Abstract
Megakaryocytes are the main members of the hematopoietic system responsible for regulating vascular homeostasis through their progeny platelets, which are generally known for maintaining hemostasis. Megakaryocytes are characterized as large polyploid cells that reside in the bone marrow but may also circulate in the vasculature. They are generated directly or through a multi-lineage commitment step from the most primitive progenitor or Hematopoietic Stem Cells (HSCs) in a process called “megakaryopoiesis”. Immature megakaryocytes enter a complicated development process defined as “thrombopoiesis” that ultimately results in the release of extended protrusions called proplatelets into bone marrow sinusoidal or lung microvessels. One of the main mediators that play an important modulatory role in hematopoiesis and hemostasis is nitric oxide (NO), a free radical gas produced by three isoforms of nitric oxide synthase within the mammalian cells. In this review, we summarize the effect of NO and its signaling on megakaryopoiesis and thrombopoiesis under both physiological and pathophysiological conditions.
1. Basic Megakaryocyte Biology
Megakaryocytes are large (50–100 µM), multi-nucleated cells that are responsible for releasing platelets into the blood [1]. They are characterized by a multilobulated nucleus accumulating 2n, 4n, 8n, 16n, 32n, up to 128n DNA content and constitute ~0.01% of bone marrow cells [2]. Recent evidence sheds light on the different roles of megakaryocytes in various physiological and pathophysiological processes. Until recently, it had been thought that megakaryocytes solely serve as progenitors or precursor cells responsible for platelet production. This simple notion may no longer be valid as recent reports have uncovered various roles of megakaryocytes in the immune response and in modulating the proliferation and differentiation of different cell lineages, particularly osteoblasts and osteoclasts within the bone marrow [3,4,5]. Megakaryocytes are capable of antigen endocytosis and, ultimately, its presentation within MHC I to CD8+ T cells [6]. Moreover, recent studies revealed that megakaryocytes release several immune-modulatory cytokines, including TGF-β and IL-1, and express co-stimulatory molecules such as CD40L and B7-2 (CD86) on their surface, suggesting they act as antigen presenting cells (APCs) within the bone marrow microenvironment [5,7,8,9,10]. Of interesting note, recent evidence suggests that megakaryocytes may act as the first line of defense against cancer metastasis to the bone [3,4]. Therefore, like platelets, which are increasingly recognized for their diverse roles beyond hemostasis [11,12,13,14], megakaryocytes may have functions beyond platelet production. As such, a greater understanding of how important chemical mediators influence megakaryocytes in platelet production and newly recognized functions is required.
According to conventional or classic hematopoiesis, hematopoietic stem cells (HSCs) give rise to megakaryocyte-biased progenitors after passing through several strict commitment points or lineage-biased steps like a hierarchical-branched tree [15,16]. However, more recent evidence demonstrates that although hematopoietic stem cells are capable of reconstituting all blood cell lineages, they may exhibit megakaryocyte or platelet-biased phenotypic and functional characteristics. Therefore, these multipotent progenitor cells may bypass differentiation pathways and directly give rise to megakaryocyte or platelet-committed progenitors at a very early step in differentiation [17,18,19,20,21,22,23]. Consistently, bone marrow transplantation in humans demonstrates that platelet reconstitution takes place earlier than that of other blood cell lineages [24].
It has been reported that HSC differentiation toward megakaryocytes and their maturation, endomitosis, and invaginated membrane system (IMS) development takes place in the osteoblastic niche, whereas a later generation of proplatelets, megakaryocytye-platelet intermediate pseudopodia-like structures, requires vascular niche localization [2]. Within these bone marrow niches HSC/megakaryocyte progenitor cell interactions with microenvironment extracellular matrix proteins help to regulate megakaryocyte differentiation and platelet production. HSC interaction with type I collagen within the osteoblastic niche via VLA-2 (integrin α2β1) promotes commitment to megakaryocyte-biased progenitor formation and maturation, but suppresses megakaryocyte terminal development, which results in proplatelet generation [25,26,27]. Similarly, megakaryocyte glycoprotein (GP) VI–collagen I interaction has inhibited proplatelet formation [28]. However, double knockout of collagen receptors (GPVI−/− integrin α2β1−/−) shows no difference in megakaryocyte distribution, size, or blood platelet levels compared to that of wild type mice, suggesting other regulatory mechanisms may exist to suppress ectopic proplatelet generation within the osteoblastic niche [29]. In contrast, the vascular niche contains extracellular matrix proteins including collagen IV, fibronectin, fibrinogen, and von Willebrand factor, which induce proplatelet generation [30,31,32,33]. Other factors, including megakaryocyte-active mitogens such as fibroblast growth factor 4 (FGF-4) and the chemokine stromal cell-derived factor 1 (SDF-1) also promote survival, maturation, and platelet production from megakaryocytes by facilitating their chemotaxis toward and affinity for bone marrow sinusoid endothelial cells [34,35]. Once in the vascular niche, several hypotheses have been proposed to explain the mechanism behind the proplatelet extension from megakaryocytes into the lumen of bone marrow blood vessels [36,37,38]. A concentration gradient of sphingosine-1 phosphate (S1P) has been shown to exist at the contact site between the megakaryocytes and sinusoidal blood, which directs proplatelets into lumens in a sphingosine-1-phosphate receptor 1 (S1prP1)-dependent manner [39]. Ultimately, blood flow shear forces facilitate proplatelet release from the megakaryocyte and their fission to produce platelets.
In addition to extracellular matrix proteins, various soluble factors have been proposed to play important roles in regulating megakaryopoiesis and thrombopoiesis. Of these, particularly important is the glycoprotein hormone thrombopoietin (TPO). Through its receptor c-mpl, which is expressed on the most primitive HSCs, TPO plays a key role in megakaryocyte differentiation from HSCs and their maturation toward platelet generation [15,40]. TPO plays a central role in maintaining platelet/megakaryocyte-biased HSCs, as TPO knockout (TPO−/−) bone marrow cells give rise to lymphoid-biased bone marrow reconstitution in irradiated recipient mice [18]. As such c-mpl and TPO knockout mice demonstrate 90% reductions in megakaryocyte and platelet numbers [41], while loss of function mutations to Mpl within humans cause congenital amegakaryocytic thrombocytopenia, resulting in a severe phenotype only rescued by bone marrow transplantation. In addition to TPO, several other factors have been identified which promote megakaryocyte proliferation and maturation, including interleukin 3(IL-3), interleukin 6(IL-6), and stem cell factor (SCF) [42,43,44].
TPO also induces megakaryocyte polyploidization, which results in the accumulation of lipids and proteins required for the constitution of a vast invaginated membrane network connected to the megakaryocyte surface membrane. This membrane network forms the surface membrane of proplatelets and the cytoskeletal ultrastructure that supports the elongation of proplatelet tubular structures [1,45,46,47]. The process of proplatelet formation and the release of platelets into the sinusoidal blood vessels in the bone marrow is highly regulated [48], During this process, cytoskeletal proteins, including β1-tubulin, dynein, F-actin, and myosin II, facilitate proplatelet generation by providing assembly lines for elongation, organelle transportation, and ultimately platelet release [35,47,49,50]. Of particular importance is the role of the transcription factor NF-E2 and its expression of β1-tubulin that plays a pivotal role in proplatelet biogenesis, structure, and function by polymerizing into microtubule bundles and coils that extend throughout these cytoplasmic extrusions. Consequently, NF-E2 and β1-tubulin knockout mice suffer from thrombocytopenia because of a significant reduction in proplatelet formation [49,51,52,53,54,55]. TPO also induces reactive oxygen species (ROS) production, which play an important role in driving HSC differentiation toward mature megakaryocytes and platelet production. This ROS generation likely involves nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and increased oxygen tension, resulting in enhanced tyrosine phosphorylation, proliferation, and polyploidization [56,57]. Moreover, NF-E2 in addition to expressing platelet genes also maintains a moderate expression of cytoprotective genes allowing for ROS accumulation during megakaryocytic maturation [58]. The initiation of platelet formation from mature megakaryocytes has also been shown to be governed by a reciprocal interplay between mitochondrial dynamics and ROS, in which increased ROS levels stimulate mitochondrial fission, leading to the production of more mitochondrial ROS [59]. Most recently, a role for ROS has also been identified in the pulling of megakaryocyte intravascular proplatelet extensions by so-called “plucking” neutrophils to enhance platelet formation [60].
However, it is worth noting that platelet generation via megakaryocyte proplatelet formation at steady state may differ mechanistically from platelet production in response to stress or injury. Stress thrombopoiesis, or the process of platelet production under inflammatory or acute thrombocytopenia conditions, can occur much faster than physiological platelet production [61,62]. This may occur in part due to the presence of platelet- or megakaryocyte-primed hematopoietic stem cells (HSCs) in the bone marrow that can bypass the traditional route of multi-step lineage-biased progenitor differentiation and give rise to platelets more quickly [18,19,23,62,63]. However, equally important to stress thrombopoiesis is whether platelet generation proceeds through or bypasses the need for TPO and classic proplatelet formation. Although proinflammatory cytokines, such as IL-1β, can upregulate the expression of TPO and other megakaryocyte-related transcription factors to further promote platelet production [64], recent studies have shown that in response to IL-1α megakaryocytes undergo rupture to rapidly produce platelets in a TPO-independent manner after platelet loss or inflammatory stimulus [65,66]. This rupture-dependent thrombopoiesis also displays caspase-3 dependence [65], and platelet generation differences at stress vs. steady state may help to explain whether or not megakaryocyte apoptosis needs to be restrained for platelet generation and which apoptotic pathways may or may not be involved [67,68,69,70].
While much is known about the roles of extracellular matrix proteins, soluble protein mediators, and even gaseous chemical mediators such as ROS in megakaryopoiesis and thrombopoiesis, relatively little is known of the role of nitric oxide (NO) in these processes. This is somewhat surprising considering NO’s pleiotropic biological activity, and the important role it plays regulating hematopoiesis and platelet function [71,72,73,74,75]. Therefore, in this mini review we summarize the role of NO and its signaling on megakaryocyte function.
2. Basic Nitric Oxide Biology
Nitric oxide (NO) is a highly diffusible free radical gas with a short half-life that plays an important role in many physiological and pathophysiological processes [76], including regulating vascular tone and signal transmission by neurons [77,78,79,80,81]. Importantly, it also plays a major role in immune function as well as within the hematopoietic system [82,83,84,85,86].
NO is produced enzymatically from the oxidation of L-arginine by NADPH-dependent family oxidation-reduction enzymes called nitric oxide synthases or NOSs [87,88,89]. These enzymes utilize flavin adenine dinucleotide (FAD), flavin mononucleotide (FMN), and (6R-)5,6,7,8-tetrahydro-L-biopterin (BH4) as cofactors to generate NO from the substrate L-arginine and co-substrates oxygen and NADPH. Three isoforms of nitric oxide synthase exist, including NOS I (nNOS, neuronal nitric oxide synthase), NOS II (iNOS, inducible nitric oxide synthase), and NOS III (eNOS, endothelial nitric oxide synthase) (Figure 1) [90,91]. Although all three enzymes bind to calmodulin (CaM), nNOS and eNOS bind CaM upon a rise in intracellular Ca2+ concentration and become activated [92,93,94]. Of further importance for eNOS regulation is its localization to cell membrane caveole wherein the caveolae coat protein caveolin-1 is a tonic inhibitor of eNOS activity and recruitment of CaM and heat shock protein 90 displaces caveolin-1, leading to eNOS activation [95,96]. eNOS activity is also widely regulated both positively and negatively via phosphorylation, with Ser1177 and Thr495 being the most widely studied of such sites. eNOS activating phosphorylation occurs in response to circulating mediators such as vascular endothelial growth factor, insulin, bradykinin, and estrogen, as well as in response to sheer stress [97]. Similar to constitutive NOS enzymes, iNOS also binds to calmodulin; however, it does so even at basal levels of intracellular Ca2+ due to its high affinity for CaM [98]. nNOS and eNOS are constitutively expressed in different cells and tissues; while, under physiological conditions iNOS expression is limited [99,100,101], but can be induced in almost any cell type by proinflammatory proteins such as IL-1, TNF-α, IFN-γ, IL-2, IL-12, IL-18, CD40 ligand and Fas-ligand and pathogen-associated molecular patterns such as lipopolysaccharide [101].
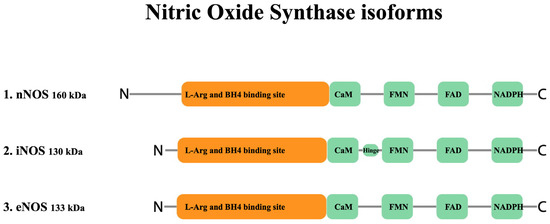
Figure 1.
Structure of nitric oxide synthase enzyme isoforms. Both nNOS (NOS I) and eNOS (NOS III) are capable of synthesizing NO in a short pulsative manner ranging from pM to nM (low) and nM to μM (moderate) concentrations, respectively. However, iNOS (NOS II) synthesizes a significantly higher amount of NO in the range of μM concentration, in a constant manner after the expression of the enzyme. (BH4: (6R-)5,6,7,8-tetrahydro-L-biopterin, a co-factor essential for NOS activity.)
NO exerts most of its biological functions through interaction with various key regulatory proteins either via direct binding to targets (cGMP-independent effects) or via cGMP-dependent signaling following its activation of soluble guanylate cyclase (sGC). Activation of sGC and cGMP generation can result in activation of cyclic nucleotide-gated ion channels as well as protein kinase G (PKG) activation and signaling, which can modulate diverse cellular processes such as regulation of enzyme activity, gene transcription, and post-translational modification [80,102,103,104,105,106]. Conversely, NO may bind to heme and regulate the activity of heme-containing enzymes such as cytochrome C oxidase or other protein/peptides via nitrosylation of thiol groups to regulate important processes such as apoptosis [97].
NO may be inactivated in a number of ways including by reacting with the heme in deoxyhemoglobin to form a stable complex and in the presence of oxygen to form methemoglobin and nitrate. It also reacts with oxygen and water to yield nitrite and nitrate in a series of reactions with a dinitrogen tetraoxide intermediate. NO also rapidly reacts with superoxide (O2−) to form peroxynitrite (ONOO−), a highly active and cytotoxic radical, which subsequently reduces NO bioavailability [107,108,109]. In high concentrations ONOO− may exert proapoptotic and cytotoxic effects through various mechanisms, including protein oxidation and tyrosine nitration, lipid peroxidation, disruption of the electron transport chain and TCA, and via single-strand breaks in DNA [109,110,111,112,113,114,115,116].
3. Nitric Oxide and Platelet Function
In the 1980s, Radomski and Moncada demonstrated that NO potently inhibits platelet adhesion and aggregation [117,118,119,120]. Subsequently, NOS and an NO signaling pathway were identified within platelets [75,121,122,123], and NO produced during aggregation was shown to inhibit further platelet recruitment [124,125]. NO mediates most of its platelet inhibitory effects via cGMP generated by sGC (Figure 2) [126,127,128]. cGMP acts on PKG, which phosphorylates vasodilator-stimulated phosphoprotein (VASP), enabling VASP binding to the platelet cytoskeleton [129,130]. Next, VASP inhibits integrin αIIbβ3 activation, preventing adhesion and aggregation [131,132]. PKG signaling is also reported to suppress intracellular Ca2+ and integrin αIIbβ3 activation via inositol-1,4,5-triphosphate receptor-associated cGMP kinase substrate signaling [133,134] and to suppress thromboxane receptor activation [135]. Platelet NOS activity has been attributed to eNOS [121,136,137,138], although a few studies report iNOS in low amounts in platelets [136,137] (there are no reports of platelet nNOS).
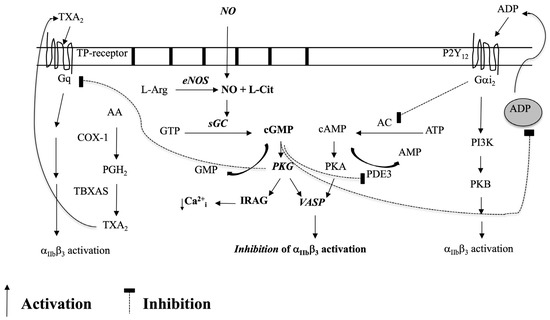
Figure 2.
Platelet eNOS-NO-sGC-PKG-signaling and its cross-talk with the PGI2/cAMP, COX-1/TXA2, and ADP/P2Y12 pathways.
In the past 20 years, however, controversies have arisen over platelet NO signaling. The most relevant questioned platelet NO production and eNOS presence [139] and whether NO also has a stimulatory role in platelet activation [140]. To address these controversies, we previously investigated the hypothesis that some of these discrepancies may be explained by differences in platelet levels with and without eNOS signaling. Recently, we identified eNOSneg/low and eNOSpos/high platelet subpopulations in blood [141]. We demonstrated that eNOSneg/low platelets do not produce NO or produce it in low amounts. This platelet subpopulation also has a down-regulated sGC-PKG-VASP signaling pathway, initiates adhesion to collagen, and more readily activates integrin αIIbβ3 than eNOSpos/high platelets. eNOSpos/high platelets contain higher protein levels of sGC, PKG, and VASP and are more abundant (~80% of total platelets). eNOSpos/high platelets also form the bulk of an aggregate via enhanced COX-1 signaling; however, they also ultimately limit aggregate size via NO generation.
Importantly, ONOO− also impacts platelet function [142], and its impacts may help explain some of the discrepant findings surrounding platelet NO function. At low concentrations, peroxynitrite was shown to mediate NO-dependent platelet inhibition; however, at higher concentrations it caused an increase in P-selectin exposure and platelet activation [143]. Consistently reducing peroxynitrite formation by suppressing NADPH oxidase, a major source of platelet superoxide generation, was shown to increase NO bioavailability and subsequent platelet inhibition [144].
Insufficient platelet NO production and a decrease in its bioavailability may also have important pathological consequences, particularly in the setting of acute coronary syndrome (ACS). Platelets from ACS patients have impaired NO production [145], and platelet NO production inversely correlates with increasing number of coronary artery disease risk factors [146]. Similarly, platelet refractoriness to the NO donor sodium nitroprusside predicts increased morbidity and mortality in patients with high-risk ACS [147]. Consistent with these findings, megakaryocytes from patients with normal coronary arteries have been reported to generate more NO in a Ca2+-dependent manner than megakaryocytes from patients with atherosclerosis, although megakaryocytes from atherosclerotic patients generate more NO in an iNOS-dependent manner [148,149,150]. That platelets have a more limited transcriptome and capacity for new protein synthesis and that iNOS protein has an extremely short half-life (<2 h) [151,152] suggests that reduced platelet NO bioavailability within coronary artery disease may reflect a reduction in megakaryocyte eNOS expression. Furthermore, NO formed from different NOS isoforms may play differing roles in megakaryocyte vs. platelet function. Hence, due to recent advances in our understanding of platelet NO biology and its significance to pathology, a closer examination of NO-signaling in megakaryocytes is also needed.
4. Nitric Oxide Synthases in Megakaryocytes
As described above, constitutive (Ca2+-dependent) and inducible NOS isoforms have been identified in both human bone marrow megakaryocytes [148] and within the Meg-01 megakaryoblastic cell line [153]. Treatment of Meg-01 with proinflammatory cytokines IL-1β and TNF-α also revealed a reciprocal relation between constitutive and inducible NOS activity consistent with an increase in iNOS expression and a down-regulation of constitutive NOS expression. The Ca2+-dependent NOS in megakaryocytes/blasts likely corresponds to eNOS as its expression has been confirmed via RT-PCR and immunostaining within Meg-01 [141]. Moreover, like in platelets, both eNOSneg/low and eNOSpos/high Meg-01 subpopulations have been identified [141,154].
5. Effect of NO on Differentiation and Proliferation of Megakaryocytes
Early research demonstrated that high concentrations (μM) of the NO donor DETA/NO induce apoptosis of bone marrow-derived CD34+ progenitor cells and that iNOS-generated NO may in part mediate hematopoietic suppression by proinflammatory cytokines IFN-γ and TNF-α [71]. Treatment of human bone marrow-derived and TPO-cultured CD34+ cells, as well as mononuclear cells, with IFN-γ and TNF-α reduces the number of CD41+ cells after 12 days of culture, while high NO-donor concentrations inhibit the outgrowth of megakaryocytes derived from these cells by inducing their apoptosis [86]. Prostacyclin treatment and cAMP signaling protect megakaryocytes outgrown from CD34+ cells from NO-induced apoptosis [155], while TPO, 5-hydroxytryptamine, and IL-11 appear to protect megakaryocytic cell lines from apoptosis induced by high NO concentrations achieved by NO donors or iNOS induction [84]. Altogether, these results suggest that in absence of protective factors and under inflammatory-like conditions up-regulation of iNOS expression and increased NO concentrations induce apoptosis of progenitor cells, preventing their differentiation toward megakaryocytes. Whether the pro-apoptotic effects of NO are mediated via cGMP-dependent or non-cGMP-dependent mechanisms remains to be fully elucidated, as does the contribution of peroxynitrite and of the apoptotic pathways involved. Moreover, potential cross-talk between pathways that retard NO-induced apoptosis of megakaryocytes and their progenitors also needs further investigation [156].
6. Effect of NO on Platelet Production by Megakaryocytes
Similar to the limited number of studies investigating the role of NO in megakaryopoiesis, there is also a paucity of data with regards to NO’s role in thrombopoiesis. Early work by Loscalzo and colleagues demonstrated that treatment of the Meg-01 cell line with high NO concentrations as achieved by utilizing the NO-donor S-nitrosoglutathione (GSNO) or by treating the Meg-01 cell line with proinflammatory cytokines (IFN-γ, TNF-α, and IL-1β) induces the generation of CD41+ platelet-sized particles in culture with a capacity to aggregate [85]. Moreover, platelet particle generation by Meg-01 is further enhanced if the Meg-01 are pretreated with TPO prior to stimulation, although TPO treatment alone was not able to promote platelet particle generation consistent with its role in megakaryocyte maturation [85]. The mechanism by which high NO concentrations induce platelet-sized particle formation was reported to be cGMP-independent, and interestingly, was associated with the generation of distinct Meg-01-derived annexin-V and propidium iodide positive apoptotic bodies. This finding led the authors to hypothesize that NO-induced apoptosis is related to the process by which megakaryocytes produce platelets, although as also noted by the authors it is not clear whether the observed apoptosis is a result of removal of spent megakaryocytes or apoptosis and platelet production are simultaneous events [84,85]. Lastly, of note, the authors identified that iNOS null mice demonstrate platelet counts nearly half of that of their wild-type or eNOS null counterparts, further exemplifying the important role of NO in platelet production.
Consistent with the findings of Loscalzo and colleagues, intravenous infusion of L-nitroarginine or N(G)-nitro-L-arginine methyl ester, both NOS inhibitors, to rats results in thrombocytopenia or decreased platelet counts [157]. More recently, CD226 whole body or platelet/megakaryocyte specific knockout mice have been shown to have elevated platelet and megakaryocyte (bone marrow and spleen) counts compared to wild-type controls [158]. Notably, the platelets from CD226−/− mice demonstrated greater aggregation response to thrombin compared to platelets from WT mice, attributed to their reduced eNOS levels and decreased ability to generate NO. Currently, it is unknown whether the potential alterations in megakaryocyte-platelet NO-signaling in these mice impact their megakaryo- or thrombopoiesis. However, considering that platelet function may be regulated by low NO concentrations attributable to eNOS activity while high NO concentrations associated with iNOS appear to have profound effects on megakaryocytes and their potential to produce platelets, it is tempting to speculate whether these two NOS isoforms have differential function in megakaryocytes vs. platelets. Specifically, the role of iNOS and its increased expression may be of particular importance to platelet production under stress such as in the case of rupture thrombopoiesis as it can be rapidly by induced by IL-1α [159] or in cases of inflammation/infection-induced secondary (reactive) thrombocytosis (Figure 3).
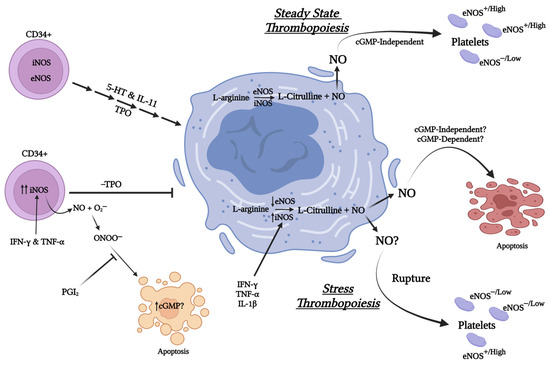
Figure 3.
Cartoon summarizing the impact of Nitric oxide derived from iNOS and eNOS on megakaryopoiesis and thrombopoiesis under stressed and non-stressed conditions. (Created with BioRender.com, accessed on 28 April 2023).
7. Summary and Conclusions
Although the role of NO in platelet biology is well studied, much less is known about its effects on megakaryocytes. To date, most studies have focused on the ability of NO at high concentrations to promote cell death of megakaryoctyes and their progenitors. Considering NO’s pleiotropic effects and its ability to influence HSC mobilization [160], future studies may need to focus on its role in regulating megakaryocyte progenitor interaction with their microenvironments within the osteoblastic and vascular niches as well as its impact on ROS-mediated signaling during megakaryocyte differentiation. Further, previous studies focusing on NO’s impact on platelet production have modeled conditions of stress rather than steady-state thrombopoiesis, implicating a role for iNOS in this process. Indeed, analogously increased iNOS expression and NO generation have recently been identified to play a role in stress erythropoiesis [161]. Considering that NO may have both pro- and anti-apoptotic roles [162], future studies delineating the role of NOS isoforms and NO in steady-state vs. stress thrombopoiesis may also clarify whether apoptosis plays a role and what role it plays in platelet production [163,164]. Lastly, further studies are also warranted to identify whether NOS-based subpopulations of megakaryocytes exist as they do for platelets [141]. The results of such studies may shed new light on the development of novel genetic and/or pharmacological tools in order to manipulate NO-signaling within megakaryocytes and their platelet progeny for therapeutic purposes.
Funding
This work was funded by grants from the Canadian Institutes of Health Research MOP-162128 to P.J.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflict of interest regarding this manuscript.
References
- Ebbe, S. Biology of megakaryocytes. Prog. Hemost. Thromb. 1976, 3, 211–229. [Google Scholar]
- Tavassoli, M.; Aoki, M. Localization of megakaryocytes in the bone marrow. Blood Cells 1989, 15, 3–14. [Google Scholar] [PubMed]
- Jackson, W., III; Sosnoski, D.M.; Ohanessian, S.E.; Chandler, P.; Mobley, A.; Meisel, K.D.; Mastro, A.M. Role of Megakaryocytes in Breast Cancer Metastasis to Bone. Cancer Res. 2017, 77, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Koh, A.J.; Wang, Z.; Soki, F.N.; Park, S.I.; Pienta, K.J.; McCauley, L.K. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J. Bone Miner. Res. 2011, 26, 125–134. [Google Scholar] [CrossRef]
- Boilard, E.; Flamand, L. The role of the megakaryocyte in immunity has gone viral. Blood 2019, 133, 2001–2002. [Google Scholar] [CrossRef]
- Zufferey, A.; Speck, E.R.; Machlus, K.R.; Aslam, R.; Guo, L.; McVey, M.J.; Kim, M.; Kapur, R.; Boilard, E.; Italiano, J.E., Jr.; et al. Mature murine megakaryocytes present antigen-MHC class I molecules to T cells and transfer them to platelets. Blood Adv. 2017, 1, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Finkielsztein, A.; Schlinker, A.C.; Zhang, L.; Miller, W.M.; Datta, S.K. Human megakaryocyte progenitors derived from hematopoietic stem cells of normal individuals are MHC class II-expressing professional APC that enhance Th17 and Th1/Th17 responses. Immunol. Lett. 2015, 163, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Kuter, D.J.; Gminski, D.M.; Rosenberg, R.D. Transforming growth factor β inhibits megakaryocyte growth and endomitosis. Blood 1992, 79, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Blair, P.; Flaumenhaft, R. Platelet α-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Levine, J.; Fu, Y.; Deng, B.; London, R.; Groopman, J.; Avraham, H. Cytokine production by primary bone marrow megakaryocytes. Blood 1994, 84, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Semple, J.W.; Italiano, J.E., Jr.; Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 2011, 11, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Gay, L.J.; Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer 2011, 11, 123–134. [Google Scholar] [CrossRef]
- Etulain, J. Platelets in wound healing and regenerative medicine. Platelets 2018, 29, 556–568. [Google Scholar] [CrossRef]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Debili, N.; Coulombel, L.; Croisille, L.; Katz, A.; Guichard, J.; Breton-Gorius, J.; Vainchenker, W. Characterization of a bipotent erythro-megakaryocytic progenitor in human bone marrow. Blood 1996, 88, 1284–1296. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Morita, Y.; Ooehara, J.; Hamanaka, S.; Onodera, M.; Rudolph, K.L.; Ema, H.; Nakauchi, H. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 2013, 154, 1112–1126. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan-Pla, A.; Macaulay, I.C.; Jensen, C.T.; Woll, P.S.; Luis, T.C.; Mead, A.; Moore, S.; Carella, C.; Matsuoka, S.; Jones, T.B.; et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 2013, 502, 232–236. [Google Scholar] [CrossRef]
- Gekas, C.; Graf, T. CD41 expression marks myeloid-biased adult hematopoietic stem cells and increases with age. Blood 2013, 121, 4463–4472. [Google Scholar] [CrossRef]
- Mansson, R.; Hultquist, A.; Luc, S.; Yang, L.; Anderson, K.; Kharazi, S.; Al-Hashmi, S.; Liuba, K.; Thoren, L.; Adolfsson, J.; et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity 2007, 26, 407–419. [Google Scholar] [CrossRef]
- Rodriguez-Fraticelli, A.E.; Wolock, S.L.; Weinreb, C.S.; Panero, R.; Patel, S.H.; Jankovic, M.; Sun, J.; Calogero, R.A.; Klein, A.M.; Camargo, F.D. Clonal analysis of lineage fate in native haematopoiesis. Nature 2018, 553, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Nakamura-Ishizu, A.; Matsumura, T.; Stumpf, P.S.; Umemoto, T.; Takizawa, H.; Takihara, Y.; O’Neil, A.; Majeed, A.; MacArthur, B.D.; Suda, T. Thrombopoietin Metabolically Primes Hematopoietic Stem Cells to Megakaryocyte-Lineage Differentiation. Cell Rep. 2018, 25, 1772–1785.e6. [Google Scholar] [CrossRef]
- Haas, S.; Hansson, J.; Klimmeck, D.; Loeffler, D.; Velten, L.; Uckelmann, H.; Wurzer, S.; Prendergast, A.M.; Schnell, A.; Hexel, K.; et al. Inflammation-Induced Emergency Megakaryopoiesis Driven by Hematopoietic Stem Cell-like Megakaryocyte Progenitors. Cell Stem Cell 2015, 17, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Vellenga, E.; van Agthoven, M.; Croockewit, A.J.; Verdonck, L.F.; Wijermans, P.J.; van Oers, M.H.; Volkers, C.P.; van Imhoff, G.W.; Kingma, T.; Uyl-de Groot, C.A.; et al. Autologous peripheral blood stem cell transplantation in patients with relapsed lymphoma results in accelerated haematopoietic reconstitution, improved quality of life and cost reduction compared with bone marrow transplantation: The Hovon 22 study. Br. J. Haematol. 2001, 114, 319–326. [Google Scholar] [CrossRef]
- Sabri, S.; Jandrot-Perrus, M.; Bertoglio, J.; Farndale, R.W.; Mas, V.M.; Debili, N.; Vainchenker, W. Differential regulation of actin stress fiber assembly and proplatelet formation by α2β1 integrin and GPVI in human megakaryocytes. Blood 2004, 104, 3117–3125. [Google Scholar] [CrossRef]
- Sabri, S.; Foudi, A.; Boukour, S.; Franc, B.; Charrier, S.; Jandrot-Perrus, M.; Farndale, R.W.; Jalil, A.; Blundell, M.P.; Cramer, E.M.; et al. Deficiency in the Wiskott-Aldrich protein induces premature proplatelet formation and platelet production in the bone marrow compartment. Blood 2006, 108, 134–140. [Google Scholar] [CrossRef]
- Zou, Z.; Schmaier, A.A.; Cheng, L.; Mericko, P.; Dickeson, S.K.; Stricker, T.P.; Santoro, S.A.; Kahn, M.L. Negative regulation of activated α-2 integrins during thrombopoiesis. Blood 2009, 113, 6428–6439. [Google Scholar] [CrossRef]
- Semeniak, D.; Kulawig, R.; Stegner, D.; Meyer, I.; Schwiebert, S.; Bosing, H.; Eckes, B.; Nieswandt, B.; Schulze, H. Proplatelet formation is selectively inhibited by collagen type I through Syk-independent GPVI signaling. J. Cell Sci. 2016, 129, 3473–3484. [Google Scholar] [CrossRef]
- Semeniak, D.; Faber, K.; Oftering, P.; Manukjan, G.; Schulze, H. Impact of Itga2-Gp6-double collagen receptor deficient mice for bone marrow megakaryocytes and platelets. PLoS ONE 2019, 14, e0216839. [Google Scholar] [CrossRef] [PubMed]
- Geddis, A.E.; Kaushansky, K. Inherited thrombocytopenias: Toward a molecular understanding of disorders of platelet production. Curr. Opin. Pediatr. 2004, 16, 15–22. [Google Scholar] [CrossRef]
- Balduini, A.; Malara, A.; Balduini, C.L.; Noris, P. Megakaryocytes derived from patients with the classical form of Bernard-Soulier syndrome show no ability to extend proplatelets in vitro. Platelets 2011, 22, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Fukai, F.; Kameda, T.; Shide, K.; Shimoda, H.; Torii, E.; Kamiunten, A.; Sekine, M.; Yamamoto, S.; Hidaka, T.; et al. Potentiated activation of VLA-4 and VLA-5 accelerates proplatelet-like formation. Ann. Hematol. 2012, 91, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Larson, M.K.; Watson, S.P. Regulation of proplatelet formation and platelet release by integrin α IIb β3. Blood 2006, 108, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Avecilla, S.T.; Hattori, K.; Heissig, B.; Tejada, R.; Liao, F.; Shido, K.; Jin, D.K.; Dias, S.; Zhang, F.; Hartman, T.E.; et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med. 2004, 10, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Nowak, R.; Billington, N.; Liu, R.; Ghosh, A.; Sellers, J.R.; Fowler, V.M. Megakaryocyte migration defects due to nonmuscle myosin IIA mutations underly thrombocytopenia in MYH9-Related Disease. Blood 2020, 135, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, H.; Calaminus, S.D.; Sinclair, A.; Monypenny, J.; Blundell, M.P.; Leon, C.; Holyoake, T.L.; Thrasher, A.J.; Michie, A.M.; Vukovic, M.; et al. Megakaryocytes assemble podosomes that degrade matrix and protrude through basement membrane. Blood 2013, 121, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Junt, T.; Schulze, H.; Chen, Z.; Massberg, S.; Goerge, T.; Krueger, A.; Wagner, D.D.; Graf, T.; Italiano, J.E., Jr.; Shivdasani, R.A.; et al. Dynamic visualization of thrombopoiesis within bone marrow. Science 2007, 317, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Dunois-Larde, C.; Capron, C.; Fichelson, S.; Bauer, T.; Cramer-Borde, E.; Baruch, D. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood 2009, 114, 1875–1883. [Google Scholar] [CrossRef]
- Zhang, L.; Orban, M.; Lorenz, M.; Barocke, V.; Braun, D.; Urtz, N.; Schulz, C.; von Bruhl, M.L.; Tirniceriu, A.; Gaertner, F.; et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J. Exp. Med. 2012, 209, 2165–2181. [Google Scholar] [CrossRef]
- Kimura, S.; Roberts, A.W.; Metcalf, D.; Alexander, W.S. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc. Natl. Acad. Sci. USA 1998, 95, 1195–1200. [Google Scholar] [CrossRef]
- Bunting, S.; Widmer, R.; Lipari, T.; Rangell, L.; Steinmetz, H.; Carver-Moore, K.; Moore, M.W.; Keller, G.-A.; de Sauvage, F.J. Normal Platelets and Megakaryocytes Are Produced In Vivo in the Absence of Thrombopoietin. Blood 1997, 90, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Segal, G.M.; Stueve, T.; Adamson, J.W. Analysis of murine megakaryocyte colony size and ploidy: Effects of interleukin-3. J. Cell. Physiol. 1988, 137, 537–544. [Google Scholar] [CrossRef]
- Kaser, A.; Brandacher, G.; Steurer, W.; Kaser, S.; Offner, F.A.; Zoller, H.; Theurl, I.; Widder, W.; Molnar, C.; Ludwiczek, O.; et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: Role in inflammatory thrombocytosis. Blood 2001, 98, 2720–2725. [Google Scholar] [CrossRef]
- Avraham, H.; Vannier, E.; Cowley, S.; Jiang, S.X.; Chi, S.; Dinarello, C.A.; Zsebo, K.M.; Groopman, J.E. Effects of the stem cell factor, c-kit ligand, on human megakaryocytic cells. Blood 1992, 79, 365–371. [Google Scholar] [CrossRef] [PubMed]
- de Sauvage, F.J.; Hass, P.E.; Spencer, S.D.; Malloy, B.E.; Gurney, A.L.; Spencer, S.A.; Darbonne, W.C.; Henzel, W.J.; Wong, S.C.; Kuang, W.J.; et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 1994, 369, 533–538. [Google Scholar] [CrossRef]
- Long, M.W.; Williams, N.; Ebbe, S. Immature megakaryocytes in the mouse: Physical characteristics, cell cycle status, and in vitro responsiveness to thrombopoietic stimulatory factor. Blood 1982, 59, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Bhatlekar, S.; Middleton, E.A.; Weyrich, A.S.; Borst, O.; Rondina, M.T. Phospho-inositide-dependent kinase 1 regulates signal dependent translation in megakaryocytes and platelets. J. Thromb. Haemost. 2020, 18, 1183–1196. [Google Scholar] [CrossRef]
- Italiano, J.E., Jr.; Lecine, P.; Shivdasani, R.A.; Hartwig, J.H. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J. Cell Biol. 1999, 147, 1299–1312. [Google Scholar] [CrossRef]
- Cerecedo, D. Platelet cytoskeleton and its hemostatic role. Blood Coagul. Fibrinolysis 2013, 24, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.L.; Shivdasani, R.A.; Boers, C.; Hartwig, J.H.; Italiano, J.E., Jr. Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood 2005, 106, 4066–4075. [Google Scholar] [CrossRef]
- Bender, M.; Thon, J.N.; Ehrlicher, A.J.; Wu, S.; Mazutis, L.; Deschmann, E.; Sola-Visner, M.; Italiano, J.E.; Hartwig, J.H. Microtubule sliding drives proplatelet elongation and is dependent on cytoplasmic dynein. Blood 2015, 125, 860–868. [Google Scholar] [CrossRef]
- Lecine, P.; Italiano, J.E., Jr.; Kim, S.-W.; Villeval, J.-L.; Shivdasani, R.A. Hematopoietic-specific β1 tubulin participates in a pathway of platelet biogenesis dependent on the transcription factor NF-E2. Blood 2000, 96, 1366–1373. [Google Scholar] [CrossRef]
- Schwer, H.D.; Lecine, P.; Tiwari, S.; Italiano, J.E., Jr.; Hartwig, J.H.; Shivdasani, R.A. A lineage-restricted and divergent β-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr. Biol. 2001, 11, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, S.; Kobayashi, R.; Itoh, T.J.; Hamaguchi, M.; Saito, H. Mutation of the β1-tubulin gene associated with congenital macrothrombocytopenia affecting microtubule assembly. Blood 2009, 113, 458–461. [Google Scholar] [CrossRef]
- Kunishima, S.; Nishimura, S.; Suzuki, H.; Imaizumi, M.; Saito, H. TUBB1 mutation disrupting microtubule assembly impairs proplatelet formation and results in congenital macrothrombocytopenia. Eur. J. Haematol. 2014, 92, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Sattler, M.; Winkler, T.; Verma, S.; Byrne, C.H.; Shrikhande, G.; Salgia, R.; Griffin, J.D. Hematopoietic growth factors signal through the formation of reactive oxygen species. Blood 1999, 93, 2928–2935. [Google Scholar] [CrossRef]
- Sardina, J.L.; López-Ruano, G.; Sánchez-Abarca, L.I.; Pérez-Simón, J.A.; Gaztelumendi, A.; Trigueros, C.; Llanillo, M.; Sánchez-Yagüe, J.; Hernández-Hernández, A. p22phox-dependent NADPH oxidase activity is required for megakaryocytic differentiation. Cell Death Differ. 2010, 17, 1842–1854. [Google Scholar] [CrossRef]
- Motohashi, H.; Kimura, M.; Fujita, R.; Inoue, A.; Pan, X.; Takayama, M.; Katsuoka, F.; Aburatani, H.; Bresnick, E.H.; Yamamoto, M. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood 2010, 115, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Poirault-Chassac, S.; Nivet-Antoine, V.; Houvert, A.; Kauskot, A.; Lauret, E.; Lai-Kuen, R.; Dusanter-Fourt, I.; Baruch, D. Mitochondrial dynamics and reactive oxygen species initiate thrombopoiesis from mature megakaryocytes. Blood Adv. 2021, 5, 1706–1718. [Google Scholar] [CrossRef]
- Petzold, T.; Zhang, Z.; Ballesteros, I.; Saleh, I.; Polzin, A.; Thienel, M.; Liu, L.; Ul Ain, Q.; Ehreiser, V.; Weber, C.; et al. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity 2022, 55, 2285–2299.e7. [Google Scholar] [CrossRef] [PubMed]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Pietras, E.M. Inflammation: A key regulator of hematopoietic stem cell fate in health and disease. Blood 2017, 130, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Noetzli, L.J.; French, S.L.; Machlus, K.R. New Insights Into the Differentiation of Megakaryocytes From Hematopoietic Progenitors. Arter. Thromb. Vasc. Biol. 2019, 39, 1288–1300. [Google Scholar] [CrossRef] [PubMed]
- Chuen, C.K.; Li, K.; Yang, M.; Fok, T.F.; Li, C.K.; Chui, C.M.; Yuen, P.M. Interleukin-1β up-regulates the expression of thrombopoietin and transcription factors c-Jun, c-Fos, GATA-1, and NF-E2 in megakaryocytic cells. J. Lab. Clin. Med. 2004, 143, 75–88. [Google Scholar] [CrossRef]
- Nishimura, S.; Nagasaki, M.; Kunishima, S.; Sawaguchi, A.; Sakata, A.; Sakaguchi, H.; Ohmori, T.; Manabe, I.; Italiano, J.E., Jr.; Ryu, T. IL-1α induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J. Cell Biol. 2015, 209, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Burzynski, L.; Humphry, M.; Pyrillou, K.; Wiggins, K.; Chan, J.; Figg, N.; Kitt, L.; Summers, C.; Tatham, K.; et al. The coagulation and immune systems are directly linked through the activation of interleukin-1α by thrombin. Immunity 2019, 50, 4. [Google Scholar]
- Zauli, G.; Vitale, M.; Falcieri, E.; Gibellini, D.; Bassini, A.; Celeghini, C.; Columbaro, M.; Capitani, S. In vitro senescence and apoptotic cell death of human megakaryocytes. Blood J. Am. Soc. Hematol. 1997, 90, 2234–2243. [Google Scholar]
- Preudhomme, C.; Sagot, C.; Boissel, N.; Cayuela, J.-M.; Tigaud, I.; de Botton, S.; Thomas, X.; Raffoux, E.; Lamandin, C.; Castaigne, S.; et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: A study from the Acute Leukemia French Association (ALFA). Blood 2002, 100, 2717–2723. [Google Scholar] [CrossRef]
- Josefsson, E.C.; James, C.; Henley, K.J.; Debrincat, M.A.; Rogers, K.L.; Dowling, M.R.; White, M.J.; Kruse, E.A.; Lane, R.M.; Ellis, S.; et al. Megakaryocytes possess a functional intrinsic apoptosis pathway that must be restrained to survive and produce platelets. J. Exp. Med. 2011, 208, 2017–2031. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Burnett, D.L.; Lebois, M.; Debrincat, M.A.; White, M.J.; Henley, K.J.; Lane, R.M.; Moujalled, D.; Preston, S.P.; O’Reilly, L.A.; et al. Platelet production proceeds independently of the intrinsic and extrinsic apoptosis pathways. Nat. Commun. 2014, 5, 3455. [Google Scholar] [CrossRef]
- Maciejewski, J.P.; Selleri, C.; Sato, T.; Cho, H.J.; Keefer, L.K.; Nathan, C.F.; Young, N.S. Nitric oxide suppression of human hematopoiesis in vitro. Contribution to inhibitory action of interferon-gamma and tumor necrosis factor-α. J. Clin. Investig. 1995, 96, 1085–1092. [Google Scholar] [CrossRef]
- Shami, P.J.; Weinberg, J.B. Differential effects of nitric oxide on erythroid and myeloid colony growth from CD34+ human bone marrow cells. Blood 1996, 87, 977–982. [Google Scholar] [CrossRef]
- North, T.E.; Goessling, W.; Peeters, M.; Li, P.; Ceol, C.; Lord, A.M.; Weber, G.J.; Harris, J.; Cutting, C.C.; Huang, P.; et al. Hematopoietic stem cell development is dependent on blood flow. Cell 2009, 137, 736–748. [Google Scholar] [CrossRef]
- Adamo, L.; Naveiras, O.; Wenzel, P.L.; McKinney-Freeman, S.; Mack, P.J.; Gracia-Sancho, J.; Suchy-Dicey, A.; Yoshimoto, M.; Lensch, M.W.; Yoder, M.C.; et al. Biomechanical forces promote embryonic haematopoiesis. Nature 2009, 459, 1131–1135. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. An L-arginine/nitric oxide pathway present in human platelets regulates aggregation. Proc. Natl. Acad. Sci. USA 1990, 87, 5193–5197. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Biosynthesis of nitric oxide from L-arginine: A pathway for the regulation of cell function and communication. Biochem. Pharmacol. 1989, 38, 1709–1715. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, T.J.; Hawkins, R.D.; Kandel, E.R.; Arancio, O. Tests of the roles of two diffusible substances in long-term potentiation: Evidence for nitric oxide as a possible early retrograde messenger. Proc. Natl. Acad. Sci. USA 1991, 88, 11285–11289. [Google Scholar] [CrossRef] [PubMed]
- Schuman, E.M.; Madison, D.V. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science 1991, 254, 1503–1506. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, R.M.; Draznin, M.B.; Murad, F. Endothelium-dependent relaxation in rat aorta may be mediated through cyclic GMP-dependent protein phosphorylation. Nature 1983, 306, 174–176. [Google Scholar] [CrossRef]
- Forstermann, U.; Mulsch, A.; Bohme, E.; Busse, R. Stimulation of soluble guanylate cyclase by an acetylcholine-induced endothelium-derived factor from rabbit and canine arteries. Circ. Res. 1986, 58, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Stamler, J.S.; Simon, D.I.; Osborne, J.A.; Mullins, M.E.; Jaraki, O.; Michel, T.; Singel, D.J.; Loscalzo, J. S-nitrosylation of proteins with nitric oxide: Synthesis and characterization of biologically active compounds. Proc. Natl. Acad. Sci. USA 1992, 89, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Karupiah, G.; Xie, Q.W.; Buller, R.M.; Nathan, C.; Duarte, C.; MacMicking, J.D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 1993, 261, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.F.; Hibbs, J.B., Jr. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 1991, 3, 65–70. [Google Scholar] [CrossRef]
- Battinelli, E.; Loscalzo, J. Nitric oxide induces apoptosis in megakaryocytic cell lines. Blood 2000, 95, 3451–3459. [Google Scholar] [CrossRef]
- Battinelli, E.; Willoughby, S.R.; Foxall, T.; Valeri, C.R.; Loscalzo, J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc. Natl. Acad. Sci. USA 2001, 98, 14458–14463. [Google Scholar] [CrossRef]
- Schattner, M.; Pozner, R.G.; Engelberger, I.; Gorostizaga, A.; Maugeri, N.; Gomez, R.; Pasqualini, A.; Torres, O.; Lazzari, M.A. Effect of nitric oxide on megakaryocyte growth induced by thrombopoietin. J. Lab. Clin. Med. 2001, 137, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.N.; Peroni, D.J.; Basclain, K.A.; Garlepp, M.J.; Thompson, P.J. Expression and activity of nitric oxide synthases in human airway epithelium. Am. J. Respir. Cell Mol. Biol. 1997, 16, 629–639. [Google Scholar] [CrossRef]
- McNaughton, L.; Puttagunta, L.; Martinez-Cuesta, M.A.; Kneteman, N.; Mayers, I.; Moqbel, R.; Hamid, Q.; Radomski, M.W. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc. Natl. Acad. Sci. USA 2002, 99, 17161–17166. [Google Scholar] [CrossRef]
- Kelley, T.J.; Drumm, M.L. Inducible nitric oxide synthase expression is reduced in cystic fibrosis murine and human airway epithelial cells. J. Clin. Investig. 1998, 102, 1200–1207. [Google Scholar] [CrossRef]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef]
- Nishimura, J.S.; Martasek, P.; McMillan, K.; Salerno, J.; Liu, Q.; Gross, S.S.; Masters, B.S. Modular structure of neuronal nitric oxide synthase: Localization of the arginine binding site and modulation by pterin. Biochem. Biophys. Res. Commun. 1995, 210, 288–294. [Google Scholar] [CrossRef]
- Noble, M.A.; Munro, A.W.; Rivers, S.L.; Robledo, L.; Daff, S.N.; Yellowlees, L.J.; Shimizu, T.; Sagami, I.; Guillemette, J.G.; Chapman, S.K. Potentiometric analysis of the flavin cofactors of neuronal nitric oxide synthase. Biochemistry 1999, 38, 16413–16418. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Xie, Q.W.; Calaycay, J.; Mumford, R.A.; Swiderek, K.M.; Lee, T.D.; Nathan, C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J. Exp. Med. 1992, 176, 599–604. [Google Scholar] [CrossRef]
- Hemmens, B.; Mayer, B. Enzymology of nitric oxide synthases. In Methods in Molecular Biology; Springer Nature: Berlin/Heidelberg, Germany, 1998; pp. 1–32. [Google Scholar] [CrossRef]
- Sowa, G.; Pypaert, M.; Sessa, W.C. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc. Natl. Acad. Sci. USA 2001, 98, 14072–14077. [Google Scholar] [CrossRef]
- Gratton, J.P.; Fontana, J.; O’Connor, D.S.; Garcia-Cardena, G.; McCabe, T.J.; Sessa, W.C. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. Evidence that hsp90 facilitates calmodulin stimulated displacement of eNOS from caveolin-1. J. Biol. Chem. 2000, 275, 22268–22272. [Google Scholar] [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. Eur. J. Physiol. 2010, 459, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837, 837a–837d. [Google Scholar] [CrossRef]
- Forstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994, 23, 1121–1131. [Google Scholar] [CrossRef]
- Nakane, M.; Schmidt, H.H.; Pollock, J.S.; Forstermann, U.; Murad, F. Cloned human brain nitric oxide synthase is highly expressed in skeletal muscle. FEBS Lett. 1993, 316, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U. Regulation of Nitric Oxide Synthase Expression and Activity. In Nitric Oxide; Mayer, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 71–91. [Google Scholar] [CrossRef]
- Khan, B.V.; Harrison, D.G.; Olbrych, M.T.; Alexander, R.W.; Medford, R.M. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 9114–9119. [Google Scholar] [CrossRef]
- Gudi, T.; Hong, G.K.; Vaandrager, A.B.; Lohmann, S.M.; Pilz, R.B. Nitric oxide and cGMP regulate gene expression in neuronal and glial cells by activating type II cGMP-dependent protein kinase. FASEB J. 1999, 13, 2143–2152. [Google Scholar] [CrossRef]
- Pantopoulos, K.; Hentze, M.W. Nitric oxide signaling to iron-regulatory protein: Direct control of ferritin mRNA translation and transferrin receptor mRNA stability in transfected fibroblasts. Proc. Natl. Acad. Sci. USA 1995, 92, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Hill, P.; Haile, D.J. Role of the ferroportin iron-responsive element in iron and nitric oxide dependent gene regulation. Blood Cells Mol. Dis. 2002, 29, 315–326. [Google Scholar] [CrossRef]
- Pozdnyakov, N.; Lloyd, A.; Reddy, V.N.; Sitaramayya, A. Nitric oxide-regulated endogenous ADP-ribosylation of rod outer segment proteins. Biochem. Biophys. Res. Commun. 1993, 192, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Campolo, N.; Trujillo, M.; Bartesaghi, S.; Carballal, S.; Romero, N.; Alvarez, B.; Radi, R. Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Chem. Rev. 2018, 118, 1338–1408. [Google Scholar] [CrossRef] [PubMed]
- Wink, D.A.; Kasprzak, K.S.; Maragos, C.M.; Elespuru, R.K.; Misra, M.; Dunams, T.M.; Cebula, T.A.; Koch, W.H.; Andrews, A.W.; Allen, J.S.; et al. DNA deaminating ability and genotoxicity of nitric oxide and its progenitors. Science 1991, 254, 1001–1003. [Google Scholar] [CrossRef]
- Fehsel, K.; Jalowy, A.; Qi, S.; Burkart, V.; Hartmann, B.; Kolb, H. Islet cell DNA is a target of inflammatory attack by nitric oxide. Diabetes 1993, 42, 496–500. [Google Scholar] [CrossRef]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Herrera, D.; Martinez, D.M.; Petruk, A.; Demicheli, V.; Trujillo, M.; Marti, M.A.; Estrin, D.A.; Radi, R. Tyrosine oxidation and nitration in transmembrane peptides is connected to lipid peroxidation. Arch. Biochem. Biophys. 2017, 622, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, J.S.; Ye, Y.Z.; Anderson, P.G.; Chen, J.; Accavitti, M.A.; Tarpey, M.M.; White, C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler 1994, 375, 81–88. [Google Scholar] [CrossRef]
- Mikkelsen, R.B.; Wardman, P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene 2003, 22, 5734–5754. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Thomas, D.D.; Mancardi, D.; Espey, M.G.; Miranda, K.M.; Paolocci, N.; Feelisch, M.; Fukuto, J.; Wink, D.A. The chemistry of nitrosative stress induced by nitric oxide and reactive nitrogen oxide species. Putting perspective on stressful biological situations. Biol. Chem. 2004, 385, 1–10. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Endogenous nitric oxide inhibits human platelet adhesion to vascular endothelium. Lancet 1987, 330, 1057–1058. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem. Biophys. Res. Commun. 1987, 148, 1482–1489. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br. J. Pharmacol. 1987, 92, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Radomski, M.W.; Palmer, R.M.; Moncada, S. The anti-aggregating properties of vascular endothelium: Interactions between prostacyclin and nitric oxide. Br. J. Pharmacol. 1987, 92, 639–646. [Google Scholar] [CrossRef]
- Radomski, M.W.; Palmer, R.M.J.; Moncada, S. Characterization of the L-arginine:nitric oxide pathway in human platelets. Br. J. Pharmacol. 1990, 101, 325–328. [Google Scholar] [CrossRef]
- Riba, R.; Sharifi, M.; Farndale, R.W.; Naseem, K.M. Regulation of platelet guanylyl cyclase by collagen: Evidence that Glycoprotein VI mediates platelet nitric oxide synthesis in response to collagen. Thromb. Haemost. 2005, 94, 395–403. [Google Scholar] [CrossRef]
- Riba, R.; Oberprieler, N.G.; Roberts, W.; Naseem, K.M. Von Willebrand factor activates endothelial nitric oxide synthase in blood platelets by a glycoprotein Ib-dependent mechanism. J. Thromb. Haemost. 2006, 4, 2636–2644. [Google Scholar] [CrossRef]
- Malinski, T.; Radomski, M.W.; Taha, Z.; Moncada, S. Direct Electrochemical Measurement of Nitric Oxide Released from Human Platelets. Biochem. Biophys. Res. Commun. 1993, 194, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E.; Loscalzo, J.; Barnard, M.R.; Alpert, C.; Keaney, J.F.; Michelson, A.D. Nitric oxide released from activated platelets inhibits platelet recruitment. J. Clin. Investig. 1997, 100, 350–356. [Google Scholar] [CrossRef]
- Moro, M.A.; Russel, R.J.; Cellek, S.; Lizasoain, I.; Su, Y.; Darley-Usmar, V.M.; Radomski, M.W.; Moncada, S. cGMP mediates the vascular and platelet actions of nitric oxide: Confirmation using an inhibitor of the soluble guanylyl cyclase. Proc. Natl. Acad. Sci. USA 1996, 93, 1480–1485. [Google Scholar] [CrossRef]
- Jurasz, P.; Sawicki, G.; Duszyk, M.; Sawicka, J.; Miranda, C.; Mayers, I.; Radomski, M.W. Matrix Metalloproteinase 2 in Tumor Cell-induced Platelet Aggregation: Regulation by Nitric Oxide. Cancer Res. 2001, 61, 376–382. [Google Scholar] [PubMed]
- Jurasz, P.; Radomski, A.; Sawicki, G.; Mayers, I.; Radomski, M.W. Nitric oxide and platelet function. In Nitric Oxide; Ignarro, L., Ed.; Raven Press: New York, NY, USA, 2000. [Google Scholar]
- Halbrugge, M.; Friedrich, C.; Eigenthaler, M.; Schanzenbacher, P.; Walter, U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J. Biol. Chem. 1990, 265, 3088–3093. [Google Scholar] [CrossRef]
- Smolenski, A.; Bachmann, C.; Reinhard, K.; Hönig-Liedl, P.; Jarchau, T.; Hoschuetzky, H.; Walter, U. Analysis and Regulation of Vasodilator-stimulated Phosphoprotein Serine 239 Phosphorylation in Vitro and in Intact Cells Using a Phosphospecific Monoclonal Antibody. J. Biol. Chem. 1998, 273, 20029–20035. [Google Scholar] [CrossRef]
- Massberg, S.; Gruner, S.; Konrad, I.; Garcia Arguinzonis, M.I.; Eigenthaler, M.; Hemler, K.; Kersting, J.; Schulz, C.; Muller, I.; Besta, F.; et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)–deficient mice. Blood 2004, 103, 136–142. [Google Scholar] [CrossRef]
- Jurasz, P.; Stewart, M.W.; Radomski, A.; Khadour, F.; Duszyk, M.; Radomski, M.W. Role of von Willebrand factor in tumour cell-induced platelet aggregation: Differential regulation by NO and prostacyclin. Br. J. Pharmacol. 2001, 134, 1104–1112. [Google Scholar] [CrossRef]
- Antl, M.; von Bruhl, M.-L.; Eiglsperger, C.; Werner, M.; Konrad, I.; Kocher, T.; Wilm, M.; Hofmann, F.; Massberg, S.; Schlossmann, J.; et al. IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood 2007, 109, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Schinner, E.; Salb, K.; Schlossmann, J. Signaling via IRAG is essential for NO/cGMP-dependent inhibition of platelet activation. Platelets 2011, 22, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-R.; Zhu, Y.; Halushka, P.V.; Lincoln, T.M.; Mendelsohn, M.E. Mechanism of platelet inhibition by nitric oxide: In vivo phosphorylation of thromboxane receptor by cyclic GMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA 1998, 95, 4888–4893. [Google Scholar] [CrossRef]
- Chen, L.Y.; Mehta, J.L. Further evidence of the presence of constitutive and inducible nitric oxide synthase isoforms in human platelets. J. Cardiovasc. Pharmacol. 1996, 27, 154–158. [Google Scholar] [CrossRef]
- Wallerath, T.; Gath, I.; Aulitzky, W.E.; Pollock, J.S.; Kleinert, H.; Förstermann, U. Identification of the NO synthase isoforms expressed in human neutrophil granules, megakaryocytes and platelets. Thromb. Haemost. 1997, 77, 163–167. [Google Scholar]
- Freedman, J.E.; Sauter, R.; Battinelli, E.M.; Ault, K.; Knowles, C.; Huang, P.L.; Loscalzo, J. Deficient Platelet-Derived Nitric Oxide and Enhanced Hemostasis in Mice Lacking the NOSIII Gene. Circ. Res. 1999, 84, 1416–1421. [Google Scholar] [CrossRef]
- Gambaryan, S.; Kobsar, A.; Hartmann, S.; Birschmann, I.; Kuhlencordt, P.J.; MÜLler-Esterl, W.; Lohmann, S.M.; Walter, U. NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J. Thromb. Haemost. 2008, 6, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, J.A.; Li, Z.; Stojanovic, A.; Du, X. Stimulatory roles of nitric-oxide synthase 3 and guanylyl cyclase in platelet activation. J. Biol. Chem. 2005, 280, 37430–37438. [Google Scholar] [CrossRef] [PubMed]
- Radziwon-Balicka, A.; Lesyk, G.; Back, V.; Fong, T.; Loredo-Calderon, E.L.; Dong, B.; El-Sikhry, H.; El-Sherbani, A.A.; El-Kadi, A.; Ogg, S.; et al. Differential eNOS-signaling by platelet subpopulations regulates adhesion and aggregation. Cardiovasc. Res. 2017, 113, 1719–1731. [Google Scholar] [CrossRef]
- Moro, M.A.; Darley-Usmar, V.M.; Goodwin, D.A.; Read, N.G.; Zamora-Pino, R.; Feelisch, M.; Radomski, M.W.; Moncada, S. Paradoxical fate and biological action of peroxynitrite on human platelets. Proc. Natl. Acad. Sci. USA 1994, 91, 6702–6706. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Moro, M.A.; Masse, J.M.; Cramer, E.M.; Radomski, M.; Darley-Usmar, V. Nitric oxide-dependent and independent effects on human platelets treated with peroxynitrite. Cardiovasc. Res. 1998, 40, 380–388. [Google Scholar]
- Clutton, P.; Miermont, A.; Freedman, J.E. Regulation of endogenous reactive oxygen species in platelets can reverse aggregation. Arter. Thromb. Vasc. Biol. 2004, 24, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.E.; Ting, B.; Hankin, B.; Loscalzo, J.; Keaney, J.F., Jr.; Vita, J.A. Impaired platelet production of nitric oxide predicts presence of acute coronary syndromes. Circulation 1998, 98, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Takajo, Y.; Murohara, T.; Ichiki, K.; Adachi, H.; Haramaki, N.; Katoh, A.; Imaizumi, T. Platelet-derived nitric oxide and coronary risk factors. Hypertension 2000, 35, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, S.R.; Stewart, S.; Holmes, A.S.; Chirkov, Y.Y.; Horowitz, J.D. Platelet nitric oxide responsiveness: A novel prognostic marker in acute coronary syndromes. Arter. Thromb. Vasc. Biol. 2005, 25, 2661–2666. [Google Scholar] [CrossRef]
- de Belder, A.; Radomski, M.; Hancock, V.; Brown, A.; Moncada, S.; Martin, J. Megakaryocytes from patients with coronary atherosclerosis express the inducible nitric oxide synthase. Arter. Thromb. Vasc. Biol. 1995, 15, 637–641. [Google Scholar] [CrossRef]
- Gnatenko, D.V.; Dunn, J.J.; McCorkle, S.R.; Weissmann, D.; Perrotta, P.L.; Bahou, W.F. Transcript profiling of human platelets using microarray and serial analysis of gene expression. Blood 2003, 101, 2285–2293. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Zimmerman, G.A. Evaluating the relevance of the platelet transcriptome. Blood 2003, 102, 1550–1551. [Google Scholar] [CrossRef]
- Kolodziejski, P.J.; Koo, J.S.; Eissa, N.T. Regulation of inducible nitric oxide synthase by rapid cellular turnover and cotranslational down-regulation by dimerization inhibitors. Proc. Natl. Acad. Sci. USA 2004, 101, 18141–18146. [Google Scholar] [CrossRef]
- Panda, K.; Chawla-Sarkar, M.; Santos, C.; Koeck, T.; Erzurum, S.C.; Parkinson, J.F.; Stuehr, D.J. Visualizing inducible nitric-oxide synthase in living cells with a heme-binding fluorescent inhibitor. Proc. Natl. Acad. Sci. USA 2005, 102, 10117–10122. [Google Scholar] [CrossRef]
- Lelchuk, R.; Radomski, M.W.; Martin, J.F.; Moncada, S. Constitutive and inducible nitric oxide synthases in human megakaryoblastic cells. J. Pharm. Exp. Ther. 1992, 262, 1220–1224. [Google Scholar]
- Lesyk, G.M.; Jurasz, P. Identification of eNOS-based Megakaryocyte Subpopulations and Their Pharmacological Characterization by IFNγ and IL-10. FASEB J. 2018, 32, lb598. [Google Scholar] [CrossRef]
- Pozner, R.G.; Negrotto, S.; D’Atri, L.P.; Kotler, M.L.; Lazzari, M.A.; Gomez, R.M.; Schattner, M. Prostacyclin prevents nitric oxide-induced megakaryocyte apoptosis. Br. J. Pharmacol. 2005, 145, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Negrotto, S.; Pacienza, N.; D’Atri, L.P.; Pozner, R.G.; Malaver, E.; Torres, O.; Lazzari, M.A.; Gómez, R.M.; Schattner, M. Activation of cyclic AMP pathway prevents CD34+ cell apoptosis. Exp. Hematol. 2006, 34, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Molnar, M.; Suto, T.; Toth, T.; Hertelendy, F. Prolonged blockade of nitric oxide synthesis in gravid rats produces sustained hypertension, proteinuria, thrombocytopenia, and intrauterine growth retardation. Am. J. Obset. Gynecol. 1994, 170, 1458–1466. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, Y.; Jiang, D.; Xie, J.; Liu, Y.; Ma, J.; Mu, Y.; Zhang, X.; Yu, C.; Zhang, Y.; et al. Deficiency of platelet adhesion molecule CD226 causes megakaryocyte development and platelet hyperactivity. FASEB J. 2020, 34, 6871–6887. [Google Scholar] [CrossRef]
- Sasu, S.; Cooper, A.L.; Beasley, D. Juxtacrine effects of IL-1α precursor promote iNOS expression in vascular smooth muscle cells. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H1615–H1623. [Google Scholar] [CrossRef]
- Gur-Cohen, S.; Itkin, T.; Chakrabarty, S.; Graf, C.; Kollet, O.; Ludin, A.; Golan, K.; Kalinkovich, A.; Ledergor, G.; Wong, E.; et al. PAR1 signaling regulates the retention and recruitment of EPCR-expressing bone marrow hematopoietic stem cells. Nat. Med. 2015, 21, 1307–1317. [Google Scholar] [CrossRef]
- Ruan, B.; Chen, Y.; Trimidal, S.; Koo, I.; Qian, F.; Cai, J.; McGuigan, J.; Hall, M.A.; Patterson, A.D.; Prabhu, K.S.; et al. Nitric oxide regulates metabolism in murine stress erythroid progenitors to promote recovery during inflammatory anemia. bioRxiv 2023. [Google Scholar] [CrossRef]
- Dimmeler, S.; Zeiher, A.M. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999, 6, 964–968. [Google Scholar] [CrossRef]
- Kile, B.T. The role of apoptosis in megakaryocytes and platelets. Br. J. Haematol. 2014, 165, 217–226. [Google Scholar] [CrossRef]
- McArthur, K.; Chappaz, S.; Kile, B.T. Apoptosis in megakaryocytes and platelets: The life and death of a lineage. Blood 2018, 131, 605–610. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).