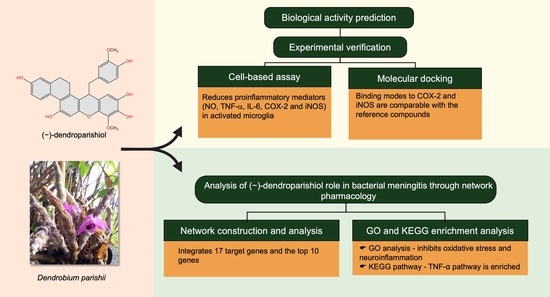
An Integrative Approach to Investigate the Mode of Action of (−)-Dendroparishiol in Bacterial Meningitis: Computer-Aided Estimation of Biological Activity and Network Pharmacology
Abstract
1. Introduction
2. Results and Discussion
2.1. Profiles of Estimated Biological Activity of (−)-Dendroparishiol
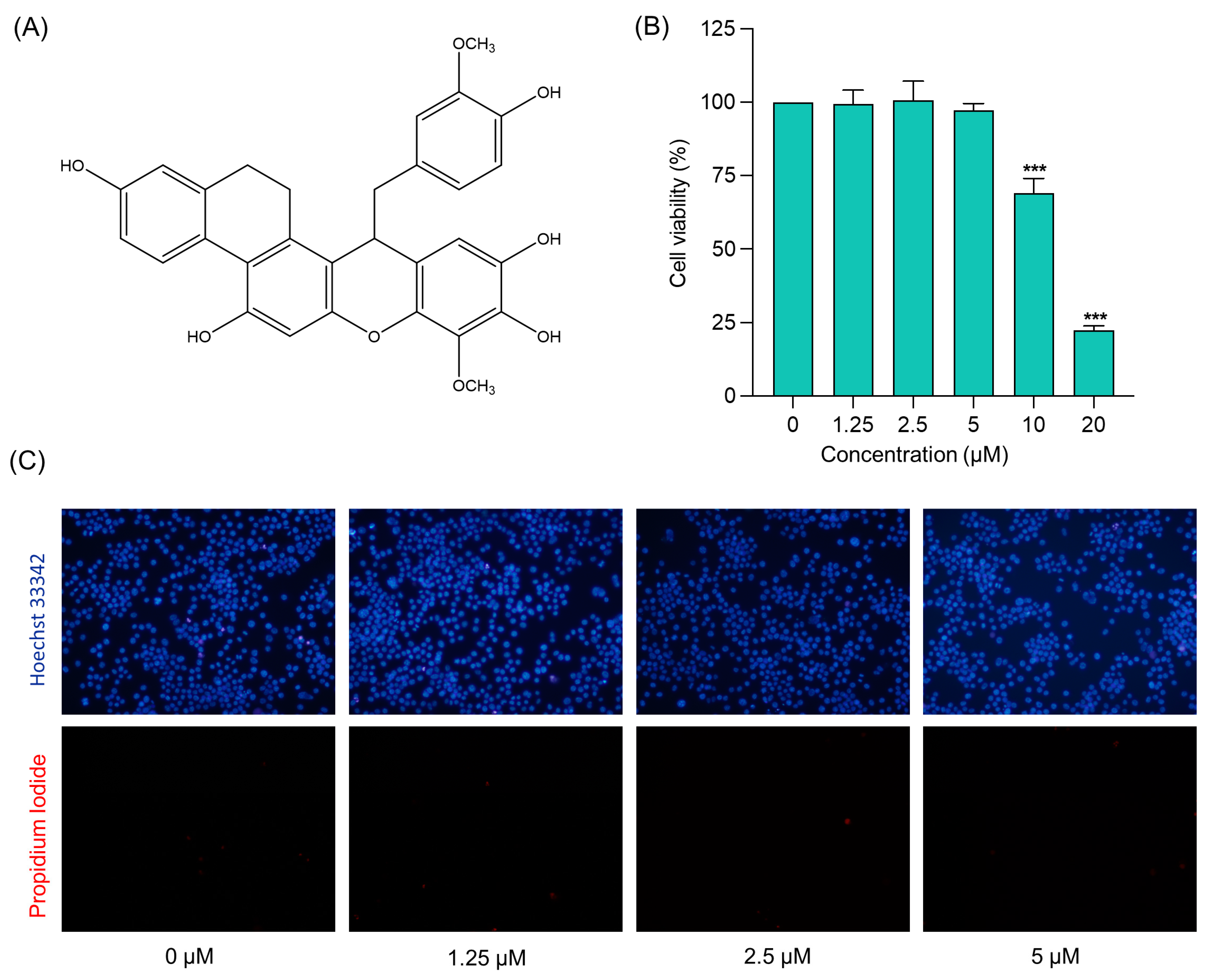
2.2. Cytotoxicity Profile of (−)-Dendroparishiol in BV-2 Microglial Cells
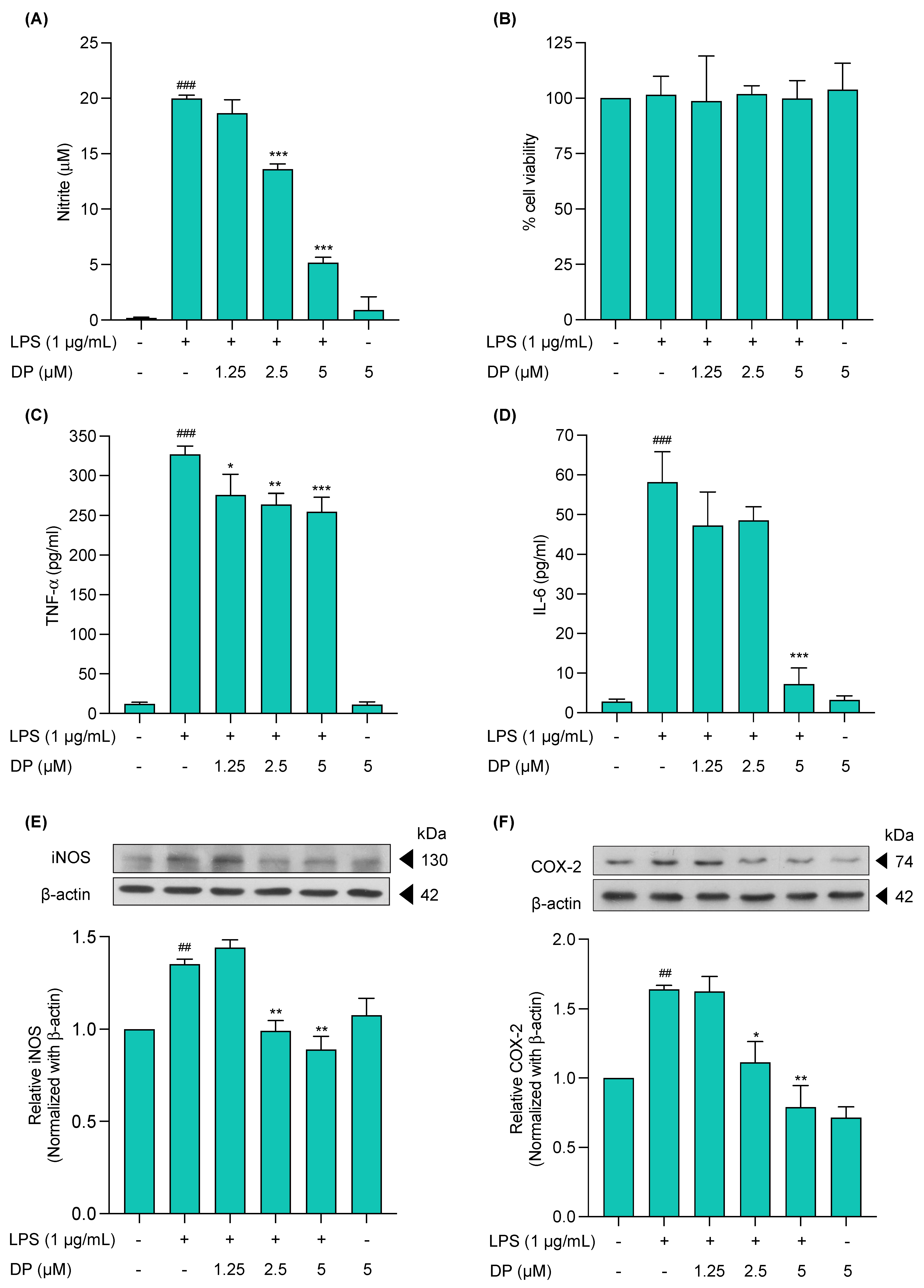
2.3. Effects of (−)-Dendroparishiol on Pro-Inflammatory Mediators in LPS-Stimulated BV-2 Cells
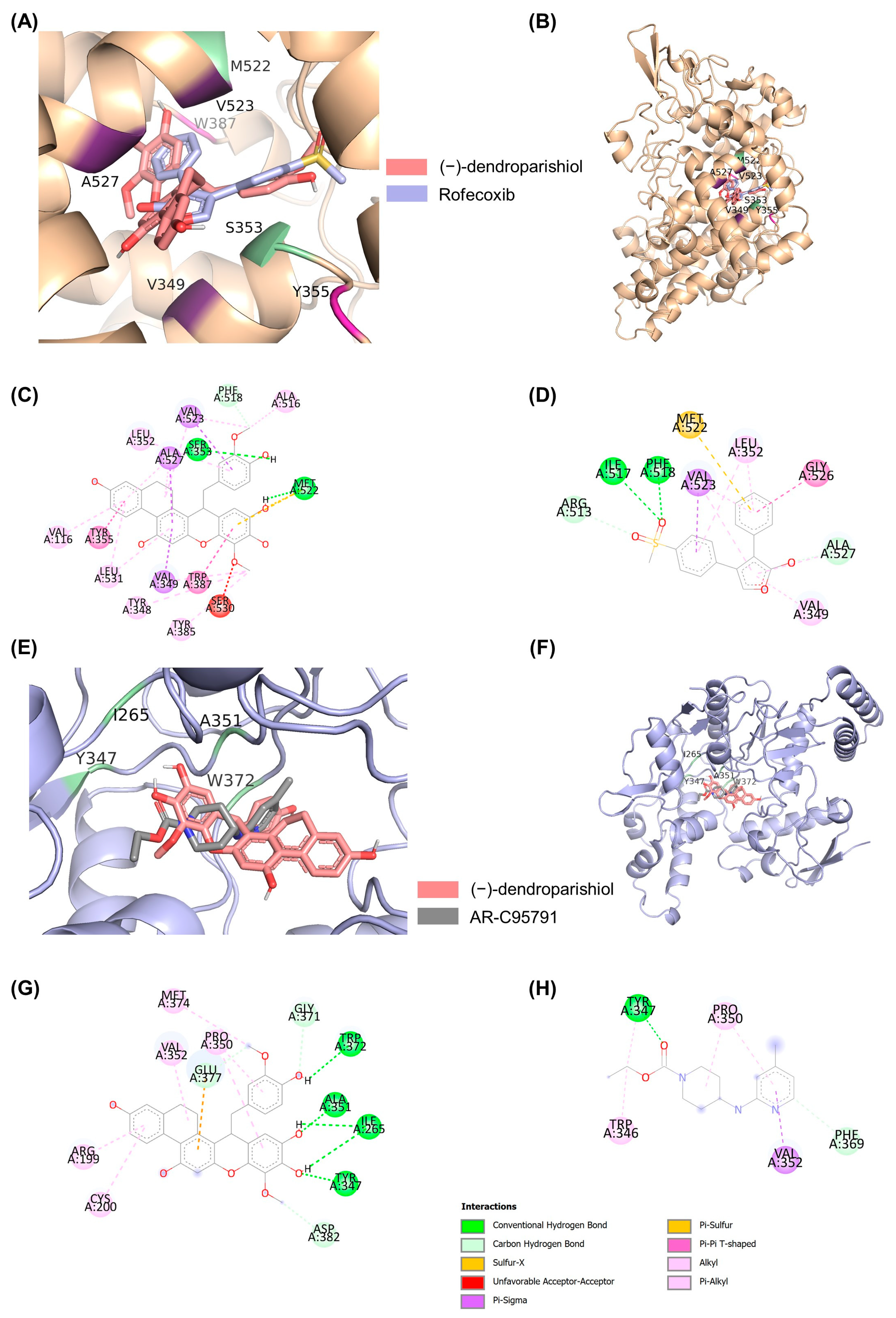
2.4. Molecular Docking to Determine the Interaction of (−)-Dendroparishiol with Proinflammatory Enzymes
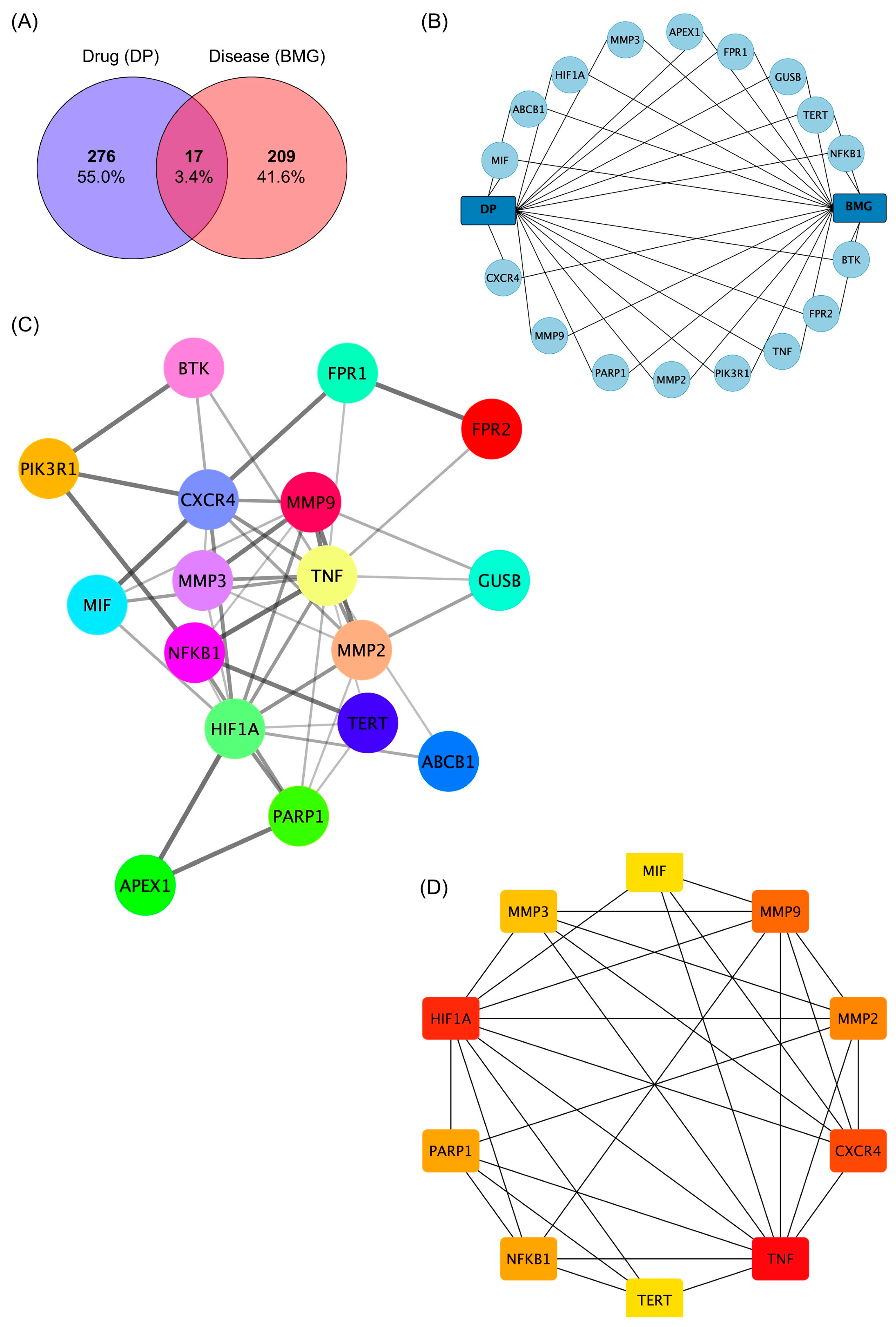
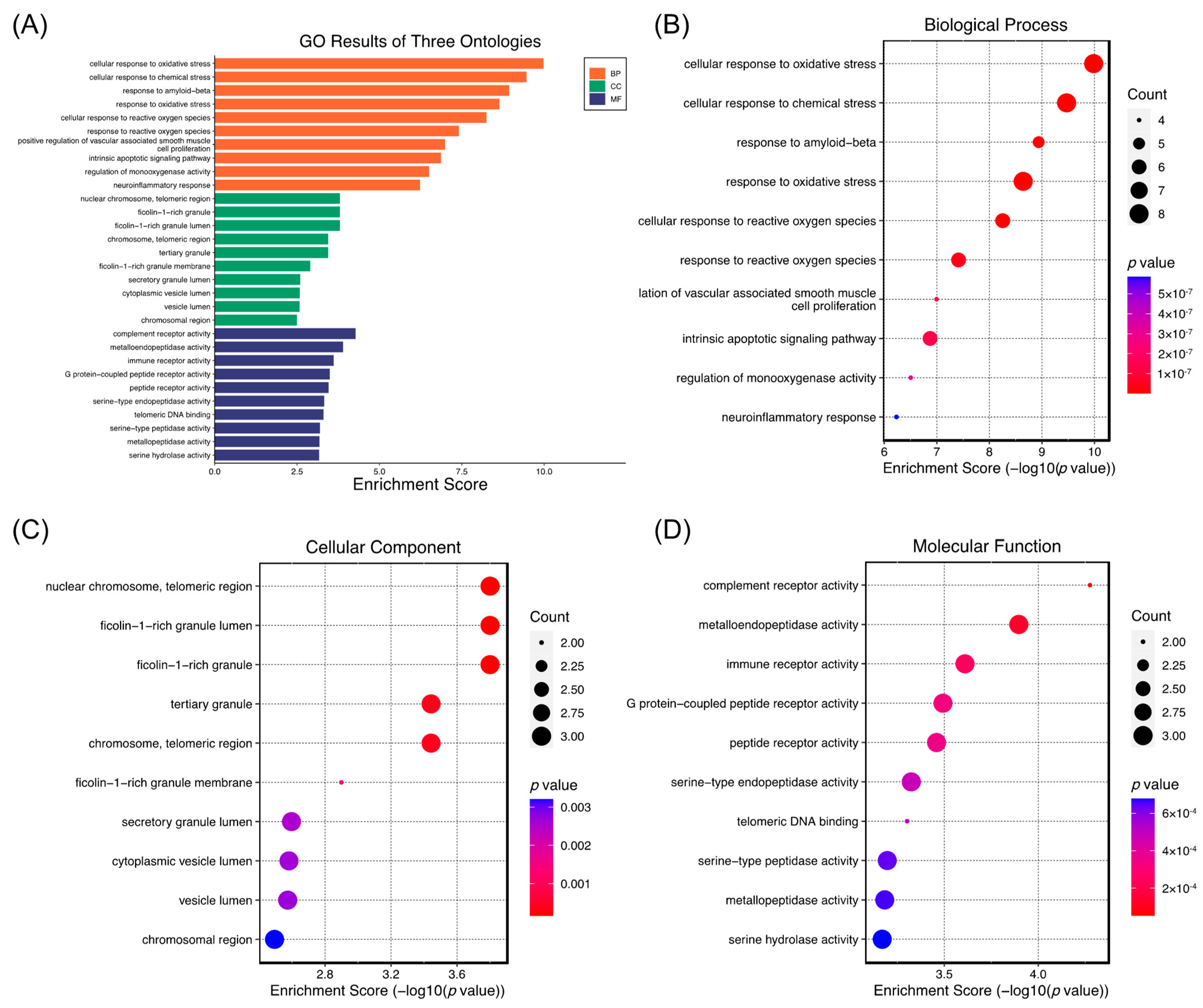
2.5. Network Pharmacology Analysis of the Role of (−)-Dendroparishiol in Bacterial Meningitis
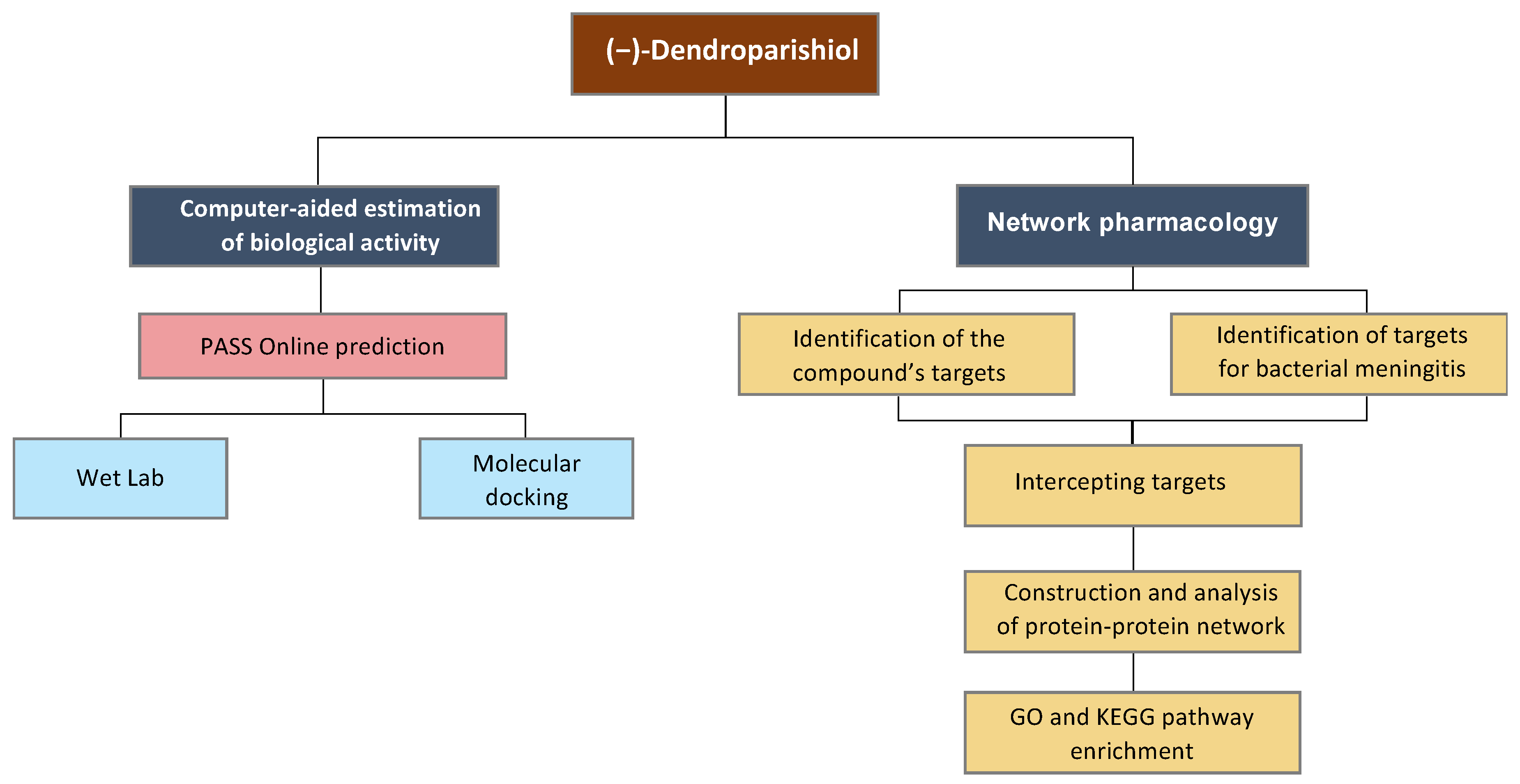
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Prediction of Biological Activity of (−)-Dendroparishiol
3.3. In Vitro Cell-Based Assay
3.3.1. Extraction and Isolation of (−)-Dendroparishiol from Dendrobium parishii
3.3.2. BV-2 Cell Culture
3.3.3. Evaluation of Cytotoxic Effects of (−)-Dendroparishiol
3.3.4. Hoechst 33342/PI Dual Staining Assay
3.3.5. Anti-Inflammatory Effects of (−)-Dendroparishiol on LPS-Stimulated BV-2 Cells
3.3.6. Nitrite Concentration Analysis Using Griess Reagent
3.3.7. Determination of IL-6 and TNF-α Levels Using ELISA
3.3.8. Western Blot Analysis
3.4. Molecular Docking
3.5. Network Pharmacology Analysis
3.5.1. Identification of (−)-Dendroparishiol-Target Genes
3.5.2. Identification of Bacterial Meningitis-Target Genes
3.5.3. Construction of Protein–Protein Interaction (PPI) Network
3.5.4. GO Enrichment and Pathway Analysis
3.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DP | Dendrobium parishii |
| NF-κB | Nuclear factor kappa |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin-6 |
| IL-1 | Interleukin-1 |
| TNF-α | Tumor necrosis factor alpha |
| NO | Nitric oxide |
| iNOS | Inducible nitric oxide synthase |
| COX-2 | Cyclooxygenase-2 |
| LPS | Lipopolysaccharide |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| CAT | Catalase |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MMPs | Matrix metalloproteinases |
| ROS | Reactive oxygen species |
| JAK2 | Janus kinase 2 |
| PGE-2 | Prostaglandin E2 |
| BMG | Bacterial meningitis |
| DMSO | Dimethyl sulfoxide |
| ELISA | Enzyme-linked immunosorbent assay |
| PPI | Protein–protein interactions |
| MCC | Maximal clique centrality |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| FDR | False discovery rate |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gels |
| TBST | Tris Buffered Saline with 0.1% Tween 20 |
| PDB | Protein data bank |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
| PBS | Phosphate buffered saline |
| PI | Propidium iodide |
| NED | N-1-Napthylenediamine dihydrochloride |
| BCA | Bicinchoninic acid |
References
- Van de Beek, D.; Brouwer, M.; Hasbun, R.; Koedel, U.; Whitney, C.G.; Wijdicks, E. Community-acquired bacterial meningitis. Nat. Rev. Dis. Prim. 2016, 2, 16074. [Google Scholar] [CrossRef] [PubMed]
- Farmen, K.; Tofiño-Vian, M.; Iovino, F. Neuronal damage and neuroinflammation, a bridge between bacterial meningitis and neurodegenerative diseases. Front. Cell. Neurosci. 2021, 15, 680858. [Google Scholar] [CrossRef] [PubMed]
- Agyeman, P.; Grandgirard, D.; Leib, S.L. Neuroinflammation in bacterial meningitis. In The Blood Brain Barrier and Inflammation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 213–252. [Google Scholar]
- Lucas, M.J.; Brouwer, M.C.; van de Beek, D. Neurological sequelae of bacterial meningitis. J. Infect. 2016, 73, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Portnoy, A.; Jit, M.; Lauer, J.; Blommaert, A.; Ozawa, S.; Stack, M.; Murray, J.; Hutubessy, R. Estimating costs of care for meningitis infections in low-and middle-income countries. Vaccine 2015, 33, A240–A247. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, K.S. Treatment of bacterial meningitis: An update. Expert Opin. Pharmacother. 2012, 13, 2189–2206. [Google Scholar] [CrossRef]
- Swartz, M.N. Bacterial meningitis—A view of the past 90 years. N. Engl. J. Med. 2004, 351, 1826–1828. [Google Scholar] [CrossRef]
- De Gans, J.; Van de Beek, D. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 2002, 347, 1549–1556. [Google Scholar] [CrossRef]
- Spreer, A.; Gerber, J.; Hanssen, M.; Schindler, S.; Hermann, C.; Lange, P.; Eiffert, H.; Nau, R. Dexamethasone increases hippocampal neuronal apoptosis in a rabbit model of Escherichia coli meningitis. Pediatr. Res. 2006, 60, 210–215. [Google Scholar] [CrossRef]
- Gu, C.; Zhang, X.; Wu, L.; Jiang, X.; Huang, L. Quality evaluation of Dendrobium based on ultra-performance liquid chromatography (UPLC) and chemometrics. J. Appl. Pharm. Sci. 2017, 7, 17–23. [Google Scholar] [CrossRef]
- Xu, J.; Han, Q.-B.; Li, S.-L.; Chen, X.-J.; Wang, X.-N.; Zhao, Z.-Z.; Chen, H.-B. Chemistry, bioactivity and quality control of Dendrobium, a commonly used tonic herb in traditional Chinese medicine. Phytochem. Rev. 2013, 12, 341–367. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, S.-Y.; Han, S.-B.; Uddin, G.M.; Kim, C.Y.; Lee, J.K. Anti-inflammatory effects of Dendrobium nobile derived phenanthrenes in LPS-stimulated murine macrophages. Arch. Pharm. Res. 2015, 38, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-M.; Jiang, H.; Zha, X.-Q.; Wu, D.-L.; Pan, L.-H.; Duan, J.; Liu, J.; Luo, J.-P. Anti-inflammatory bibenzyls from the stems of Dendrobium huoshanense via bioassay guided isolation. Nat. Prod. Res. 2020, 34, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, J.-H.; Kim, Y. Dendrobium moniliforme Stem Extract Inhibits Lipoteichoic Acid-Induced Inflammatory Responses by Upregulation of Heme Oxygenase-1. J. Microbiol. Biotechnol. 2018, 28, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Kongkatitham, V.; Muangnoi, C.; Kyokong, N.; Thaweesest, W.; Likhitwitayawuid, K.; Rojsitthisak, P.; Sritularak, B. Anti-oxidant and anti-inflammatory effects of new bibenzyl derivatives from Dendrobium parishii in hydrogen peroxide and lipopolysaccharide treated RAW264.7 cells. Phytochem. Lett. 2018, 24, 31–38. [Google Scholar] [CrossRef]
- Agamah, F.E.; Mazandu, G.K.; Hassan, R.; Bope, C.D.; Thomford, N.E.; Ghansah, A.; Chimusa, E.R. Computational/in silico methods in drug target and lead prediction. Brief. Bioinform. 2020, 21, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Martínez, F.D.; López-López, E.; Juárez-Mercado, K.E.; Medina-Franco, J.L. Computational drug design methods—Current and future perspectives. In Silico Drug Design; Academic Press: London, UK, 2019; pp. 19–44. [Google Scholar]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the Biological Activity Spectra of Organic Compounds Using the Pass Online Web Resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Y.; Dai, E.; Du, H.; Wang, L. Mechanism of action of celastrol against rheumatoid arthritis: A network pharmacology analysis. Int. Immunopharmacol. 2019, 74, 105725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lai, X.; Wang, X.; Yao, X.; Wang, W.; Li, S. Network pharmacology to explore the anti-inflammatory mechanism of Xuebijing in the treatment of sepsis. Phytomedicine 2021, 85, 153543. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wang, X.; Liu, Y.; Ye, J.; Liu, Q.; Li, Y.; Li, S. Integrating network pharmacology and metabolomics study on anti-rheumatic mechanisms and antagonistic effects against methotrexate-induced toxicity of Qing-Luo-Yin. Front. Pharmacol. 2018, 9, 1472. [Google Scholar] [CrossRef]
- Kim, K.S. Mechanisms of microbial traversal of the blood–brain barrier. Nat. Rev. Microbiol. 2008, 6, 625–634. [Google Scholar] [CrossRef]
- Rodríguez, A.M.; Rodríguez, J.; Giambartolomei, G.H. Microglia at the Crossroads of Pathogen-Induced Neuroinflammation. ASN Neuro 2022, 14, 17590914221104566. [Google Scholar] [CrossRef] [PubMed]
- Hasriadi; Dasuni Wasana, P.W.; Vajragupta, O.; Rojsitthisak, P.; Towiwat, P. Mechanistic insight into the effects of curcumin on neuroinflammation-driven chronic pain. Pharmaceuticals 2021, 14, 777. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Goularte, J.A.; Petronilho, F.; Saigal, P.; Badawy, M.; Quevedo, J. Role of microglial activation in the pathophysiology of bacterial meningitis. Mol. Neurobiol. 2016, 53, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 2004, 17, 942–964. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.-Y.; Liu, C.; Liu, Z.-Q.; Deng, Y.-F.; Hou, T.-J.; Cao, D.-S. Comprehensive assessment of nine target prediction web services: Which should we choose for target fishing? Brief. Bioinform. 2023, 24, bbad014. [Google Scholar] [CrossRef]
- Gallo, K.; Goede, A.; Preissner, R.; Gohlke, B.-O. SuperPred 3.0: Drug classification and target prediction—A machine learning approach. Nucleic Acids Res. 2022, 50, W726–W731. [Google Scholar] [CrossRef]
- Gerber, J.; Nau, R. Mechanisms of injury in bacterial meningitis. Curr. Opin. Neurol. 2010, 23, 312–318. [Google Scholar] [CrossRef]
- Barichello, T.; Dos Santos, I.; Savi, G.D.; Florentino, A.F.; Silvestre, C.; Comim, C.M.; Feier, G.; Sachs, D.; Teixeira, M.M.; Teixeira, A.L. Tumor necrosis factor alpha (TNF-α) levels in the brain and cerebrospinal fluid after meningitis induced by Streptococcus pneumoniae. Neurosci. Lett. 2009, 467, 217–219. [Google Scholar] [CrossRef]
- Meli, D.N.; Loeffler, J.M.; Baumann, P.; Neumann, U.; Buhl, T.; Leppert, D.; Leib, S.L. In pneumococcal meningitis a novel water-soluble inhibitor of matrix metalloproteinases and TNF-α converting enzyme attenuates seizures and injury of the cerebral cortex. J. Neuroimmunol. 2004, 151, 6–11. [Google Scholar] [CrossRef]
- Li, S. Network pharmacology evaluation method guidance-draft. World J. Tradit. Chin. Med. 2021, 7, 146. [Google Scholar] [CrossRef]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating protein pharmacology by ligand chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Günther, S.; Kuhn, M.; Dunkel, M.; Campillos, M.; Senger, C.; Petsalaki, E.; Ahmed, J.; Urdiales, E.G.; Gewiess, A.; Jensen, L.J. SuperTarget and Matador: Resources for exploring drug-target relationships. Nucleic Acids Res. 2007, 36, D919–D922. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]

| Pa | Pi | Activity |
|---|---|---|
| 0.806 | 0.008 | JAK2 expression inhibitor |
| 0.620 | 0.004 | NOS2 expression inhibitor |
| 0.437 | 0.041 | MMP9 expression inhibitor |
| 0.335 | 0.010 | Nitric oxide antagonist |
| 0.405 | 0.093 | Anti-inflammatory |
| 0.333 | 0.083 | TNF expression inhibitor |
| 0.285 | 0.048 | Non-steroidal anti-inflammatory agent |
| 0.213 | 0.024 | Transcription factor NF kappa B inhibitor |
| 0.187 | 0.020 | Lipoxygenase inhibitor |
| 0.172 | 0.014 | Cytokine release inhibitor |
| 0.175 | 0.040 | Leukotriene synthesis inhibitor |
| 0.145 | 0.055 | Interleukin 4 antagonist |
| 0.107 | 0.023 | 5-Lipoxygenase inhibitor |
| 0.210 | 0.129 | Phospholipase C inhibitor |
| 0.158 | 0.095 | Nitric oxide scavenger |
| 0.111 | 0.076 | Cyclooxygenase inhibitor |
| 0.076 | 0.072 | Cyclooxygenase 2 inhibitor |
| Protein Targets | Ligands | ∆G (kcal/mol) | Interacting Residues | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H-Bond | C-H Bond | Pi-Sulfur | Pi-Anion | Pi-Pi | Amide-Pi | Pi-Sigma | Hydrophobic | |||
| COX-2 | (−)-dendroparishiol | −11.24 | SER353 MET522 | PHE518 | MET522 | - | TYR355 TRP387 | - | VAL349 VAL523 ALA527 | VAL116 TYR348 LEU352 TYR385 ALA516 LEU531 |
Rofecoxib | −10.70 | ILE517 PHE518 | ARG513 ALA527 | MET522 | - | - | GLY526 | VAL523 | VAL349 LEU352 | |
| iNOS | (−)-dendroparishiol | −7.09 | ILE265 TYR347 ALA351 TRP372 | GLY371 GLU377 ASP382 | - | GLU377 | - | - | - | ARG199 CYS200 PRO350 VAL352 MET374 |
AR-C95791 | −6.62 | TYR347 | PHE369 | - | - | - | - | VAL352 | TRP346 PRO350 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limcharoen, T.; Dasuni Wasana, P.W.; Hasriadi; Angsuwattana, P.; Muangnoi, C.; Warinhomhoun, S.; Ongtanasup, T.; Sritularak, B.; Vajragupta, O.; Rojsitthisak, P.; et al. An Integrative Approach to Investigate the Mode of Action of (−)-Dendroparishiol in Bacterial Meningitis: Computer-Aided Estimation of Biological Activity and Network Pharmacology. Int. J. Mol. Sci. 2023, 24, 8072. https://doi.org/10.3390/ijms24098072
Limcharoen T, Dasuni Wasana PW, Hasriadi, Angsuwattana P, Muangnoi C, Warinhomhoun S, Ongtanasup T, Sritularak B, Vajragupta O, Rojsitthisak P, et al. An Integrative Approach to Investigate the Mode of Action of (−)-Dendroparishiol in Bacterial Meningitis: Computer-Aided Estimation of Biological Activity and Network Pharmacology. International Journal of Molecular Sciences. 2023; 24(9):8072. https://doi.org/10.3390/ijms24098072
Chicago/Turabian StyleLimcharoen, Thanchanok, Peththa Wadu Dasuni Wasana, Hasriadi, Pornpoom Angsuwattana, Chawanphat Muangnoi, Sakan Warinhomhoun, Tassanee Ongtanasup, Boonchoo Sritularak, Opa Vajragupta, Pornchai Rojsitthisak, and et al. 2023. "An Integrative Approach to Investigate the Mode of Action of (−)-Dendroparishiol in Bacterial Meningitis: Computer-Aided Estimation of Biological Activity and Network Pharmacology" International Journal of Molecular Sciences 24, no. 9: 8072. https://doi.org/10.3390/ijms24098072
APA StyleLimcharoen, T., Dasuni Wasana, P. W., Hasriadi, Angsuwattana, P., Muangnoi, C., Warinhomhoun, S., Ongtanasup, T., Sritularak, B., Vajragupta, O., Rojsitthisak, P., & Towiwat, P. (2023). An Integrative Approach to Investigate the Mode of Action of (−)-Dendroparishiol in Bacterial Meningitis: Computer-Aided Estimation of Biological Activity and Network Pharmacology. International Journal of Molecular Sciences, 24(9), 8072. https://doi.org/10.3390/ijms24098072