Uncovering the Cryptic Gene Cluster ahb for 3-amino-4-hydroxybenzoate Derived Ahbamycins, by Searching SARP Regulator Encoding Genes in the Streptomyces argillaceus Genome
Abstract
1. Introduction
2. Results
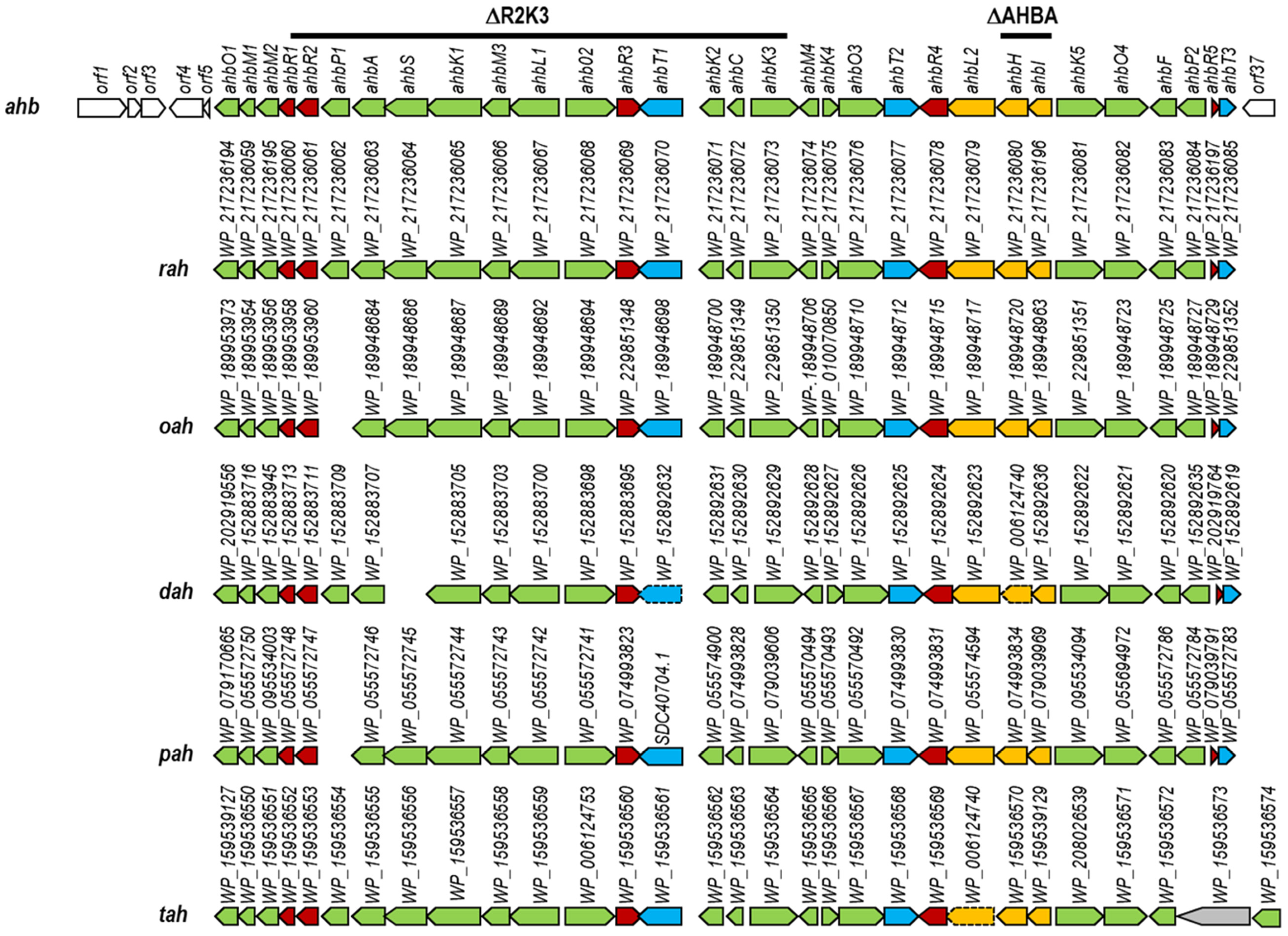
2.1. Identification of the ahb Biosynthesis Gene Cluster
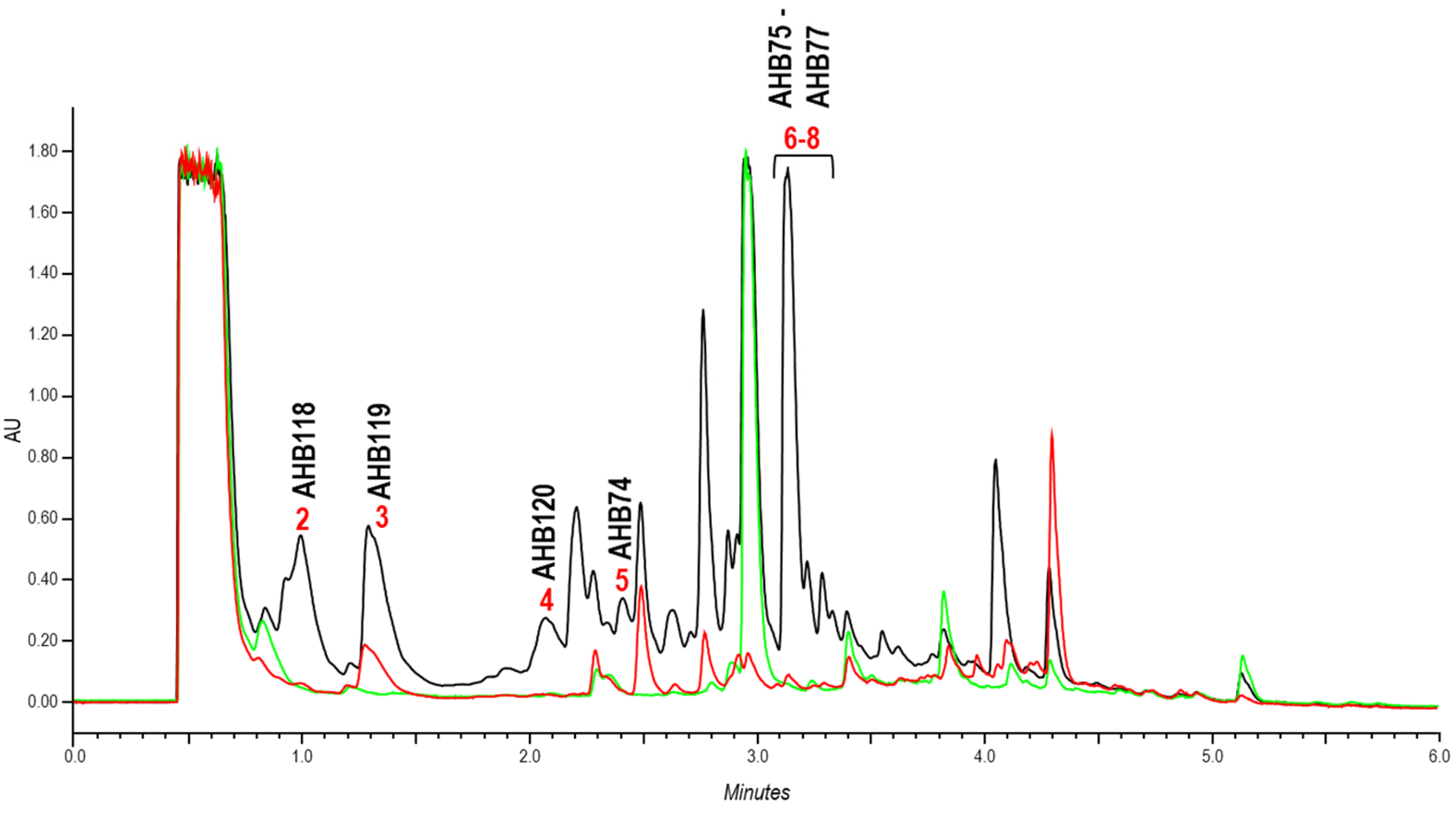
2.2. Identification of Compounds Encoded by the ahb Cluster
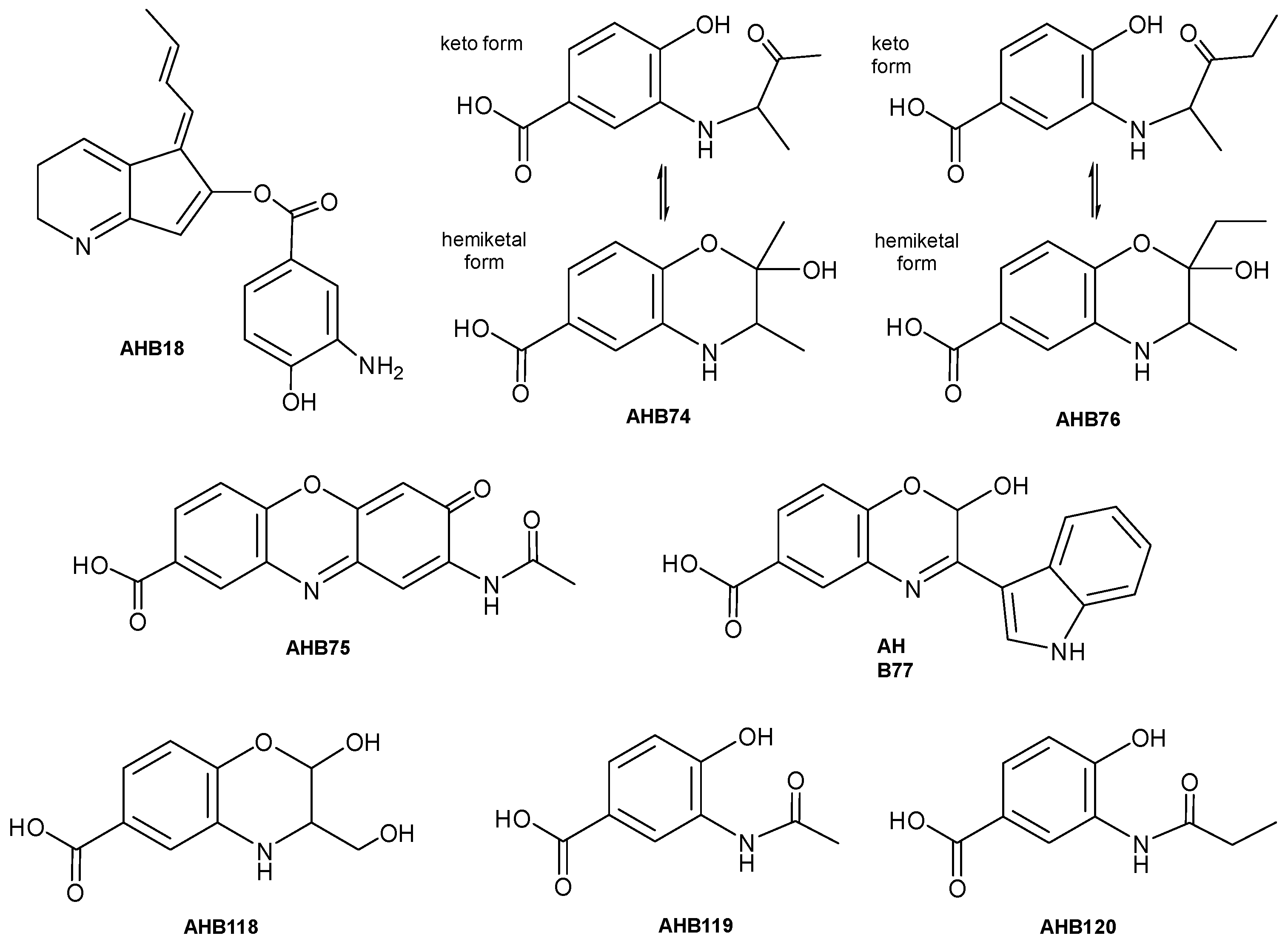
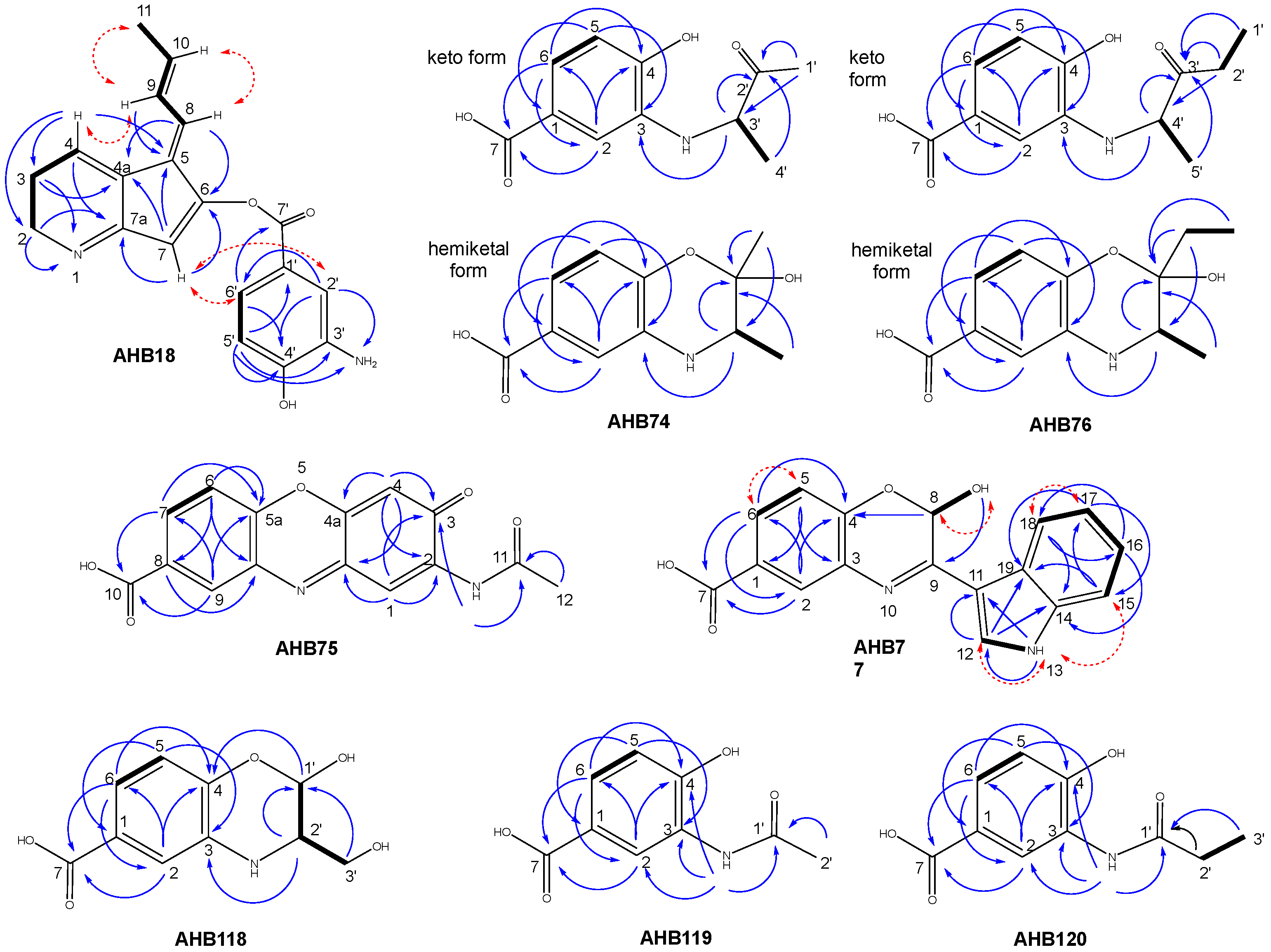
2.3. Structural Elucidation and Bioactivity of Ahbamycins
3. Discussion
4. Materials and Methods
4.1. Strains, Culture Conditions, Plasmids, and DNA Manipulations
4.2. Plasmid Constructs to Generate Mutant Strains
- pHZΔasu: This plasmid was used for jointly deleting ahbH and ahbI. First, a 2.11 kb DNA fragment containing the 5′-end of ahbR4, ahbL2 and the 3′-end of ahbH was amplified using oligonucleotides Delta ASU A fwd and Delta ASU A rev, digested with EcoRI and PstI and cloned upstream the apramycin resistance cassette in pUO9090 digested with the same enzymes. Secondly, a 2 kb DNA fragment containing the 5′-end of ahbI, ahbK5 and the 5′-end of ahbO4 was amplified using oligonucleotides Delta ASU B fwd and Delta ASU B rev, digested with BamHI and EcoRV and cloned downstream the apramycin resistance cassette in the above pUO9090-derivative generated plasmid that was digested with the same enzymes. Finally, the whole insert in pUO9090 was recovered as a SpeI fragment and cloned into the XbaI site of pHZ1358, generating pHZΔasu.
- pHZΔasu1705: This plasmid was used for deleting a DNA region from ahbR1 to ahbK3. First, a 1.9 kb DNA fragment containing the 5′-end of ahbO1, ahbM1, ahbM2 and the 3′-end of ahbR1 was amplified using oligonucleotides Delta 1705A fwd and Delta 1705A rev, digested with EcoRI and PstI (this last cutting within ahbR1) and cloned upstream the apramycin resistance cassette in pUO9090 digested with the same enzymes. Additionally, a 2 kb DNA fragment containing the 3′-end of ahbK3, ahbM4, ahbK4 and the 5′-end of ahbO3 was amplified using oligonucleotides Delta 1705B fwd and Delta 1705B rev, digested with BamHI and EcoRV and cloned downstream the apramycin resistance cassette in the pUO9090 plasmid containing the first fragment and digested with the same enzymes. Finally, the whole fragment was released as a SpeI fragment and cloned into the XbaI site of pHZ1358, generating pHZΔasu1705.
4.3. Plasmid Constructs for Gene Expression
- pEM4T-AHBA: This plasmid was used to overexpress ahbI, ahbH, and ahbL2. These genes were amplified as a 3.84 kb fragment using oligonucleotides AHBAermE fwd and AHBAermE rev, digested with BamHI and EcoRI, and cloned downstream of the erythromycin resistance promoter in pEM4T digested with the same enzymes. The final plasmid pEM4T-AHBA was introduced into S. argillaceus WT to generate S. argillaceus WT-pEM4T-AHBA strain.
- pREGT: This plasmid was used for overexpressing ahbR2, ahbR3, and ahbR4. First, ahbR2 was amplified as a 0.92 kb fragment using oligonucleotides SARP1304 fwd and SARP1304 rev, cloned into pCR-Blunt, recovered as a PstI fragment, and cloned into the PstI site of pEM4 downstream of the erythromycin resistance promoter, generating pEM4-ahbR2. Second, ahbR3 was amplified as a 1 kb fragment using oligonucleotides SARP1705 fwd and SARP1705 rev, cloned into pCR-Blunt, recovered as a XbaI fragment, and cloned into the XbaI site, in the right orientation, downstream of pEM4-ahbR2 to generate pEM4-ahbR2R3. Third, ahbR4 was amplified as a 1.26 kb fragment using oligonucleotides 1705araC fwd and 1705araC rev, cloned into pCR-Blunt, recovered as an EcoRI fragment, and cloned in the right orientation, in the EcoRI site of the downstream of ahbR3 in pEM4-ahbR2R3, generating pREG. Finally, the oriT fragment was recovered from pEM4T as a PstI fragment, cloned into pCR-Blunt, recovered as a HindIII fragment, and cloned into the same site of the pREG. The final plasmid pREGT was introduced into S. argillaceus WT, ΔAHBA and ΔR2K3 to generate S. argillaceus WT-pREGT, ΔAHBA-pREGT and ΔR2K3-pREGT, respectively.
- pSETeAHBAHyg: This plasmid was used to complement S. argillaceus ΔAHBA. Genes ahbI, ahbH, and ahbL2 were amplified as a 3.84 kb fragment using oligonucleotides AHBAermE fwd and AHBAermE rev, cloned into pCR-Blunt, and recovered as a BamHI-EcoRI fragment to be cloned in the right orientation downstream of the erythromycin resistance promoter in pSETe digested with the same enzymes, to generate pSETeAHBA. Then, a hygromycin resistance cassette was obtained from pLHyg as an EcoRV fragment and cloned in pSETeAHBA digested with NheI and filled ends with Klenow. The final plasmid pSETeAHBAHyg was introduced into S. argillaceus ΔAHBA-pREGT to generate S. argillaceus ΔAHBA-pREGT-pSETeAHBAHyg strain.
4.4. Feeding Experiments
4.5. Extraction, UPLC Analysis and Purification of Ahbamycins
4.6. Structural Elucidation of Ahbamycins
4.7. Accession Codes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Van Keulen, G.; Dyson, P.J. Production of specialized metabolites by Streptomyces coelicolor A3(2). Adv. Appl. Microbiol. 2014, 89, 217–266. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Baltz, R.H. Gifted microbes for genome mining and natural product discovery. J. Ind. Microbiol. Biotechnol. 2017, 44, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Bentley, S.D.; Chater, K.F.; Cerdeño-Tárraga, A.M.; Challis, G.L.; Thomson, N.R.; James, K.D.; Harris, D.E.; Quail, M.A.; Kieser, H.; Harper, D.; et al. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 2002, 417, 141–147. [Google Scholar] [CrossRef]
- Ikeda, H.; Ishikawa, J.; Hanamoto, A.; Shinose, M.; Kikuchi, H.; Shiba, T.; Sakaki, Y.; Hattori, M.; Omura, S. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 2003, 21, 526–531. [Google Scholar] [CrossRef]
- Blin, K.; Medema, M.H.; Kazempour, D.; Fischbach, M.A.; Breitling, R.; Takano, E.; Weber, T. antiSMASH 2.0--a versatile platform for genome mining of secondary metabolite producers. Nucleic Acids Res. 2013, 41, W204–W212. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Weber, T.; Blin, K.; Duddela, S.; Krug, D.; Kim, H.U.; Bruccoleri, R.; Lee, S.Y.; Fischbach, M.A.; Müller, R.; Wohlleben, W.; et al. antiSMASH 3.0-a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. 2015, 43, W237–W243. [Google Scholar] [CrossRef]
- Baltz, R.H. Natural product drug discovery in the genomic era: Realities, conjectures, misconceptions, and opportunities. J. Ind. Microbiol. Biotechnol. 2019, 46, 281–299. [Google Scholar] [CrossRef]
- Medema, M.H.; de Rond, T.; Moore, B.S. Mining genomes to illuminate the specialized chemistry of life. Nat. Rev. Genet. 2021, 22, 553–571. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Shi, J.; Molle, V.; Sohlberg, B.; Weaver, D.; Bibb, M.J.; Karoonuthaisiri, N.; Lih, C.J.; Kao, C.M.; Buttner, M.J.; et al. Cross-regulation among disparate antibiotic biosynthetic pathways of Streptomyces coelicolor. Mol. Microbiol. 2005, 58, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular regulation of antibiotic biosynthesis in streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. Engineering of regulatory cascades and networks controlling antibiotic biosynthesis in Streptomyces. Curr. Opin. Microbiol. 2010, 13, 263–273. [Google Scholar] [CrossRef]
- Xia, H.; Zhan, X.; Mao, X.M.; Li, Y.Q. The regulatory cascades of antibiotic production in Streptomyces. World J. Microbiol. Biotechnol. 2020, 36, 13. [Google Scholar] [CrossRef] [PubMed]
- Wietzorrek, A.; Bibb, M. A novel family of proteins that regulates antibiotic production in streptomycetes appears to contain an OmpR-like DNA-binding fold. Mol. Microbiol. 1997, 25, 1181–1184. [Google Scholar] [CrossRef]
- Ye, S.; Molloy, B.; Braña, A.F.; Zabala, D.; Olano, C.; Cortés, J.; Morís, F.; Salas, J.A.; Méndez, C. Identification by Genome Mining of a Type I Polyketide Gene Cluster from Streptomyces argillaceus Involved in the Biosynthesis of Pyridine and Piperidine Alkaloids Argimycins P. Front. Microbiol. 2017, 8, 194. [Google Scholar] [CrossRef]
- Lombó, F.; Menéndez, N.; Salas, J.A.; Méndez, C. The aureolic acid family of antitumor compounds: Structure, mode of action, biosynthesis, and novel derivatives. Appl. Microbiol. Biotechnol. 2006, 73, 1–14. [Google Scholar] [CrossRef]
- Becerril, A.; Álvarez, S.; Braña, A.F.; Rico, S.; Díaz, M.; Santamaría, R.I.; Salas, J.A.; Méndez, C. Uncovering production of specialized metabolites by Streptomyces argillaceus: Activation of cryptic biosynthesis gene clusters using nutritional and genetic approaches. PLoS ONE 2018, 13, e0198145. [Google Scholar] [CrossRef]
- Becerril, A.; Pérez-Victoria, I.; Ye, S.; Braña, A.F.; Martín, J.; Reyes, F.; Salas, J.A.; Méndez, C. Discovery of Cryptic Largimycins in Streptomyces Reveals Novel Biosynthetic Avenues Enriching the Structural Diversity of the Leinamycin Family. ACS Chem. Biol. 2020, 15, 1541–1553. [Google Scholar] [CrossRef]
- Price, M.N.; Arkin, A.P. Curated BLAST for Genomes. mSystems 2019, 4, e00072-19. [Google Scholar] [CrossRef]
- Altschul, S.F.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Ceniceros, A.; Cuervo, L.; Méndez, C.; Salas, J.A.; Olano, C.; Malmierca, M.G. A Multidisciplinary Approach to Unraveling the Natural Product Biosynthetic Potential of a Streptomyces Strain Collection Isolated from Leaf-Cutting Ants. Microorganisms 2021, 9, 2225. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohnishi, Y.; Furusho, Y.; Sakuda, S.; Horinouchi, S. Novel benzene ring biosynthesis from C(3) and C(4) primary metabolites by two enzymes. J. Biol. Chem. 2006, 281, 36944–36951. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Braña, A.F.; González-Sabín, J.; Morís, F.; Olano, C.; Salas, J.A.; Méndez, C. New Insights into the Biosynthesis Pathway of Polyketide Alkaloid Argimycins P in Streptomyces argillaceus. Front. Microbiol. 2018, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Ballin, G.; Pérez-Victoria, I.; Braña, A.F.; Martín, J.; Reyes, F.; Salas, J.A.; Méndez, C. Combinatorial biosynthesis yields novel hybrid argimycin P alkaloids with diverse scaffolds in Streptomyces argillaceus. Microb. Biotechnol. 2022, 15, 2905–2916. [Google Scholar] [CrossRef] [PubMed]
- Dictionary of Natural Products on USB v. 31:2; CRC Press: Boca Raton, FL, USA, 2022.
- Abdelfattah, M.S. A new bioactive aminophenoxazinone alkaloid from a marine-derived actinomycete. Nat. Prod. Res. 2013, 27, 2126–2131. [Google Scholar] [CrossRef]
- Zeeck, A.; Breiding-Mack, S.; Grabley, S.; Voelskow, H.; Seibert, G. Preparation of Phenoxazinone Derivatives by Fermentation with Streptomyces DSM 3813 and Their Use as Parasiticides. Eur Patent EP26 0486, 23 March 1988. [Google Scholar]
- Ohnishi, Y.; Furusho, Y.; Higashi, T.; Chun, H.K.; Furihata, K.; Sakuda, S.; Horinouchi, S. Structures of grixazone A and B, A-factor-dependent yellow pigments produced under phosphate depletion by Streptomyces griseus. J. Antibiot. 2004, 57, 218–223. [Google Scholar] [CrossRef]
- Bertasso, M.; Holzenkämpfer, M.; Zeeck, A.; Dall’Antonia, F.; Fiedler, H.P. Bagremycin A and B, novel antibiotics from Streptomyces sp. Tü 4128. J. Antibiot. 2001, 54, 730–736. [Google Scholar] [CrossRef]
- Rui, Z.; Petrícková, K.; Skanta, F.; Pospísil, S.; Yang, Y.; Chen, C.Y.; Tsai, S.F.; Floss, H.G.; Petrícek, M.; Yu, T.W. Biochemical and genetic insights into asukamycin biosynthesis. J. Biol. Chem. 2010, 285, 24915–24924. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.J.; Pechersky, Y.; Wang, P.; Wang, J.X.; Balskus, E.P. The Cremeomycin Biosynthetic Gene Cluster Encodes a Pathway for Diazo Formation. Chembiochem 2015, 16, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Walsh, C.T.; Haynes, S.W.; Ames, B.D. Aminobenzoates as building blocks for natural product assembly lines. Nat. Prod. Rep. 2012, 29, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.D.; Dong, L.B.; Shen, B. Platensimycin and platencin: Inspirations for chemistry, biology, enzymology, and medicine. Biochem. Pharmacol. 2017, 133, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Kalkreuter, E.; Pan, G.; Cepeda, A.J.; Shen, B. Targeting Bacterial Genomes for Natural Product Discovery. Trends Pharmacol. Sci. 2020, 41, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Ohnishi, Y.; Horinouchi, S. GriC and GriD constitute a carboxylic acid reductase involved in grixazone biosynthesis in Streptomyces griseus. J. Antibiot. 2007, 60, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Furusho, Y.; Higashi, T.; Ohnishi, Y.; Horinouchi, S. A novel o-aminophenol oxidase responsible for formation of the phenoxazinone chromophore of grixazone. J. Biol. Chem. 2006, 281, 824–833. [Google Scholar] [CrossRef]
- Barry, C.E., 3rd; Nayar, P.G.; Begley, T.P. Phenoxazinone synthase: Mechanism for the formation of the phenoxazinone chromophore of actinomycin. Biochemistry 1989, 28, 6323–6333. [Google Scholar] [CrossRef]
- Le Roes-Hill, M.; Goodwin, C.; Burton, S. Phenoxazinone synthase: What’s in a name? Trends Biotechnol. 2009, 27, 248–258. [Google Scholar] [CrossRef]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics; The John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Fernández, E.; Weissbach, U.; Sánchez Reillo, C.; Braña, A.F.; Méndez, C.; Rohr, J.; Salas, J.A. Identification of two genes from Streptomyces argillaceus encoding glycosyltransferases involved in transfer of a disaccharide during biosynthesis of the antitumor drug mithramycin. J. Bacteriol. 1998, 180, 4929–4937. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, X.; Liu, J.; Bao, K.; Zhang, G.; Tu, G.; Kieser, T.; Deng, Z. ‘Streptomyces nanchangensis’, a producer of the insecticidal polyether antibiotic nanchangmycin and the antiparasitic macrolide meilingmycin, contains multiple polyketide gene clusters. Microbiology 2002, 148, 361–371. [Google Scholar] [CrossRef]
- Menéndez, N.; Nur-e-Alam, M.; Fischer, C.; Braña, A.F.; Salas, J.A.; Rohr, J.; Méndez, C. Deoxysugar transfer during chromomycin A3 biosynthesis in Streptomyces griseus subsp. griseus: New derivatives with antitumor activity. Appl. Environ. Microbiol. 2006, 72, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Quirós, L.M.; Aguirrezabalaga, I.; Olano, C.; Méndez, C.; Salas, J.A. Two glycosyltransferases and a glycosidase are involved in oleandomycin modification during its biosynthesis by Streptomyces antibioticus. Mol. Microbiol. 1998, 28, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Cano-Prieto, C.; García-Salcedo, R.; Sánchez-Hidalgo, M.; Braña, A.F.; Fiedler, H.P.; Méndez, C.; Salas, J.A.; Olano, C. Genome Mining of Streptomyces sp. Tü 6176: Characterization of the Nataxazole Biosynthesis Pathway. Chembiochem 2015, 16, 1461–1473. [Google Scholar] [CrossRef]
- Olano, C.; Wilkinson, B.; Sánchez, C.; Moss, S.J.; Sheridan, R.; Math, V.; Weston, A.J.; Braña, A.F.; Martin, C.J.; Oliynyk, M.; et al. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tü4055: Cluster analysis and assignment of functions. Chem. Biol. 2004, 11, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Alanjary, M.; Steinke, K.; Ziemert, N. AutoMLST: An automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res. 2019, 47, W276–W282. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Crespo, G.; González-Menéndez, V.; Pérez-Moreno, G.; Sánchez-Carrasco, P.; Pérez-Victoria, I.; Ruiz-Pérez, L.M.; González-Pacanowska, D.; Vicente, F.; Genilloud, O.; et al. MDN-0104, an antiplasmodial betaine lipid from Heterospora chenopodii. J. Nat. Prod. 2014, 77, 2118–2123. [Google Scholar] [CrossRef]
- Pérez-Victoria, I.; Martín, J.; Reyes, F. Combined LC/UV/MS and NMR Strategies for the Dereplication of Marine Natural Products. Planta Med. 2016, 82, 857–871. [Google Scholar] [CrossRef] [PubMed]


| Mutant Strain | Plasmid | Deleted Genes |
|---|---|---|
| ΔAHBA | pHZΔasu | ahbH, ahbI |
| ΔR2K3 | pHZΔasu1705 | ahbR1, ahbR2, ahbP1, ahbA, ahbS, ahbK1, ahbM3, ahbL1, ahbO2, ahbR3, ahbT1, ahbK2, ahbC, ahbK3 |
| Recombinant Strain | Plasmid | Expressed Genes |
| WT-pEM4T | pEM4T | - |
| WT-pEM4T-AHBA | pEM4T-AHBA | ahbI, ahbH, ahbL2 |
| WT-pREGT | pREGT | ahbR2, ahbR3, ahbR4 |
| ΔAHBA-pREGT | pREGT | ahbR2, ahbR3, ahbR4 |
| ΔR2K3-pREGT | pREGT | ahbR2, ahbR3, ahbR4 |
| ΔAHBA-pREG-pSETeAHBAHyg | pREGT pSETeAHBAHyg | ahbR2, ahbR3, ahbR4 ahbI, ahbH, ahbL2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, S.; Molloy, B.; Pérez-Victoria, I.; Montero, I.; Braña, A.F.; Olano, C.; Arca, S.; Martín, J.; Reyes, F.; Salas, J.A.; et al. Uncovering the Cryptic Gene Cluster ahb for 3-amino-4-hydroxybenzoate Derived Ahbamycins, by Searching SARP Regulator Encoding Genes in the Streptomyces argillaceus Genome. Int. J. Mol. Sci. 2023, 24, 8197. https://doi.org/10.3390/ijms24098197
Ye S, Molloy B, Pérez-Victoria I, Montero I, Braña AF, Olano C, Arca S, Martín J, Reyes F, Salas JA, et al. Uncovering the Cryptic Gene Cluster ahb for 3-amino-4-hydroxybenzoate Derived Ahbamycins, by Searching SARP Regulator Encoding Genes in the Streptomyces argillaceus Genome. International Journal of Molecular Sciences. 2023; 24(9):8197. https://doi.org/10.3390/ijms24098197
Chicago/Turabian StyleYe, Suhui, Brian Molloy, Ignacio Pérez-Victoria, Ignacio Montero, Alfredo F. Braña, Carlos Olano, Sonia Arca, Jesús Martín, Fernando Reyes, José A. Salas, and et al. 2023. "Uncovering the Cryptic Gene Cluster ahb for 3-amino-4-hydroxybenzoate Derived Ahbamycins, by Searching SARP Regulator Encoding Genes in the Streptomyces argillaceus Genome" International Journal of Molecular Sciences 24, no. 9: 8197. https://doi.org/10.3390/ijms24098197
APA StyleYe, S., Molloy, B., Pérez-Victoria, I., Montero, I., Braña, A. F., Olano, C., Arca, S., Martín, J., Reyes, F., Salas, J. A., & Méndez, C. (2023). Uncovering the Cryptic Gene Cluster ahb for 3-amino-4-hydroxybenzoate Derived Ahbamycins, by Searching SARP Regulator Encoding Genes in the Streptomyces argillaceus Genome. International Journal of Molecular Sciences, 24(9), 8197. https://doi.org/10.3390/ijms24098197










