Potential Biomarkers to Distinguish Type 1 Myocardial Infarction in Troponin-Elevated Diseases
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
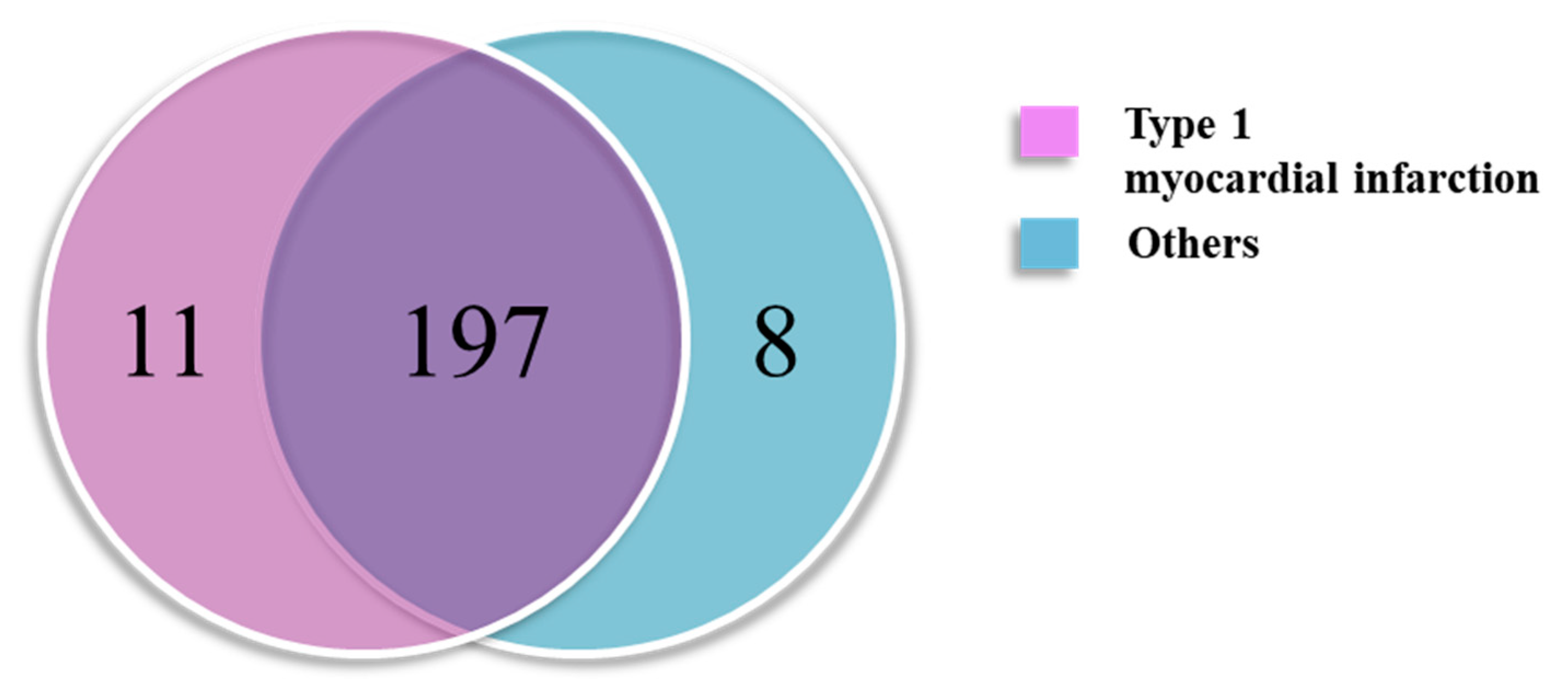
2.2. Finding Type 1 Myocardial-Infarction-Specific Protein Markers
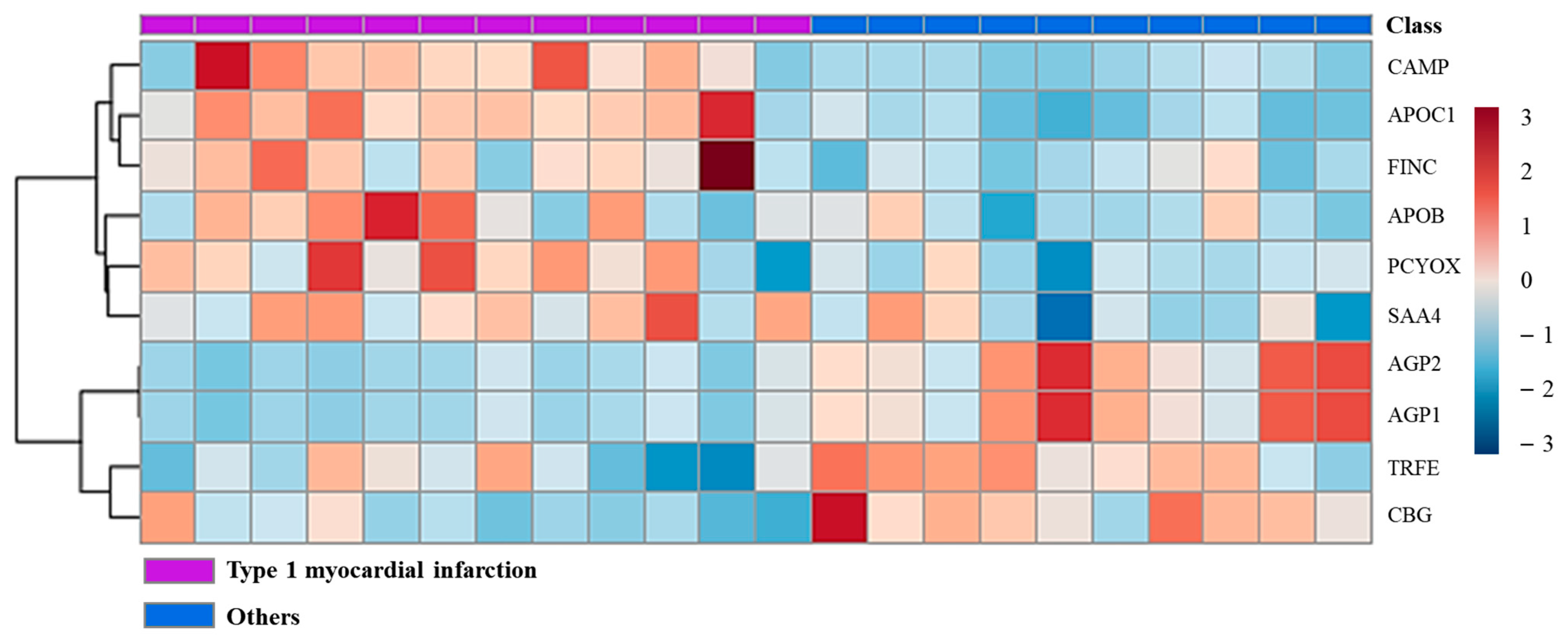
2.3. Quantitative Comparison of Protein Groups Differentiating Type 1 Myocardial Infarction
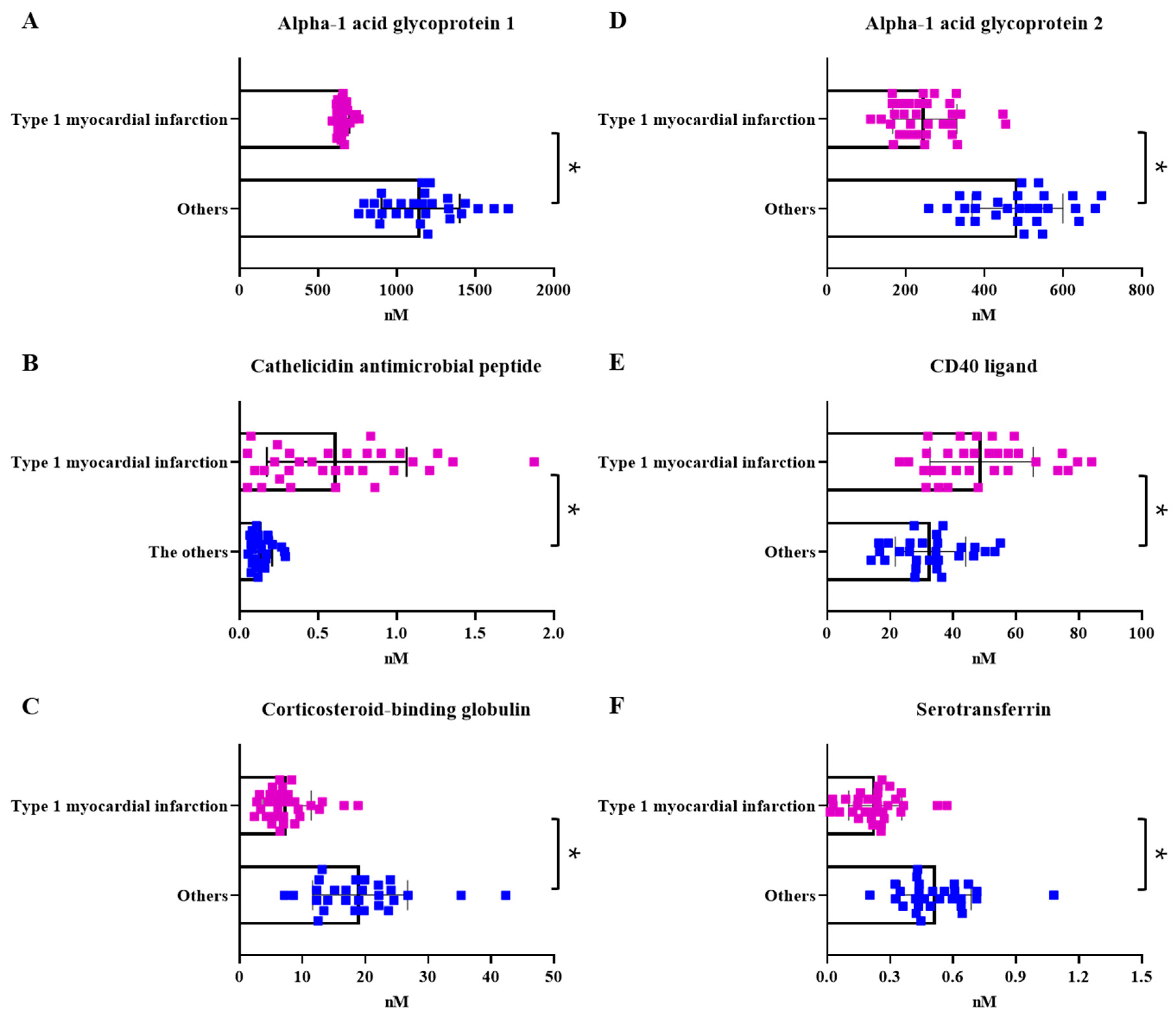
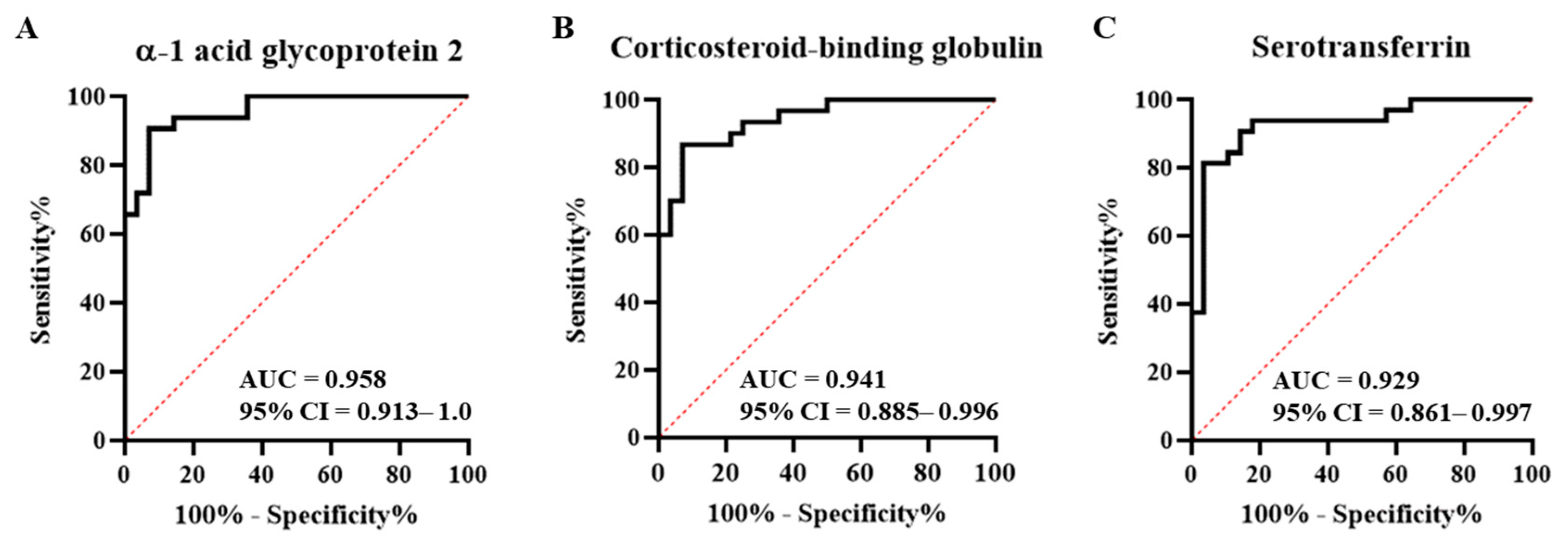
2.4. Comparison with the Healthy Control Group and Marker Selection
3. Discussion
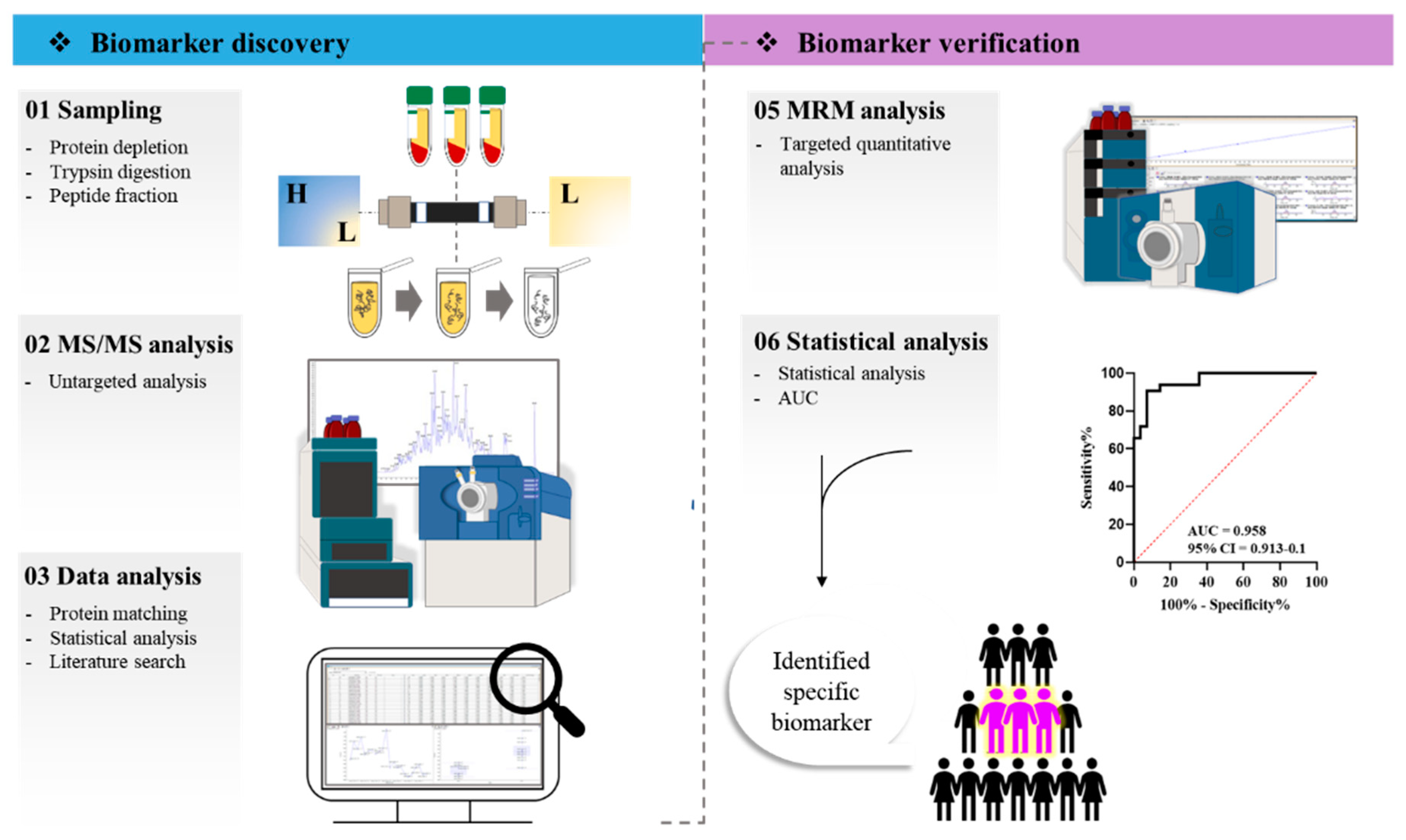
4. Materials and Methods
4.1. Study Population
4.2. High-Abundance Protein Depletion
4.3. Low-Abundance Protein Digestion
4.4. Peptide Fractionation
4.5. LC-MS/MS Setup
4.6. Data Processing
4.7. Peptide Selection for MRM
4.8. Analysis of Parameter Settings and MRM Method Development
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bracey, A.M.H. Posterior Myocardial Ischemia; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Neumann, J.T.; Weimann, J.; Sörensen, N.A.; Hartikainen, T.S.; Haller, P.M.; Lehmacher, J.; Brocks, C.; Tenhaeff, S.; Karakas, M.; Renné, T.; et al. A Biomarker Model to Distinguish Types of Myocardial Infarction and Injury. J. Am. Coll. Cardiol. 2021, 78, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, K.H.; Lee, J.M.; Kim, H.K.; Hwang, D.; Rhee, T.M.; Kim, J.; Park, T.K.; Yang, J.H.; Song, Y.B.; et al. Prognostic Implications of Door-to-Balloon Time and Onset-to-Door Time on Mortality in Patients With ST -Segment-Elevation Myocardial Infarction Treated With Primary Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e012188. [Google Scholar] [CrossRef] [PubMed]
- Tilea, I.; Varga, A.; Serban, R.C. Past, Present, and Future of Blood Biomarkers for the Diagnosis of Acute Myocardial Infarction-Promises and Challenges. Diagnostics 2021, 11, 881. [Google Scholar] [CrossRef]
- Collinson, P. High sensitivity troponin, analytical advantages, clinical benefits and clinical challenges—An update. Clin. Biochem. 2021, 91, 1–8. [Google Scholar] [CrossRef]
- Garg, P.; Morris, P.; Fazlanie, A.L.; Vijayan, S.; Dancso, B.; Dastidar, A.G.; Plein, S.; Mueller, C.; Haaf, P. Cardiac biomarkers of acute coronary syndrome: From history to high-sensitivity cardiac troponin. Intern. Emerg. Med. 2017, 12, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Adamson, P.D.; Shah, A.S.V.; Anand, A.; Strachan, F.E.; Ferry, A.V.; Lee, K.K.; Berry, C.; Findlay, I.; Cruikshank, A.; et al. High-Sensitivity Cardiac Troponin and the Universal Definition of Myocardial Infarction. Circulation 2020, 141, 161–171. [Google Scholar] [CrossRef]
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 42, 1289–1367. [Google Scholar] [CrossRef]
- Sandoval, Y.; Thordsen, S.E.; Smith, S.W.; Schulz, K.M.; Murakami, M.M.; Pearce, L.A.; Apple, F.S. Cardiac troponin changes to distinguish type 1 and type 2 myocardial infarction and 180-day mortality risk. Eur. Heart J. Acute Cardiovasc. Care 2014, 3, 317–325. [Google Scholar] [CrossRef]
- Eggers, K.M.; Baron, T.; Gard, A.; Lindahl, B. Clinical and prognostic implications of high-sensitivity cardiac troponin T concentrations in type 2 non-ST elevation myocardial infarction. IJC Heart Vasc. 2022, 39, 100972. [Google Scholar] [CrossRef]
- Wereski, R.; Kimenai, D.M.; Taggart, C.; Doudesis, D.; Lee, K.K.; Lowry, M.T.H.; Bularga, A.; Lowe, D.J.; Fujisawa, T.; Apple, F.S.; et al. Cardiac Troponin Thresholds and Kinetics to Differentiate Myocardial Injury and Myocardial Infarction. Circulation 2021, 144, 528–538. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Qamar, A.; Wheeler, E.N.; Levine, A.L.; Misenheimer, J.A.; Cavender, M.A.; Stouffer, G.A.; Kaul, P. Impact of Type 2 Myocardial Infarction (MI) on Hospital-Level MI Outcomes: Implications for Quality and Public Reporting. J. Am. Heart Assoc. 2018, 7, e008661. [Google Scholar] [CrossRef]
- Nestelberger, T.; Boeddinghaus, J.; Lopez-Ayala, P.; Kaier, T.E.; Marber, M.; Gysin, V.; Koechlin, L.; Sanchez, A.Y.; Giménez, M.R.; Wussler, D.; et al. Cardiovascular Biomarkers in the Early Discrimination of Type 2 Myocardial Infarction. JAMA Cardiol. 2021, 6, 771–780. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Wettersten, N.; Patel, M.P.; Mueller, C.; Neath, S.X.; Christenson, R.H.; Morgenthaler, N.G.; McCord, J.; Nowak, R.M.; Vilke, G.M.; et al. Biomarkers Enhance Discrimination and Prognosis of Type 2 Myocardial Infarction. Circulation 2020, 142, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.W. The Role of Complement in Myocardial Infarction Reperfusion Injury: An Underappreciated Therapeutic Target. Front. Cell Dev. Biol. 2020, 8, 606407. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Upadhye, S.; Worster, A. Understanding receiver operating characteristic (ROC) curves. CJEM 2006, 8, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Sajic, T.; Liu, Y.; Aebersold, R. Using data-independent, high-resolution mass spectrometry in protein biomarker research: Perspectives and clinical applications. Proteom. Clin. Appl. 2015, 9, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.E.; Borchers, C.H. Mass spectrometry based biomarker discovery, verification, and validation–quality assurance and control of protein biomarker assays. Mol. Oncol. 2014, 8, 840–858. [Google Scholar] [CrossRef]
- Crutchfield, C.A.; Thomas, S.N.; Sokoll, L.J.; Chan, D.W. Advances in mass spectrometry-based clinical biomarker discovery. Clin. Proteom. 2016, 13, 1. [Google Scholar] [CrossRef]
- Gianazza, E.; Tremoli, E.; Banfi, C. The selected reaction monitoring/multiple reaction monitoring-based mass spectrometry approach for the accurate quantitation of proteins: Clinical applications in the cardiovascular diseases. Expert Rev. Proteom. 2014, 11, 771–788. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E.J.C.R. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of atherothrombosis: Mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Silvain, J.; Collet, J.P.; Nagaswami, C.; Beygui, F.; Edmondson, K.E.; Bellemain-Appaix, A.; Cayla, G.; Pena, A.; Brugier, D.; Barthelemy, O.; et al. Composition of coronary thrombus in acute myocardial infarction. J. Am. Coll. Cardiol. 2011, 57, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Nishi, K.; Chuang, V.T.G.; Maruyama, T.; Otagiri, M. Molecular aspects of human alpha-1 acid glycoprotein—structure and function. Acute Phase Proteins 2013, 139–162. [Google Scholar]
- Fournier, T.; Medjoubi-N, N.; Porquet, D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 2000, 1482, 157–171. [Google Scholar] [CrossRef]
- Siegel, R.; Fishbein, C.; Said, J.; Tokes, Z.; Shell, W. Localization of alpha-1 acid glycoproteins in human myocardium. Lab. Investig. 1985, 52, 107–112. [Google Scholar] [PubMed]
- Luo, Z.; Lei, H.; Sun, Y.; Liu, X.; Su, D.F. Orosomucoid, an acute response protein with multiple modulating activities. J. Physiol. Biochem. 2015, 71, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.; Kurose, K.; Koizumi, T.; Katori, N.; Sawada, J.; Matsumura, Y.; Saijo, N.; Yamamoto, N.; Tamura, T.; Okuda, H.; et al. Distal promoter regions are responsible for differential regulation of human orosomucoid-1 and -2 gene expression and acute phase responses. Biol. Pharm. Bull 2014, 37, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The immune system and the remodeling infarcted heart: Cell biological insights and therapeutic opportunities. J. Cardiovasc. Pharmacol. 2014, 63, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, S.; Basingab, F.; Alrahimi, J.; Alharbi, M.; Mufawwaz, A.; Alotaibi, K. The Role of Alpha-1-acid Glycoprotein 2 Protein and the Underlying Orosomucoid 2 Gene in Different Diseases. J. Pharm. Res. Int. 2022, 34, 15–29. [Google Scholar]
- Gulfo, J.; Castel, R.; Ledda, A.; Romero, M.d.M.; Esteve, M.; Grasa, M. Corticosteroid-Binding Globulin is expressed in the adrenal gland and its absence impairs corticosterone synthesis and secretion in a sex-dependent manner. Sci. Rep. 2019, 9, 14018. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, H.H.; Gebhart, V.M.; Hertel, K.; Jirikowski, G.F. Expression of corticosteroid-binding globulin CBG in the human heart. Horm. Metab. Res. 2015, 47, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.G.; Bagley, C.J.; Elder, P.A.; Bachmann, A.W.; Torpy, D.J. Plasma free cortisol fraction reflects levels of functioning corticosteroid-binding globulin. Clin. Chim. Acta 2005, 359, 189–194. [Google Scholar] [CrossRef]
- Busillo, J.M.; Cidlowski, J.A. The five Rs of glucocorticoid action during inflammation: Ready, reinforce, repress, resolve, and restore. Trends Endocrinol. Metab. 2013, 24, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Keenan, D.M.; Roelfsema, F.; Veldhuis, J.D. Endogenous ACTH concentration-dependent drive of pulsatile cortisol secretion in the human. Am. J. Physiol.-Endocrinol. Metab. 2004, 287, E652–E661. [Google Scholar] [CrossRef]
- Chan, W.L.; Carrell, R.W.; Zhou, A.; Read, R.J. How changes in affinity of corticosteroid-binding globulin modulate free cortisol concentration. J. Clin. Endocrinol. Metab. 2013, 98, 3315–3322. [Google Scholar] [CrossRef]
- Burford, N.G.; Webster, N.A.; Cruz-Topete, D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int. J. Mol. Sci. 2017, 18, 2150. [Google Scholar] [CrossRef]
- Mendel, C.M. The free hormone hypothesis: A physiologically based mathematical model. Endocr. Rev. 1989, 10, 232–274. [Google Scholar] [CrossRef]
- Hammond, G.L.; Smith, C.L.; Paterson, N.A.; Sibbald, W.J. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J. Clin. Endocrinol. Metab. 1990, 71, 34–39. [Google Scholar] [CrossRef]
- Perogamvros, I.; Ray, D.W.; Trainer, P.J. Regulation of cortisol bioavailability—Effects on hormone measurement and action. Nat. Rev. Endocrinol. 2012, 8, 717–727. [Google Scholar] [CrossRef]
- Hammond, G.L. Plasma steroid-binding proteins: Primary gatekeepers of steroid hormone action. J. Endocrinol. 2016, 230, R13–R25. [Google Scholar] [CrossRef] [PubMed]
- Zouaghi, H.; Savu, L.; Guerot, C.; Gryman, R.; Coulon, A.; Nunez, E.A. Total and unbound cortisol-, progesterone-, oestrone- and transcortin-binding activities in sera from patients with myocardial infarction: Evidence for differential responses of good and bad prognostic cases. Eur. J. Clin. Investig. 1985, 15, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.A.; Bodnar, T.S.; Weinberg, J.; Hammond, G.L. Corticosteroid-binding globulin is a biomarker of inflammation onset and severity in female rats. J. Endocrinol. 2016, 230, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.T.; Liu, Y.K.; Song, H.Y.; Dai, Z.; Qin, L.X.; Almofti, M.R.; Fang, C.Y.; Lu, H.J.; Yang, P.Y.; Tang, Z.Y. Heat-shock protein 27: A potential biomarker for hepatocellular carcinoma identified by serum proteome analysis. Proteomics 2005, 5, 4581–4588. [Google Scholar] [CrossRef]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Ogun, A.S.; Adeyinka, A. Biochemistry, Transferrin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Iqbal, M.P.; Mehboobali, N.; Tareen, A.K.; Yakub, M.; Iqbal, S.P.; Iqbal, K.; Haider, G. Association of body iron status with the risk of premature acute myocardial infarction in a Pakistani population. PLoS ONE 2013, 8, e67981. [Google Scholar] [CrossRef]
- Bi, Y.; Ajoolabady, A.; Demillard, L.J.; Yu, W.; Hilaire, M.L.; Zhang, Y.; Ren, J. Dysregulation of iron metabolism in cardiovascular diseases: From iron deficiency to iron overload. Biochem. Pharmacol. 2021, 190, 114661. [Google Scholar] [CrossRef]
- Paterek, A.; Mackiewicz, U.; Mączewski, M. Iron and the heart: A paradigm shift from systemic to cardiomyocyte abnormalities. J. Cell Physiol. 2019, 234, 21613–21629. [Google Scholar] [CrossRef]
- Day, S.M.; Duquaine, D.; Mundada, L.V.; Menon, R.G.; Khan, B.V.; Rajagopalan, S.; Fay, W.P. Chronic iron administration increases vascular oxidative stress and accelerates arterial thrombosis. Circulation 2003, 107, 2601–2606. [Google Scholar] [CrossRef]
- Liu, M.; Chen, M.; Hao, Z.; Li, Q.; Feng, Y.; Li, Y.; Li, R. Erythrocyte Fraction in Thrombi Is Increased with Serum Iron by Influencing Fibrin Networks via Oxidative Stress. Oxid. Med. Cell Longev. 2021, 2021, 3673313. [Google Scholar] [CrossRef]
- Fuentes, E.; Moore-Carrasco, R.; de Andrade Paes, A.M.; Trostchansky, A. Role of Platelet Activation and Oxidative Stress in the Evolution of Myocardial Infarction. J. Cardiovasc. Pharmacol. Ther. 2019, 24, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Mury, P.; Chirico, E.N.; Mura, M.; Millon, A.; Canet-Soulas, E.; Pialoux, V. Oxidative Stress and Inflammation, Key Targets of Atherosclerotic Plaque Progression and Vulnerability: Potential Impact of Physical Activity. Sport. Med. 2018, 48, 2725–2741. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, S.; Sarkisian, L.; Saaby, L.; Poulsen, T.S.; Gerke, O.; Hosbond, S.; Diederichsen, A.C.P.; Thygesen, K.; Mickley, H. Different Causes of Death in Patients with Myocardial Infarction Type 1, Type 2, and Myocardial Injury. Am. J. Med. 2018, 131, 548–554. [Google Scholar] [CrossRef]
- Wereski, R.; Kimenai, D.M.; Bularga, A.; Taggart, C.; Lowe, D.J.; Mills, N.L.; Chapman, A.R.; on behalf of the High, S.I. Risk factors for type 1 and type 2 myocardial infarction. Eur. Heart J. 2022, 43, 127–135. [Google Scholar] [CrossRef]
- Saaby, L.; Poulsen, T.S.; Hosbond, S.; Larsen, T.B.; Pyndt Diederichsen, A.C.; Hallas, J.; Thygesen, K.; Mickley, H. Classification of myocardial infarction: Frequency and features of type 2 myocardial infarction. Am. J. Med. 2013, 126, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Vaidya, S.R.; Arora, S.; Bahekar, A.; Devarapally, S.R. Type 2 versus type 1 myocardial infarction: A comparison of clinical characteristics and outcomes with a meta-analysis of observational studies. Cardiovasc. Diagn. Ther. 2017, 7, 348–358. [Google Scholar] [CrossRef]
| All (n = 60) | Discovery (n = 22) | Verification (n = 60) | |||||
|---|---|---|---|---|---|---|---|
| T1MI (n = 12) | Others a (n = 10) | p-Value | T1MI (n = 32) | Others a (n = 28) | p-Value | ||
| Age (year) | 73 (60.5, 80) | 69.6 ± 11.6 | 70.1 ± 17.5 | 0.935 | 74 (62.0, 79.8) | 72.5 (59, 80.8) | 0.805 |
| Male, n (%) | 37 (61.7) | 10 (83.3) | 4 (40) | 0.074 | 22 (68.8) | 15 (53.6) | 0.291 |
| BMI, kg/m2 | 23.5 (21.5, 26) | 23.1 ± 2.9 | 25.4 ± 2.6 | 0.044 | 23.4 (21.6, 25.6) | 24.2 (21.5, 26.5) | 0.800 |
| Systolic BP, mmHg | 130.4 ± 19.9 | 135.3 ± 14.4 | 133.5 ± 12.7 | 0.768 | 126.9 ± 18.1 | 134.4 ± 21.3 | 0.149 |
| Diastolic BP, mmHg | 78.5 (69, 89.8) | 78.5 (71.3, 88.8) | 79.5 (70, 84.8) | 0.936 | 74 (67.5, 88.5) | 80 (70, 90) | 0.394 |
| Risk Factors | |||||||
| Past smoker | 9 (15) | 2 (16.7) | 2 (20) | >0.999 | 4 (12.5) | 5 (17.9) | 0.721 |
| Current smoker | 13 (21.7) | 4 (33.3) | 0 (0) | 0.096 | 13 (40.6) | 4 (14.3) | 0.043 |
| Hypertension | 32 (53.3) | 5 (41.7) | 7 (70) | 0.231 | 16 (50.0) | 16 (57.1) | 0.613 |
| Hyperlipidemia | 19 (31.7) | 3 (25) | 5 (50) | 0.377 | 9 (28.1) | 10 (35.7) | 0.586 |
| Diabetes | 25 (41.7) | 3 (25) | 6 (60) | 0.192 | 11 (34.4) | 14 (50.0) | 0.296 |
| CAD family history | 6 (10) | 1 (8.3) | 1 (10) | >0.999 | 4 (12.5) | 2 (7.1) | 0.675 |
| Past Medical History | |||||||
| Previous myocardial infarction | 5 (8.3) | 1 (8.3) | 1 (10) | >0.999 | 3 (9.4) | 2 (7.1) | >0.999 |
| Previous heart failure | 19 (31.7) | 3 (25) | 2 (20) | >0.999 | 10 (31.3) | 9 (32.1) | >0.999 |
| Previous revascularization | 9 (15) | 2 (16.7) | 1 (10) | >0.999 | 6 (18.8) | 3 (10.7) | 0.192 |
| ECG | |||||||
| ST-depression | 15 (25) | 5 (41.7) | 0 (0) | 0.040 | 12 (37.5) | 3 (10.7) | 0.020 |
| ST elevation | 18 (30) | 6 (50) | 1 (10.0) | 0.074 | 14 (43.8) | 4 (14.3) | 0.007 |
| Nonspecific change | 27 (45) | 1 (8.3) | 9 (90.0) | 0.0003 | 6 (18.8) | 21 (75.0) | <0.0001 |
| Laboratory Findings | |||||||
| Hemoglobin, g/dL | 12.6 ± 2 | 13.19 ± 2 | 11.9 ± 2.1 | 0.186 | 12.9 ± 2 | 12.1 ± 2 | 0.115 |
| Peak troponin, ng/mL | 0.9 (0.3, 2.5) | 1.4 (0.5, 2.1) | 0.3 (0.2, 0.5) | 0.014 | 2.7 ± 2.6 | 1.90 ± 1.2 | 0.002 |
| CK-MB, ng/mL | 23 (7.7, 64.9) | 31.7 (10.8, 104.7) | 7.3 (5.0, 18.8) | 0.009 | 51.0 (10.7, 137.6) | 13.5 (5.6, 36.1) | 0.002 |
| CRP | 0.9 (0.3, 3.1) | 0.9 (0.2, 3.4) | 2.4 (0.2, 5.3) | 0.528 | 0.6 (0.3, 2.5) | 1.3 (0.7, 3.4) | 0.178 |
| Protein | Peptide | Transition | RT (min) | CE | DP |
|---|---|---|---|---|---|
| -1-acid glycoprotein 1 | YVGGQEHFAHLLILR | 877.0/235.3 | 7.3 | 45.0 | 74.6 |
| -1-acid glycoprotein 2 | EHVAHLLFLR | 617.9/267.1 | 6.3 | 76.2 | 35.1 |
| Apolipoprotein B-100 | GFEPTLEALFGK | 654.9/205.1 | 12.8 | 78.9 | 29.4 |
| Apolipoprotein C-I | TPDVSSALDK | 516.8/466.2 | 3.3 | 68.8 | 23.5 |
| Cathelicidin antimicrobial peptide | FALLGDFFR | 543.3/219.0 | 14.1 | 82.4 | 31.9 |
| Corticosteroid-binding globulin | GTWTQPFDLASTR | 740.4/906.5 | 8.5 | 85.1 | 37.5 |
| Fibronectin | WLPSSSPVTGYR | 675.4/525.7 | 5.7 | 80.3 | 30.2 |
| Prenylcysteine oxidase 1 | LFLSYDYAVK | 609.8/261.1 | 8.3 | 75.6 | 25.8 |
| Serum amyloid oxidase 1 | EALQGVGDMGR | 566.7/691.3 | 3.5 | 72.4 | 30.3 |
| Serotransferrin | EGYYGYTGAFR | 642.3/551.4 | 5.0 | 79.5 | 47.4 |
| CD40 ligand | SQFEGFVK | 471.2/249.2 | 4.6 | 77.8 | 41.3 |
| Matrix metallopeptidase 9 | AVIDDAFAR | 978.2/465.2 | 4.9 | 216.0 | 63.0 |
| Myeloperoxidase | IANVFTNAFR | 576.8/755.4 | 8.4 | 73.2 | 30.6 |
| Pregnancy-associated plasma protein A | EQVDFQHHQLAEAFK | 458.2/449.2 | 4.2 | 90.6 | 17.0 |
| Protein | Sensitivity | Specificity | Cut-off Value (nM a) |
|---|---|---|---|
| -1 acid glycoprotein 2 | 90.63 (75.8–96.8) | 89.3 (72.8–96.3) | <337.3 |
| Corticosteroid-binding globulin | 86.7 (70.3–94.7) | 85.7 (68.5–94.3) | <12.4 |
| Serotransferrin | 84.4 (68.3–93.1) | 85.7 (68.5–94.3) | <0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Park, S.-H.; Mun, S.; Lee, J.; Kang, H.-G. Potential Biomarkers to Distinguish Type 1 Myocardial Infarction in Troponin-Elevated Diseases. Int. J. Mol. Sci. 2023, 24, 8097. https://doi.org/10.3390/ijms24098097
Kwon S, Park S-H, Mun S, Lee J, Kang H-G. Potential Biomarkers to Distinguish Type 1 Myocardial Infarction in Troponin-Elevated Diseases. International Journal of Molecular Sciences. 2023; 24(9):8097. https://doi.org/10.3390/ijms24098097
Chicago/Turabian StyleKwon, Sohyen, Sang-Hyun Park, Sora Mun, Jiyeong Lee, and Hee-Gyoo Kang. 2023. "Potential Biomarkers to Distinguish Type 1 Myocardial Infarction in Troponin-Elevated Diseases" International Journal of Molecular Sciences 24, no. 9: 8097. https://doi.org/10.3390/ijms24098097
APA StyleKwon, S., Park, S.-H., Mun, S., Lee, J., & Kang, H.-G. (2023). Potential Biomarkers to Distinguish Type 1 Myocardial Infarction in Troponin-Elevated Diseases. International Journal of Molecular Sciences, 24(9), 8097. https://doi.org/10.3390/ijms24098097






