochem. Biophys. Res. Commun. 1998, 242, 176–180. [Google Scholar] [CrossRef]
- Inoue, H.; Nomiyama, J.; Nakai, K.; Matsutani, A.; Tanizawa, Y.; Oka, Y. Isolation of full-length cDNA of mouse PAX4 gene and identification of its human homologue. Biochem. Biophys. Res. Commun. 1998, 243, 628–633. [Google Scholar] [CrossRef]
- Smith, S.B.; Ee, H.C.; Conners, J.R.; German, M.S. Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol. Cell Biol. 1999, 19, 8272–8280. [Google Scholar] [CrossRef]
- Shimajiri, Y.; Sanke, T.; Furuta, H.; Hanabusa, T.; Nakagawa, T.; Fujitani, Y.; Kajimoto, Y.; Takasu, N.; Nanjo, K. A missense mutation of Pax4 gene (R121W) is associated with type 2 diabetes in Japanese. Diabetes 2001, 50, 2864–2869. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Smith, S.B.; Le May, C.; Leal, S.M.; Gautier, J.F.; Molokhia, M.; Riveline, J.P.; Rajan, A.S.; Kevorkian, J.P.; Zhang, S.; et al. PAX4 gene variations predispose to ketosis-prone diabetes. Hum. Mol. Genet. 2004, 13, 3151–3159. [Google Scholar] [CrossRef]
- Plengvidhya, N.; Kooptiwut, S.; Songtawee, N.; Doi, A.; Furuta, H.; Nishi, M.; Nanjo, K.; Tantibhedhyangkul, W.; Boonyasrisawat, W.; Yenchitsomanus, P.T.; et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J. Clin. Endocrinol. Metab. 2007, 92, 2821–2826. [Google Scholar] [CrossRef]
- Sujjitjoon, J.; Kooptiwut, S.; Chongjaroen, N.; Tangjittipokin, W.; Plengvidhya, N.; Yenchitsomanus, P.T. Aberrant mRNA splicing of paired box 4 (PAX4) IVS7-1G>A mutation causing maturity-onset diabetes of the young, type 9. Acta Diabetol. 2016, 53, 205–216. [Google Scholar] [CrossRef]
- Rath, M.F.; Bailey, M.J.; Kim, J.S.; Coon, S.L.; Klein, D.C.; Moller, M. Developmental and daily expression of the Pax4 and Pax6 homeobox genes in the rat retina: Localization of Pax4 in photoreceptor cells. J. Neurochem. 2009, 108, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.F.; Bailey, M.J.; Kim, J.S.; Ho, A.K.; Gaildrat, P.; Coon, S.L.; Moller, M.; Klein, D.C. Developmental and diurnal dynamics of Pax4 expression in the mammalian pineal gland: Nocturnal down-regulation is mediated by adrenergic-cyclic adenosine 3’,5’-monophosphate signaling. Endocrinology 2009, 150, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Pineda, B.; Chowdhury, K.; Torres, M.; Oliver, G.; Gruss, P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 1997, 386, 399–402. [Google Scholar] [CrossRef]
- Xu, W.; Murphy, L.J. Cloning of the mouse Pax4 gene promoter and identification of a pancreatic beta cell specific enhancer. Mol. Cell Endocrinol. 2000, 170, 79–89. [Google Scholar] [CrossRef]
- Wang, J.; Elghazi, L.; Parker, S.E.; Kizilocak, H.; Asano, M.; Sussel, L.; Sosa-Pineda, B. The concerted activities of Pax4 and Nkx2.2 are essential to initiate pancreatic beta-cell differentiation. Dev. Biol. 2004, 266, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Gauthier, B.R. A focus on the role of Pax4 in mature pancreatic islet beta-cell expansion and survival in health and disease. J. Mol. Endocrinol. 2008, 40, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.I.; Fuente-Martin, E.; Brun, T.; Cobo-Vuilleumier, N.; Jimenez-Moreno, C.M.; Herrera Gomez, I.G.; Lopez Noriega, L.; Mellado-Gil, J.M.; Martin-Montalvo, A.; Soria, B.; et al. PAX4 Defines an Expandable beta-Cell Subpopulation in the Adult Pancreatic Islet. Sci. Rep. 2015, 5, 15672. [Google Scholar] [CrossRef]
- Brink, C.; Chowdhury, K.; Gruss, P. Pax4 regulatory elements mediate beta cell specific expression in the pancreas. Mech. Dev. 2001, 100, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Brink, C.; Gruss, P. DNA sequence motifs conserved in endocrine promoters are essential for Pax4 expression. Dev. Dyn. 2003, 228, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Elayat, A.A.; El-Naggar, M.M.; Tahir, M. An immunocytochemical and morphometric study of the rat pancreatic islets. J. Anat. 1995, 186 Pt 3, 629–637. [Google Scholar]
- Collombat, P.; Hecksher-Sørensen, J.; Serup, P.; Mansouri, A. Specifying pancreatic endocrine cell fates. Mech. Dev. 2006, 123, 501–512. [Google Scholar] [CrossRef]
- Pictet, R.L.; Clark, W.R.; Williams, R.H.; Rutter, W.J. An ultrastructural analysis of the developing embryonic pancreas. Dev. Biol. 1972, 29, 436–467. [Google Scholar] [CrossRef]
- Le Douarin, N.M. On the origin of pancreatic endocrine cells. Cell 1988, 53, 169–171. [Google Scholar] [CrossRef]
- Kordowich, S.; Serup, P.; Collombat, P.; Mansouri, A. Generation of animals allowing the conditional inactivation of the Pax4 gene. Transgenic Res. 2012, 21, 1215–1220. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Song, Y.; Deng, J.; Chen, M.; Ouyang, H.; Lai, L.; Li, Z. Generation and Phenotype Identification of PAX4 Gene Knockout Rabbit by CRISPR/Cas9 System. G3 2018, 8, 2833–2840. [Google Scholar] [CrossRef]
- Djiotsa, J.; Verbruggen, V.; Giacomotto, J.; Ishibashi, M.; Manning, E.; Rinkwitz, S.; Manfroid, I.; Voz, M.L.; Peers, B. Pax4 is not essential for beta-cell differentiation in zebrafish embryos but modulates alpha-cell generation by repressing arx gene expression. BMC Dev. Biol. 2012, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Collombat, P.; Hecksher-Sorensen, J.; Broccoli, V.; Krull, J.; Ponte, I.; Mundiger, T.; Smith, J.; Gruss, P.; Serup, P.; Mansouri, A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development 2005, 132, 2969–2980. [Google Scholar] [CrossRef]
- Collombat, P.; Mansouri, A.; Hecksher-Sorensen, J.; Serup, P.; Krull, J.; Gradwohl, G.; Gruss, P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003, 17, 2591–2603. [Google Scholar] [CrossRef]
- Petersen, H.V.; Jorgensen, M.C.; Andersen, F.G.; Jensen, J.; F-Nielsen, T.; Jorgensen, R.; Madsen, O.D.; Serup, P. Pax4 represses pancreatic glucagon gene expression. Mol. Cell Biol. Res. Commun. 2000, 3, 249–254. [Google Scholar] [CrossRef]
- Swisa, A.; Avrahami, D.; Eden, N.; Zhang, J.; Feleke, E.; Dahan, T.; Cohen-Tayar, Y.; Stolovich-Rain, M.; Kaestner, K.H.; Glaser, B.; et al. PAX6 maintains beta cell identity by repressing genes of alternative islet cell types. J. Clin. Investig. 2017, 127, 230–243. [Google Scholar] [CrossRef]
- Ashery-Padan, R.; Zhou, X.; Marquardt, T.; Herrera, P.; Toube, L.; Berry, A.; Gruss, P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev. Biol. 2004, 269, 479–488. [Google Scholar] [CrossRef]
- Sander, M.; Neubuser, A.; Kalamaras, J.; Ee, H.C.; Martin, G.R.; German, M.S. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997, 11, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, L.; Sosa-Pineda, B.; Chowdhury, K.; Mansouri, A.; Gruss, P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature 1997, 387, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Ritz-Laser, B.; Estreicher, A.; Gauthier, B.R.; Mamin, A.; Edlund, H.; Philippe, J. The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia 2002, 45, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Cragg, H.; Elrick, L.J.; Macfarlane, W.M.; Shennan, K.I.; Docherty, K. Inhibitory effect of pax4 on the human insulin and islet amyloid polypeptide (IAPP) promoters. FEBS Lett. 1999, 463, 53–57. [Google Scholar] [CrossRef]
- Brun, T.; Hu He, K.H.; Lupi, R.; Boehm, B.; Wojtusciszyn, A.; Sauter, N.; Donath, M.; Marchetti, P.; Maedler, K.; Gauthier, B.R. The diabetes-linked transcription factor Pax4 is expressed in human pancreatic islets and is activated by mitogens and GLP-1. Hum. Mol. Genet. 2008, 17, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Jang, H.; Kim, Y.Y.; Choe, S.S.; Kong, J.; Hwang, I.; Park, J.; Im, S.S.; Kim, J.B. SREBP1c-PAX4 Axis Mediates Pancreatic beta-Cell Compensatory Responses Upon Metabolic Stress. Diabetes 2019, 68, 81–94. [Google Scholar] [CrossRef]
- Brun, T.; Franklin, I.; St-Onge, L.; Biason-Lauber, A.; Schoenle, E.J.; Wollheim, C.B.; Gauthier, B.R. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J. Cell Biol. 2004, 167, 1123–1135. [Google Scholar] [CrossRef]
- Mellado-Gil, J.M.; Jimenez-Moreno, C.M.; Martin-Montalvo, A.; Alvarez-Mercado, A.I.; Fuente-Martin, E.; Cobo-Vuilleumier, N.; Lorenzo, P.I.; Bru-Tari, E.; Herrera-Gomez Ide, G.; Lopez-Noriega, L.; et al. PAX4 preserves endoplasmic reticulum integrity preventing beta cell degeneration in a mouse model of type 1 diabetes mellitus. Diabetologia 2016, 59, 755–765. [Google Scholar] [CrossRef]
- Hu He, K.H.; Lorenzo, P.I.; Brun, T.; Jimenez Moreno, C.M.; Aeberhard, D.; Vallejo Ortega, J.; Cornu, M.; Thorel, F.; Gjinovci, A.; Thorens, B.; et al. In vivo conditional Pax4 overexpression in mature islet beta-cells prevents stress-induced hyperglycemia in mice. Diabetes 2011, 60, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- Brun, T.; Duhamel, D.L.; Hu He, K.H.; Wollheim, C.B.; Gauthier, B.R. The transcription factor PAX4 acts as a survival gene in INS-1E insulinoma cells. Oncogene 2007, 26, 4261–4271. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, K.R.; Zhang, Y.; Cao, A.M.; Wang, H.; Fonseca, V.A.; Wu, H. Pax4 Gene Delivery Improves Islet Transplantation Efficacy by Promoting beta Cell Survival and alpha-to-beta Cell Transdifferentiation. Cell Transplant. 2020, 29, 963689720958655. [Google Scholar] [CrossRef] [PubMed]
- Collombat, P.; Xu, X.; Ravassard, P.; Sosa-Pineda, B.; Dussaud, S.; Billestrup, N.; Madsen, O.D.; Serup, P.; Heimberg, H.; Mansouri, A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009, 138, 449–462. [Google Scholar] [CrossRef]
- Al-Hasani, K.; Pfeifer, A.; Courtney, M.; Ben-Othman, N.; Gjernes, E.; Vieira, A.; Druelle, N.; Avolio, F.; Ravassard, P.; Leuckx, G.; et al. Adult duct-lining cells can reprogram into beta-like cells able to counter repeated cycles of toxin-induced diabetes. Dev. Cell 2013, 26, 86–100. [Google Scholar] [CrossRef]
- Zhang, Y.; Fava, G.E.; Wang, H.; Mauvais-Jarvis, F.; Fonseca, V.A.; Wu, H. PAX4 Gene Transfer Induces alpha-to-beta Cell Phenotypic Conversion and Confers Therapeutic Benefits for Diabetes Treatment. Mol. Ther. 2016, 24, 251–260. [Google Scholar] [CrossRef]
- Druelle, N.; Vieira, A.; Shabro, A.; Courtney, M.; Mondin, M.; Rekima, S.; Napolitano, T.; Silvano, S.; Navarro-Sanz, S.; Hadzic, B.; et al. Ectopic expression of Pax4 in pancreatic delta cells results in beta-like cell neogenesis. J. Cell Biol. 2017, 216, 4299–4311. [Google Scholar] [CrossRef] [PubMed]
- Biason-Lauber, A.; Boehm, B.; Lang-Muritano, M.; Gauthier, B.R.; Brun, T.; Wollheim, C.B.; Schoenle, E.J. Association of childhood type 1 diabetes mellitus with a variant of PAX4: Possible link to beta cell regenerative capacity. Diabetologia 2005, 48, 900–905. [Google Scholar] [CrossRef]
- Cheung, C.Y.; Tang, C.S.; Xu, A.; Lee, C.H.; Au, K.W.; Xu, L.; Fong, C.H.; Kwok, K.H.; Chow, W.S.; Woo, Y.C.; et al. Exome-chip association analysis reveals an Asian-specific missense variant in PAX4 associated with type 2 diabetes in Chinese individuals. Diabetologia 2017, 60, 107–115. [Google Scholar] [CrossRef]
- Jo, W.; Endo, M.; Ishizu, K.; Nakamura, A.; Tajima, T. A novel PAX4 mutation in a Japanese patient with maturity-onset diabetes of the young. Tohoku J. Exp. Med. 2011, 223, 113–118. [Google Scholar] [CrossRef]
- Kooptiwut, S.; Plengvidhya, N.; Chukijrungroat, T.; Sujjitjoon, J.; Semprasert, N.; Furuta, H.; Yenchitsomanus, P.T. Defective PAX4 R192H transcriptional repressor activities associated with maturity onset diabetes of the young and early onset-age of type 2 diabetes. J. Diabetes Complicat. 2012, 26, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Chapla, A.; Mruthyunjaya, M.D.; Asha, H.S.; Varghese, D.; Varshney, M.; Vasan, S.K.; Venkatesan, P.; Nair, V.; Mathai, S.; Paul, T.V.; et al. Maturity onset diabetes of the young in India—A distinctive mutation pattern identified through targeted next-generation sequencing. Clin. Endocrinol. 2015, 82, 533–542. [Google Scholar] [CrossRef]
- Abreu, G.M.; Soares, C.; Tarantino, R.M.; da Fonseca, A.C.P.; de Souza, R.B.; Pereira, M.F.C.; Cabello, P.H.; Rodacki, M.; Zajdenverg, L.; Zembrzuski, V.M.; et al. Identification of the First PAX4-MODY Family Reported in Brazil. Diabetes Metab. Syndr. Obes. 2020, 13, 2623–2631. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, C.; Yang, W.; Piao, Y.; Ren, L.; Sang, Y.C. 487C>T mutation in PAX4 gene causes MODY9: A case report and literature review. Medicine 2022, 101, e32461. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Gu, B.; Zhang, J.; Fang, C.; Su, J.; Li, H.; Han, R.; Ye, L.; Wang, W.; Ning, G.; et al. Missense Variants in PAX4 Are Associated with Early-Onset Diabetes in Chinese. Diabetes Ther. 2021, 12, 289–300. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M. Incretins in obesity and diabetes. Ann. N. Y. Acad. Sci. 2020, 1461, 104–126. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell Metab. 2018, 27, 740–756. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.J.; Abrahamson, M.J.; Barzilay, J.I.; Blonde, L.; Bloomgarden, Z.T.; Bush, M.A.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Fonseca, V.A.; et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2018 Executive Summary. Endocr. Pract. 2018, 24, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.I.; Blau, J.E.; Rother, K.I.; Beitelshees, A.L. SGLT2 inhibitors as adjunctive therapy for type 1 diabetes: Balancing benefits and risks. Lancet Diabetes Endocrinol. 2019, 7, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Silver, B.; Ramaiya, K.; Andrew, S.B.; Fredrick, O.; Bajaj, S.; Kalra, S.; Charlotte, B.M.; Claudine, K.; Makhoba, A. EADSG Guidelines: Insulin Therapy in Diabetes. Diabetes Ther. 2018, 9, 449–492. [Google Scholar] [CrossRef]
- Dong, S.; Wu, H. Regenerating beta cells of the pancreas—Potential developments in diabetes treatment. Expert Opin. Biol. Ther. 2018, 18, 175–185. [Google Scholar] [CrossRef]
- Lorenzo, P.I.; Cobo-Vuilleumier, N.; Gauthier, B.R. Therapeutic potential of pancreatic PAX4-regulated pathways in treating diabetes mellitus. Curr. Opin. Pharmacol. 2018, 43, 1–10. [Google Scholar] [CrossRef]
- Blyszczuk, P.; Czyz, J.; Kania, G.; Wagner, M.; Roll, U.; St-Onge, L.; Wobus, A.M. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc. Natl. Acad. Sci. USA 2003, 100, 998–1003. [Google Scholar] [CrossRef]
- Lin, H.T.; Kao, C.L.; Lee, K.H.; Chang, Y.L.; Chiou, S.H.; Tsai, F.T.; Tsai, T.H.; Sheu, D.C.; Ho, L.L.; Ku, H.H. Enhancement of insulin-producing cell differentiation from embryonic stem cells using pax4-nucleofection method. World J. Gastroenterol. 2007, 13, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Liew, C.G.; Shah, N.N.; Briston, S.J.; Shepherd, R.M.; Khoo, C.P.; Dunne, M.J.; Moore, H.D.; Cosgrove, K.E.; Andrews, P.W. PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS ONE 2008, 3, e1783. [Google Scholar] [CrossRef] [PubMed]
- Gage, B.K.; Baker, R.K.; Kieffer, T.J. Overexpression of PAX4 reduces glucagon expression in differentiating hESCs. Islets 2014, 6, e29236. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, C.; Zhou, S.; Liu, X.; Wang, J.; Liu, X.; Qian, S.; Xin, Y.; Gao, Y.; Zhu, Y.; et al. PAX4 promotes PDX1-induced differentiation of mesenchymal stem cells into insulin-secreting cells. Am. J. Transl. Res. 2017, 9, 874–886. [Google Scholar] [PubMed]
- Ferber, S.; Halkin, A.; Cohen, H.; Ber, I.; Einav, Y.; Goldberg, I.; Barshack, I.; Seijffers, R.; Kopolovic, J.; Kaiser, N.; et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat. Med. 2000, 6, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Berneman-Zeitouni, D.; Molakandov, K.; Elgart, M.; Mor, E.; Fornoni, A.; Dominguez, M.R.; Kerr-Conte, J.; Ott, M.; Meivar-Levy, I.; Ferber, S. The temporal and hierarchical control of transcription factors-induced liver to pancreas transdifferentiation. PLoS ONE 2014, 9, e87812. [Google Scholar] [CrossRef]
- Gefen-Halevi, S.; Rachmut, I.H.; Molakandov, K.; Berneman, D.; Mor, E.; Meivar-Levy, I.; Ferber, S. NKX6.1 promotes PDX-1-induced liver to pancreatic beta-cells reprogramming. Cell Reprogram. 2010, 12, 655–664. [Google Scholar] [CrossRef]
- Tang, D.Q.; Cao, L.Z.; Chou, W.; Shun, L.; Farag, C.; Atkinson, M.A.; Li, S.W.; Chang, L.J.; Yang, L.J. Role of Pax4 in Pdx1-VP16-mediated liver-to-endocrine pancreas transdifferentiation. Lab. Investig. 2006, 86, 829–841. [Google Scholar] [CrossRef]
- Tang, D.Q.; Lu, S.; Sun, Y.P.; Rodrigues, E.; Chou, W.; Yang, C.; Cao, L.Z.; Chang, L.J.; Yang, L.J. Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab. Investig. 2006, 86, 83–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.; Fonseca, V.A.; Wu, H. Pax4 in Health and Diabetes. Int. J. Mol. Sci. 2023, 24, 8283. https://doi.org/10.3390/ijms24098283
Ko J, Fonseca VA, Wu H. Pax4 in Health and Diabetes. International Journal of Molecular Sciences. 2023; 24(9):8283. https://doi.org/10.3390/ijms24098283
Chicago/Turabian StyleKo, Jenna, Vivian A. Fonseca, and Hongju Wu. 2023. "Pax4 in Health and Diabetes" International Journal of Molecular Sciences 24, no. 9: 8283. https://doi.org/10.3390/ijms24098283
APA StyleKo, J., Fonseca, V. A., & Wu, H. (2023). Pax4 in Health and Diabetes. International Journal of Molecular Sciences, 24(9), 8283. https://doi.org/10.3390/ijms24098283





