Animal Models for the Investigation of P2X7 Receptors
Abstract
1. Introduction
2. Uses of Animals to Study P2X7
2.1. Antibodies and Nanobodies
2.2. Recombinant Receptors
2.3. Pharmacokinetic Studies and Radioligands
| Species | Length (Amino Acid Residues) 1 | Identity to Human P2X7 (%) 1 | ATP EC50 (μM) 2,3 | BzATP EC50 (μM) 3 | Reference |
|---|---|---|---|---|---|
| Human | 595 | 100 | 779 4 | 52 4 | [80] |
| Rhesus macaque | 595 | 97 | 802 | 58 | [96] |
| Dog | 595 | 86 | 3162 | 501 | [97] |
| Giant panda | 595 | 85 | 122 | N.R. | [83] |
| Mouse | 595 | 81 | 734 | 90 | [98] |
| Rat | 595 | 80 | 115 | 7 | [37] |
| Guinea pig | 594 | 77 | 603 | >100 | [99] |
| Seabream | 576 | 46 | 1840 | 130 | [100] |
| Japanese flounder | 580 | 46 | 790 | 743 | [101] |
| African clawed frog | 553 | 45 | 2600 | 139 | [102] |
| Zebrafish | 596 | 42 | 109 5 | 19 5 | [103] |
2.4. Physiology and Pathophysiology Studies
3. Mice
3.1. Preclinical Mouse Models
3.1.1. Selection of Antagonists
3.1.2. Dosing of Antagonists
3.2. Polymorphic P2X7 Variants in Mice
| Pro451 1 | 451Leu 1 |
|---|---|
| 129/J, 129S1, 129X1/SvJ, A/He, A/J, BALB/c, BALB/cAnNCrl, BALB/cByJ, BUB/Bn, New Zealand White (NZW), LG, LP, nonobese diabetic (NOD), MRL/Mp | AKR/J, B10.D2, C3H/HeJ, CALB/RkJ, C57BL/6, C57BL/6NCrl, C57BL/10, C57L/J, DBA/1, DBA/2, DDY/J, FVB/N, New Zealand Black (NZB), SJL/J, SM/J, SWR/J |
3.3. P2rx7 Gene Knockout Mouse Models
3.4. P2X7 Reporter Mouse Models
3.5. Humanized Mouse Models
4. Rats
4.1. Preclinical Rat Models
4.2. Polymorphic P2X7 Variants in Rats
4.3. P2rx7 Gene Knockout Rat Models
5. Guinea Pigs
6. Rabbits
7. Monkeys
8. Dogs
9. Cats
10. Zebrafish
| Disease | Key Findings 1 | Reference |
|---|---|---|
| Metastasis (MDA-MB-435s, EO33) | A-438079 ↓ MDA-MB-435s but not EO33 cell invasion | [298] |
| Metastasis (MDA-MB-435s, MDA-MB-468) | Emodin ↓ MDA-MB-435s but not MDA-MB-468 cell invasion | [299] |
| Metastasis (MDA-MB-231) | P2X7 antagonists ↓ cell invasion | [44] |
| Polycystic kidney disease (pkd2 morphant) | pkd2 morphant ↑ p2rx7 expression, OxATP, A438079 and p2rx7 knockdown ↓ cyst formation | [300] |
| Seizure (pentylenetetrazol-induced) | Probenecid and A-438079 ↓ seizure activity | [301] |
| Tissue injury (tail transection) | KN-62 and BBG ↓ neutrophil and macrophage recruitment, and il1b mRNA expression | [302] |
| Inflammation (CuS04-induced) | Probenecid but not A-740003 ↓ inflammation | [303] |
| Pain (acetic acid-induced) | Probenecid but not A-740003 ↓ pain | [304] |
| Hepatic steatosis (ethanol-induced) | Quercetin ↓ p2rx7 expression | [305] |
| Heavy metal toxicity (HgCl2-induced) | HgCl ↓ P2rx7 expression, HgCl+ATP ↓ survival (A740003 ↑ survival) | [306] |
| Retinal degeneration (CoCl2-induced) | BzATP ↑ degeneration (↓ by A-740003), CoCl2 ↑ p2rx7 expression, A-740003 ↑ CoCl2-induced degeneration | [307] |
| Mycobacterium marium infection | Clemastine ↓ mycobacterium growth | [308] |
11. Other Fish Species
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Z.; Xie, N.; Illes, P.; Di Virgilio, F.; Ulrich, H.; Semyanov, A.; Verkhratsky, A.; Sperlagh, B.; Yu, S.-G.; Huang, C.; et al. From purines to purinergic signalling: Molecular functions and human diseases. Signal Transduct. Target. Ther. 2021, 6, 162. [Google Scholar] [CrossRef]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Agrawal, A.; Gartland, A. P2X7 receptors: Role in bone cell formation and function. J. Mol. Endocrinol. 2015, 54, R75–R88. [Google Scholar] [CrossRef]
- Shokoples, B.G.; Paradis, P.; Schiffrin, E.L. P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 186–199. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, Z.; Liu, X.; Yin, H.-Y.; Tang, Y.; Cao, X. P2X7 Receptor-Mediated Inflammation in Cardiovascular Disease. Front. Pharmacol. 2021, 12, 654425. [Google Scholar] [CrossRef]
- Déchelle-Marquet, P.-A.; Guillonneau, X.; Sennlaub, F.; Delarasse, C. P2X7-dependent immune pathways in retinal diseases. Neuropharmacology 2022, 223, 109332. [Google Scholar] [CrossRef]
- Solini, A.; Novak, I. Role of the P2X7 receptor in the pathogenesis of type 2 diabetes and its microvascular complications. Curr. Opin. Pharmacol. 2019, 47, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Zhang, L.; Liu, L. Understanding the Role of Purinergic P2X7 Receptors in the Gastrointestinal System: A Systematic Review. Front. Pharmacol. 2021, 12, 786579. [Google Scholar] [CrossRef]
- Hillman, K.; Burnstock, G.; Unwin, R. The P2X7 ATP Receptor in the Kidney: A Matter of Life or Death? Nephron Exp. Nephrol. 2005, 101, e24–e30. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; Di Vincenzo, A.; Pagano, C.; El Hadi, H.; Vettor, R. The P2X7 Receptor and NLRP3 Axis in Non-Alcoholic Fatty Liver Disease: A Brief Review. Cells 2020, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A. New insights of P2X7 receptor signaling pathway in alveolar functions. J. Biomed. Sci. 2013, 20, 26. [Google Scholar] [CrossRef]
- Andrejew, R.; Oliveira-Giacomelli, Á.; Ribeiro, D.E.; Glaser, T.; Arnaud-Sampaio, V.F.; Lameu, C.; Ulrich, H. The P2X7 Receptor: Central Hub of Brain Diseases. Front. Mol. Neurosci. 2020, 13, 124. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Ortega, F.; Gómez-Villafuertes, R.; Gualix, J.; Pérez-Sen, R.; Delicado, E.G. P2X7 receptors in the central nervous system. Biochem. Pharmacol. 2021, 187, 114472. [Google Scholar] [CrossRef] [PubMed]
- Górecki, D.C. P2X7 purinoceptor as a therapeutic target in muscular dystrophies. Curr. Opin. Pharmacol. 2019, 47, 40–45. [Google Scholar] [CrossRef]
- Geraghty, N.J.; Watson, D.; Adhikary, S.R.; Sluyter, R. P2X7 receptor in skin biology and diseases. World J. Dermatol. 2016, 5, 72–83. [Google Scholar] [CrossRef]
- Lara, R.; Adinolfi, E.; Harwood, C.A.; Philpott, M.; Barden, J.A.; Di Virgilio, F.; McNulty, S. P2X7 in Cancer: From Molecular Mechanisms to Therapeutics. Front. Pharmacol. 2020, 11, 793. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Mazziotta, C.; Lanzillotti, C.; Stefani, C.; Badiale, G.; Campione, G.; Martini, F.; Tognon, M. The Role of Purinergic P2X7 Receptor in Inflammation and Cancer: Novel Molecular Insights and Clinical Applications. Cancers 2022, 14, 1116. [Google Scholar] [CrossRef]
- Ren, W.-J.; Illes, P. Involvement of P2X7 receptors in chronic pain disorders. Purinergic Signal. 2022, 18, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Filippin, K.J.; de Souza, K.F.S.; de Araujo Júnior, R.T.; Torquato, H.F.V.; Dias, D.A.; Parisotto, E.B.; Ferreira, A.T.; Paredes-Gamero, E.J. Involvement of P2 receptors in hematopoiesis and hematopoietic disorders, and as pharmacological targets. Purinergic Signal. 2020, 16, 1–15. [Google Scholar] [CrossRef]
- Coccurello, R.; Volonté, C. P2X7 Receptor in the Management of Energy Homeostasis: Implications for Obesity, Dyslipidemia, and Insulin Resistance. Front. Endocrinol. 2020, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R. The P2X7 Receptor. Adv. Exp. Med. Biol. 2017, 1051, 17–53. [Google Scholar] [CrossRef]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef]
- Miras-Portugal, M.T.; Sebastián-Serrano, Á.; de Diego García, L.; Díaz-Hernández, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J. Neurosci. 2017, 37, 7063–7072. [Google Scholar] [CrossRef] [PubMed]
- Nicke, A. Homotrimeric complexes are the dominant assembly state of native P2X7 subunits. Biochem. Biophys. Res. Commun. 2008, 377, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Prudic, K.; Pippel, A.; Klapperstück, M.; Braam, U.; Müller, C.E.; Schmalzing, G.; Markwardt, F. Interaction of Purinergic P2X4 and P2X7 Receptor Subunits. Front. Pharmacol. 2017, 8, 860. [Google Scholar] [CrossRef]
- Trang, M.; Schmalzing, G.; Müller, C.E.; Markwardt, F. Dissection of P2X4 and P2X7 Receptor Current Components in BV-2 Microglia. Int. J. Mol. Sci. 2020, 21, 8489. [Google Scholar] [CrossRef]
- Guo, C.; Masin, M.; Qureshi, O.S.; Murrell-Lagnado, R.D. Evidence for Functional P2X4/P2X7 Heteromeric Receptors. Mol. Pharmacol. 2007, 72, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Rump, A.; Smolander, O.-P.; Boudinot, S.R.; Kanellopoulos, J.M.; Boudinot, P. Evolutionary Origin of the P2X7 C-ter Region: Capture of an Ancient Ballast Domain by a P2X4-like Gene in Ancient Jawed Vertebrates. Front. Immunol. 2020, 11, 113. [Google Scholar] [CrossRef]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandonà, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef]
- Cheewatrakoolpong, B.; Gilchrest, H.; Anthes, J.C.; Greenfeder, S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem. Biophys. Res. Commun. 2005, 332, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.-H.; Li, X.; Wang, L.; Zhou, L.; Gorodeski, G.I. A Truncated P2X7 Receptor Variant (P2X7-j) Endogenously Expressed in Cervical Cancer Cells Antagonizes the Full-length P2X7 Receptor through Hetero-oligomerization. J. Biol. Chem. 2006, 281, 17228–17237. [Google Scholar] [CrossRef] [PubMed]
- Skarratt, K.K.; Gu, B.J.; Lovelace, M.D.; Milligan, C.J.; Stokes, L.; Glover, R.; Petrou, S.; Wiley, J.S.; Fuller, S.J. A P2RX7 single nucleotide polymorphism haplotype promotes exon 7 and 8 skipping and disrupts receptor function. FASEB J. 2020, 34, 3884–3901. [Google Scholar] [CrossRef]
- Kido, Y.; Kawahara, C.; Terai, Y.; Ohishi, A.; Kobayashi, S.; Hayakawa, M.; Kamatsuka, Y.; Nishida, K.; Nagasawa, K. Regulation of activity of P2X7 receptor by its splice variants in cultured mouse astrocytes. Glia 2014, 62, 440–451. [Google Scholar] [CrossRef]
- Nicke, A.; Kuan, Y.-H.; Masin, M.; Rettinger, J.; Marquez-Klaka, B.; Bender, O.; Górecki, D.C.; Murrell-Lagnado, R.D.; Soto, F. A Functional P2X7 Splice Variant with an Alternative Transmembrane Domain 1 Escapes Gene Inactivation in P2X7 Knock-out Mice. J. Biol. Chem. 2009, 284, 25813–25822. [Google Scholar] [CrossRef]
- Gargett, C.E.; Cornish, J.E.; Wiley, J.S. ATP, a partial agonist for the P2Z receptor of human lymphocytes. Br. J. Pharmacol. 1997, 122, 911–917. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Seman, M.; Adriouch, S.; Scheuplein, F.; Krebs, C.; Freese, D.; Glowacki, G.; Deterre, P.; Haag, F.; Koch-Nolte, F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity 2003, 19, 571–582. [Google Scholar] [CrossRef]
- Liao, S.D.; Puro, D.G. NAD+-Induced Vasotoxicity in the Pericyte-Containing Microvasculature of the Rat Retina: Effect of Diabetes. Investig. Opthalmology Vis. Sci. 2006, 47, 5032–5038. [Google Scholar] [CrossRef]
- Adriouch, S.; Bannas, P.; Schwarz, N.; Fliegert, R.; Guse, A.H.; Seman, M.; Haag, F.; Koch-Noke, F. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. 2008, 22, 861–869. [Google Scholar] [CrossRef]
- Koch-Nolte, F.; Kernstock, S.; Mueller-Dieckmann, C.; Weiss, M.S.; Haag, F. Mammalian ADP-ribosyltransferases and ADP-ribosylhydrolases. Front. Biosci. 2008, 13, 6716–6729. [Google Scholar] [CrossRef]
- Schwarz, N.; Drouot, L.; Nicke, A.; Fliegert, R.; Boyer, O.; Guse, A.H.; Haag, F.; Adriouch, S.; Koch-Nolte, F. Alternative Splicing of the N-Terminal Cytosolic and Transmembrane Domains of P2X7 Controls Gating of the Ion Channel by ADP-Ribosylation. PLoS ONE 2012, 7, e41269. [Google Scholar] [CrossRef]
- Xu, X.J.; Boumechache, M.; Robinson, L.E.; Marschall, V.; Gorecki, D.; Masin, M.; Murrell-Lagnado, R.D. Splice-variants of the P2X7 receptor reveal differential agonist-dependence and functional coupling with pannexin-1. J. Cell Sci. 2012, 125, 3776–3789. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Williams, D.R.; Lee, J.-H.; Lee, S.-D.; Lee, J.-H.; Ko, H.; Lee, G.-E.; Kim, S.; Lee, J.-M.; Abdelrahman, A.; et al. Potent Suppressive Effects of 1-Piperidinylimidazole Based Novel P2X7 Receptor Antagonists on Cancer Cell Migration and Invasion. J. Med. Chem. 2016, 59, 7410–7430. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Gu, B.J.; Avula, P.; Wiley, J.S. Assays to Measure Purinoceptor Pore Dilation. Methods Mol. Biol. 2020, 2041, 323–334. [Google Scholar] [CrossRef]
- Zhou, Y.; Fei, M.; Zhang, G.; Liang, W.-C.; Lin, W.; Wu, Y.; Piskol, R.; Ridgway, J.; McNamara, E.; Huang, H.; et al. Blockade of the Phagocytic Receptor MerTK on Tumor-Associated Macrophages Enhances P2X7R-Dependent STING Activation by Tumor-Derived cGAMP. Immunity 2020, 52, 357–373.e359. [Google Scholar] [CrossRef] [PubMed]
- Kopp, R.; Krautloher, A.; Ramírez-Fernández, A.; Nicke, A. P2X7 Interactions and Signaling—Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem. Pharmacol. 2021, 187, 114385. [Google Scholar] [CrossRef]
- Pupovac, A.; Sluyter, R. Roles of extracellular nucleotides and P2 receptors in ectodomain shedding. Cell Mol. Life Sci. 2016, 73, 4159–4173. [Google Scholar] [CrossRef]
- Orioli, E.; De Marchi, E.; Giuliani, A.L.; Adinolfi, E. P2X7 Receptor Orchestrates Multiple Signalling Pathways Triggering Inflammation, Autophagy and Metabolic/Trophic Responses. Curr. Med. Chem. 2017, 24, 2261–2275. [Google Scholar] [CrossRef]
- Savio, L.E.B.; de Andrade Mello, P.; Da Silva, C.G.; Coutinho-Silva, R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.J.; Wiley, J.S. P2X7 as a scavenger receptor for innate phagocytosis in the brain. Br. J. Pharmacol. 2018, 175, 4195–4208. [Google Scholar] [CrossRef]
- Bartlett, R.; Stokes, L.; Sluyter, R. The P2X7 Receptor Channel: Recent Developments and the Use of P2X7 Antagonists in Models of Disease. Pharmacol. Rev. 2014, 66, 638–675. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Knight, G.E. Cellular Distribution and Functions of P2 Receptor Subtypes in Different Systems. Int. Rev. Cytol. 2004, 240, 31–304. [Google Scholar] [CrossRef] [PubMed]
- Stähler, T.; Danquah, W.; Demeules, M.; Gondé, H.; Hardet, R.; Haag, F.; Adriouch, S.; Koch-Nolte, F.; Menzel, S. Development of Antibody and Nanobody Tools for P2X7. Methods Mol. Biol. 2022, 2510, 99–127. [Google Scholar] [CrossRef] [PubMed]
- Adriouch, S.; Dubberke, G.; Diessenbacher, P.; Rassendren, F.; Seman, M.; Haag, F.; Koch-Nolte, F. Probing the expression and function of the P2X7 purinoceptor with antibodies raised by genetic immunization. Cell. Immunol. 2005, 236, 72–77. [Google Scholar] [CrossRef]
- Collo, G.; Neidhart, S.; Kawashima, E.; Kosco-Vilbois, M.; North, R.; Buell, G. Tissue distribution of the P2X7 receptor. Neuropharmacology 1997, 36, 1277–1283. [Google Scholar] [CrossRef]
- Buell, G.; Chessell, I.; Michel, A.; Collo, G.; Salazzo, M.; Herren, S.; Gretener, D.; Grahames, C.; Kaur, R.; Kosco-Vilbois, M.; et al. Blockade of Human P2X7 Receptor Function with a Monoclonal Antibody. Blood 1998, 92, 3521–3528. [Google Scholar] [CrossRef]
- Li, M.; Luo, S.; Zhang, Y.; Jia, L.; Yang, C.; Peng, X.; Zhao, R. Production, characterization, and application of a monoclonal antibody specific for the extracellular domain of human P2X7R. Appl. Microbiol. Biotechnol. 2020, 104, 2017–2028. [Google Scholar] [CrossRef]
- Kurashima, Y.; Amiya, T.; Nochi, T.; Fujisawa, K.; Haraguchi, T.; Iba, H.; Tsutsui, H.; Sato, S.; Nakajima, S.; Iijima, H.; et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nat. Commun. 2012, 3, 1034. [Google Scholar] [CrossRef]
- Elhage, A.; Turner, R.J.; Cuthbertson, P.; Watson, D.; Sluyter, R. Preparation of the Murine Anti-Human P2X7 Receptor Monoclonal Antibody (Clone L4). Methods Mol. Biol. 2022, 2510, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.L.; Berchan, M.; Sanz, J.M.; Passaro, A.; Pizzicotti, S.; Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. The P2X7 Receptor Is Shed into Circulation: Correlation with C-Reactive Protein Levels. Front. Immunol. 2019, 10, 793. [Google Scholar] [CrossRef]
- Martínez-García, J.J.; Martínez-Banaclocha, H.; Angosto-Bazarra, D.; de Torre-Minguela, C.; Baroja-Mazo, A.; Alarcón-Vila, C.; Martínez-Alarcón, L.; Amores-Iniesta, J.; Martín-Sánchez, F.; Ercole, G.A.; et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat. Commun. 2019, 10, 2711. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Chen, P.; Guo, X.; Li, X.; Wu, Y.; Liu, W.; Jiang, F.; Liu, H.; Wang, L. Correlations between Serum P2X7, Vitamin A, 25-hydroxy Vitamin D, and Mycoplasma Pneumoniae Pneumonia. J. Clin. Lab. Anal. 2021, 35, e23760. [Google Scholar] [CrossRef]
- Shi, X.-X.; Zheng, K.-C.; Shan, P.-R.; Zhang, L.; Wu, S.-J.; Huang, Z.-Q. Elevated circulating level of P2X7 receptor is related to severity of coronary artery stenosis and prognosis of acute myocardial infarction. Cardiol. J. 2021, 28, 453–459. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, J.; Hurtado-Navarro, L.; Peñín-Franch, A.; Molina-López, C.; Martínez-Alarcón, L.; Angosto-Bazarra, D.; Baroja-Mazo, A.; Pelegrin, P. Soluble P2X7 Receptor Is Elevated in the Plasma of COVID-19 Patients and Correlates with Disease Severity. Front. Immunol. 2022, 13, 894470. [Google Scholar] [CrossRef]
- Conte, G.; Menéndez-Méndez, A.; Bauer, S.; El-Naggar, H.; Alves, M.; Nicke, A.; Delanty, N.; Rosenow, F.; Henshall, D.C.; Engel, T. Circulating P2X7 Receptor Signaling Components as Diagnostic Biomarkers for Temporal Lobe Epilepsy. Cells 2021, 10, 2444. [Google Scholar] [CrossRef]
- Kristóf, Z.; Baranyi, M.; Tod, P.; Mut-Arbona, P.; Demeter, K.; Bitter, I.; Sperlágh, B. Elevated Serum Purine Levels in Schizophrenia: A Reverse Translational Study to Identify Novel Inflammatory Biomarkers. Int. J. Neuropsychopharmacol. 2022, 25, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Danquah, W.; Meyer-Schwesinger, C.; Rissiek, B.; Pinto, C.; Serracant-Prat, A.; Amadi, M.; Iacenda, D.; Knop, J.H.; Hammel, A.; Bergmann, P.; et al. Nanobodies that block gating of the P2X7 ion channel ameliorate inflammation. Sci. Transl. Med. 2016, 8, 366ra162. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. Elife 2018, 7, e36217. [Google Scholar] [CrossRef] [PubMed]
- Jooss, T.; Zhang, J.; Zimmer, B.; Rezzonico-Jost, T.; Rissiek, B.; Pelczar, P.F.; Seehusen, F.; Koch-Nolte, F.; Magnus, T.; Zierler, S.; et al. Macrophages and glia are the dominant P2X7-expressing cell types in the gut nervous system—No evidence for the role of neuronal P2X7 receptors in colitis. Mucosal Immunol. 2023, 16, 180–193. [Google Scholar] [CrossRef]
- Winzer, R.; Serracant-Prat, A.; Brock, V.J.; Pinto-Espinoza, C.; Rissiek, B.; Amadi, M.; Eich, N.; Rissiek, A.; Schneider, E.; Magnus, T.; et al. P2X7 is expressed on human innate-like T lymphocytes and mediates susceptibility to ATP-induced cell death. Eur. J. Immunol. 2022, 52, 1805–1818. [Google Scholar] [CrossRef]
- Wilmes, M.; Espinoza, C.P.; Ludewig, P.; Stabernack, J.; Liesz, A.; Nicke, A.; Gelderblom, M.; Gerloff, C.; Falzoni, S.; Tolosa, E.; et al. Blocking P2X7 by intracerebroventricular injection of P2X7-specific nanobodies reduces stroke lesions. J. Neuroinflammation 2022, 19, 256. [Google Scholar] [CrossRef] [PubMed]
- Demeules, M.; Scarpitta, A.; Hardet, R.; Gondé, H.; Abad, C.; Blandin, M.; Menzel, S.; Duan, Y.; Rissiek, B.; Magnus, T.; et al. Evaluation of nanobody-based biologics targeting purinergic checkpoints in tumor models in vivo. Front. Immunol. 2022, 13, 1012534. [Google Scholar] [CrossRef] [PubMed]
- Koch-Nolte, F.; Eichhoff, A.; Pinto-Espinoza, C.; Schwarz, N.; Schäfer, T.; Menzel, S.; Haag, F.; Demeules, M.; Gondé, H.; Adriouch, S. Novel biologics targeting the P2X7 ion channel. Curr. Opin. Pharmacol. 2019, 47, 110–118. [Google Scholar] [CrossRef]
- Gondé, H.; Demeules, M.; Hardet, R.; Scarpitta, A.; Junge, M.; Pinto-Espinoza, C.; Varin, R.; Koch-Nolte, F.; Boyer, O.; Adriouch, S. A Methodological Approach Using rAAV Vectors Encoding Nanobody-Based Biologics to Evaluate ARTC2.2 and P2X7 In Vivo. Front. Immunol. 2021, 12, 704408. [Google Scholar] [CrossRef]
- Pinto-Espinoza, C.; Guillou, C.; Rissiek, B.; Wilmes, M.; Javidi, E.; Schwarz, N.; Junge, M.; Haag, F.; Liaukouskaya, N.; Wanner, N.; et al. Effective targeting of microglial P2X7 following intracerebroventricular delivery of nanobodies and nanobody-encoding AAVs. Front. Pharmacol. 2022, 13, 1029236. [Google Scholar] [CrossRef]
- DeMeules, M.; Scarpitta, A.; Abad, C.; Gondé, H.; Hardet, R.; Pinto-Espinoza, C.; Eichhoff, A.M.; Schäfer, W.; Haag, F.; Koch-Nolte, F.; et al. Evaluation of P2X7 Receptor Function in Tumor Contexts Using rAAV Vector and Nanobodies (AAVnano). Front. Oncol. 2020, 10, 1699. [Google Scholar] [CrossRef]
- Rassendren, F.; Buell, G.N.; Virginio, C.; Collo, G.; North, R.A.; Surprenant, A. The Permeabilizing ATP Receptor, P2X7. J. Biol. Chem. 1997, 272, 5482–5486. [Google Scholar] [CrossRef]
- North, R.A.; Surprenant, A. Pharmacology of Cloned P2X Receptors. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 563–580. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Baldwin, J.M.; Eroger, S.; Baldwin, S.A. Insights into the Molecular Mechanisms Underlying Mammalian P2X7 Receptor Functions and Contributions in Diseases, Revealed by Structural Modeling and Single Nucleotide Polymorphisms. Front. Pharmacol. 2013, 4, 55. [Google Scholar] [CrossRef]
- Karasawa, A.; Kawate, T. Structural basis for subtype-specific inhibition of the P2X7 receptor. Elife 2016, 5, e22153. [Google Scholar] [CrossRef]
- McCarthy, A.E.; Yoshioka, C.; Mansoor, S.E. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell 2019, 179, 659–670.e13. [Google Scholar] [CrossRef] [PubMed]
- Sander, S.; Müller, I.; Garcia-Alai, M.M.; Nicke, A.; Tidow, H. New insights into P2X7 receptor regulation: Ca2+-calmodulin and GDP bind to the soluble P2X7 ballast domain. J. Biol. Chem. 2022, 298, 102495. [Google Scholar] [CrossRef] [PubMed]
- Durner, A.; Durner, E.; Nicke, A. Improved ANAP incorporation and VCF analysis reveal details of P2X7 current facilitation and a limited conformational interplay between ATP binding and the intracellular ballast domain. Elife 2023, 12, e82479. [Google Scholar] [CrossRef]
- Duplantier, A.J.; Dombroski, M.A.; Subramanyam, C.; Beaulieu, A.M.; Chang, S.-P.; Gabel, C.A.; Jordan, C.; Kalgutkar, A.S.; Kraus, K.G.; Labasi, J.M.; et al. Optimization of the physicochemical and pharmacokinetic attributes in a 6-azauracil series of P2X7 receptor antagonists leading to the discovery of the clinical candidate CE-224,535. Bioorganic Med. Chem. Lett. 2011, 21, 3708–3711. [Google Scholar] [CrossRef] [PubMed]
- Stock, T.C.; Bloom, B.J.; Wei, N.; Ishaq, S.; Park, W.; Wang, X.; Gupta, P.; Mebus, C.A. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J. Rheumatol. 2012, 39, 720–727. [Google Scholar] [CrossRef]
- Chrovian, C.C.; Rech, J.C.; Bhattacharya, A.; Letavic, M.A. P2X7 antagonists as potential therapeutic agents for the treatment of CNS disorders. Prog. Med. Chem. 2014, 53, 65–100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.-H. Radioligands targeting purinergic P2X7 receptor. Bioorganic Med. Chem. Lett. 2020, 30, 127169. [Google Scholar] [CrossRef]
- Han, J.; Liu, H.; Liu, C.; Jin, H.; Perlmutter, J.S.; Egan, T.M.; Tu, Z. Pharmacologic characterizations of a P2X7 receptor-specific radioligand, [11C]GSK1482160 for neuroinflammatory response. Nucl. Med. Commun. 2017, 38, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Barret, O.; Bhattacharya, A.; Chen, G.; Constantinescu, C.; Huang, C.; Letavic, M.; Tamagnan, G.; Xia, C.A.; Zhang, W.; et al. Preclinical Evaluation and Nonhuman Primate Receptor Occupancy Study of 18F-JNJ-64413739, a PET Radioligand for P2X7 Receptors. J. Nucl. Med. 2019, 60, 1154–1159. [Google Scholar] [CrossRef]
- Territo, P.R.; Meyer, J.A.; Peters, J.S.; Riley, A.A.; McCarthy, B.P.; Gao, M.; Wang, M.; Green, M.A.; Zheng, Q.-H.; Hutchins, G.D. Characterization of 11C-GSK1482160 for Targeting the P2X7 Receptor as a Biomarker for Neuroinflammation. J. Nucl. Med. 2016, 58, 458–465. [Google Scholar] [CrossRef]
- Fu, Z.; Lin, Q.; Hu, B.; Zhang, Y.; Chen, W.; Zhu, J.; Zhao, Y.; Choi, H.S.; Shi, H.; Cheng, D. P2X7 PET Radioligand 18F-PTTP for Differentiation of Lung Tumor from Inflammation. J. Nucl. Med. 2019, 60, 930–936. [Google Scholar] [CrossRef]
- Fu, Z.; Lin, Q.; Xu, Z.; Zhao, Y.; Cheng, Y.; Shi, D.; Fu, W.; Yang, T.; Shi, H.; Cheng, D. P2X7 receptor-specific radioligand 18F-FTTM for atherosclerotic plaque PET imaging. Eur. J. Nucl. Med. 2022, 49, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Bradley, H.J.; Browne, L.E.; Yang, W.; Jiang, L. Pharmacological properties of the rhesus macaque monkey P2X7 receptor. Br. J. Pharmacol. 2011, 164, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Roman, S.; Cusdin, F.; Fonfria, E.; Goodwin, J.; Reeves, J.; Lappin, S.; Chambers, L.; Walter, D.; Clay, W.; Michel, A. Cloning and pharmacological characterization of the dog P2X7 receptor. Br. J. Pharmacol. 2009, 158, 1513–1526. [Google Scholar] [CrossRef]
- Chessell, I.; Simon, J.; Hibell, A.; Michel, A.; Barnard, E.; Humphrey, P. Cloning and functional characterisation of the mouse P2X7 receptor. FEBS Lett. 1998, 439, 26–30. [Google Scholar] [CrossRef]
- Fonfria, E.; Clay, W.C.; Levy, D.S.; Goodwin, J.A.; Roman, S.; Smith, G.D.; Condreay, J.P.; Michel, A.D. Cloning and pharmacological characterization of the guinea pig P2X7 receptor orthologue. Br. J. Pharmacol. 2008, 153, 544–556. [Google Scholar] [CrossRef]
- López-Castejón, G.; Young, M.T.; Meseguer, J.; Surprenant, A.; Mulero, V. Characterization of ATP-gated P2X7 receptors in fish provides new insights into the mechanism of release of the leaderless cytokine interleukin-1β. Mol. Immunol. 2007, 44, 1286–1299. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Coddou, C.; Geng, X.; Wei, J.; Sun, J. Molecular characterization and expression analysis of ATP-gated P2X7 receptor involved in Japanese flounder (Paralichthys olivaceus) innate immune response. PLoS ONE 2014, 9, e96625. [Google Scholar] [CrossRef] [PubMed]
- Paukert, M.; Hidayat, S.; Gründer, S. The P2X7 receptor from Xenopus laevis: Formation of a large pore in Xenopus oocytes. FEBS Lett. 2002, 513, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Kucenas, S.; Li, Z.; Cox, J.; Egan, T.; Voigt, M. Molecular characterization of the zebrafish P2X receptor subunit gene family. Neuroscience 2003, 121, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.I. Animal experimentation: Implementation and application of the 3Rs. Emerg. Top. Life Sci. 2019, 3, 675–679. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Ludolph, A.C.; Bendotti, C.; Blaugrund, E.; Chio, A.; Greensmith, L.; Loeffler, J.-P.; Mead, R.; Niessen, H.G.; Petri, S.; Pradat, P.-F.; et al. Guidelines for preclinical animal research in ALS/MND: A consensus meeting. Amyotroph. Lateral Scler. 2010, 11, 38–45. [Google Scholar] [CrossRef]
- Ludolph, A.C.; Bendotti, C.; Blaugrund, E.; Hengerer, B.; Löffler, J.; Martin, J.; Meininger, V.; Meyer, T.; Moussaoui, S.; Robberecht, W.; et al. Guidelines for the preclinical in vivo evaluation of pharmacological active drugs for ALS/MND: Report on the 142nd ENMC international workshop. Amyotroph. Lateral Scler. 2007, 8, 217–223. [Google Scholar] [CrossRef]
- Vollert, J.; Schenker, E.; Macleod, M.; Bespalov, A.; Wuerbel, H.; Michel, M.; Dirnagl, U.; Potschka, H.; Waldron, A.-M.; Wever, K.; et al. Systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments involving laboratory animals. BMJ Open Sci. 2020, 44, e100046. [Google Scholar] [CrossRef]
- Young, C.N.J.; Górecki, D.C. P2RX7 Purinoceptor as a Therapeutic Target—The Second Coming? Front. Chem. 2018, 6, 248. [Google Scholar] [CrossRef]
- Jiang, L.-H.; Mackenzie, A.B.; North, R.A.; Surprenant, A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol. Pharmacol. 2000, 58, 82–88. [Google Scholar] [CrossRef]
- Peng, W.; Cotrina, M.L.; Han, X.; Yu, H.; Bekar, L.; Blum, L.; Takano, T.; Tian, G.-F.; Goldman, S.A.; Nedergaard, M. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 106, 12489–12493. [Google Scholar] [CrossRef]
- Georgiou, C.D.; Grintzalis, K.; Zervoudakis, G.; Papapostolou, I. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 2008, 391, 391–403. [Google Scholar] [CrossRef]
- Bo, X.; Jiang, L.-H.; Wilson, H.L.; Kim, M.; Burnstock, G.; Surprenant, A.; North, R.A. Pharmacological and biophysical properties of the human P2X5 receptor. Mol. Pharmacol. 2003, 63, 1407–1416. [Google Scholar] [CrossRef]
- Qiu, F.; Dahl, G. A permeant regulating its permeation pore: Inhibition of pannexin 1 channels by ATP. Am. J. Physiol. Physiol. 2009, 296, C250–C255. [Google Scholar] [CrossRef]
- Jo, S.; Bean, B.P. Inhibition of neuronal voltage-gated sodium channels by Brilliant Blue G. Mol. Pharmacol. 2011, 80, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Iwamaru, Y.; Takenouchi, T.; Murayama, Y.; Okada, H.; Imamura, M.; Shimizu, Y.; Hashimoto, M.; Mohri, S.; Yokoyama, T.; Kitani, H. Anti-prion activity of Brilliant Blue G. PLoS ONE 2012, 7, e37896. [Google Scholar] [CrossRef] [PubMed]
- How, S.-C.; Hsin, A.; Chen, G.-Y.; Hsu, W.-T.; Yang, S.-M.; Chou, W.-L.; Chou, S.-H.; Wang, S.S. Exploring the influence of Brilliant Blue G on amyloid fibril formation of lysozyme. Int. J. Biol. Macromol. 2019, 138, 37–48. [Google Scholar] [CrossRef]
- Lee, D.; Lee, E.-K.; Lee, J.-H.; Chang, C.-S.; Paik, S.R. Self-oligomerization and protein aggregation of α-synuclein in the presence of Coomassie Brilliant Blue. Eur. J. Biochem. 2001, 268, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Biber, K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016, 64, 1772–1787. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Bartlett, R.; Ly, D.; Yerbury, J.J. P2X7 receptor antagonism in amyotrophic lateral sclerosis. Neural Regen. Res. 2017, 12, 749–750. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wang, Q.; Ao, H.; Shoblock, J.R.; Lord, B.; Aluisio, L.; Fraser, I.; Nepomuceno, D.; Neff, R.A.; Welty, N.; et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br. J. Pharmacol. 2013, 170, 624–640. [Google Scholar] [CrossRef]
- Cuthbertson, P.; Elhage, A.; Al-Rifai, D.; Sophocleous, R.A.; Turner, R.J.; Aboelela, A.; Majed, H.; Bujaroski, R.S.; Jalilian, I.; Kelso, M.J.; et al. 6-Furopyridine Hexamethylene Amiloride Is a Non-Selective P2X7 Receptor Antagonist. Biomolecules 2022, 12, 1309. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.V.; Poputnikov, D.M.; Zhernosek, N.; Melenchuk, E.V.; Gerstberger, R.; Spyer, K.M.; Gourine, V.N. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br. J. Pharmacol. 2005, 146, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Matute, C.; Torre, I.; Pérez-Cerdá, F.; Pérez-Samartín, A.; Alberdi, E.; Etxebarria, E.; Arranz, A.M.; Ravid, R.; Rodríguez-Antigüedad, A.; Sánchez-Gómez, M.; et al. P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 2007, 27, 9525–9533. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Hernández, M.; Díez-Zaera, M.; Sánchez-Nogueiro, J.; Gómez-Villafuertes, R.; Canals, J.M.; Alberch, J.; Miras-Portugal, M.T.; Lucas, J.J. Altered P2X7-receptor level and function in mouse models of Huntington’s disease and therapeutic efficacy of antagonist administration. FASEB J. 2009, 23, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Apolloni, S.; Amadio, S.; Parisi, C.; Matteucci, A.; Potenza, R.L.; Armida, M.; Popoli, P.; D’Ambrosi, N.; Volonté, C. Spinal cord pathology is ameliorated by P2X7 antagonism in SOD1-G93A mouse model of amyotrophic lateral sclerosis. Dis. Model. Mech. 2014, 7, 1101–1109. [Google Scholar] [CrossRef]
- Bartlett, R.; Sluyter, V.; Watson, D.; Sluyter, R.; Yerbury, J.J. P2X7 antagonism using Brilliant Blue G reduces body weight loss and prolongs survival in female SOD1G93A amyotrophic lateral sclerosis mice. PeerJ 2017, 5, e3064. [Google Scholar] [CrossRef]
- Cervetto, C.; Frattaroli, D.; Maura, G.; Marcoli, M. Motor neuron dysfunction in a mouse model of ALS: Gender-dependent effect of P2X7 antagonism. Toxicology 2013, 311, 69–77. [Google Scholar] [CrossRef]
- Cuthbertson, P.; Geraghty, N.J.; Adhikary, S.R.; Casolin, S.; Watson, D.; Sluyter, R. P2X7 receptor antagonism increases regulatory T cells and reduces clinical and histological graft-versus-host disease in a humanised mouse model. Clin. Sci. 2021, 135, 495–513. [Google Scholar] [CrossRef]
- Cuthbertson, P.; Geraghty, N.J.; Adhikary, S.R.; Bird, K.M.; Fuller, S.J.; Watson, D.; Sluyter, R. Purinergic Signalling in Allogeneic Haematopoietic Stem Cell Transplantation and Graft-versus-Host Disease. Int. J. Mol. Sci. 2021, 22, 8343. [Google Scholar] [CrossRef]
- Geraghty, N.J.; Belfiore, L.; Ly, D.; Adhikary, S.R.; Fuller, S.J.; Varikatt, W.; Sanderson-Smith, M.L.; Sluyter, V.; Alexander, S.I.; Watson, D. The P2X7 receptor antagonist Brilliant Blue G reduces serum human interferon-γ in a humanized mouse model of graft-versus-host disease. Clin. Exp. Immunol. 2017, 190, 79–95. [Google Scholar] [CrossRef] [PubMed]
- Chessell, I.P.; Michel, A.D.; Humphrey, P.P. Properties of the pore-forming P2X7 purinoceptor in mouse NTW8 microglial cells. Br. J. Pharmacol. 1997, 121, 1429–1437. [Google Scholar] [CrossRef]
- Michel, A.D.; Chambers, L.J.; Clay, W.C.; Condreay, J.P.; Walter, D.S.; Chessell, I.P. Direct labelling of the human P2X7 receptor and identification of positive and negative cooperativity of binding. Br. J. Pharmacol. 2007, 151, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, N.J.; Elhage, A.; Cuthbertson, P.; Watson, D.; Sluyter, R. The P2X7 Receptor Antagonist AZ10606120 Does Not Alter Graft-Versus-Host Disease Development and Increases Serum Human Interferon-γ in a Humanized Mouse Model. OBM Transplant. 2022, 6, 18. [Google Scholar] [CrossRef]
- Douguet, L.; Janho Dit Hreich, S.; Benzaquen, J.; Seguin, L.; Juhel, T.; Dezitter, X.; Duranton, C.; Ryffel, B.; Kanellopoulos, J.; Delarasse, C.; et al. A small-molecule P2RX7 activator promotes anti-tumor immune responses and sensitizes lung tumor to immunotherapy. Nat. Commun. 2021, 12, 653. [Google Scholar] [CrossRef]
- Adriouch, S.; Dox, C.; Welge, V.; Seman, M.; Koch-Nolte, F.; Haag, F. Cutting edge: A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J. Immunol. 2002, 169, 4108–4112. [Google Scholar] [CrossRef] [PubMed]
- Young, M.T.; Pelegrin, P.; Surprenant, A. Identification of Thr283 as a key determinant of P2X7 receptor function. Br. J. Pharmacol. 2006, 149, 261–268. [Google Scholar] [CrossRef]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, M.; Griffiths, R.; Dewitt, S.; Knauper, V.; Aeschlimann, D. P2X7 receptor activation regulates rapid unconventional export of transglutaminase-2. J. Cell Sci. 2015, 128, 4615–4628. [Google Scholar] [CrossRef]
- Sorge, R.E.; Trang, T.; Dorfman, R.; Smith, S.B.; Beggs, S.; Ritchie, J.; Austin, J.-S.; Zaykin, D.V.; Vander Meulen, H.; Costigan, M.; et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 2012, 18, 595–599. [Google Scholar] [CrossRef]
- Syberg, S.; Schwarz, P.; Petersen, S.; Steinberg, T.H.; Jensen, J.-E.B.; Teilmann, J.; Jørgensen, N.R. Association between P2X7 Receptor Polymorphisms and Bone Status in Mice. J. Osteoporos. 2012, 2012, 637986. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, C.; Li, Y.; Pearce, R.; Bell, E.W.; Zhang, Y. Folding non-homologous proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep. Methods 2021, 1, 100014. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef]
- Sehnal, D.; Bittrich, S.; Deshpande, M.; Svobodová, R.; Berka, K.; Bazgier, V.; Velankar, S.; Burley, S.K.; Koča, J.; Rose, A.S. Mol* Viewer: Modern web app for 3D visualization and analysis of large biomolecular structures. Nucleic Acids Res. 2021, 49, W431–W437. [Google Scholar] [CrossRef]
- Elliott, J.I.; McVey, J.H.; Higgins, C.F. The P2X7 receptor is a candidate product of murine and human lupus susceptibility loci: A hypothesis and comparison of murine allelic products. Thromb. Haemost. 2005, 7, R468–R475. [Google Scholar] [CrossRef]
- Le Stunff, H.; Auger, R.; Kanellopoulos, J.; Raymond, M.-N. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J. Biol. Chem. 2004, 279, 16918–16926. [Google Scholar] [CrossRef]
- Er-Lukowiak, M.; Duan, Y.; Rassendren, F.; Ulmann, L.; Nicke, A.; Ufer, F.; Friese, M.A.; Koch-Nolte, F.; Magnus, T.; Rissiek, B. A P2rx7 Passenger Mutation Affects the Vitality and Function of T cells in Congenic Mice. iScience 2020, 23, 101870. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.N.; Poon, W.; Lyssenko, V.; Groop, L.; Nichols, B.; Wilmot, M.; Robson, S.; Enjyoji, K.; Herman, M.; Hu, C.; et al. Variation in glucose homeostasis traits associated with P2RX7 polymorphisms in mice and humans. J. Clin. Endocrinol. Metab. 2015, 100, E688–E696. [Google Scholar] [CrossRef] [PubMed]
- Syberg, S.; Petersen, S.; Beck Jensen, J.E.; Gartland, A.; Teilmann, J.; Chessell, I.; Steinberg, T.H.; Schwarz, P.; Jørgensen, N.R. Genetic Background Strongly Influences the Bone Phenotype of P2X7 Receptor Knockout Mice. J. Osteoporos. 2012, 2012, 391097. [Google Scholar] [CrossRef]
- Ellegaard, M.; Hegner, T.; Ding, M.; Ulmann, L.; Jørgensen, N.R. Bone phenotype of P2X4 receptor knockout mice: Implication of a P2X7 receptor mutation? Purinergic Signal. 2021, 17, 241–246. [Google Scholar] [CrossRef]
- Tian, T.; Heine, M.; Evangelakos, I.; Jaeckstein, M.Y.; Schaltenberg, N.; Stähler, T.; Koch-Nolte, F.; Kumari, M.; Heeren, J. The P2X7 ion channel is dispensable for energy and metabolic homeostasis of white and brown adipose tissues. Purinergic Signal. 2020, 16, 529–542. [Google Scholar] [CrossRef]
- Sikora, A.; Liu, J.; Brosnan, C.; Buell, G.; Chessel, I.; Bloom, B.R. Cutting edge: Purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J. Immunol. 1999, 163, 558–561. [Google Scholar] [CrossRef]
- Chessell, I.P.; Hatcher, J.P.; Bountra, C.; Michel, A.D.; Hughes, J.P.; Green, P.; Egerton, J.; Murfin, M.; Richardson, J.; Peck, W.L.; et al. Disruption of the P2X7 purinoceptor gene abolishes chronic inflammatory and neuropathic pain. Pain 2005, 114, 386–396. [Google Scholar] [CrossRef]
- Sim, J.A.; Young, M.T.; Sung, H.-Y.; North, R.A.; Surprenant, A. Reanalysis of P2X7 receptor expression in rodent brain. J. Neurosci. 2004, 24, 6307–6314. [Google Scholar] [CrossRef]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered cytokine production in mice lacking P2X(7) receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Labasi, J.M.; Petrushova, N.; Donovan, C.; McCurdy, S.; Lira, P.; Payette, M.M.; Brissette, W.; Wicks, J.R.; Audoly, L.; Gabel, C.A. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J. Immunol. 2002, 168, 6436–6445. [Google Scholar] [CrossRef] [PubMed]
- Basso, A.M.; Bratcher, N.A.; Harris, R.R.; Jarvis, M.F.; Decker, M.W.; Rueter, L.E. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: Relevance for neuropsychiatric disorders. Behav. Brain Res. 2009, 198, 83–90. [Google Scholar] [CrossRef]
- Zhang, C.; He, H.; Wang, L.; Zhang, N.; Huang, H.; Xiong, Q.; Yan, Y.; Wu, N.; Ren, H.; Han, H.; et al. Virus-Triggered ATP Release Limits Viral Replication through Facilitating IFN-β Production in a P2X7-Dependent Manner. J. Immunol. 2017, 199, 1372–1381. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Z.; Xie, G.; Zhou, T.; Hu, W.; Liu, C.; Liu, X.; Wang, X.; Qian, M.; Ni, B. The effects of P2X7 receptor knockout on emotional conditions over the lifespan of mice. Neuroreport 2018, 29, 1479–1486. [Google Scholar] [CrossRef]
- Delic, S.; Streif, S.; Deussing, J.M.; Weber, P.; Ueffing, M.; Hölter, S.M.; Wurst, W.; Kühn, R. Genetic mouse models for behavioral analysis through transgenic RNAi technology. Genes Brain Behav. 2008, 7, 821–830. [Google Scholar] [CrossRef]
- Felix, K.M.; Teng, F.; Bates, N.A.; Ma, H.; Jaimez, I.A.; Sleiman, K.C.; Tran, N.L.; Wu, H.J. P2RX7 Deletion in T Cells Promotes Autoimmune Arthritis by Unleashing the Tfh Cell Response. Front. Immunol. 2019, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Arkhipov, S.N.; Potter, D.L.; Geurts, A.M.; Pavlov, T.S.; Mehrotra, P.; Ullah, M.; Collett, J.A.; Myers, S.L.; Dwinell, M.R.; Basile, D.P. Knockout of P2rx7 purinergic receptor attenuates cyst growth in a rat model of ARPKD. Am. J. Physiol. Physiol. 2019, 317, F1649–F1655. [Google Scholar] [CrossRef]
- Prendecki, M.; McAdoo, S.P.; Turner-Stokes, T.; Garcia-Diaz, A.; Orriss, I.; Woollard, K.J.; Behmoaras, J.; Cook, H.T.; Unwin, R.; Pusey, C.D.; et al. Glomerulonephritis and autoimmune vasculitis are independent of P2RX7 but may depend on alternative inflammasome pathways. J. Pathol. 2022, 257, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Nespoux, J.; Monaghan, M.T.; Jones, N.K.; Denby, L.; Czopek, A.; Mullins, J.J.; Menzies, R.I.; Baker, A.H.; Bailey, M.A. Sex Difference in Renal Artery Contractility in a Novel CRISPR/Cas9-Generated P2X7 Knockout Rat. FASEB J. 2022, 36, R5740. [Google Scholar] [CrossRef]
- Ke, H.Z.; Qi, H.; Weidema, A.F.; Zhang, Q.; Panupinthu, N.; Crawford, D.T.; Grasser, W.A.; Paralkar, V.M.; Li, M.; Audoly, L.P.; et al. Deletion of the P2X7 nucleotide receptor reveals its regulatory roles in bone formation and resorption. Mol. Endocrinol. 2003, 17, 1356–1367. [Google Scholar] [CrossRef]
- Labrousse, V.F.; Costes, L.; Aubert, A.; Darnaudery, M.; Ferreira, G.; Amédée, T.; Layé, S. Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice. PLoS ONE 2009, 4, e6006. [Google Scholar] [CrossRef]
- Smith, K.L.; Todd, S.M.; Boucher, A.; Bennett, M.R.; Arnold, J.C. P2X7 receptor knockout mice display less aggressive biting behaviour correlating with increased brain activation in the piriform cortex. Neurosci. Lett. 2020, 714, 134575. [Google Scholar] [CrossRef]
- Vessey, K.A.; Fletcher, E.L. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS ONE 2012, 7, e29990. [Google Scholar] [CrossRef]
- Mankus, C.; Chi, C.; Rich, C.; Ren, R.; Trinkaus-Randall, V. The P2X7 receptor regulates proteoglycan expression in the corneal stroma. Mol. Vis. 2012, 18, 128–138. [Google Scholar]
- Vessey, K.A.; Gu, B.J.; Jobling, A.I.; Phipps, J.A.; Greferath, U.; Tran, M.X.; Dixon, M.A.; Baird, P.N.; Guymer, R.H.; Wiley, J.S.; et al. Loss of Function of P2X7 Receptor Scavenger Activity in Aging Mice: A Novel Model for Investigating the Early Pathogenesis of Age-Related Macular Degeneration. Am. J. Pathol. 2017, 187, 1670–1685. [Google Scholar] [CrossRef]
- Haanes, K.A.; Schwab, A.; Novak, I. The P2X7 receptor supports both life and death in fibrogenic pancreatic stellate cells. PLoS ONE 2012, 7, e51164. [Google Scholar] [CrossRef]
- Beaucage, K.L.; Xiao, A.; Pollmann, S.I.; Grol, M.W.; Beach, R.J.; Holdsworth, D.W.; Sims, S.M.; Darling, M.R.; Dixon, S.J. Loss of P2X7 nucleotide receptor function leads to abnormal fat distribution in mice. Purinergic Signal. 2014, 10, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Arguin, G.; Bourzac, J.-F.; Placet, M.; Molle, C.M.; Paquette, M.; Beaudoin, J.-F.; Rousseau, J.A.; Lecomte, R.; Plourde, M.; Gendron, F.-P. The loss of P2X7 receptor expression leads to increase intestinal glucose transit and hepatic steatosis. Sci. Rep. 2017, 7, 12917. [Google Scholar] [CrossRef] [PubMed]
- Giacovazzo, G.; Apolloni, S.; Coccurello, R. Loss of P2X7 receptor function dampens whole body energy expenditure and fatty acid oxidation. Purinergic Signal. 2018, 14, 299–305. [Google Scholar] [CrossRef]
- Faroni, A.; Smith, R.; Procacci, P.; Castelnovo, L.; Puccianti, E.; Reid, A.; Magnaghi, V.; Verkhratsky, A. Purinergic signaling mediated by P2X7 receptors controls myelination in sciatic nerves. J. Neurosci. Res. 2014, 92, 1259–1269. [Google Scholar] [CrossRef]
- Gao, L.; Lin, Z.; Hu, W.; Liu, C.; Zhou, T.; Xie, G.; Qian, M.; Ni, B. Age-specific effects of P2X7 receptors on olfactory function in mice. Neuroreport 2019, 30, 1055–1061. [Google Scholar] [CrossRef]
- Tung, L.T.; Wang, H.; Belle, J.I.; Petrov, J.C.; Langlais, D.; Nijnik, A. p53-dependent induction of P2X7 on hematopoietic stem and progenitor cells regulates hematopoietic response to genotoxic stress. Cell Death Dis. 2021, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Hubert, S.; Rissiek, B.; Klages, K.; Huehn, J.; Sparwasser, T.; Haag, F.; Koch-Nolte, F.; Boyer, O.; Seman, M.; Adriouch, S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2–P2X7 pathway. J. Exp. Med. 2010, 207, 2561–2568. [Google Scholar] [CrossRef] [PubMed]
- Frascoli, M.; Marcandalli, J.; Schenk, U.; Grassi, F. Purinergic P2X7 receptor drives T cell lineage choice and shapes peripheral γδ cells. J. Immunol. 2012, 189, 174–180. [Google Scholar] [CrossRef]
- Proietti, M.; Cornacchione, V.; Jost, T.R.; Romagnani, A.; Faliti, C.E.; Perruzza, L.; Rigoni, R.; Radaelli, E.; Caprioli, F.; Preziuso, S.; et al. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer’s patches to promote host-microbiota mutualism. Immunity 2014, 41, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Perruzza, L.; Strati, F.; Gargari, G.; D’erchia, A.M.; Fosso, B.; Pesole, G.; Guglielmetti, S.; Grassi, F. Enrichment of intestinal Lactobacillus by enhanced secretory IgA coating alters glucose homeostasis in P2rx7−/− mice. Sci. Rep. 2019, 9, 9315. [Google Scholar] [CrossRef]
- Perruzza, L.; Gargari, G.; Proietti, M.; Fosso, B.; D’erchia, A.M.; Faliti, C.E.; Rezzonico-Jost, T.; Scribano, D.; Mauri, L.; Colombo, D.; et al. T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP. Cell Rep. 2017, 18, 2566–2575. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.A.; Bawden, E. Gut Microbiome. Nutr. Clin. Pract. 2015, 30, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Masin, M.; Young, C.; Lim, K.; Barnes, S.J.; Xu, X.J.; Marschall, V.; Brutkowski, W.; Mooney, E.R.; Gorecki, D.C.; Murrell-Lagnado, R. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: Re-evaluation of P2X7 knockouts. Br. J. Pharmacol. 2012, 165, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Boumechache, M.; Masin, M.; Edwardson, J.M.; Górecki, D.C.; Murrell-Lagnado, R. Analysis of assembly and trafficking of native P2X4 and P2X7 receptor complexes in rodent immune cells. J. Biol. Chem. 2009, 284, 13446–13454. [Google Scholar] [CrossRef]
- Taylor, S.R.; Gonzalez-Begne, M.; Sojka, D.K.; Richardson, J.C.; Sheardown, S.A.; Harrison, S.M.; Pusey, C.D.; Tam, F.W.; Elliott, J.I. Lymphocytes from P2X7-deficient mice exhibit enhanced P2X7 responses. J. Leukoc. Biol. 2009, 85, 978–986. [Google Scholar] [CrossRef]
- Scheuplein, F.; Schwarz, N.; Adriouch, S.; Krebs, C.; Bannas, P.; Rissiek, B.; Seman, M.; Haag, F.; Koch-Nolte, F. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J. Immunol. 2009, 182, 2898–2908. [Google Scholar] [CrossRef]
- Borges da Silva, H.; Wang, H.; Qian, L.J.; Hogquist, K.A.; Jameson, S.C. ARTC2.2/P2RX7 Signaling during Cell Isolation Distorts Function and Quantification of Tissue-Resident CD8+ T Cell and Invariant NKT Subsets. J. Immunol. 2019, 202, 2153–2163. [Google Scholar] [CrossRef] [PubMed]
- Rissiek, B.; Danquah, W.; Haag, F.; Koch-Nolte, F. Technical Advance: A new cell preparation strategy that greatly improves the yield of vital and functional Tregs and NKT cells. J. Leukoc. Biol. 2014, 95, 543–549. [Google Scholar] [CrossRef]
- Rissiek, B.; Lukowiak, M.; Raczkowski, F.; Magnus, T.; Mittrücker, H.-W.; Koch-Nolte, F. In Vivo Blockade of Murine ARTC2.2 during Cell Preparation Preserves the Vitality and Function of Liver Tissue-Resident Memory T Cells. Front. Immunol. 2018, 9, 1580. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Ganesan, J.; Müller, T.; Dürr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Jüttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef]
- Chen, L.; Brosnan, C.F. Exacerbation of experimental autoimmune encephalomyelitis in P2X7R−/− mice: Evidence for loss of apoptotic activity in lymphocytes. J. Immunol. 2006, 176, 3115–3126. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Vieira, R.P.; Grimm, M.; Dürk, T.; Cicko, S.; Zeiser, R.; Jakob, T.; Martin, S.F.; Blumenthal, B.; Sorichter, S.; et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am. J. Respir. Cell Mol. Biol. 2011, 44, 456–464. [Google Scholar] [CrossRef]
- Lucattelli, M.; Cicko, S.; Müller, T.; Lommatzsch, M.; De Cunto, G.; Cardini, S.; Sundas, W.; Grimm, M.; Zeiser, R.; Dürk, T.; et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am. J. Respir. Cell Mol. Biol. 2011, 44, 423–429. [Google Scholar] [CrossRef]
- Cicko, S.; Köhler, T.C.; Ayata, C.K.; Müller, T.; Ehrat, N.; Meyer, A.; Hossfeld, M.; Zech, A.; Di Virgilio, F.; Idzko, M. Extracellular ATP is a danger signal activating P2X7 receptor in a LPS mediated inflammation (ARDS/ALI). Oncotarget 2018, 9, 30635–30648. [Google Scholar] [CrossRef]
- Csölle, C.; Andó, R.D.; Kittel, Á.; Gölöncsér, F.; Baranyi, M.; Soproni, K.; Zelena, D.; Haller, J.; Németh, T.; Mócsai, A.; et al. The absence of P2X7 receptors (P2rx7) on non-haematopoietic cells leads to selective alteration in mood-related behaviour with dysregulated gene expression and stress reactivity in mice. Int. J. Neuropsychopharmacol. 2013, 16, 213–233. [Google Scholar] [CrossRef]
- Adinolfi, E.; Capece, M.; Franceschini, A.; Falzoni, S.; Giuliani, A.L.; Rotondo, A.; Sarti, A.C.; Bonora, M.; Syberg, S.; Corigliano, D.; et al. Accelerated tumor progression in mice lacking the ATP receptor P2X7. Cancer Res 2015, 75, 635–644. [Google Scholar] [CrossRef]
- Huang, S.W.; Walker, C.; Pennock, J.; Else, K.; Muller, W.; Daniels, M.J.; Pellegrini, C.; Brough, D.; Lopez-Castejon, G.; Cruickshank, S.M. P2X7 receptor-dependent tuning of gut epithelial responses to infection. Immunol. Cell Biol. 2017, 95, 178–188. [Google Scholar] [CrossRef]
- Koo, T.Y.; Lee, J.-G.; Yan, J.-J.; Jang, J.Y.; Ju, K.D.; Han, M.; Oh, K.-H.; Ahn, C.; Yang, J. The P2X7 receptor antagonist, oxidized adenosine triphosphate, ameliorates renal ischemia-reperfusion injury by expansion of regulatory T cells. Kidney Int. 2017, 92, 415–431. [Google Scholar] [CrossRef]
- Qian, Y.; Qian, C.; Xie, K.; Fan, Q.; Yan, Y.; Lu, R.; Wang, L.; Zhang, M.; Wang, Q.; Mou, S.; et al. P2X7 receptor signaling promotes inflammation in renal parenchymal cells suffering from ischemia-reperfusion injury. Cell Death Dis. 2021, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Csóka, B.; Németh, Z.H.; Törő, G.; Idzko, M.; Zech, A.; Koscsó, B.; Spolarics, Z.; Antonioli, L.; Cseri, K.; Erdélyi, K.; et al. Extracellular ATP protects against sepsis through macrophage P2X7 purinergic receptors by enhancing intracellular bacterial killing. FASEB J. 2015, 29, 3626–3637. [Google Scholar] [CrossRef]
- Bomfim, C.C.B.; Amaral, E.P.; Cassado, A.D.A.; Salles, É.M.; Nascimento, R.S.D.; Lasunskaia, E.; Hirata, M.H.; Álvarez, J.M.; D’império-Lima, M.R. P2X7 Receptor in Bone Marrow-Derived Cells Aggravates Tuberculosis Caused by Hypervirulent Mycobacterium bovis. Front. Immunol. 2017, 8, 435. [Google Scholar] [CrossRef]
- Furlan-Freguia, C.; Marchese, P.; Gruber, A.; Ruggeri, Z.M.; Ruf, W. P2X7 receptor signaling contributes to tissue factor–dependent thrombosis in mice. J. Clin. Investig. 2011, 121, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Skarnes, W.C.; Rosen, B.; West, A.P.; Koutsourakis, M.; Bushell, W.; Iyer, V.; Mujica, A.O.; Thomas, M.; Harrow, J.; Cox, T.; et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011, 474, 337–342. [Google Scholar] [CrossRef]
- Faliti, C.E.; Gualtierotti, R.; Rottoli, E.; Gerosa, M.; Perruzza, L.; Romagnani, A.; Pellegrini, G.; De Ponte Conti, B.; Rossi, R.L.; Idzko, M.; et al. P2X7 receptor restrains pathogenic Tfh cell generation in systemic lupus erythematosus. J. Exp. Med. 2019, 216, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.B.; Peng, C.; Wang, H.; Wanhainen, K.M.; Ma, C.; Lopez, S.; Khoruts, A.; Zhang, N.; Jameson, S.C. Sensing of ATP via the Purinergic Receptor P2RX7 Promotes CD8+ Trm Cell Generation by Enhancing Their Sensitivity to the Cytokine TGF-β. Immunity 2020, 53, 158–171.e156. [Google Scholar] [CrossRef]
- Becher, B.; Waisman, A.; Lu, L.-F. Conditional Gene-Targeting in Mice: Problems and Solutions. Immunity 2018, 48, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Song, A.J.; Palmiter, R.D. Detecting and Avoiding Problems When Using the Cre-lox System. Trends Genet. 2018, 34, 333–340. [Google Scholar] [CrossRef]
- Rumney, R.M.H.; Róg, J.; Chira, N.; Kao, A.P.; Al-Khalidi, R.; Górecki, D.C. P2X7 Purinoceptor Affects Ectopic Calcification of Dystrophic Muscles. Front. Pharmacol. 2022, 13, 935804. [Google Scholar] [CrossRef]
- Engel, T.; Gomez-Villafuertes, R.; Tanaka, K.; Mesuret, G.; Sanz-Rodriguez, A.; Garcia-Huerta, P.; Miras-Portugal, M.T.; Henshall, D.C.; Diaz-Hernandez, M. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012, 26, 1616–1628. [Google Scholar] [CrossRef]
- Jimenez-Pacheco, A.; Diaz-Hernandez, M.; Arribas-Blázquez, M.; Sanz-Rodriguez, A.; Olivos-Oré, L.A.; Artalejo, A.R.; Alves, M.; Letavic, M.; Miras-Portugal, M.T.; Conroy, R.M.; et al. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. J. Neurosci. 2016, 36, 5920–5932. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Pacheco, A.; Mesuret, G.; Sanz-Rodriguez, A.; Tanaka, K.; Mooney, C.; Conroy, R.; Miras-Portugal, M.T.; Diaz-Hernandez, M.; Henshall, D.C.; Engel, T. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia 2013, 54, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Sebastián-Serrano, Á.; Engel, T.; De Diego-García, L.; Olivos-Oré, L.A.; Arribas-Blázquez, M.; Martínez-Frailes, C.; Pérez-Díaz, C.; Millán, J.L.; Artalejo, A.R.; Miras-Portugal, M.T.; et al. Neurodevelopmental alterations and seizures developed by mouse model of infantile hypophosphatasia are associated with purinergic signalling deregulation. Hum. Mol. Genet. 2016, 25, 4143–4156. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, Y.; Ikeda-Matsuo, Y.; Notomi, S.; Enaida, H.; Kinouchi, H.; Koizumi, S. Astrocyte-mediated ischemic tolerance. J. Neurosci. 2015, 35, 3794–3805. [Google Scholar] [CrossRef]
- Martínez-Frailes, C.; Di Lauro, C.; Bianchi, C.; De Diego-García, L.; Sebastián-Serrano, Á.; Boscá, L.; Díaz-Hernández, M. Amyloid Peptide Induced Neuroinflammation Increases the P2X7 Receptor Expression in Microglial Cells, Impacting on Its Functionality. Front. Cell. Neurosci. 2019, 13, 143. [Google Scholar] [CrossRef]
- García-Huerta, P.; Díaz-Hernandez, M.; Delicado, E.G.; Pimentel-Santillana, M.; Miras-Portugal, M.T.; Gómez-Villafuertes, R. The specificity protein factor Sp1 mediates transcriptional regulation of P2X7 receptors in the nervous system. J. Biol. Chem. 2012, 287, 44628–44644. [Google Scholar] [CrossRef]
- Ortega, F.; Gomez-Villafuertes, R.; Benito-León, M.; Martínez de la Torre, M.; Olivos-Oré, L.A.; Arribas-Blazquez, M.; Gomez-Gaviro, M.V.; Azcorra, A.; Desco, M.; Artalejo, A.R.; et al. Salient brain entities labelled in P2rx7-EGFP reporter mouse embryos include the septum, roof plate glial specializations and circumventricular ependymal organs. Anat. Embryol. 2021, 226, 715–741. [Google Scholar] [CrossRef]
- Morgan, J.; Alves, M.; Conte, G.; Menéndez-Méndez, A.; De Diego-Garcia, L.; De Leo, G.; Beamer, E.; Smith, J.; Nicke, A.; Engel, T. Characterization of the Expression of the ATP-Gated P2X7 Receptor Following Status Epilepticus and during Epilepsy Using a P2X7-EGFP Reporter Mouse. Neurosci. Bull. 2020, 36, 1242–1258. [Google Scholar] [CrossRef]
- Calovi, S.; Mut-Arbona, P.; Tod, P.; Iring, A.; Nicke, A.; Mato, S.; Vizi, E.S.; Tønnesen, J.; Sperlagh, B. P2X7 Receptor-Dependent Layer-Specific Changes in Neuron-Microglia Reactivity in the Prefrontal Cortex of a Phencyclidine Induced Mouse Model of Schizophrenia. Front. Mol. Neurosci. 2020, 13, 566251. [Google Scholar] [CrossRef]
- Beamer, E.; Morgan, J.; Alves, M.; Méndez, A.M.; Morris, G.; Zimmer, B.; Conte, G.; Diego-Garcia, L.; Alarcón-Vila, C.; Yiu Ng, N.K.; et al. Increased expression of the ATP-gated P2X7 receptor reduces responsiveness to anti-convulsants during status epilepticus in mice. Br. J. Pharmacol. 2022, 179, 2986–3006. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Fernández, A.; Urbina-Treviño, L.; Conte, G.; Alves, M.; Rissiek, B.; Durner, A.; Scalbert, N.; Zhang, J.; Magnus, T.; Koch-Nolte, F.; et al. Deviant reporter expression and P2X4 passenger gene overexpression in the soluble EGFP BAC transgenic P2X7 reporter mouse model. Sci. Rep. 2020, 10, 19876. [Google Scholar] [CrossRef]
- Prades, S.; Heard, G.; Gale, J.E.; Engel, T.; Kopp, R.; Nicke, A.; Smith, K.E.; Jagger, D.J. Functional P2X(7) Receptors in the Auditory Nerve of Hearing Rodents Localize Exclusively to Peripheral Glia. J. Neurosci. 2021, 41, 2615–2629. [Google Scholar] [CrossRef]
- Sluyter, R.; Watson, D. Use of Humanized Mouse Models to Investigate the Roles of Purinergic Signaling in Inflammation and Immunity. Front. Pharmacol. 2020, 11, 596357. [Google Scholar] [CrossRef]
- Urbina-Treviño, L.; von Mücke-Heim, I.-A.; Deussing, J.M. P2X7 Receptor-Related Genetic Mouse Models—Tools for Translational Research in Psychiatry. Front. Neural Circuits 2022, 16, 876304. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.W.; Walser, S.M.; Aprile-Garcia, F.; Dedic, N.; Chen, A.; Holsboer, F.; Arzt, E.; Wurst, W.; Deussing, J.M. Genetically dissecting P2rx7 expression within the central nervous system using conditional humanized mice. Purinergic Signal. 2017, 13, 153–170. [Google Scholar] [CrossRef]
- Metzger, M.W.; Walser, S.M.; Dedic, N.; Aprile-Garcia, F.; Jakubcakova, V.; Adamczyk, M.; Webb, K.J.; Uhr, M.; Refojo, D.; Schmidt, M.V.; et al. Heterozygosity for the Mood Disorder-Associated Variant Gln460Arg Alters P2X7 Receptor Function and Sleep Quality. J. Neurosci. 2017, 37, 11688–11700. [Google Scholar] [CrossRef] [PubMed]
- Barden, N.; Harvey, M.; Gagné, B.; Shink, E.; Tremblay, M.; Raymond, C.; Labbé, M.; Villeneuve, A.; Rochette, D.; Bordeleau, L.; et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2006, 141b, 374–382. [Google Scholar] [CrossRef]
- Lucae, S.; Salyakina, D.; Barden, N.; Harvey, M.; Gagné, B.; Labbé, M.; Binder, E.B.; Uhr, M.; Paez-Pereda, M.; Sillaber, I.; et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 2006, 15, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Deussing, J.M.; Arzt, E. P2X7 Receptor: A Potential Therapeutic Target for Depression? Trends Mol. Med. 2018, 24, 736–747. [Google Scholar] [CrossRef]
- Fuller, S.J.; Stokes, L.; Skarratt, K.K.; Gu, B.J.; Wiley, J.S. Genetics of the P2X7 receptor and human disease. Purinergic Signal. 2009, 5, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Stokes, L. Significance of P2X7 receptor variants to human health and disease. Recent Pat. DNA Gene Seq. 2011, 5, 41–54. [Google Scholar] [CrossRef]
- Adinolfi, E.; Raffaghello, L.; Giuliani, A.L.; Cavazzini, L.; Capece, M.; Chiozzi, P.; Bianchi, G.; Kroemer, G.; Pistoia, V.; Di Virgilio, F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 2012, 72, 2957–2969. [Google Scholar] [CrossRef]
- Chong, J.-H.; Zheng, G.-G.; Ma, Y.-Y.; Zhang, H.-Y.; Nie, K.; Lin, Y.-M.; Wu, K.-F. The hyposensitive N187D P2X7 mutant promotes malignant progression in nude mice. J. Biol. Chem. 2010, 285, 36179–36187. [Google Scholar] [CrossRef]
- Feng, W.; Yang, X.; Wang, L.; Wang, R.; Yang, F.; Wang, H.; Liu, X.; Ren, Q.; Zhang, Y.; Zhu, X.; et al. P2X7 promotes the progression of MLL-AF9 induced acute myeloid leukemia by upregulation of Pbx3. Haematologica 2021, 106, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-J.; Luo, C.; Huang, C.; Pu, F.-Q.; Zhu, J.-F.; Zhu, Z.-M. PI3K/Akt/GSK-3β signal pathway is involved in P2X7 receptor-induced proliferation and EMT of colorectal cancer cells. Eur. J. Pharmacol. 2021, 899, 174041. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Wang, L.; Lou, Y. A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor. Biochem. Biophys. Res. Commun. 2021, 558, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ji, Y.G.; Ko, J.J.; Cho, H.J.; Lee, D.H. Activating P2X7 Receptors Increases Proliferation of Human Pancreatic Cancer Cells via ERK1/2 and JNK. Pancreas 2018, 47, 643–651. [Google Scholar] [CrossRef]
- Giannuzzo, A.; Saccomano, M.; Napp, J.; Ellegaard, M.; Alves, F.; Novak, I. Targeting of the P2X7 receptor in pancreatic cancer and stellate cells. Int. J. Cancer 2016, 139, 2540–2552. [Google Scholar] [CrossRef]
- Pegoraro, A.; Orioli, E.; De Marchi, E.; Salvestrini, V.; Milani, A.; Di Virgilio, F.; Curti, A.; Adinolfi, E. Differential sensitivity of acute myeloid leukemia cells to daunorubicin depends on P2X7A versus P2X7B receptor expression. Cell Death Dis. 2020, 11, 876. [Google Scholar] [CrossRef]
- Salvestrini, V.; Orecchioni, S.; Talarico, G.; Reggiani, F.; Mazzetti, C.; Bertolini, F.; Orioli, E.; Adinolfi, E.; Di Virgilio, F.; Pezzi, A.; et al. Extracellular ATP induces apoptosis through P2X7R activation in acute myeloid leukemia cells but not in normal hematopoietic stem cells. Oncotarget 2017, 8, 5895–5908. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Y.; Wu, W.; Ouyang, N.; Chen, J.; Li, H.; Liu, X.; Su, F.; Lin, L.; Yao, Y. miR-150 promotes human breast cancer growth and malignant behavior by targeting the pro-apoptotic purinergic P2X7 receptor. PLoS ONE 2013, 8, e80707. [Google Scholar] [CrossRef]
- Zhou, J.Z.; Riquelme, M.A.; Gao, X.; Ellies, L.G.; Sun, L.Z.; Jiang, J.X. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene 2015, 34, 1831–1842. [Google Scholar] [CrossRef]
- Li, C.-F.; Chan, T.-C.; Pan, C.-T.; Vejvisithsakul, P.P.; Lai, J.-C.; Chen, S.-Y.; Hsu, Y.-W.; Shiao, M.-S.; Shiue, Y.-L. EMP2 induces cytostasis and apoptosis via the TGFβ/SMAD/SP1 axis and recruitment of P2RX7 in urinary bladder urothelial carcinoma. Cell. Oncol. 2021, 44, 1133–1150. [Google Scholar] [CrossRef]
- Adinolfi, E.; De Marchi, E.; Orioli, E.; Pegoraro, A.; Di Virgilio, F. Role of the P2X7 receptor in tumor-associated inflammation. Curr. Opin. Pharmacol. 2019, 47, 59–64. [Google Scholar] [CrossRef]
- Watson, D.; Adhikary, S.R.; Cuthbertson, P.; Geraghty, N.J.; Bird, K.M.; Elhage, A.; Sligar, C.; Sluyter, R. Humanized Mouse Model to Study the P2X7 Receptor in Graft-Versus-Host Disease. Methods Mol. Biol. 2022, 2510, 315–340. [Google Scholar] [CrossRef]
- Cuthbertson, P.; Adhikary, S.R.; Geraghty, N.J.; Guy, T.V.; Hadjiashrafi, A.; Fuller, S.J.; Ly, D.; Watson, D.; Sluyter, R. Increased P2X7 expression in the gastrointestinal tract and skin in a humanised mouse model of graft-versus-host disease. Clin. Sci. 2020, 134, 207–223. [Google Scholar] [CrossRef]
- Sluyter, R.; Cuthbertson, P.; Elhage, A.; Sligar, C.; Watson, D. Purinergic signalling in graft-versus-host disease. Curr. Opin. Pharmacol. 2023, 68, 102346. [Google Scholar] [CrossRef] [PubMed]
- Adhikary, S.R.; Geraghty, N.J.; Cuthbertson, P.; Sluyter, R.; Watson, D. Altered donor P2X7 activity in human leukocytes correlates with P2RX7 genotype but does not affect the development of graft-versus-host disease in humanised mice. Purinergic Signal. 2019, 15, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Koldej, R.M.; Perera, T.; Collins, J.; Ritchie, D.S. Association between P2X7 Polymorphisms and Post-Transplant Outcomes in Allogeneic Haematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2020, 21, 3772. [Google Scholar] [CrossRef] [PubMed]
- Honore, P.; Donnelly-Roberts, D.; Namovic, M.T.; Hsieh, G.; Zhu, C.Z.; Mikusa, J.P.; Hernandez, G.; Zhong, C.; Gauvin, D.M.; Chandran, P.; et al. A-740003 [N-(1-{[(cyanoimino)(5-quinolinylamino) methyl]amino}-2,2-dimethylpropyl)-2-(3,4-dimethoxyphenyl)acetamide], a novel and selective P2X7 receptor antagonist, dose-dependently reduces neuropathic pain in the rat. J. Pharmacol. Exp. Ther. 2006, 319, 1376–1385. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Namovic, M.T.; Donnelly-Roberts, D.L.; Harris, R.R.; Zhang, X.-F.; Shieh, C.-C.; Wismer, C.T.; Zhu, C.Z.; Gauvin, D.M.; et al. P2X7-related modulation of pathological nociception in rats. Neuroscience 2007, 146, 1817–1828. [Google Scholar] [CrossRef]
- Lord, B.; Aluisio, L.; Shoblock, J.; Neff, R.A.; Varlinskaya, E.; Ceusters, M.; Lovenberg, T.W.; Carruthers, N.; Bonaventure, P.; Letavic, M.A.; et al. Pharmacology of a novel central nervous system-penetrant P2X7 antagonist JNJ-42253432. J. Pharmacol. Exp. Ther. 2014, 351, 628–641. [Google Scholar] [CrossRef]
- Swanson, D.M.; Savall, B.M.; Coe, K.J.; Schoetens, F.; Koudriakova, T.; Skaptason, J.; Wall, J.; Rech, J.; Deng, X.; De Angelis, M.; et al. Identification of (R)-(2-Chloro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyridin-2-yl)-4-methyl-6,7-dihydro-1H-imidazo[4,5-c]pyridin-5(4H)-yl)methanone (JNJ 54166060), a Small Molecule Antagonist of the P2X7 receptor. J. Med. Chem. 2016, 59, 8535–8548. [Google Scholar] [CrossRef]
- Hopper, A.T.; Juhl, M.; Hornberg, J.; Badolo, L.; Kilburn, J.P.; Thougaard, A.; Smagin, G.; Song, D.; Calice, L.; Menon, V.; et al. Synthesis and Characterization of the Novel Rodent-Active and CNS-Penetrant P2X7 Receptor Antagonist Lu AF27139. J. Med. Chem. 2021, 64, 4891–4902. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, H.; Xing, J.; Shi, X.; Huang, H.; Huang, J.; Xu, C. Silencing P2X7R Alleviates Diabetic Neuropathic Pain Involving TRPV1 via PKCε/P38MAPK/NF-κB Signaling Pathway in Rats. Int. J. Mol. Sci. 2022, 23, 14141. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.; Punaro, G.R.; Serralha, R.S.; Lima, D.Y.; Mouro, M.G.; Oliveira, L.G.G.; Casarini, D.E.; Rodrigues, A.M.; Higa, E.M.S. Inhibition of the P2X7 receptor improves renal function via renin-angiotensin system and nitric oxide on diabetic nephropathy in rats. Life Sci. 2020, 251, 117640. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Serralha, R.; Lima, D.Y.; Punaro, G.R.; Visona, I.; Fernandes, M.J.S.; Higa, E.M.S. P2X7 siRNA targeted to the kidneys increases klotho and delays the progression of experimental diabetic nephropathy. Purinergic Signal. 2020, 16, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.P.; Araújo, M.G.L.; Valero, J.; Lopes-Cendes, I.; Pascoal, V.D.B.; Malva, J.O.; da Silva Fernandes, M.J. Silencing of P2X7R by RNA interference in the hippocampus can attenuate morphological and behavioral impact of pilocarpine-induced epilepsy. Purinergic Signal. 2017, 13, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Ding, R.; Fu, Z.; Yang, S.; Deng, X.; Zeng, J. P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: Involvement of peroxynitrite. J. Neuroinflammation 2015, 12, 190. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Li, X.-Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef]
- Deplano, S.; Cook, H.T.; Russell, R.; Franchi, L.; Schneiter, S.; Bhangal, G.; Unwin, R.J.; Pusey, C.D.; Tam, F.W.K.; Behmoaras, J. P2X7 receptor-mediated Nlrp3-inflammasome activation is a genetic determinant of macrophage-dependent crescentic glomerulonephritis. J. Leukoc. Biol. 2013, 93, 127–134. [Google Scholar] [CrossRef]
- Li, X.; Wan, A.; Liu, Y.; Li, M.; Zhu, Z.; Luo, C.; Tao, J. P2X7R Mediates the Synergistic Effect of ATP and MSU Crystals to Induce Acute Gouty Arthritis. Oxidative Med. Cell. Longev. 2023, 2023, 3317307. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.-Z.; Gao, N.; Lin, Z.; Gao, C.; Liu, S.; Ren, J.; Xia, Y.; Wood, J.D. P2X7 receptors in the enteric nervous system of guinea-pig small intestine. J. Comp. Neurol. 2001, 440, 299–310. [Google Scholar] [CrossRef]
- Menzies, J.; Paul, A.; Kennedy, C. P2X7 subunit-like immunoreactivity in the nucleus of visceral smooth muscle cells of the guinea pig. Auton. Neurosci. 2003, 106, 103–109. [Google Scholar] [CrossRef]
- Valdez-Morales, E.; Guerrero-Alba, R.; Liñán-Rico, A.; Espinosa-Luna, R.; Zarazua-Guzman, S.; Miranda-Morales, M.; Montaño, L.M.; Barajas-López, C. P2X7 receptors contribute to the currents induced by ATP in guinea pig intestinal myenteric neurons. Eur. J. Pharmacol. 2011, 668, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Creed, K.E.; Loxley, R.A.; Phillips, J.K. Functional expression of muscarinic and purinoceptors in the urinary bladder of male and female rats and guinea pigs. J. Smooth Muscle Res. 2010, 46, 201–215. [Google Scholar] [CrossRef]
- Chávez, J.; Vargas, M.H.; Martínez-Zúñiga, J.; Falfán-Valencia, R.; Ambrocio-Ortiz, E.; Carbajal, V.; Sandoval-Roldán, R. Allergic sensitization increases the amount of extracellular ATP hydrolyzed by guinea pig leukocytes. Purinergic Signal. 2019, 15, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Szücs, A.; Szappanos, H.; Tóth, A.; Farkas, Z.; Panyi, G.; Csernoch, L.; Sziklai, I. Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear. Res. 2004, 196, 2–7. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Yu, N.; Fleming, C.R. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc. Natl. Acad. Sci. USA 2005, 102, 18724–18729. [Google Scholar] [CrossRef]
- Szucs, A.; Szappanos, H.; Batta, T.J.; Tóth, A.; Szigeti, G.P.; Panyi, G.; Csernoch, L.; Sziklai, I. Changes in purinoceptor distribution and intracellular calcium levels following noise exposure in the outer hair cells of the guinea pig. J. Membr. Biol. 2006, 213, 135–141. [Google Scholar] [CrossRef]
- Sueta, T.; Paki, B.; Everett, A.; Robertson, D. Purinergic receptors in auditory neurotransmission. Hear. Res. 2003, 183, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Vlajkovic, S.M.; Thorne, P.R. Purinergic Signalling in the Cochlea. Int. J. Mol. Sci. 2022, 23, 14874. [Google Scholar] [CrossRef] [PubMed]
- Pulvirenti, T.J.; Yin, J.L.; Chaufour, X.; McLachlan, C.; Hambly, B.D.; Bennett, M.R.; Barden, J.A. P2X (purinergic) receptor redistribution in rabbit aorta following injury to endothelial cells and cholesterol feeding. J. Neurocytol. 2000, 29, 623–631. [Google Scholar] [CrossRef]
- Ma, W.; Korngreen, A.; Weil, S.; Cohen, E.B.; Priel, A.; Kuzin, L.; Silberberg, S.D. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J. Physiol. 2006, 571, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Naemsch, L.N.; Dixon, S.J.; Sims, S.M. Activity-dependent development of P2X7 current and Ca2+ entry in rabbit osteoclasts. J. Biol. Chem. 2001, 276, 39107–39114. [Google Scholar] [CrossRef]
- Korcok, J.; Raimundo, L.N.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. Extracellular nucleotides act through P2X7 receptors to activate NF-kappaB in osteoclasts. J. Bone Miner. Res. 2004, 19, 642–651. [Google Scholar] [CrossRef]
- Tanigawa, H.; Toyoda, F.; Kumagai, K.; Okumura, N.; Maeda, T.; Matsuura, H.; Imai, S. P2X7 ionotropic receptor is functionally expressed in rabbit articular chondrocytes and mediates extracellular ATP cytotoxicity. Purinergic Signal. 2018, 14, 245–258. [Google Scholar] [CrossRef]
- Zhang, Q.; Siroky, M.; Yang, J.-H.; Zhao, Z.; Azadzoi, K. Effects of ischemia and oxidative stress on bladder purinoceptors expression. Urology 2014, 84, 1249.e1–1249.e7. [Google Scholar] [CrossRef]
- Osgood, M.J.; Sexton, K.; Voskresensky, I.; Hocking, K.; Song, J.; Komalavilas, P.; Brophy, C.; Cheung-Flynn, J. Use of Brilliant Blue FCF during vein graft preparation inhibits intimal hyperplasia. J. Vasc. Surg. 2016, 64, 471–478. [Google Scholar] [CrossRef]
- Wang, J.; Jackson, D.G.; Dahl, G. The food dye FD&C Blue No. 1 is a selective inhibitor of the ATP release channel Panx1. J. Gen. Physiol. 2013, 141, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Oku, H.; Komori, A.; Ikeda, T. Effect of P2X7 receptor activation on the retinal blood velocity of diabetic rabbits. Arch. Ophthalmol. 2006, 124, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Hanau, S.; Pizzo, P.; Rippa, M.; Di Virgilio, F. Oxidized ATP. An irreversible inhibitor of the macrophage purinergic P2Z receptor. J. Biol. Chem. 1993, 268, 8199–8203. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Pouzaud, F.; Larosche, I.; Brignole-Baudouin, F.; Warnet, J.-M.; Rat, P. Fluoroquinolone eye drop-induced cytotoxicity: Role of preservative in P2X7 cell death receptor activation and apoptosis. Investig. Opthalmology Vis. Sci. 2006, 47, 2812–2819. [Google Scholar] [CrossRef]
- Pauloin, T.; Dutot, M.; Liang, H.; Chavinier, E.; Warnet, J.-M.; Rat, P. Corneal protection with high-molecular-weight hyaluronan against in vitro and in vivo sodium lauryl sulfate-induced toxic effects. Cornea 2009, 28, 1032–1041. [Google Scholar] [CrossRef]
- Ishii, K.; Kaneda, M.; Li, H.; Rockland, K.S.; Hashikawa, T. Neuron-specific distribution of P2X7 purinergic receptors in the monkey retina. J. Comp. Neurol. 2003, 459, 267–277. [Google Scholar] [CrossRef]
- Burm, S.M.; Zuiderwijk-Sick, E.A.; Weert, P.M.; Bajramovic, J.J. ATP-induced IL-1β secretion is selectively impaired in microglia as compared to hematopoietic macrophages. Glia 2016, 64, 2231–2246. [Google Scholar] [CrossRef]
- Sluyter, R.; Sophocleous, R.A.; Stokes, L. P2X receptors: Insights from the study of the domestic dog. Neuropharmacology 2023, 224, 109358. [Google Scholar] [CrossRef]
- Birder, L.A.; Ruan, H.Z.; Chopra, B.; Xiang, Z.; Barrick, S.; Buffington, C.A.; Roppolo, J.R.; Ford, A.P.; de Groat, W.C.; Burnstock, G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am. J. Physiol. Renal. Physiol. 2004, 287, F1084–F1091. [Google Scholar] [CrossRef]
- Ruan, H.Z.; Birder, L.A.; Xiang, Z.; Chopra, B.; Buffington, T.; Tai, C.; Roppolo, J.R.; de Groat, W.C.; Burnstock, G. Expression of P2X and P2Y receptors in the intramural parasympathetic ganglia of the cat urinary bladder. Am. J. Physiol. Renal. Physiol. 2006, 290, F1143–F1152. [Google Scholar] [CrossRef]
- Ruan, H.-Z.; Birder, L.A.; De Groat, W.C.; Tai, C.; Roppolo, J.; Buffington, C.A.; Burnstock, G. Localization of P2X and P2Y receptors in dorsal root ganglia of the cat. J. Histochem. Cytochem. 2005, 53, 1273–1282. [Google Scholar] [CrossRef]
- Barden, J.A.; Sluyter, R.; Gu, B.J.; Wiley, J.S. Specific detection of non-functional human P2X7 receptors in HEK293 cells and B-lymphocytes. FEBS Lett. 2003, 538, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Barden, J.A.; Gidley-Baird, A.; Teh, L.C.; Rajasekariah, G.-H.; Pedersen, J.; Christensen, N.I.; Spielman, D.; Ashley, D.M. Therapeutic Targeting of the Cancer-Specific Cell Surface Biomarker nfP2X7. J. Clin. Cell. Immunol. 2016, 7, 3. [Google Scholar] [CrossRef]
- Gilbert, S.M.; Gidley Baird, A.; Glazer, S.; Barden, J.A.; Glazer, A.; Teh, L.C.; King, J. A phase I clinical trial demonstrates that nfP2X7-targeted antibodies provide a novel, safe and tolerable topical therapy for basal cell carcinoma. Br. J. Dermatol. 2017, 177, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, S.M.; Oliphant, C.J.; Hassan, S.; Peille, A.L.; Bronsert, P.; Falzoni, S.; Di Virgilio, F.; McNulty, S.; Lara, R. ATP in the tumour microenvironment drives expression of nfP2X7, a key mediator of cancer cell survival. Oncogene 2019, 38, 194–208. [Google Scholar] [CrossRef]
- Nabinger, D.D.; Altenhofen, S.; Bonan, C.D. Zebrafish models: Gaining insight into purinergic signaling and neurological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 98, 109770. [Google Scholar] [CrossRef] [PubMed]
- Boué-Grabot, E.; Akimenko, M.A.; Séguéla, P. Unique functional properties of a sensory neuronal P2X ATP-gated channel from zebrafish. J. Neurochem. 2000, 75, 1600–1607. [Google Scholar] [CrossRef]
- Jelassi, B.; Chantôme, A.; Alcaraz-Pérez, F.; Baroja-Mazo, A.; Cayuela, M.L.; Pelegrin, P.; Surprenant, A.; Roger, S. P2X7 receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 2011, 30, 2108–2122. [Google Scholar] [CrossRef]
- Jelassi, B.; Anchelin, M.; Chamouton, J.; Cayuela, M.L.; Clarysse, L.; Li, J.; Goré, J.; Jiang, L.H.; Roger, S. Anthraquinone emodin inhibits human cancer cell invasiveness by antagonizing P2X7 receptors. Carcinogenesis 2013, 34, 1487–1496. [Google Scholar] [CrossRef]
- Chang, M.Y.; Lu, J.K.; Tian, Y.C.; Chen, Y.C.; Hung, C.C.; Huang, Y.H.; Chen, Y.H.; Wu, M.S.; Yang, C.W.; Cheng, Y.C. Inhibition of the P2X7 receptor reduces cystogenesis in PKD. J. Am. Soc. Nephrol. 2011, 22, 1696–1706. [Google Scholar] [CrossRef]
- Whyte-Fagundes, P.; Taskina, D.; Safarian, N.; Zoidl, C.; Carlen, P.L.; Donaldson, L.W.; Zoidl, G.R. Panx1 channels promote both anti- and pro-seizure-like activities in the zebrafish via p2rx7 receptors and ATP signaling. Commun. Biol. 2022, 5, 472. [Google Scholar] [CrossRef] [PubMed]
- Ogryzko, N.V.; Hoggett, E.E.; Solaymani-Kohal, S.; Tazzyman, S.; Chico, T.J.; Renshaw, S.A.; Wilson, H.L. Zebrafish tissue injury causes up-regulation of interleukin-1 and caspase dependent amplification of the inflammatory response. Dis. Models Mech. 2014, 7, 259–264. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, F.; Cruz, F.; Menezes, F.; Kist, L.; Bogo, M.; Morrone, F. P2X7R and PANX-1 channel relevance in a zebrafish larvae copper-induced inflammation model. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 223, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Gusso, D.; Cruz, F.F.; Fritsch, P.M.; Gobbo, M.O.; Morrone, F.B.; Bonan, C.D. Pannexin channel 1, P2X7 receptors, and Dimethyl Sulfoxide mediate pain responses in zebrafish. Behav. Brain Res. 2022, 423, 113786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gong, L.; Wang, C.; Liu, M.; Hu, N.; Dai, X.; Peng, C.; Li, Y. Quercetin mitigates ethanol-induced hepatic steatosis in zebrafish via P2X7R-mediated PI3K/Keap1/Nrf2 signaling pathway. J. Ethnopharmacol. 2021, 268, 113569. [Google Scholar] [CrossRef]
- Cruz, F.F.; Leite, C.E.; Pereira, T.C.; Bogo, M.R.; Bonan, C.D.; Battastini, A.M.; Campos, M.M.; Morrone, F.B. Assessment of mercury chloride-induced toxicity and the relevance of P2X7 receptor activation in zebrafish larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 158, 159–164. [Google Scholar] [CrossRef]
- Medrano, M.P.; Pisera-Fuster, A.; Bernabeu, R.O.; Faillace, M.P. P2X7 and A2A receptor endogenous activation protects against neuronal death caused by CoCl2-induced photoreceptor toxicity in the zebrafish retina. J. Comp. Neurol. 2020, 528, 2000–2020. [Google Scholar] [CrossRef] [PubMed]
- Matty, M.A.; Knudsen, D.R.; Walton, E.M.; Beerman, R.W.; Cronan, M.R.; Pyle, C.J.; Hernandez, R.E.; Tobin, D.M. Potentiation of P2RX7 as a host-directed strategy for control of mycobacterial infection. Elife 2019, 8, e39123. [Google Scholar] [CrossRef]
- Miller, C.M.; Boulter, N.R.; Fuller, S.J.; Zakrzewski, A.M.; Lees, M.P.; Saunders, B.M.; Wiley, J.S.; Smith, N.C. The role of the P2X7 receptor in infectious diseases. PLoS Pathog. 2011, 7, e1002212. [Google Scholar] [CrossRef]
- Nörenberg, W.; Hempel, C.; Urban, N.; Sobottka, H.; Illes, P.; Schaefer, M. Clemastine potentiates the human P2X7 receptor by sensitizing it to lower ATP concentrations. J. Biol. Chem. 2011, 286, 11067–11081. [Google Scholar] [CrossRef]
- Monette, M.M.; Evans, D.L.; Krunkosky, T.; Camus, A.; Jaso-Friedmann, L. Nonspecific cytotoxic cell antimicrobial protein (NCAMP-1): A novel alarmin ligand identified in zebrafish. PLoS ONE 2015, 10, e0116576. [Google Scholar] [CrossRef]
- Pelegrȷn, P.; Chaves-Pozo, E.; Mulero, V.; Meseguer, J. Production and mechanism of secretion of interleukin-1β from the marine fish gilthead seabream. Dev. Comp. Immunol. 2004, 28, 229–237. [Google Scholar] [CrossRef]
- López-Castejón, G.; Sepulcre, M.P.; Mulero, I.; Pelegrín, P.; Meseguer, J.; Mulero, V. Molecular and functional characterization of gilthead seabream Sparus aurata caspase-1: The first identification of an inflammatory caspase in fish. Mol. Immunol. 2008, 45, 49–57. [Google Scholar] [CrossRef]
- He, Y.Q.; Chen, J.; Lu, X.J.; Shi, Y.H. Characterization of P2X7R its function in the macrophages of ayu, Plecoglossus altivelis. PLoS ONE 2013, 8, e57505. [Google Scholar] [CrossRef]
- Li, C.H.; Lu, X.J.; Li, M.Y.; Chen, J. Cathelicidin modulates the function of monocytes/macrophages via the P2X7 receptor in a teleost, Plecoglossus altivelis. Fish Shellfish Immunol. 2015, 47, 878–885. [Google Scholar] [CrossRef] [PubMed]
- McEwan, T.B.; Sanderson-Smith, M.L.; Sluyter, R. Purinergic Signalling in Group A Streptococcus Pathogenesis. Front. Immunol. 2022, 13, 872053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.J.; Wang, P.; Zhang, N.; Chen, D.D.; Nie, P.; Li, J.L.; Zhang, Y.A. B Cell Functions Can Be Modulated by Antimicrobial Peptides in Rainbow Trout Oncorhynchus mykiss: Novel Insights into the Innate Nature of B Cells in Fish. Front. Immunol. 2017, 8, 388. [Google Scholar] [CrossRef] [PubMed]
- Paredes, C.; Li, S.; Chen, X.; Coddou, C. Divalent metal modulation of Japanese flounder (Paralichthys olivaceus) purinergic P2X7 receptor. FEBS Open Bio 2018, 8, 383–389. [Google Scholar] [CrossRef]
- Li, S.; Peng, W.; Li, J.; Hao, G.; Geng, X.; Sun, J. Characterization of Japanese flounder (Paralichthys olivaceus) Caspase1 involved in extracellular ATP-mediated immune signaling in fish. Fish Shellfish Immunol. 2017, 67, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Peng, W.; Hao, G.; Sun, J. Characterization of the responses of the caspase 2, 3, 6 and 8 genes to immune challenges and extracellular ATP stimulation in the Japanese flounder (Paralichthys olivaceus). BMC Vet. Res. 2019, 15, 20. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Wang, N.; Li, J.; Feng, Y.; Sun, J. Identification and characterization of ecto-nucleoside triphosphate diphosphohydrolase 1 (CD39) involved in regulating extracellular ATP-mediated innate immune responses in Japanese flounder (Paralichthys olivaceus). Mol. Immunol. 2019, 112, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Yáñez, E.; Barrera-Avalos, C.; Parra-Tello, B.; Briceño, P.; Rosemblatt, M.V.; Saavedra-Almarza, J.; Rosemblatt, M.; Acuña-Castillo, C.; Bono, M.R.; Sauma, D. P2X7 Receptor at the Crossroads of T Cell Fate. Int. J. Mol. Sci. 2020, 21, 4937. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, X.; Li, J.; Li, X.; Zhang, T.; Hao, G.; Sun, J. Extracellular ATP is a potent signaling molecule in the activation of the Japanese flounder (Paralichthys olivaceus) innate immune responses. Innate Immun. 2020, 26, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Feng, Y.; Li, S.; Sun, J. Identification and characterization of apoptosis-related gene serine/threonine kinase 17A (STK17A) from Japanese flounder Paralichthys olivaceus. Fish Shellfish Immunol. 2020, 98, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
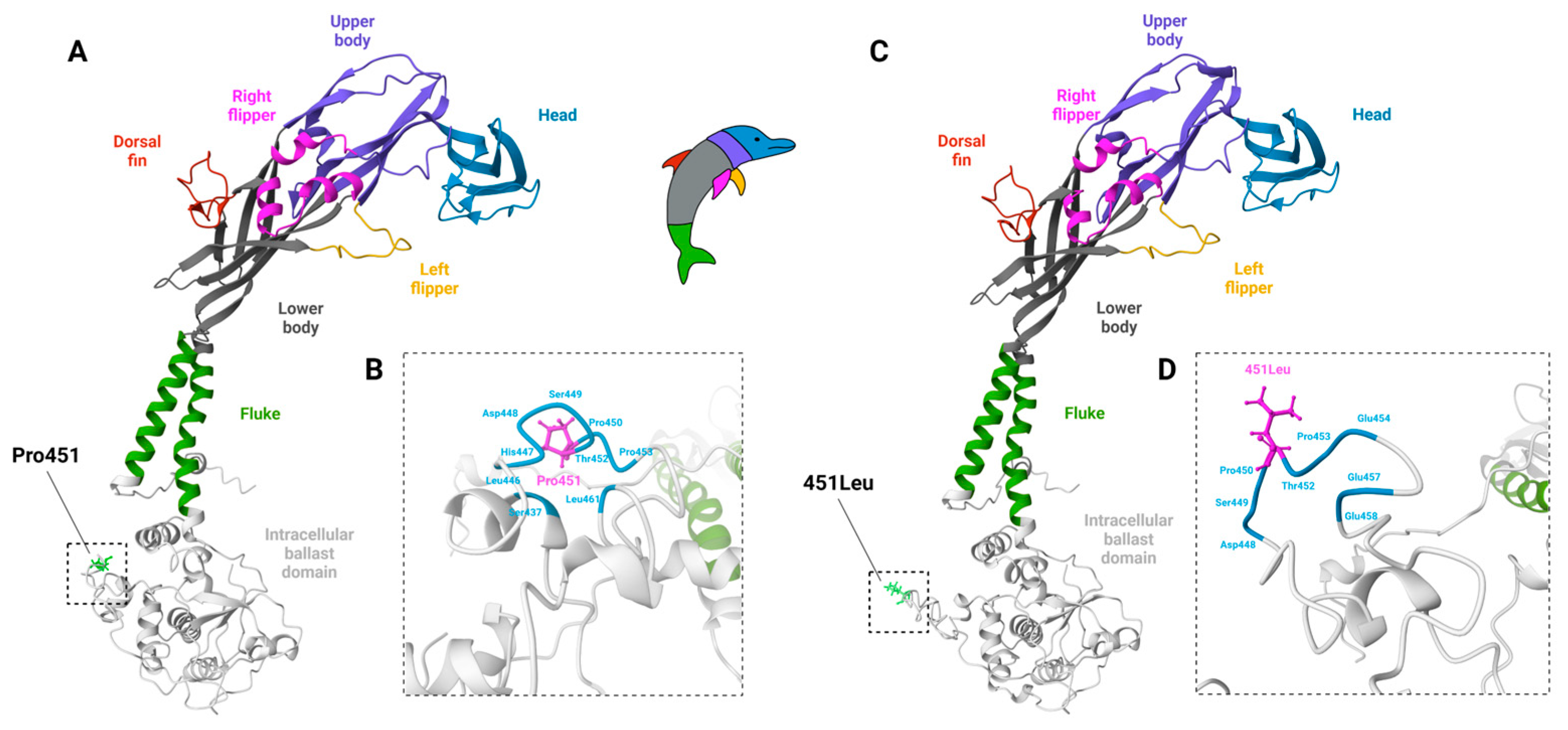
| Compound or Biologic 1 | |
|---|---|
| Agonists | ATP, BzATP, NAD+ |
| Partial agonists | Adenosine-5′-O-(3-thio) triphosphate, 2-methylthio-ATP |
| Positive modulators | Clemastine, HEI3090, anti-murine nanobody (14D5) |
| Non-selective antagonists | BB FCF, BBG, emodin, KN-62, OxATP, PPADS, probenecid |
| Selective antagonists | A-438079, A-740003, AZ101606120, CE-224,535, JNJ-42253432, JNJ-47965567, JNJ-54166060, Lu AF27139, 1-piperidinylmidazole-based antagonists 2 |
| Inhibitory antibodies | Anti-ayu P2X7 polyclonal antibody (aEPAb), anti-human P2X7 mAb (clone L4), anti-murine P2X7 mAb (clone 1F11) |
| Inhibitory nanobodies | Anti-human nanobody (Dano1), anti-murine nanobody (13A7) |
| Species | Technology | Target Site (Defect) | Reference |
|---|---|---|---|
| Mouse | LacZ-neomycin cassette 1 | Exon 1 (deletion) | [153] |
| Mouse | Neomycin cassette | Exon 13 (truncation) | [156] |
| Mouse | LacZ-neomycin cassette | Exon 2 and 3 (substitution) | [158] |
| Mouse | Short hairpin cassette | Exon 3 (knockdown) | [161] |
| Mouse | CRISPR/Cas9 | Exon 2 (deletion) | [159] |
| Rat | CRISPR/Cas9 | Exon 2 (frameshift mutation) | [163] |
| Rat | ZFN 2 | Exon 10 (2 bp insertion) | [164] |
| Rat | CRISPR/Cas9 | Exon 2 (stop codon) | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sluyter, R.; Adriouch, S.; Fuller, S.J.; Nicke, A.; Sophocleous, R.A.; Watson, D. Animal Models for the Investigation of P2X7 Receptors. Int. J. Mol. Sci. 2023, 24, 8225. https://doi.org/10.3390/ijms24098225
Sluyter R, Adriouch S, Fuller SJ, Nicke A, Sophocleous RA, Watson D. Animal Models for the Investigation of P2X7 Receptors. International Journal of Molecular Sciences. 2023; 24(9):8225. https://doi.org/10.3390/ijms24098225
Chicago/Turabian StyleSluyter, Ronald, Sahil Adriouch, Stephen J. Fuller, Annette Nicke, Reece A. Sophocleous, and Debbie Watson. 2023. "Animal Models for the Investigation of P2X7 Receptors" International Journal of Molecular Sciences 24, no. 9: 8225. https://doi.org/10.3390/ijms24098225
APA StyleSluyter, R., Adriouch, S., Fuller, S. J., Nicke, A., Sophocleous, R. A., & Watson, D. (2023). Animal Models for the Investigation of P2X7 Receptors. International Journal of Molecular Sciences, 24(9), 8225. https://doi.org/10.3390/ijms24098225







